Abstract
Osteochondral defects involve both the articular cartilage and the underlying subchondral bone. If left untreated, they may lead to osteoarthritis. Advanced biomaterial-guided delivery of gene vectors has recently emerged as an attractive therapeutic concept for osteochondral repair. The goal of this review is to provide an overview of the variety of biomaterials employed as nonviral or viral gene carriers for osteochondral repair approaches both in vitro and in vivo, including hydrogels, solid scaffolds, and hybrid materials. The data show that a site-specific delivery of therapeutic gene vectors in the context of acellular or cellular strategies allows for a spatial and temporal control of osteochondral neotissue composition in vitro. In vivo, implantation of acellular hydrogels loaded with nonviral or viral vectors has been reported to significantly improve osteochondral repair in translational defect models. These advances support the concept of scaffold-mediated gene delivery for osteochondral repair.
1. Introduction
Articular cartilage, the gliding tissue covering the ends of all joints, has a reduced ability for repair [1]. Osteochondral defects are areas of joint damage that involve both the articular cartilage and the underlying subchondral bone (Figure 1). Such defects often result from an acute traumatic injury to the joint or are caused by an underlying disorder of the subchondral bone, for example osteochondritis dissecans (OCD) that secondarily affects the cartilage.
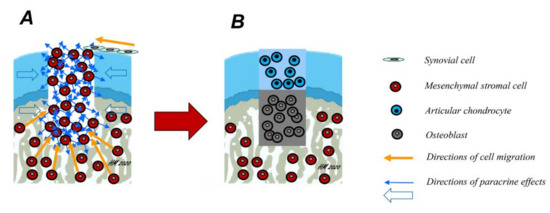
Figure 1.
Mechanisms of osteochondral repair. Osteochondral defects involve, by definition, both the articular cartilage and the subchondral bone. Spontaneously, they are mainly repaired by mesenchymal stromal cells (MSCs) arising from the subchondral bone marrow (A, yellow arrows). However, some cell migration into the defect from synovial cells is also possible. Over time, these cells differentiate either into chondrocytes or osteoblasts and deposit their extracellular matrix (ECM), depending on their location within the osteochondral defect, a mechanism possibly regulated in part by paracrine effects of the cells in the adjacent osteochondral unit (blue arrows). Ideally, a fibrocartilaginous repair tissue forms in the upper part of the defect (B), while the subchondral bone is repaired with new bone.
Surgical repair techniques for osteochondral defects focus on simultaneously restoring the subchondral bone and a cartilaginous repair tissue [2]. While the quality of (osteo)chondral repair is often regarded as a sole outcome and criterion for success, translational evidence shows that even small lesions can be the starting point of osteoarthritis (OA) development in the vicinity of the defect [3]. OA originating from such defects may encroach on formerly unaffected areas of the affected compartment and progressively involve the entire joint [3]. Such OA is challenging as it may be present long before arising to a clinically symptomatic state [4]. Long-term clinical evaluations attest to the high rate of OA in the case of untreated large osteochondral defects such as those occurring in OCD [5]. Surgical restoration of the osteochondral unit leads to good long-term outcomes in such cases [6,7]. However, many patients suffering from advanced OA may require total joint arthroplasty, a surgical end-stage treatment using implants that over time may pose problems such as loosening or infection. To avoid arthroplasty, especially in younger patients, much effort has been dedicated to the treatment of osteochondral defects at early stages to provide for long-term repair and prevent the development and progression of secondary OA.
Emerging treatments include cell-based and acellular, scaffold-based tissue engineering and gene therapy. The standard cell-based therapy to repair cartilage defects is the autologous chondrocyte implantation (ACI), with good long-term outcomes [8]. Mesenchymal stromal/stem cells (MSCs) from the bone marrow or from connective tissues like fat are being pursued as alternatives for cartilage repair and are applied via intra-articular administration in patients with knee OA. Early-phase clinical studies provide some promising insight into their efficacy, but the mechanisms of action involved remains unclear [9].
A potential advantage of gene therapy is the local delivery of gene sequences coding for therapeutic factors with a known ability to promote both cartilage and bone reparative processes. Herein, multiple growth factors have been identified as potent mediators to promote chondrogenesis, osteogenesis, and/or angiogenesis [10,11,12]. In recent years, the successful genetic modification of cells of the musculoskeletal system, among which articular chondrocytes and MSCs, either using viral or nonviral vectors, has been achieved to efficiently deliver therapeutic genes, enhancing their regenerative capacities [13,14]. Moreover, different scaffolds have been used to support the delivery of recombinant genes and gene combinations via gene transfer using both nonviral and viral vectors to target cells relevant of osteochondral tissue engineering and repair in vitro and in vivo. The development of such bioactive osteochondral implants that circumvent the need for ex vivo tissue generation allows for an in situ tissue engineering based on the active transgene product in vivo. The goal of the present article is to provide an overview of the current advances in scaffold-mediated gene delivery for osteochondral repair.
2. Candidate Genes for Osteochondral Repair
Polypeptide growth factors including the transforming growth factor beta (TGF-β) [15,16,17], the insulin-like growth factor I (IGF-I) [18,19], and the basic fibroblast growth factor (FGF-2) [20,21] play important roles in bone and cartilage repair by modulating ossification and enhancing the expression of cartilage major ECM components (type-II collagen, aggrecan). Due to their potent osteogenic effects, the members from the bone morphogenetic protein (BMP) superfamily, and particularly BMP-2, have been applied to trigger osteogenesis and mineralization leading to the expression of bone-related markers (osteopontin, osteocalcin, alkaline phosphatase) [22]. The BMP-2 isoform [15], as well as BMP-7 [23], also plays important roles in chondrogenesis by stimulating cell differentiation and the production of the cartilage ECM. In addition, angiogenic factors such as the vascular endothelial growth factor (VEGF) [24,25] and the platelet-derived growth factor (PDGF) [26,27] have also been described to promote successful bone and cartilage healing via the induction of osteochondral progenitors proliferation and proteoglycan deposition. Transcription factors as the cartilage-specific sex determining region Y-boxes (SOX) 5, 6, and 9 (SOX5, SOX6, and SOX9, or SOX trio) [28] are also potential candidates for osteochondral repair. These factors are critically involved in the formation and maintenance of the cartilage by activating the expression of major matrix components. Other factors include the bone-specific Cbfa-1/runt-related transcription factor 2 (RUNX2) [29] that modulates osteoblast differentiation with endochondral and membranous ossification, and osterix (OSX) that is required for bone maintenance in synergism with RUNX2 [30]. More recently, the use of messenger ribonucleic acids (mRNAs) for these various factors has been evoked as tools for therapy, being potentially amenable to scaffold-mediated delivery in particular for strategies that aim at initiating osteochondral repair [31,32,33,34]. Figure 2 presents an overview of the pathways targeted by these various candidates.
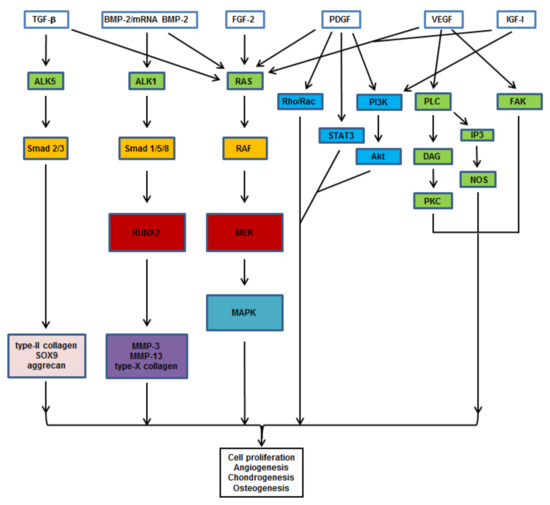
Figure 2.
Signaling pathways triggered by therapeutic candidates for osteochondral repair. TGF-β promotes chondrogenesis by activating the PI3K, Smad 2/3, and RhoA pathways. FGF-2, BMP-2, and mRNA BMP-2 induce osteo-/chondrogenesis via the RAS/RAF/MEK/MAPK and Smad pathways. PDGF activates angiogenesis via crosstalks between the MAPK, Rho/Rac, STAT3, and PI3K pathways. IGF-I induces the MAPK and PI3K pathways. VEGF induces angiogenesis by activating the PLC, IP3, and FAK pathways. Abbreviations: TGF-β: transforming growth factor beta; BMP-2: bone morphogenetic protein 2; mRNA: messenger ribonucleic acid; FGF-2: basic fibroblast growth factor; PDGF: platelet-derived growth factor; VEGF: vascular endothelial growth factor; IGF-I: insulin-like growth factor I; ALK: activin receptor-like kinase; RAS: Rat sarcoma; RhoA: Ras homolog gene family, member A; Rac: Ras-related C3 botulinum toxin substrate; PI3K: phosphatidylinositol-4,5-bisphosphate 3-kinase; PLC: phosphoinositide phospholipase C; FAK: focal adhesion kinase; STAT3: signal transducer and activator of transcription 3; IP3: inositol 1,4,5-trisphosphate; Smad: suppressor of mothers against decapentaplegic; RAF: rapidly accelerated fibrosarcoma; Akt/PKB: protein kinase B; DAG: diacylglycerol; NOS: nitric oxide synthase; PKC: protein kinase C; RUNX2: Cbfa-1/runt-related transcription factor 2; MEK: mitogen-activated protein kinase kinase; MAPK: MAPK: mitogen-activated protein kinase; SOX9: sex determining region Y-box 9; MMP: matrix metalloproteinase.
