Extended Intake of Mulberry Leaf Extract Delayed Metformin Elimination via Inhibiting the Organic Cation Transporter 2
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Materials
2.2. Animals
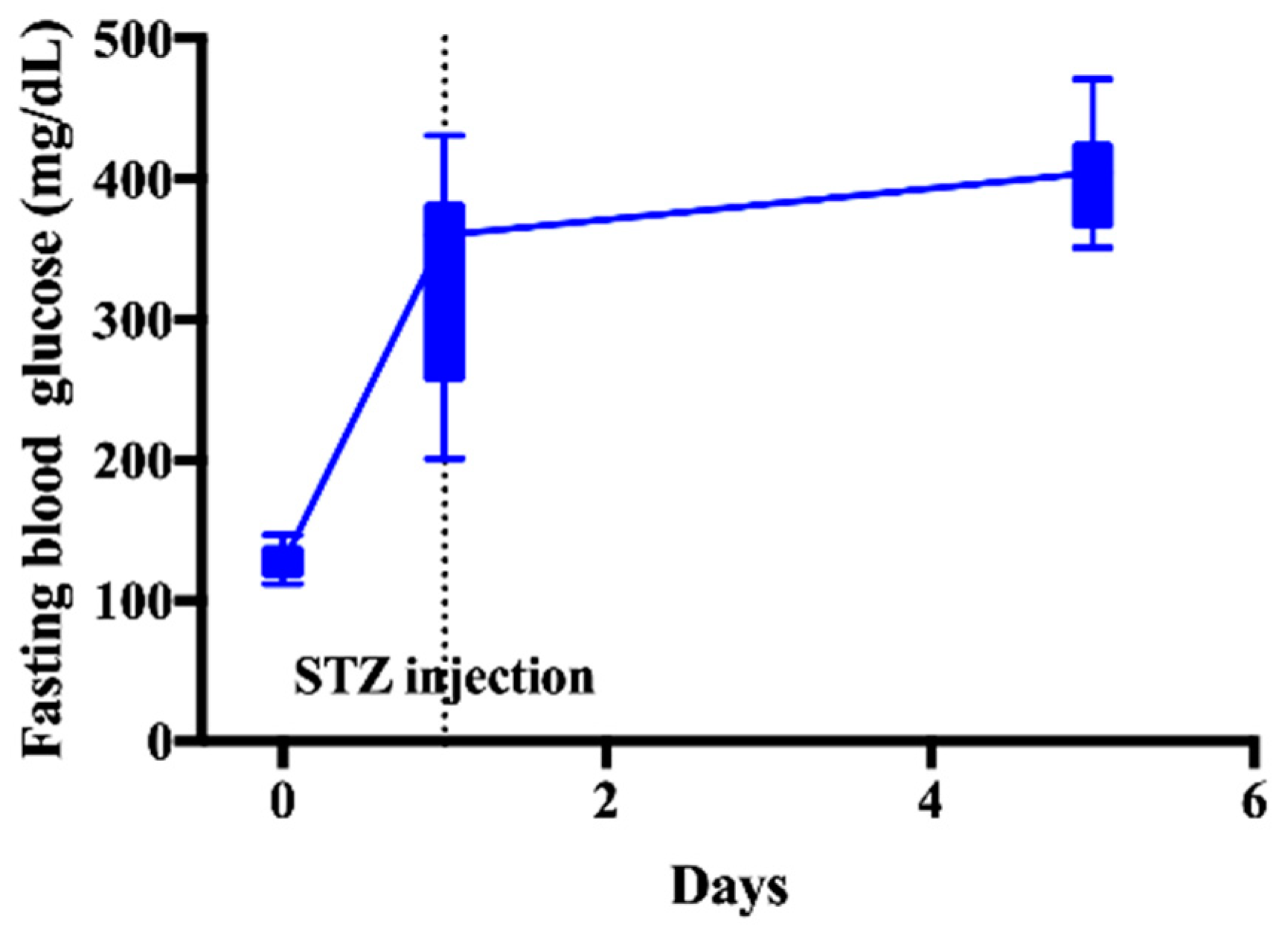
2.3. Induction of DM
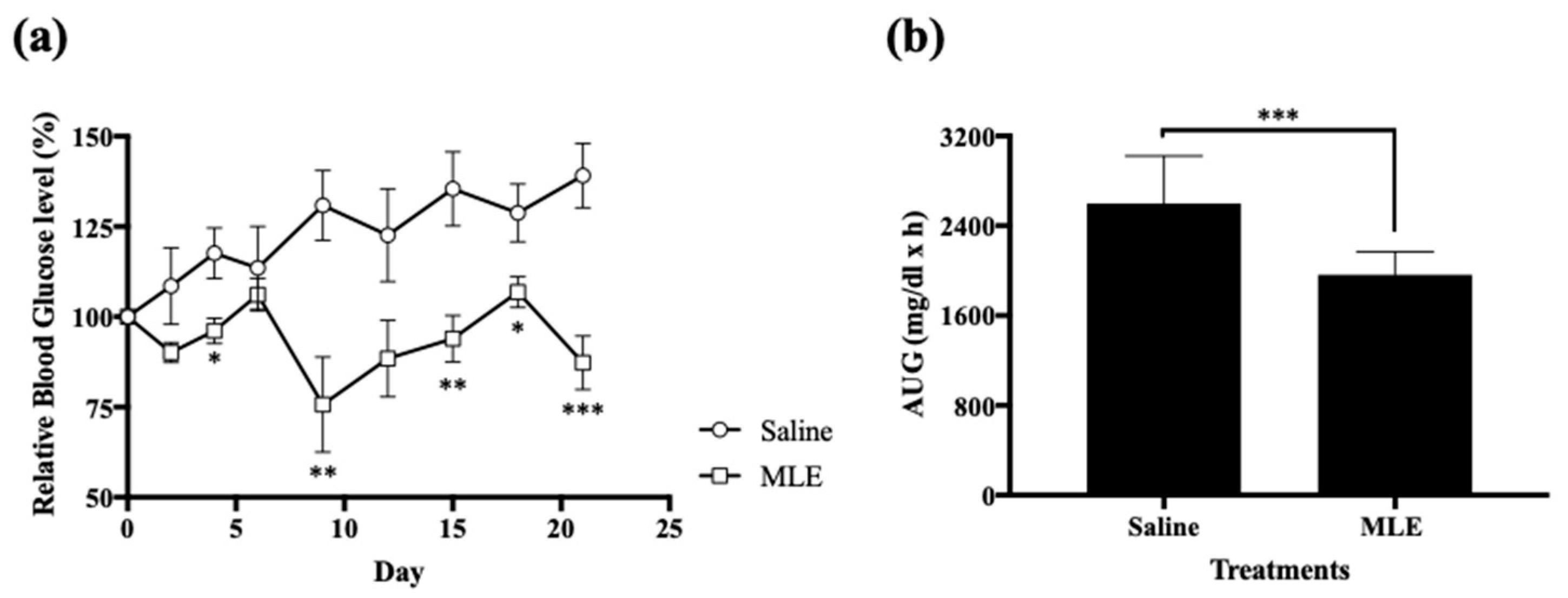
2.4. Anti-Hyperglycemic Effect of Repeated Administrations of MLE
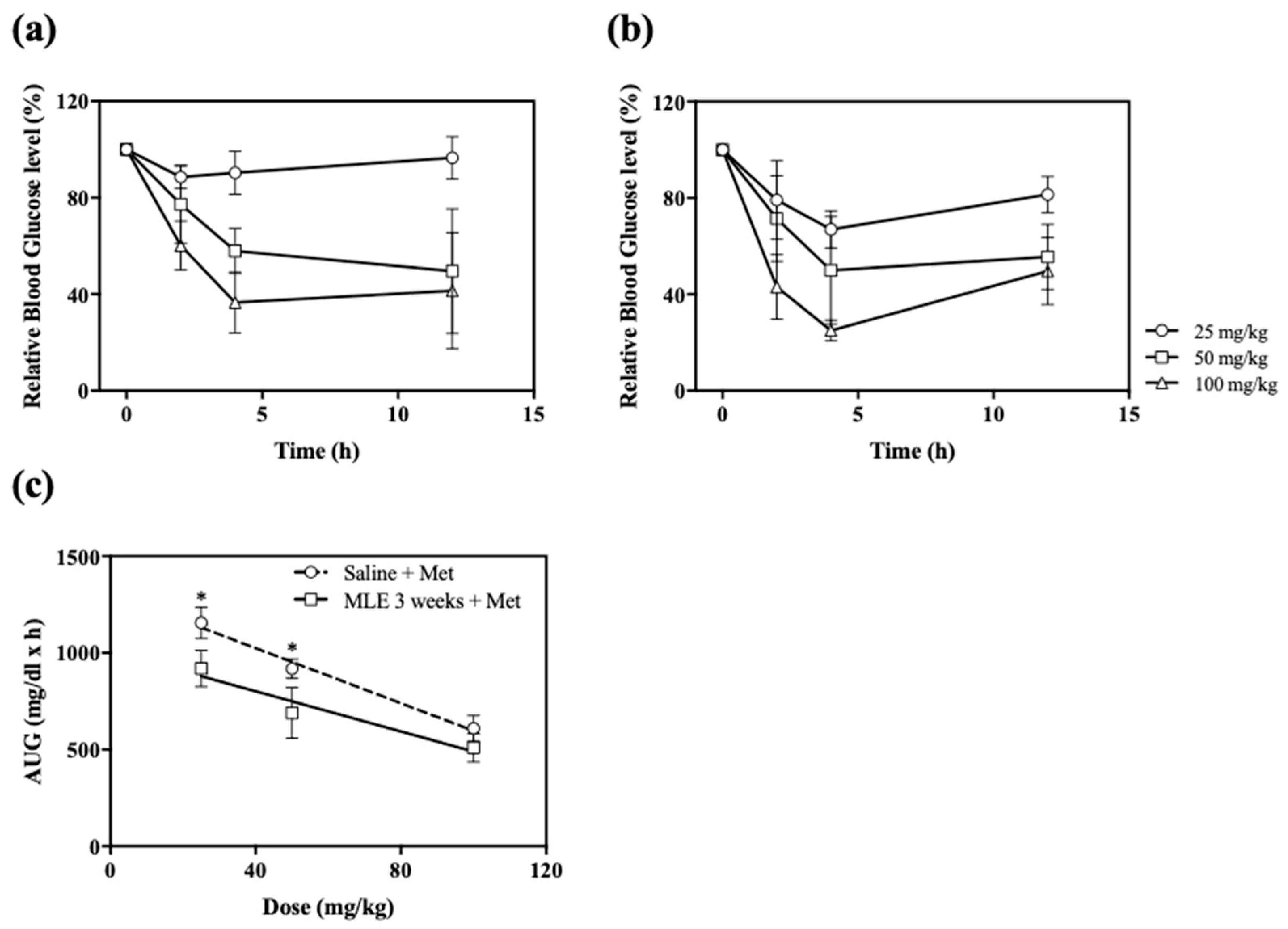
2.5. Impact of Repeated Administrations of MLE on the Anti-Hyperglycemic Effect of Various Doses of Met
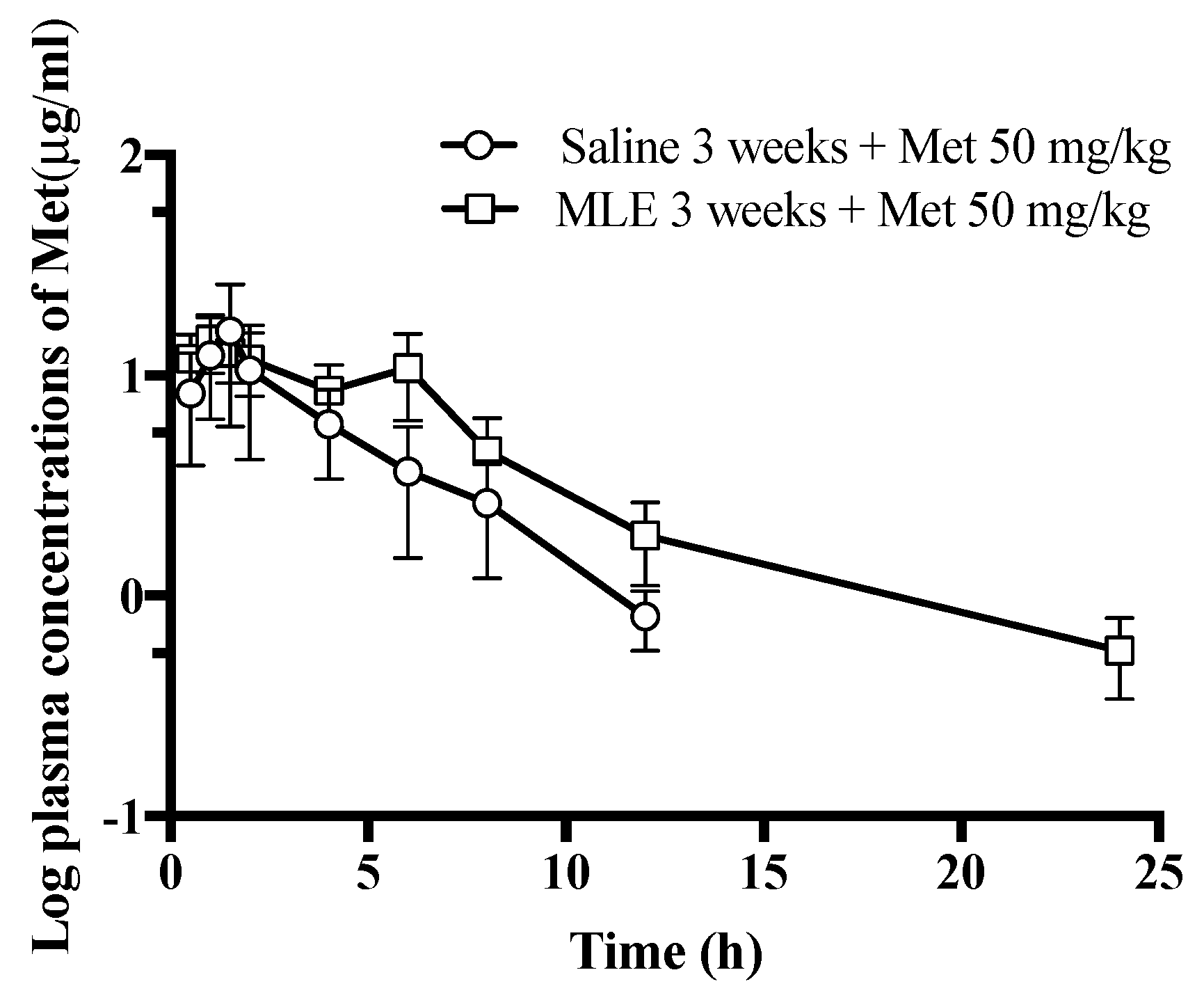
2.6. Influence of Repeated Administrations of MLE to the Pharmacokinetics of Met
2.7. Interference of MLE to Met Analysis in Rat Plasma
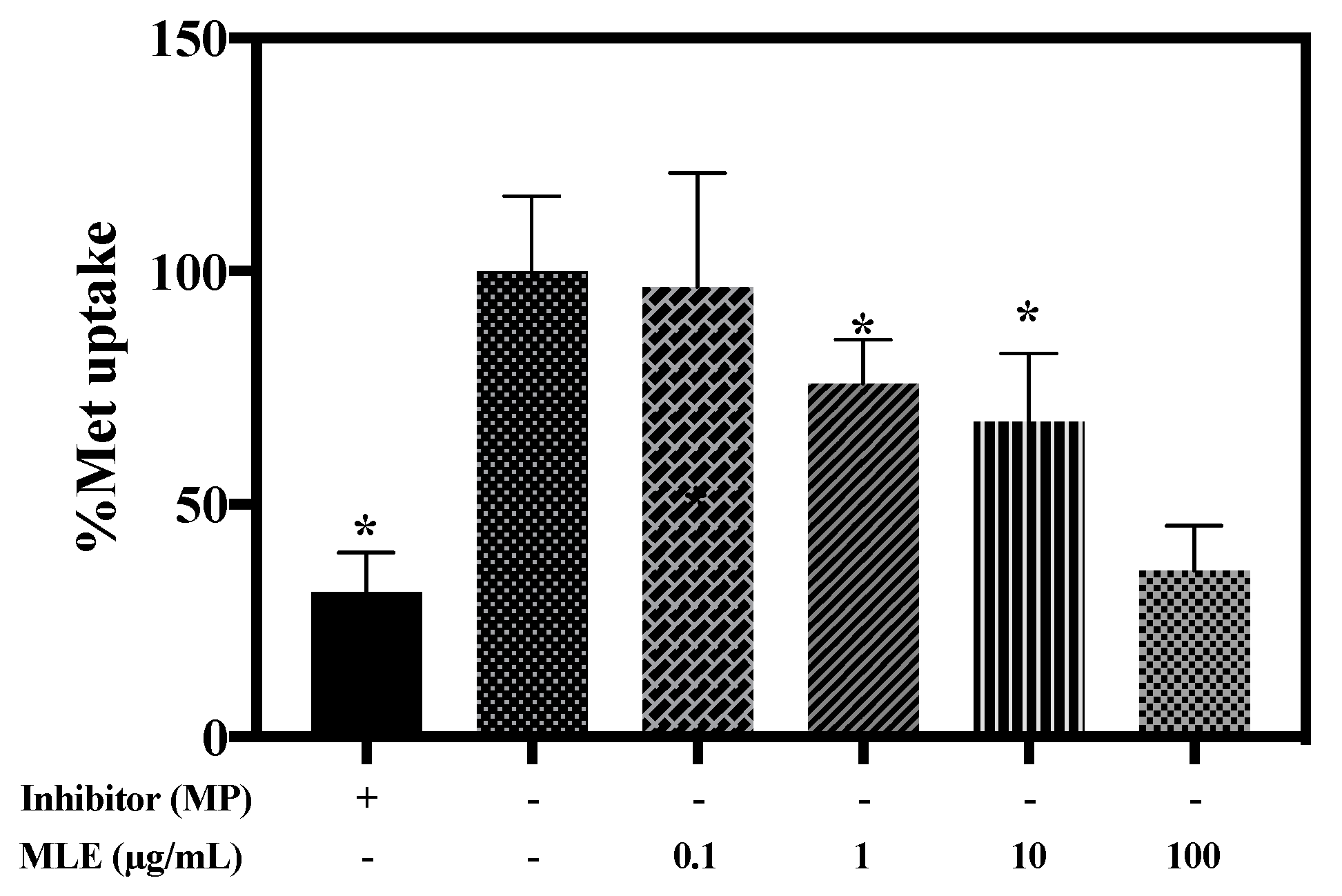