4. Viral Vectors
Recombinant viral vectors are divided into different groups according to the original type of virus they are based upon: adenovirus, retrovirus, baculovirus, and adeno-associated virus (AAV) [49] (Table 1).
4.1. Adenoviral Vectors
Among the viral systems employed for gene therapy, adenoviruses have been used often because of their high transduction efficiencies and transgene expression in various types of cells, potentially important for in vivo approaches. More than 50 adenovirus serotypes are available for gene therapy approaches. Adenovirus serotype 5 (Ad5) has been briefly used in both in vitro and in vivo studies. Adenoviral vectors have been used to transfer growth factor genes (TGF-β, FGF-2, IGF-I, BMPs, and the growth and differentiation factor 5—GDF-5) into cells of the musculoskeletal system [50,51,52,53,54,55,56,57,58,59]. Direct delivery via adenoviral-mediated transduction of IGF-I in synovial tissue in the metacarpophalangeal joints of horses [60] and of BMP-2 injected directly in osteochondral defects in vivo [61], or together with a decalcified cortical bone matrix as scaffold containing the vector particles has been achieved [62]. However, the major challenges limiting the success of adenoviral approaches are the considerable immune responses [63] particularly with a view towards a clinical applications.
4.2. Retroviral Vectors
Retroviruses have the advantage of integrating their DNA into the host genome, allowing them to maintain gene expression for longer periods of time [64]. In comparison to the adenoviral vectors, fewer studies employed retroviral vectors for the delivery of growth factors, such as TGF-β, SOXs, BMPs, or VEGF inhibitors, both in vitro and in vivo [65,66,67,68]. The main problem of this kind of vector is the risk of insertional mutagenesis and the potential activation of oncogenes. In addition, retroviral vectors transduce only dividing cells with a restricted host range and low efficacy.
4.3. Baculoviral Vectors
Baculoviruses show no pathogenicity toward humans and can be used under biosafety level 2 conditions. Baculoviral vectors, like adenoviral vectors, have been shown to transduce both dividing and non-dividing mammalian cells, including articular chondrocytes and adipose-derived MSCs with TGF-β [69] and BMPs [70] in vitro and in vivo. However, baculoviral vectors are not able to replicate and do not integrate their DNA into the chromosomes of transduced mammalian cells, resulting in a transient transgene expression of less than 1 week. For these reasons, baculoviral vectors have attracted less research interests and their clinical application is not permitted.
4.4. Recombinant Adeno-Associated Viral (rAAV) Vectors
AAV is a small, non-pathogenic human parvovirus that is defective for replication. It has been genetically manipulated to form recombinant particles that lack all viral sequences and contain instead a transgene cassette. This feature therefore makes rAAV much less immunogenic than adenoviral gene vehicles that are not fully devoid of viral coding sequences. AAV are generally kept as stable episomes in the target cells, which allows them to support the long-term expression of the transgenes they carry (months to years). This characteristic further prevents the activation of oncogenes upon insertional mutagenesis as noted when using integrative retroviral vectors. rAAV target dividing and also non-dividing cells both at very high efficiencies, which enables direct gene transfer protocols in vivo. For cells that remain refractory to rAAV gene transfer, research has been developed to replace conventional rAAV vectors by pseudotyped, chimeric, hybrid, and self-complementary (scAAV) constructs to overcome the slow viral genome conversion from single-stranded to double-stranded DNA. Finally, the relatively limited gene delivery ability of rAAV (~4.7 kb) has been tackled by using the aptitude of the virus to form concatemers. Therefore, rAAV became a preferred gene transfer system for cartilage and osteochondral repair in vivo [21,53,71,72,73].
5. Scaffolds for Osteochondral Repair
The ideal scaffold for osteochondral repair is biocompatible, biodegradable and provides a three-dimensional (3D) environment, mimicking structural and biological cues of the native osteochondral unit, aiming to support both cartilaginous and subchondral bone repair in a bioinspired spatio-temporal fashion. Due to the differences in the mechanical properties and biological structure of the articular cartilage and the subchondral bone, the design of scaffolds for osteochondral repair needs to fulfill the requirements of both tissues [74]. In this scenario, the development of composite scaffolds is centered on the design of biomaterials with adequate mechanical properties to support subchondral bone restoration, while maintaining a comparably weaker structure allowing for cartilaginous repair [75,76]. Such composites may comprise bilayer and multilayer scaffolds, and also continuous gradient scaffolds [77].
Initially, the scaffold should provide a biomechanically strong support, with an adapted porous structure to permit cellular activities, together with an appropriate in vivo degradation rate that is in parallel to the ECM deposition. Combination of biomaterials with advanced technologies [78,79,80,81,82,83] has allowed for developing a new generation of 3D scaffolds with adapted features for cartilage and bone repair [84]. The technique of 3D printing has been also applied to generate osteochondral constructs that may be tailored in the future to match the often irregular osteochondral defects. A recent study engineered biphasic osteochondral constructs from 3D-printed fiber networks that mechanically reinforces alginate hydrogels whilst simultaneously supporting MSC chondrogenesis [83].
A variety of biomaterials have been employed in osteochondral tissue engineering, including hydrogels [34,82], solid scaffolds [85], and hybrid scaffolds [76]. Each are made of either natural cell-compatible materials or of synthetic compounds with more controllable properties as mono- or multiphasic systems (Table 2). While cells or gene vectors can be encapsulated in the 3D hydrogels, they are usually attached to the porous structures of solid scaffolds. Hydrogels are well adapted for cartilage repair as they have high water contents mimicking cartilage-based ECM glycosaminoglycans [86] and biocompatibility, often with lower mechanical properties compared with solid scaffolds [87,88]. Natural polymer biomaterials such as collagen [89], alginate [90], gellan gum [91], or silk fibroin [92] have been studied as scaffolds for cartilage repair due to their biocompatible nature promoting proliferation and differentiation of the encapsulated cells which makes them promising systems in various tissue engineering approaches [74,92,93]. Solid scaffolds are highly porous structures, allowing for migration and infiltration of cells from the surrounding tissue. They may originate from natural polymers such as poly-glycolic acid (PGA), poly (L, D-lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), polyurethane (PU), and polyethylene terephthalate. Synthetic biomaterials synthetic as PGA and their poly(lactide-co-glycolide) copolymers (PLGA) exhibit more reproducible physical and chemical properties but their degradation by-products may be toxic [94]. Inorganic materials like hydroxyapatite (HAp) have been mostly used in bone regeneration approaches, due to its exceptional mechanical stiffness and osteoinductivity [74,76]. Metallic scaffolds based on tantalum or titanium are also used as subchondral bone substitutes alone or in combination with other biomaterials in composite scaffolds [95,96,97]. Their lack of degradation and the possibility of releasing corrosion products are concerns to be considered [74]. Finally, a variety of hybrid scaffolds based on solid scaffolds and hydrogels has been prepared, for example by combining fibrin hydrogels with solid PU scaffolds.

Table 2.
Properties of scaffolds used for osteochondral repair.
Noteworthily, biomaterial scaffolds may have a significant impact on immune responses and foreign body reactions due to their physical, chemical, and biological properties. Herein, both the form of biomaterial (hydrogel, solid matrix, or micro/nanoparticles), degradation profile, level of crosslinking, hydrophobicity, topography, ad biomaterial origin (natural versus synthetic) are important parameters to consider when designing an ideal scaffold for osteochondral repair [98].
Scaffold-guided gene transfer for the goal of cartilage repair (Figure 3) has been attempted using hydrogels and polymeric micellar systems which are able to condense DNA by forming a polyplex micelle through polyion complex formation [99,100], similarly to poloxamer PF68 and poloxamine T908 polymeric micelles, alginate-, self-assembling RAD16-I peptides- or polypseudorotaxane gels [101], and PU scaffolds carrying rAAV vectors [102]. Such systems were employed to overexpress TGF-β [103], an interleukin-1(IL-1) receptor antagonist (IL-1Ra), and SOX9 [102,103] as a means to safely target human MSCs (hMSCs) [102,103] and enhance their potential for chondrogenesis and immunomodulation [103], applied to experimental models of cartilage defects in situ for biological joint resurfacing. Moreover, mechanical loading of these structures showed to be an advantageous strategy to promote the formation of an ECM cartilage-like tissue [102].
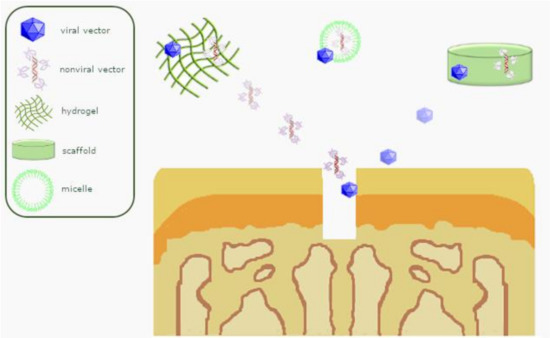
Figure 3.
Overview of scaffold-guided gene transfer mechanisms for osteochondral repair.
8. Scaffold-Mediated Viral In Vitro Gene Delivery for Osteochondral Repair
Many studies immobilize active viral vectors to solid scaffolds, for example by using PLL, creating a biomechanically functional scaffold system (Table 4). Guilak et al generated a self-contained bioactive scaffold capable of mediating stem cell differentiation and formation of a cartilaginous ECM by immobilizing lentiviral vectors on woven PCL scaffolds, an FDA-approved biocompatible aliphatic polyester [138,139]. Such scaffold-mediated gene delivery of TGF-β3 induced robust cartilaginous ECM formation by hMSCs and was as effective as traditional differentiation protocols involving medium supplementation with TGF-β3 protein [140]. A doxycycline-inducible lentiviral vector was capable to transduce MSCs in monolayer or in a 3D arrangement within woven PCL scaffolds to enable a tunable IL-1Ra production as an anti-inflammatory actor. In the presence of IL-1, the IL-1Ra-overexpressing engineered cartilage produced cartilage-specific ECM, while resisting the IL-1-induced upregulation of matrix metalloproteinase (MMP) and, at the same time, maintaining mechanical properties similar to native articular cartilage. In a continuation from this study, the same group engineered functional cartilage anatomically shaped scaffolds capable of inducible and tunable expression of IL-1Ra. Thus, 3D hemispherical scaffolds based on woven PCL fibers were fabricated and seeded with human adipose-derived stromal cells (ASCs). Doxycycline (dox)-inducible lentiviral vectors encoding for eGFP or IL-1Ra transgenes were then immobilized into the PCL scaffolds and constructs were cultured in chondrogenic medium for 28 days. Constructs showed uniform tissue growth and adapted cartilage properties while maintaining their anatomic architecture throughout culture. IL-1Ra-overexpressing constructs produced IL-1Ra (~1 μg/mL) as a result of dox-controlled induction. Likewise, a significant increase of MMP activity was observed in the conditioned media of eGFP-expressing constructs upon IL-1 treatment, but not in IL-1Ra-overexpressing constructs [141]. Chondrogenesis in PCL scaffolds was also induced in MSCs from human bone marrow by rAAV vector gene transfer of SOX9 upon. Prolonged, effective SOX9 expression was reported in the constructs for at least 3 weeks in vitro, leading to enhanced chondrogenic activities by deposition of proteoglycans and increased type-II collagen content in the cells without affecting it proliferative activities. These findings reveal the therapeutic potential of providing rAAV-modified marrow concentrates within 3D-woven PCL scaffolds [142]. Among the large variety of solid scaffolds available for cartilage repair [143], PCL scaffolds present significant advantages as the surface of this low immunogenic, biodegradable compound can be grafted with poly(sodium styrene sulfonate) (pNaSS), a bioactive polymer that facilitates protein adsorption and stimulates reparative cellular responses (adhesion, proliferation) [144]. Overexpression of sox9 in human bone marrow aspirates via rAAV vectors delivered by PCL films functionalized via grafting with pNaSS increased chondrogenic differentiation activities in the aspirates while containing premature osteogenesis and hypertrophy without impacting cell proliferation, with more potent effects noted when using pNaSS-grafted films in vitro [145]. Another study investigated the combined effect of complex mechanical stimulation and adenoviral-mediated overexpression of BMP-2 on hMSC chondrogenesis. Human MSCs transduced with Ad.BMP-2 were encapsulated in a fibrin hydrogel seeded into biodegradable PU scaffolds were stimulated mechanically for 7 or 28 days in chondrogenic medium without growth factors to mimics an in vivo environment, while controls cells were left un-transduced [146]. Transduction with Ad.BMP-2 led to a notable expression of the chondrogenic genes aggrecan and Sox9 upon mechanical stimulation. Besides, the glycosaminoglycans (GAGs)/DNA ratios were reduced following BMP-2 overexpression upon mechanical stimulation [146].

Table 4.
Scaffold-mediated viral gene delivery.
Cartilage-derived matrix (CDM) is another interesting scaffold material due to its chondroinductive capacity and its ability to support endochondral ossification. A recent study aimed to engineer anatomically-shaped cartilage and bone CDM constructs with the ability to inhibit inflammatory processes. Controlled induction of IL-1Ra expression following scaffold-mediated lentiviral gene delivery protected CDM hemispheres from inflammation-mediated degradation, and supported robust bone and cartilage tissue formation even in the presence of IL-1. Moreover, concentric CDM hemispheres resembling the femoral head overexpressing the chondrogenic TGF-β3, or the osteogenic BMP-2 transgenes could be fused into a single bi-layered osteochondral construct [147].
Using hydrogels as biomaterials, controlled delivery via polymeric micelles of rAAV vectors enhanced their temporal and spatial presentation into their targets. Delivering of rAAV vectors via PEO and PPO (poloxamer and poloxamine) polymeric micelles as a means to overexpress TGF-β in human OA chondrocytes resulted in increased proteoglycan deposition and higher cell numbers, thus providing potential tools to remodel human OA cartilage [148]. Another study examined whether a fibrin PU hybrid scaffold provides a favorable environment for the effective chondrogenic differentiation of hMSCs overexpressing SOX9 via rAAV-mediated gene transfer when cultured in rotating bioreactors in vitro. hMSCs could be modified via rAAV to overexpress SOX9 over an extended period within these scaffolds, leading to an improved cell chondrogenic differentiation in such a hydrodynamic environment relative to control (reporter lacZ) vector treatment [112].
9. Scaffold-Mediated Viral In Vivo Gene Delivery for Osteochondral Repair
Although several groups have applied transduced cells for musculoskeletal repair in solid [54,69,149,150] or hydrogel scaffolds for cartilage [59] and bone repair [149], comparably few have used viral vectors immobilized to scaffolds without ex vivo transduced cells for in vivo applications (Table 4). So far, only one published study performed a biomaterial-guided in vivo delivery of a gene vector in an orthotopic large animal model of osteochondral repair. In this study, a thermosensitive hydrogel based on PEO-PPO-PEO poloxamers, capable of controlled release of a rAAV vector overexpressing SOX9 was applied to a full-thickness chondral defect treated with microfracture in a minipig model. PEO-PPO-PEO (PF127) hydrogels carrying either the candidate rAAV-FLAG-hsox9 vector (sox9/hydrogel) or a control rAAV-lacZ vector (lacZ/hydrogel) were directly applied into defects treated with microfracture. Four weeks postoperatively, the individual histological scoring parameters “integration”, “cellular morphology”, “matrix staining” and the total cartilage repair score were significantly improved following the sox9/hydrogel application relative to all other groups, together with an increased deposition of type-II collagen in the sox9/hydrogel versus lacZ/hydrogel defects or when applying the lacZ vector in a hydrogel-free form. Next, the apparent absence of an immune response in all defects (lack of expression of CD3 for T-lymphocytes, of CD11b for activated macrophages, and of human leukocyte antigen isotype DR alpha—HLA-DRα—for class II major histocompatibility complex—MHC—antigens) supported the use of such PEO-PPO-PEO poloxamers to protect rAAV-mediated gene transfer from neutralization by antibodies directed against the AAV capsid. Although not directly applied to an osteochondral defect model, this study showed by a comprehensive analyses of the entire osteochondral unit that rAAV-FLAG-hsox9/PEO-PPO-PEO hydrogel-augmented microfracture significantly improves osteochondral repair [151].
10. Clinical Scaffolds for Osteochondral Repair
No scaffold is currently in routine clinical use that is capable of delivering a gene vector to sites of osteochondral damage. Also, entry in clinical trials to treat osteochondral defects has been granted to only a few scaffolds so far, among which are a nanocomposite three-layered collagen-HAp scaffold, a PLGA-calcium-sulfate bilayer scaffold, and an aragonite-based scaffold [152]. Clinical results were either not satisfying, or limited to a few reported case series, necessitating more high-level studies with longer follow-up [152].
In contrast, a variety of scaffolds are in clinical development to deliver articular chondrocytes in the context of ACI. These scaffolds can also be used either with or without cells to cover an osteochondral defect when the bony part of the defect is filled with a bone graft or bone substitutes. Classically, solid scaffolds are used in such clinical situations. They are composed of materials such as type-I/III collagen (MACI™, Novocart 3D™), HA (Hyalograft® C) and PGA, polylactic acid (PLA), and polydioxanone (BioSeed C™). More recently, hydrogels have emerged as a viable alternative, among them type-I collagen (atelocollagen) hydrogels (Koken Atelocollagen Implant), HA (CARTISTEM™) hydrogels, albumin and HA hydrogels (Novocart Inject™), fibrin (Chondron™), and agarose and alginate hydrogels (Cartipatch™). Currently, not all commercial products are available for clinical use. While these scaffolds have been used largely to deliver articular chondrocytes, they may also be used alone as a cell-free approach.
11. Conclusions
A variety of biomaterials have been employed as nonviral or viral gene carriers for steochondral repair in vitro, including hydrogels, solid scaffolds and hybrid scaffolds, supporting the concept of advanced biomaterial-guided delivery of gene carriers as an attractive therapeutic option for osteochondral repair in vivo. Such biomaterial-mediated gene therapy provides both a template for endogenous cell migration, infiltration and tissue formation while simultaneously promoting overexpression of therapeutic proteins in a sustained and locally determined fashion [76]. As demonstrated, a site-specific delivery of inducible transgenes confers spatial and temporal control over both scaffold remodeling and osteochondral neotissue composition [147]. Of note, a combined gene delivery approach may also provide immunomodulatory properties that allow for chondrogenesis in the presence of pathologic levels of degenerative factors among which IL-1, a critical step that may enhance the long-term success of osteochondral repair in the case of injuries or the presence of OA [153]. Since the techniques of scaffold design are highly sophisticated, such scaffold-mediated gene delivery may be potentially used to generate both large anatomically shaped but also cartilage constructs individualized to the 3D defect morphology while possessing the capability for a controlled gene delivery [141]. Moreover, the PEO-PPO-PEO copolymers controlling the release of the gene vectors are promising biomaterials for in vivo rAAV delivery, supporting repair in conditions where protection against potentially damaging host immune responses may be needed.
From a clinical point of view, the proven capability to deliver thermosensitive hydrogels that display a sol-gel transition at body temperature while simultaneously controlling the release of therapeutic gene vectors conceptually supports minimally invasive in vivo application strategies, an attractive feature for osteochondral defects that are located in joints that are more difficult to access via an arthrotomy, for example, the hip joint. These current advances support the concept of a scaffold-mediated gene delivery for osteochondral repair in the future.
Author Contributions
Conceptualization, H.M., J.K.V., A.R.-R. and M.C.; Validation, H.M., J.K.V., N.C.-P., A.R.-R. and M.C.; Resources, H.M., J.K.V., A.R.-R. and M.C.; Writing-Original Draft Preparation, H.M., J.K.V., N.C.-P., A.R.-R. and M.C.; Writing-Review & Editing, H.M., J.K.V., N.C.-P., A.R.-R. and M.C.; Supervision, M.C.; Funding Acquisition, H.M., J.K.V., A.R.-R. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deutsche Forschungsgemeinschaft (DFG VE 1099/1-1 to JKV, HM, and MC), the Ministerio de Ciencia e Innovación (RTI2018-099389-A-100 to A.R.-R.), and the Ministerio de Ciencia e Innovación for a Ramón y Cajal Fellowship (RYC2018-025617-I to A.R.-R.).
Acknowledgments
Work supported by the Deutsche Forschungsgemeinschaft (DFG VE 1099/1-1 to J.K.V., H.M., and M.C.) and the Ministerio de Ciencia e Innovación (RTI2018-099389-A-100 to A.R.-R.). A.R.-R. also acknowledges the Ministerio de Ciencia e Innovación for a Ramón y Cajal Fellowship (RYC2018-025617-I).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buckwalter, J.A. Articular cartilage: Injuries and potential for healing. J. Orthop. Sports Phys. Ther. 1998, 28, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N.; Hu, J.C.; Athanasiou, K.A. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 2019, 15, 550–570. [Google Scholar] [CrossRef] [PubMed]
- Schinhan, M.; Gruber, M.; Vavken, P.; Dorotka, R.; Samouh, L.; Chiari, C.; Gruebl-Barabas, R.; Nehrer, S. Critical-size defect induces unicompartmental osteoarthritis in a stable ovine knee. J. Orthop. Res. 2012, 30, 214–220. [Google Scholar] [CrossRef]
- Orth, P.; Eldracher, M.; Cucchiarini, M.; Madry, H. Small-diameter subchondral drilling improves DNA and proteoglycan content of the cartilaginous repair tissue in a large animal model of a full-thickness chondral defect. J. Clin. Med. 2020, 9, 1903. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Kon, E.; Condello, V.; Peretti, G.M.; Steinwachs, M.; Seil, R.; Berruto, M.; Engebretsen, L.; Filardo, G.; Angele, P. Early osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1753–1762. [Google Scholar] [CrossRef]
- Perelli, S.; Molina Romoli, A.R.; Costa-Paz, M.; Erquicia, J.I.; Gelber, P.E.; Monllau, J.C. Internal fixation of osteochondritis dissecans of the knee leads to good long-term outcomes and high degree of healing without differences between fixation devices. J. Clin. Med. 2019, 8, 1934. [Google Scholar] [CrossRef]
- Sanders, T.L.; Pareek, A.; Obey, M.R.; Johnson, N.R.; Carey, J.L.; Stuart, M.J.; Krych, A.J. High rate of osteoarthritis after osteochondritis dissecans fragment excision compared with surgical restoration at a mean 16-year follow-up. Am. J. Sports Med. 2017, 45, 1799–1805. [Google Scholar] [CrossRef]
- Saris, D.B.; Vanlauwe, J.; Victor, J.; Haspl, M.; Bohnsack, M.; Fortems, Y.; Vandekerckhove, B.; Almqvist, K.F.; Claes, T.; Handelberg, F.; et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am. J. Sports Med. 2008, 36, 235–246. [Google Scholar] [CrossRef]
- Barry, F.; Murphy, M. Mesenchymal stem cells in joint disease and repair. Nat. Rev. Rheumatol. 2013, 9, 584–594. [Google Scholar] [CrossRef]
- Van den Berg, W.B.; van der Kraan, P.M.; Scharstuhl, A.; van Beuningen, H.M. Growth factors and cartilage repair. Clin. Orthop. Relat Res. 2001, S244–S250. [Google Scholar] [CrossRef]
- Devescovi, V.; Leonardi, E.; Ciapetti, G.; Cenni, E. Growth factors in bone repair. Chir. Organi. Mov. 2008, 92, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The role of growth factors in cartilage repair. Clin. Orthop. Relat Res. 2011, 469, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; Madry, H. The potential of gene transfer for the treatment of osteoarthritis. Regen. Med. 2014, 9, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.J.; Kim, T.H.; Choi, S.J.; Park, J.H.; Wall, I.B.; Kim, H.W. Gene delivery techniques for adult stem cell-based regenerative therapy. Nanomedicine 2013, 8, 1875–1891. [Google Scholar] [CrossRef]
- Hanada, K.; Solchaga, L.A.; Caplan, A.I.; Hering, T.M.; Goldberg, V.M.; Yoo, J.U.; Johnstone, B. BMP-2 induction and TGF-beta 1 modulation of rat periosteal cell chondrogenesis. J. Cell Biochem. 2001, 81, 284–294. [Google Scholar] [CrossRef]
- Lee, K.H.; Song, S.U.; Hwang, T.S.; Yi, Y.; Oh, I.S.; Lee, J.Y.; Choi, K.B.; Choi, M.S.; Kim, S.J. Regeneration of hyaline cartilage by cell-mediated gene therapy using transforming growth factor beta 1-producing fibroblasts. Hum. Gene Ther. 2001, 12, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.P. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- Nixon, A.J.; Fortier, L.A.; Williams, J.; Mohammed, H. Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. J. Orthop. Res. 1999, 17, 475–487. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Wildemann, B.; Heeger, J.; Gabelein, T.; Flyvbjerg, A.; Bail, H.J.; Raschke, M. Improvement of fracture healing by systemic administration of growth hormone and local application of insulin-like growth factor-1 and transforming growth factor-beta1. Bone 2002, 31, 165–172. [Google Scholar] [CrossRef]
- Jentzsch, K.D.; Wellmitz, G.; Heder, G.; Petzold, E.; Buntrock, P.; Oehme, P. A bovine brain fraction with fibroblast growth factor activity inducing articular cartilage regeneration in vivo. Acta Biol. Med. Ger. 1980, 39, 967–971. [Google Scholar]
- Cucchiarini, M.; Madry, H.; Ma, C.; Thurn, T.; Zurakowski, D.; Menger, M.D.; Kohn, D.; Trippel, S.B.; Terwilliger, E.F. Improved tissue repair in articular cartilage defects in vivo by rAAV-mediated overexpression of human fibroblast growth factor 2. Mol. Ther. 2005, 12, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Klein-Nulend, J.; Louwerse, R.T.; Heyligers, I.C.; Wuisman, P.I.; Semeins, C.M.; Goei, S.W.; Burger, E.H. Osteogenic protein (OP-1, BMP-7) stimulates cartilage differentiation of human and goat perichondrium tissue in vitro. J. Biomed. Mater. Res. 1998, 40, 614–620. [Google Scholar] [CrossRef]
- Street, J.; Bao, M.; deGuzman, L.; Bunting, S.; Peale, F.V., Jr.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.S.; Sable, R.B.; Kothari, R.M. Occurrence, biochemical profile of vascular endothelial growth factor (VEGF) isoforms and their functions in endochondral ossification. J Cell Physiol. 2012, 227, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.B.; Chen, E.H.; Lynch, S.E. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthr. Cartil. 2006, 14, 403–412. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. PDGF in bone formation and regeneration: New insights into a novel mechanism involving MSCs. J. Orthop. Res. 2011, 29, 1795–1803. [Google Scholar] [CrossRef]
- Lefebvre, V.; Behringer, R.R.; de Crombrugghe, B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthr. Cartil. 2001, 9 (Suppl. A), S69–S75. [Google Scholar] [CrossRef]
- Inada, M.; Yasui, T.; Nomura, S.; Miyake, S.; Deguchi, K.; Himeno, M.; Sato, M.; Yamagiwa, H.; Kimura, T.; Yasui, N.; et al. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev. Dyn. 1999, 214, 279–290. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Raisin, S.; Belamie, E.; Morille, M. Non-viral gene activated matrices for mesenchymal stem cells based tissue engineering of bone and cartilage. Biomaterials 2016, 104, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Athirasala, A.; Menezes, P.P.; Ashwanikumar, N.; Zou, T.; Sahay, G.; Bertassoni, L.E. Messenger RNA delivery for tissue engineering and regenerative medicine applications. Tissue Eng. Part A 2019, 25, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.C.; Raftery, R.M.; Curtin, C.M.; O’Driscoll, C.M.; O’Brien, F.J. Scaffold-based delivery of nucleic acid therapeutics for enhanced bone and cartilage repair. J. Orthop. Res. 2019, 37, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Pedrares, N.; Fuentes-Boquete, I.; Díaz-Prado, S.; Rey-Rico, A. Hydrogel-based localized nonviral gene delivery in regenerative medicine approaches-an overview. Pharmaceutics 2020, 12, 752. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Oskuee, R.K.; Nassirli, H.; Malaekeh-Nikouei, B. Progress in the development of lipopolyplexes as efficient non-viral gene delivery systems. J. Control Release 2016, 236, 1–14. [Google Scholar] [CrossRef]
- Bono, N.; Ponti, F.; Mantovani, D.; Candiani, G. Non-viral in vitro gene delivery: It is now time to set the bar! Pharmaceutics 2020, 12, 183. [Google Scholar] [CrossRef]
- Al Qtaish, N.; Gallego, I.; Villate-Beitia, I.; Sainz-Ramos, M.; Lopez-Mendez, T.B.; Grijalvo, S.; Eritja, R.; Soto-Sanchez, C.; Martinez-Navarrete, G.; Fernandez, E.; et al. Niosome-based approach for in situ gene delivery to retina and brain cortex as immune-privileged tissues. Pharmaceutics 2020, 12, 198. [Google Scholar] [CrossRef]
- Rai, R.; Alwani, S.; Badea, I. Polymeric nanoparticles in gene therapy: New avenues of design and optimization for delivery applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef]
- Sum, C.H.; Shortall, S.M.; Wong, S.; Wettig, S.D. Non-viral gene delivery. Exp. Suppl. 2018, 110, 3–68. [Google Scholar] [CrossRef]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet 2003, 4, 346–358. [Google Scholar] [CrossRef]
- Tokushige, K.; Moradpour, D.; Wakita, T.; Geissler, M.; Hayashi, N.; Wands, J.R. Comparison between cytomegalovirus promoter and elongation factor-1 alpha promoter-driven constructs in the establishment of cell lines expressing hepatitis C virus core protein. J. Virol. Methods 1997, 64, 73–80. [Google Scholar] [CrossRef]
- Chung, S.; Andersson, T.; Sonntag, K.C.; Bjorklund, L.; Isacson, O.; Kim, K.S. Analysis of different promoter systems for efficient transgene expression in mouse embryonic stem cell lines. Stem Cells 2002, 20, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Raup, A.; Jerome, V.; Freitag, R.; Synatschke, C.V.; Muller, A.H. Promoter, transgene, and cell line effects in the transfection of mammalian cells using PDMAEMA-based nano-stars. Biotechnol. Rep. 2016, 11, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Sum, C.H.; Wettig, S.; Slavcev, R.A. Impact of DNA vector topology on non-viral gene therapeutic safety and efficacy. Curr. Gene Ther. 2014, 14, 309–329. [Google Scholar] [CrossRef]
- Nafissi, N.; Alqawlaq, S.; Lee, E.A.; Foldvari, M.; Spagnuolo, P.A.; Slavcev, R.A. DNA ministrings: Highly safe and effective gene delivery vectors. Mol. Ther. Nucleic Acids 2014, 3, e165. [Google Scholar] [CrossRef]
- Aronovich, E.L.; McIvor, R.S.; Hackett, P.B. The Sleeping Beauty transposon system: A non-viral vector for gene therapy. Hum. Mol. Genet 2011, 20, R14–R20. [Google Scholar] [CrossRef]
- Qian, Q.; Che, J.; Ye, L.; Zhong, B. [The improvement and application of piggyBac transposon system in mammals]. Yi Chuan 2014, 36, 965–973. [Google Scholar] [CrossRef]
- Vargas, J.E.; Chicaybam, L.; Stein, R.T.; Tanuri, A.; Delgado-Canedo, A.; Bonamino, M.H. Retroviral vectors and transposons for stable gene therapy: Advances, current challenges and perspectives. J. Transl. Med. 2016, 14, 288–303. [Google Scholar] [CrossRef]
- Cucchiarini, M. Human gene therapy: Novel approaches to improve the current gene delivery systems. Discov. Med. 2016, 21, 495–506. [Google Scholar]
- Shi, S.; Chan, A.G.; Mercer, S.; Eckert, G.J.; Trippel, S.B. Endogenous versus exogenous growth factor regulation of articular chondrocytes. J. Orthop. Res. 2014, 32, 54–60. [Google Scholar] [CrossRef]
- Shi, S.; Mercer, S.; Eckert, G.J.; Trippel, S.B. Regulation of articular chondrocyte aggrecan and collagen gene expression by multiple growth factor gene transfer. J. Orthop. Res. 2012, 30, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Cheng, Y.; Yuan, Q.; Li, J. IGF-1 and BMP-2 induces differentiation of adipose-derived mesenchymal stem cells into chondrocytes-like cells. Ann. Biomed. Eng. 2010, 38, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Pagnotto, M.R.; Wang, Z.; Karpie, J.C.; Ferretti, M.; Xiao, X.; Chu, C.R. Adeno-associated viral gene transfer of transforming growth factor-beta1 to human mesenchymal stem cells improves cartilage repair. Gene. Ther. 2007, 14, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Jin, Y.Q.; Kretlow, J.D.; Liu, W.; Ding, W.; Sun, H.; Zhou, G.; Zhang, W.; Cao, Y. Adenoviral transduction of hTGF-beta1 enhances the chondrogenesis of bone marrow derived stromal cells. Biotechnol. Lett. 2009, 31, 639–646. [Google Scholar] [CrossRef]
- Garza-Veloz, I.; Romero-Diaz, V.J.; Martinez-Fierro, M.L.; Marino-Martinez, I.A.; Gonzalez-Rodriguez, M.; Martinez-Rodriguez, H.G.; Espinoza-Juarez, M.A.; Bernal-Garza, D.A.; Ortiz-Lopez, R.; Rojas-Martinez, A. Analyses of chondrogenic induction of adipose mesenchymal stem cells by combined co-stimulation mediated by adenoviral gene transfer. Arthr. Res. Ther. 2013, 15, R80–R82. [Google Scholar] [CrossRef]
- Neumann, A.J.; Gardner, O.F.; Williams, R.; Alini, M.; Archer, C.W.; Stoddart, M.J. Human articular cartilage progenitor cells are responsive to mechanical stimulation and adenoviral-mediated overexpression of bone-morphogenetic protein 2. PLoS ONE 2015, 10, e0136229. [Google Scholar] [CrossRef]
- Chopra, I.; Hodgson, J.; Metcalf, B.; Poste, G. New approaches to the control of infections caused by antibiotic-resistant bacteria. An industry perspective. JAMA 1996, 275, 401–403. [Google Scholar] [CrossRef]
- Evans, C.H.; Liu, F.J.; Glatt, V.; Hoyland, J.A.; Kirker-Head, C.; Walsh, A.; Betz, O.; Wells, J.W.; Betz, V.; Porter, R.M.; et al. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur. Cell Mater. 2009, 18, 96–111. [Google Scholar] [CrossRef]
- Goodrich, L.R.; Hidaka, C.; Robbins, P.D.; Evans, C.H.; Nixon, A.J. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J. Bone Joint Surg. Br. 2007, 89, 672–685. [Google Scholar] [CrossRef]
- Goodrich, L.R.; Brower-Toland, B.D.; Warnick, L.; Robbins, P.D.; Evans, C.H.; Nixon, A.J. Direct adenovirus-mediated IGF-I gene transduction of synovium induces persisting synovial fluid IGF-I ligand elevations. Gene Ther. 2006, 13, 1253–1262. [Google Scholar] [CrossRef]
- Menendez, M.I.; Clark, D.J.; Carlton, M.; Flanigan, D.C.; Jia, G.; Sammet, S.; Weisbrode, S.E.; Knopp, M.V.; Bertone, A.L. Direct delayed human adenoviral BMP-2 or BMP-6 gene therapy for bone and cartilage regeneration in a pony osteochondral model. Osteoarthr. Cartil. 2011, 19, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, Z.; Liu, P.; Ma, Y.; Lin, L.; Lang, N.; Fu, X.; Zhang, J.; Ma, K.; Chen, P.; et al. The synergistic effects of microfracture, perforated decalcified cortical bone matrix and adenovirus-bone morphogenetic protein-4 in cartilage defect repair. Biomaterials 2008, 29, 4616–4629. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E. Gene therapy death prompts review of adenovirus vector. Science 1999, 286, 2244–2245. [Google Scholar] [CrossRef] [PubMed]
- Ueblacker, P.; Wagner, B.; Vogt, S.; Salzmann, G.; Wexel, G.; Kruger, A.; Plank, C.; Brill, T.; Specht, K.; Hennig, T.; et al. In vivo analysis of retroviral gene transfer to chondrocytes within collagen scaffolds for the treatment of osteochondral defects. Biomaterials 2007, 28, 4480–4487. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, R.; Usas, A.; Kubo, S.; Corsi, K.; Peng, H.; Rose, T.; Cummins, J.; Fu, F.H.; Huard, J. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthr. Rheum. 2006, 54, 433–442. [Google Scholar] [CrossRef]
- Kubo, S.; Cooper, G.M.; Matsumoto, T.; Phillippi, J.A.; Corsi, K.A.; Usas, A.; Li, G.; Fu, F.H.; Huard, J. Blocking vascular endothelial growth factor with soluble Flt-1 improves the chondrogenic potential of mouse skeletal muscle-derived stem cells. Arthr. Rheum. 2009, 60, 155–165. [Google Scholar] [CrossRef]
- Lee, J.M.; Im, G.I. SOX trio-co-transduced adipose stem cells in fibrin gel to enhance cartilage repair and delay the progression of osteoarthritis in the rat. Biomaterials 2012, 33, 2016–2024. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, S.B.; Somaiya, D.; Noh, M.J.; Choi, K.B.; Lim, C.L.; Lee, H.Y.; Lee, Y.J.; Yi, Y.; Lee, K.H. Type II collagen and glycosaminoglycan expression induction in primary human chondrocyte by TGF-beta1. BMC Musculoskelet Disord. 2015, 16, 141–152. [Google Scholar] [CrossRef]
- Lu, C.H.; Yeh, T.S.; Yeh, C.L.; Fang, Y.H.; Sung, L.Y.; Lin, S.Y.; Yen, T.C.; Chang, Y.H.; Hu, Y.C. Regenerating cartilages by engineered ASCs: Prolonged TGF-beta3/BMP-6 expression improved articular cartilage formation and restored zonal structure. Mol. Ther. 2014, 22, 186–195. [Google Scholar] [CrossRef]
- Chen, H.C.; Chang, Y.H.; Chuang, C.K.; Lin, C.Y.; Sung, L.Y.; Wang, Y.H.; Hu, Y.C. The repair of osteochondral defects using baculovirus-mediated gene transfer with de-differentiated chondrocytes in bioreactor culture. Biomaterials 2009, 30, 674–681. [Google Scholar] [CrossRef]
- Cucchiarini, M.; Thurn, T.; Weimer, A.; Kohn, D.; Terwilliger, E.F.; Madry, H. Restoration of the extracellular matrix in human osteoarthritic articular cartilage by overexpression of the transcription factor SOX9. Arthr. Rheum. 2007, 56, 158–167. [Google Scholar] [CrossRef]
- Watanabe, S.; Imagawa, T.; Boivin, G.P.; Gao, G.; Wilson, J.M.; Hirsch, R. Adeno-associated virus mediates long-term gene transfer and delivery of chondroprotective IL-4 to murine synovium. Mol. Ther. 2000, 2, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.G.; Xie, J.; Yang, P.; Wang, Y.; Xu, L.; Liu, D.; Hsu, H.C.; Zhou, T.; Edwards, C.K., 3rd; Mountz, J.D. Adeno-associated virus production of soluble tumor necrosis factor receptor neutralizes tumor necrosis factor alpha and reduces arthritis. Hum. Gene Ther. 2000, 11, 2431–2442. [Google Scholar] [CrossRef] [PubMed]
- Nooeaid, P.; Salih, V.; Beier, J.P.; Boccaccini, A.R. Osteochondral tissue engineering: Scaffolds, stem cells and applications. J. Cell Mol. Med. 2012, 16, 2247–2270. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Keeney, M.; Pandit, A. The osteochondral junction and ist repair via bi-phasic tissue engineering scaffolds. Tissue Eng Part B Rev. 2009, 15, 55–73. [Google Scholar] [CrossRef]
- Lopa, S.; Madry, H. Bioinspired scaffolds for osteochondral regeneration. Tissue Eng. Part A 2014, 20, 2052–2076. [Google Scholar] [CrossRef]
- Shpichka, A.; Koroleva, A.; Kuznetsova, D.; Dmitriev, R.I.; Timashev, P. Fabrication and handling of 3D scaffolds based on polymers and decellularized tissues. Adv. Exp. Med. Biol. 2017, 1035, 71–81. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludag, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- De Mori, A.; Pena Fernandez, M.; Blunn, G.; Tozzi, G.; Roldo, M. 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering. Polymers 2018, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Gao, L.; Venkatesan, J.K.; Wang, G.; Madry, H.; Cucchiarini, M. Translational applications of photopolymerizable hydrogels for cartilage repair. J. Exp. Orthop. 2019, 6, 47–58. [Google Scholar] [CrossRef]
- Critchley, S.; Sheehy, E.J.; Cunniffe, G.; Diaz-Payno, P.; Carroll, S.F.; Jeon, O.; Alsberg, E.; Brama, P.A.J.; Kelly, D.J. 3D printing of fibre-reinforced cartilaginous templates for the regeneration of osteochondral defects. Acta Biomater. 2020, 113, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Camarero-Espinosa, S.; Cooper-White, J. Tailoring biomaterial scaffolds for osteochondral repair. Int. J. Pharm. 2017, 523, 476–489. [Google Scholar] [CrossRef]
- Cassaro, A. Fracture resistance of prosthetic abutments reconstructed with different methods. Stomatol. Mediterr. 1988, 8, 133–138. [Google Scholar] [PubMed]
- Zhu, C.; Wu, Q.; Wang, F.; Zhang, X.; Chen, F.; Liu, X.; Yang, Q.; Zhu, L. Animal models used for testing hydrogels in cartilage regeneration. Curr. Stem Cell Res. Ther. 2018, 13, 517–525. [Google Scholar] [CrossRef]
- Verrier, S.; Alini, M.; Alsberg, E.; Buchman, S.R.; Kelly, D.; Laschke, M.W.; Menger, M.D.; Murphy, W.L.; Stegemann, J.P.; Schutz, M.; et al. Tissue engineering and regenerative approaches to improving the healing of large bone defects. Eur. Cell. Mater. 2016, 32, 87–110. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Rothrauff, B.B.; Tuan, R.S. Tendon and ligament regeneration and repair: Clinical relevance and developmental paradigm. Birth Defects Res. C Embryo Today 2013, 99, 203–222. [Google Scholar] [CrossRef]
- Chajra, H.; Rousseau, C.F.; Cortial, D.; Ronziere, M.C.; Herbage, D.; Mallein-Gerin, F.; Freyria, A.M. Collagen-based biomaterials and cartilage engineering. Application to osteochondral defects. Biomed. Mater. Eng. 2008, 18, S33–S45. [Google Scholar]
- Hishimura, R.; Onodera, T.; Hontani, K.; Baba, R.; Homan, K.; Matsubara, S.; Joutoku, Z.; Kim, W.; Nonoyama, T.; Kurokawa, T.; et al. Osteochondral autograft transplantation technique augmented by an ultrapurified alginate gel enhances osteochondral repair in a rabbit model. Am. J. Sports Med. 2019, 47, 468–478. [Google Scholar] [CrossRef]
- Costa, L.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Gellan gum-based hydrogels for osteochondral repair. Adv. Exp. Med. Biol. 2018, 1058, 281–304. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.P.; Pina, S.; Oliveira, J.M.; Reis, R.L. Silk fibroin-based hydrogels and scaffolds for osteochondral repair and regeneration. Adv. Exp. Med. Biol. 2018, 1058, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, J.; Ma, L.; Li, F.; Tu, Z.; Gao, C. Fabrication of poly(lactide-co-glycolide) scaffold filled with fibrin gel, mesenchymal stem cells, and poly(ethylene oxide)-b-poly(L-lysine)/TGF-beta1 plasmid DNA complexes for cartilage restoration in vivo. J. Biomed. Mater. Res. A 2013, 101, 3097–3108. [Google Scholar] [CrossRef] [PubMed]
- Iulian, A.; Dan, L.; Camelia, T.; Claudia, M.; Sebastian, G. Synthetic materials for osteochondral tissue engineering. Adv. Exp. Med. Biol. 2018, 1058, 31–52. [Google Scholar] [CrossRef]
- Xie, H.; Ji, Y.; Tian, Q.; Wang, X.; Zhang, N.; Zhang, Y.; Xu, J.; Wang, N.; Yan, J. Autogenous bone particle/titanium fiber composites for bone regeneration in a rabbit radius critical-size defect model. Connect Tissue Res. 2017, 58, 553–561. [Google Scholar] [CrossRef]
- Freitas, G.P.; Lopes, H.B.; Almeida, A.L.G.; Abuna, R.P.F.; Gimenes, R.; Souza, L.E.B.; Covas, D.T.; Beloti, M.M.; Rosa, A.L. Potential of osteoblastic cells derived from bone marrow and adipose tissue associated with a polymer/ceramic composite to repair bone tissue. Calcif. Tissue Int. 2017, 101, 312–320. [Google Scholar] [CrossRef]
- Kawai, T.; Matsui, K.; Ezoe, Y.; Kajii, F.; Suzuki, O.; Takahashi, T.; Kamakura, S. Efficacy of octacalcium phosphate collagen composite for titanium dental implants in dogs. Materials 2018, 11, 229. [Google Scholar] [CrossRef]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef]
- Akagi, D.; Oba, M.; Koyama, H.; Nishiyama, N.; Fukushima, S.; Miyata, T.; Nagawa, H.; Kataoka, K. Biocompatible micellar nanovectors achieve efficient gene transfer to vascular lesions without cytotoxicity and thrombus formation. Gene Ther. 2007, 14, 1029–1038. [Google Scholar] [CrossRef]
- Wang, H.; Ding, S.; Zhang, Z.; Wang, L.; You, Y. Cationic micelle: A promising nanocarrier for gene delivery with high transfection efficiency. J. Gene Med. 2019, 21, e3101. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Venkatesan, J.K.; Frisch, J.; Rial-Hermida, I.; Schmitt, G.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. PEO-PPO-PEO micelles as effective rAAV-mediated gene delivery systems to target human mesenchymal stem cells without altering their differentiation potency. Acta Biomater. 2015, 27, 42–52. [Google Scholar] [CrossRef]
- Venkatesan, J.K.; Gardner, O.; Rey-Rico, A.; Eglin, D.; Alini, M.; Stoddart, M.J.; Cucchiarini, M.; Madry, H. Improved chondrogenic differentiation of rAAV SOX9-modified human MSCs seeded in fibrin-polyurethane scaffolds in a hydrodynamic environment. Int. J. Mol. Sci. 2018, 19, 2635. [Google Scholar] [CrossRef]
- Frisch, J.; Rey-Rico, A.; Venkatesan, J.K.; Schmitt, G.; Madry, H.; Cucchiarini, M. rAAV-mediated overexpression of sox9, TGF-beta and IGF-I in minipig bone marrow aspirates to enhance the chondrogenic processes for cartilage repair. Gene Ther. 2016, 23, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; Madry, H. Biomaterial-guided delivery of gene vectors for targeted articular cartilage repair. Nat. Rev. Rheumatol. 2019, 15, 18–29. [Google Scholar] [CrossRef] [PubMed]
- He, C.X.; Zhang, T.Y.; Miao, P.H.; Hu, Z.J.; Han, M.; Tabata, Y.; Hu, Y.L.; Gao, J.Q. TGF-beta1 gene-engineered mesenchymal stem cells induce rat cartilage regeneration using nonviral gene vector. Biotechnol. Appl. Biochem. 2012, 59, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Lv, L.; Dai, Y.; Wu, G.; Zhao, H.; Zhang, F. Porous chitosan scaffolds with embedded hyaluronic acid/chitosan/plasmid-DNA nanoparticles encoding TGF-beta1 induce DNA controlled release, transfected chondrocytes, and promoted cell proliferation. PLoS ONE 2013, 8, e69950. [Google Scholar] [CrossRef]
- Elangovan, S.; D’Mello, S.R.; Hong, L.; Ross, R.D.; Allamargot, C.; Dawson, D.V.; Stanford, C.M.; Johnson, G.K.; Sumner, D.R.; Salem, A.K. The enhancement of bone regeneration by gene activated matrix encoding for platelet derived growth factor. Biomaterials 2014, 35, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Curtin, C.M.; Tierney, E.G.; McSorley, K.; Cryan, S.A.; Duffy, G.P.; O’Brien, F.J. Combinatorial gene therapy accelerates bone regeneration: Non-viral dual delivery of VEGF and BMP2 in a collagen-nanohydroxyapatite scaffold. Adv. Healthc. Mater. 2015, 4, 223–227. [Google Scholar] [CrossRef]
- D’Mello, S.R.; Elangovan, S.; Hong, L.; Ross, R.D.; Sumner, D.R.; Salem, A.K. A pilot study evaluating combinatorial and simultaneous delivery of polyethylenimine-plasmid DNA complexes encoding for VEGF and PDGF for bone regeneration in calvarial bone defects. Curr. Pharm. Biotechnol. 2015, 16, 655–660. [Google Scholar] [CrossRef]
- Keeney, M.; Chung, M.T.; Zielins, E.R.; Paik, K.J.; McArdle, A.; Morrison, S.D.; Ransom, R.C.; Barbhaiya, N.; Atashroo, D.; Jacobson, G.; et al. Scaffold-mediated BMP-2 minicircle DNA delivery accelerated bone repair in a mouse critical-size calvarial defect model. J. Biomed. Mater. Res. A 2016, 104, 2099–2107. [Google Scholar] [CrossRef]
- Plonka, A.B.; Khorsand, B.; Yu, N.; Sugai, J.V.; Salem, A.K.; Giannobile, W.V.; Elangovan, S. Effect of sustained PDGF nonviral gene delivery on repair of tooth-supporting bone defects. Gene Ther. 2017, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Khorsand, B.; Nicholson, N.; Do, A.V.; Femino, J.E.; Martin, J.A.; Petersen, E.; Guetschow, B.; Fredericks, D.C.; Salem, A.K. Regeneration of bone using nanoplex delivery of FGF-2 and BMP-2 genes in diaphyseal long bone radial defects in a diabetic rabbit model. J. Control Release 2017, 248, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Raftery, R.M.; Mencia Castano, I.; Chen, G.; Cavanagh, B.; Quinn, B.; Curtin, C.M.; Cryan, S.A.; O’Brien, F.J. Translating the role of osteogenic-angiogenic coupling in bone formation: Highly efficient chitosan-pDNA activated scaffolds can accelerate bone regeneration in critical-sized bone defects. Biomaterials 2017, 149, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Raftery, R.M.; Mencia-Castano, I.; Sperger, S.; Chen, G.; Cavanagh, B.; Feichtinger, G.A.; Redl, H.; Hacobian, A.; O’Brien, F.J. Delivery of the improved BMP-2-advanced plasmid DNA within a gene-activated scaffold accelerates mesenchymal stem cell osteogenesis and critical size defect repair. J. Control Release 2018, 283, 20–31. [Google Scholar] [CrossRef]
- Tierney, E.G.; Duffy, G.P.; Hibbitts, A.J.; Cryan, S.A.; O’Brien, F.J. The development of non-viral gene-activated matrices for bone regeneration using polyethyleneimine (PEI) and collagen-based scaffolds. J. Control Release 2012, 158, 304–311. [Google Scholar] [CrossRef]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef]
- Saravanan, S.; Leena, R.S.; Selvamurugan, N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1354–1365. [Google Scholar] [CrossRef]
- Wang, W.; Li, B.; Li, Y.; Jiang, Y.; Ouyang, H.; Gao, C. In vivo restoration of full-thickness cartilage defects by poly(lactide-co-glycolide) sponges filled with fibrin gel, bone marrow mesenchymal stem cells and DNA complexes. Biomaterials 2010, 31, 5953–5965. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Li, P.; Diao, H.; Zhu, S.; Dong, L.; Wang, R.; Guo, T.; Zhao, J.; Zhang, J. Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials 2011, 32, 4793–4805. [Google Scholar] [CrossRef]
- Li, B.; Li, F.; Ma, L.; Yang, J.; Wang, C.; Wang, D.; Gao, C. Poly(lactide-co-glycolide)/fibrin gel construct as a 3D model to evaluate gene therapy of cartilage in vivo. Mol. Pharm. 2014, 11, 2062–2070. [Google Scholar] [CrossRef]
- Lee, Y.H.; Wu, H.C.; Yeh, C.W.; Kuan, C.H.; Liao, H.T.; Hsu, H.C.; Tsai, J.C.; Sun, J.S.; Wang, T.W. Enzyme-crosslinked gene-activated matrix for the induction of mesenchymal stem cells in osteochondral tissue regeneration. Acta. Biomater 2017, 63, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Ledo, A.M.; Senra, A.; Rilo-Alvarez, H.; Borrajo, E.; Vidal, A.; Alonso, M.J.; Garcia-Fuentes, M. mRNA-activated matrices encoding transcription factors as primers of cell differentiation in tissue engineering. Biomaterials 2020, 247, 120016–120027. [Google Scholar] [CrossRef] [PubMed]
- Balmayor, E.R.; Geiger, J.P.; Koch, C.; Aneja, M.K.; van Griensven, M.; Rudolph, C.; Plank, C. Modified mRNA for BMP-2 in combination with biomaterials serves as a transcript-activated matrix for effectively inducing osteogenic pathways in stem cells. Stem Cells Dev. 2017, 26, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.D.; Salter, E.; Chen, E.; Sutter, K.A.; Alsberg, E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J. Biomed. Mater. Res. A 2010, 92, 1131–1138. [Google Scholar] [CrossRef]
- Wegman, F.; Bijenhof, A.; Schuijff, L.; Oner, F.C.; Dhert, W.J.; Alblas, J. Osteogenic differentiation as a result of BMP-2 plasmid DNA based gene therapy in vitro and in vivo. Eur. Cell Mater. 2011, 21, 230–242. [Google Scholar] [CrossRef]
- Leng, P.; Ding, C.R.; Zhang, H.N.; Wang, Y.Z. Reconstruct large osteochondral defects of the knee with hIGF-1 gene enhanced Mosaicplasty. Knee 2012, 19, 804–811. [Google Scholar] [CrossRef]
- Wegman, F.; Geuze, R.E.; van der Helm, Y.J.; Cumhur Oner, F.; Dhert, W.J.; Alblas, J. Gene delivery of bone morphogenetic protein-2 plasmid DNA promotes bone formation in a large animal model. J. Tissue Eng. Regen Med. 2014, 8, 763–770. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Tierney, E.G.; Cunniffe, G.M.; O’Brien, F.J.; Kelly, D.J. Gene delivery of TGF-beta3 and BMP2 in an MSC-laden alginate hydrogel for articular cartilage and endochondral bone tissue engineering. Tissue Eng. Part A 2016, 22, 776–787. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Rathan, S.; Hobbs, C.; Pitacco, P.; Freeman, F.E.; Cunniffe, G.M.; Dunne, N.J.; McCarthy, H.O.; Nicolosi, V.; O’Brien, F.J.; et al. Pore-forming bioinks to enable spatio-temporally defined gene delivery in bioprinted tissues. J. Control Release 2019, 301, 13–27. [Google Scholar] [CrossRef]
- Loozen, L.D.; Wegman, F.; Oner, F.C.; Dhert, W.J.A.; Alblas, J. Porous bioprinted constructs in BMP-2 non-viral gene therapy for bone tissue engineering. J. Mater. Chem. B 2013, 1, 6619–6626. [Google Scholar] [CrossRef]
- Yang, H.N.; Park, J.S.; Jeon, S.Y.; Park, K.H. Carboxymethylcellulose (CMC) formed nanogels with branched poly(ethyleneimine) (bPEI) for inhibition of cytotoxicity in human MSCs as a gene delivery vehicles. Carbohydr. Polym. 2015, 122, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Huang, Z.; Cai, X.; Xiang, J.; Zhu, Y.A.; Wang, R.; Chen, J.; Zhang, J. Localized delivery of antisense oligonucleotides by cationic hydrogel suppresses TNF-alpha expression and endotoxin-induced osteolysis. Pharm. Res. 2011, 28, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Pan, J.F.; Ji, Q.X.; Yu, X.B.; Liu, L.S.; Li, H.; Jiao, X.J.; Wang, L. Characterization and cytocompatibility of thermosensitive hydrogel embedded with chitosan nanoparticles for delivery of bone morphogenetic protein-2 plasmid DNA. J. Mater. Sci. Mater. Med. 2016, 27, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ji, Q.; Chen, X.; Sun, Y.; Xu, Q.; Deng, P.; Hu, F.; Yang, J. Accelerated bony defect healing based on chitosan thermosensitive hydrogel scaffolds embedded with chitosan nanoparticles for the delivery of BMP2 plasmid DNA. J. Biomed. Mater. Res. A 2017, 105, 265–273. [Google Scholar] [CrossRef]
- Komatsu, K.; Shibata, T.; Shimada, A.; Ideno, H.; Nakashima, K.; Tabata, Y.; Nifuji, A. Cationized gelatin hydrogels mixed with plasmid DNA induce stronger and more sustained gene expression than atelocollagen at calvarial bone defects in vivo. J. Biomater. Sci. Polym. Ed. 2016, 27, 419–430. [Google Scholar] [CrossRef]
- Needham, C.J.; Shah, S.R.; Dahlin, R.L.; Kinard, L.A.; Lam, J.; Watson, B.M.; Lu, S.; Kasper, F.K.; Mikos, A.G. Osteochondral tissue regeneration through polymeric delivery of DNA encoding for the SOX trio and RUNX2. Acta Biomater. 2014, 10, 4103–4112. [Google Scholar] [CrossRef]
- Wehrhan, F.; Amann, K.; Molenberg, A.; Lutz, R.; Neukam, F.W.; Schlegel, K.A. Critical size defect regeneration using PEG-mediated BMP-2 gene delivery and the use of cell occlusive barrier membranes—The osteopromotive principle revisited. Clin. Oral Implants Res. 2013, 24, 910–920. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Li, Z.; Tan, B.H. Towards the development of polycaprolactone based amphiphilic block copolymers: Molecular design, self-assembly and biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 620–634. [Google Scholar] [CrossRef]
- Brunger, J.M.; Huynh, N.P.; Guenther, C.M.; Perez-Pinera, P.; Moutos, F.T.; Sanchez-Adams, J.; Gersbach, C.A.; Guilak, F. Scaffold-mediated lentiviral transduction for functional tissue engineering of cartilage. Proc. Natl. Acad. Sci. USA 2014, 111, E798–E806. [Google Scholar] [CrossRef]
- Moutos, F.T.; Glass, K.A.; Compton, S.A.; Ross, A.K.; Gersbach, C.A.; Guilak, F.; Estes, B.T. Anatomically shaped tissue-engineered cartilage with tunable and inducible anticytokine delivery for biological joint resurfacing. Proc. Natl. Acad. Sci. USA 2016, 113, E4513–E4522. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.K.; Moutos, F.T.; Rey-Rico, A.; Estes, B.T.; Frisch, J.; Schmitt, G.; Madry, H.; Guilak, F.; Cucchiarini, M. Chondrogenic differentiation processes in human bone-marrow aspirates seeded in three-dimensional-woven poly(ε-caprolactone) scaffolds enhanced by recombinant adeno-associated virus-mediated SOX9 gene transfer. Hum. Gene Ther. 2018, 29, 1277–1286. [Google Scholar] [CrossRef]
- Johnstone, B.; Alini, M.; Cucchiarini, M.; Dodge, G.R.; Eglin, D.; Guilak, F.; Madry, H.; Mata, A.; Mauck, R.L.; Semino, C.E.; et al. Tissue engineering for articular cartilage repair—The state of the art. Eur. Cell Mater. 2013, 25, 248–267. [Google Scholar] [CrossRef] [PubMed]
- Rohman, G.; Huot, S.; Vilas-Boas, M.; Radu-Bostan, G.; Castner, D.G.; Migonney, V. The grafting of a thin layer of poly(sodium styrene sulfonate) onto poly(ε-caprolactone) surface can enhance fibroblast behavior. J. Mater. Sci. Mater. Med. 2015, 26, 206–215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Venkatesan, J.K.; Meng, W.; Rey-Rico, A.; Schmitt, G.; Speicher-Mentges, S.; Falentin-Daudre, C.; Leroux, A.; Madry, H.; Migonney, V.; Cucchiarini, M. Enhanced chondrogenic differentiation activities in human bone marrow aspirates via sox9 overexpression mediated by pNaSS-grafted PCL film-guided rAAV gene transfer. Pharmaceutics 2020, 12, 280. [Google Scholar] [CrossRef]
- Neumann, A.J.; Alini, M.; Archer, C.W.; Stoddart, M.J. Chondrogenesis of human bone marrow-derived mesenchymal stem cells is modulated by complex mechanical stimulation and adenoviral-mediated overexpression of bone morphogenetic protein 2. Tissue Eng. Part A 2013, 19, 1285–1294. [Google Scholar] [CrossRef]
- Rowland, C.R.; Glass, K.A.; Ettyreddy, A.R.; Gloss, C.C.; Matthews, J.R.L.; Huynh, N.P.T.; Guilak, F. Regulation of decellularized tissue remodeling via scaffold-mediated lentiviral delivery in anatomically-shaped osteochondral constructs. Biomaterials 2018, 177, 161–175. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Venkatesan, J.K.; Schmitt, G.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. rAAV-mediated overexpression of TGF-beta via vector delivery in polymeric micelles stimulates the biological and reparative activities of human articular chondrocytes in vitro and in a human osteochondral defect model. Int. J. Nanomed. 2017, 12, 6985–6996. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Han, R.; He, C.; Wang, G.; Wang, J.; Zheng, J.; Pei, M.; Wei, L. Demineralized bone matrix combined bone marrow mesenchymal stem cells, bone morphogenetic protein-2 and transforming growth factor-beta3 gene promoted pig cartilage defect repair. PLoS ONE 2014, 9, e116061. [Google Scholar] [CrossRef]
- Cao, L.; Yang, F.; Liu, G.; Yu, D.; Li, H.; Fan, Q.; Gan, Y.; Tang, T.; Dai, K. The promotion of cartilage defect repair using adenovirus mediated Sox9 gene transfer of rabbit bone marrow mesenchymal stem cells. Biomaterials 2011, 32, 3910–3920. [Google Scholar] [CrossRef]
- Madry, H.; Gao, L.; Rey-Rico, A.; Venkatesan, J.K.; Muller-Brandt, K.; Cai, X.; Goebel, L.; Schmitt, G.; Speicher-Mentges, S.; Zurakowski, D.; et al. Thermosensitive hydrogel based on PEO-PPO-PEO poloxamers for a controlled in situ release of recombinant adeno-associated viral vectors for effective gene therapy of cartilage defects. Adv. Mater. 2020, 32, e1906508. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Perdisa, F.; Venieri, G.; Marcacci, M. Clinical results of multilayered biomaterials for osteochondral regeneration. J. Exp. Orthop. 2014, 1, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Glass, K.A.; Link, J.M.; Brunger, J.M.; Moutos, F.T.; Gersbach, C.A.; Guilak, F. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials 2014, 35, 5921–5931. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).