2.8. Effects of MLE on Met Uptake in HEK-293 Cells Overexpressing hOCT2
2.9. Hepatotoxicity of the Extended Intake of MLE
2.10. HPLC and LC-MS/MS Analysis
2.11. Statistical Analysis
3. Results
3.1. Anti-Hyperglycemic Effect of Repeated Administrations of MLE
3.2. Impact of Repeated Administrations of MLE on the Anti-Hyperglycemic Effect of Various Doses of Met
3.3. Influence of Repeated Administrations of MLE to the Pharmacokinetics of Met
3.4. Interference of MLE to Met Analysis in Rat Plasma
3.5. Effects of MLE on Met Uptake in HEK-293 Cells Overexpressing hOCT2
3.6. Hepatotoxicity of the Extended Intake of MLE
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marniemi, J.; Parkki, M.G. Radiochemical assay of glutathione S-epoxide transferase and its enhancement by phenobarbital in rat liver in vivo. Biochem. Pharmacol. 1975, 24, 1569–1572. [Google Scholar] [CrossRef]
- Amos, A.F.; McCarty, D.J.; Zimmet, P. The rising global burden of diabetes and its complications: Estimates and projections to the year 2010. Diabet. Med. 1997, 14 (Suppl. 5), S1–S85. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, Z. Current views on type 2 diabetes. J. Endocrinol. 2009, 204, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.Y.Y. Oral antihyperglycemic therapy for type 2 diabetes mellitus. Can. Med. Assoc. J. 2005, 172, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Brunton, L.; Knollman, B.; Hilal-Dandan, R. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 13th ed.; McGraw Hill Professional: New York, NY, USA, 2017. [Google Scholar]
- Ren, C.; Zhang, Y.; Cui, W.; Lu, G.; Wang, Y.; Gao, H.; Huang, L.; Mu, Z. A polysaccharide extract of mulberry leaf ameliorates hepatic glucose metabolism and insulin signaling in rats with type 2 diabetes induced by high fat-diet and streptozotocin. Int. J. Biol. Macromol. 2015, 72, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, C.; Lu, G.; Mu, Z.; Cui, W.; Gao, H.; Wang, Y. Anti-hyperglycemic effect of mulberry leaf polysaccharide by inhibiting pancreatic islet cell apoptosis and ameliorating insulin secretory capacity in diabetic rats. Int. Immunopharmacol. 2014, 22, 248–257. [Google Scholar] [CrossRef]
- Ştefănuţ, M.N.; Căta, A.; Pop, R.; Tănasie, C.; Boc, D.; Ienaşcu, I.; Ordodi, V. Anti-hyperglycemic effect of bilberry, blackberry and mulberry ultrasonic extracts on diabetic rats. Plant Foods Hum. Nutr. 2013, 68, 378–384. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, L.; Wang, C.; Tang, C.; He, X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (morus alba L.) polyphenol enhanced extract. PLoS ONE 2013, 8, e71144. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Flaczyk, E.; Jeszka, J.; Krejpcio, Z.; Król, E.; Buchowski, M.S. Mulberry leaf extract intake reduces hyperglycaemia in streptozotocin (STZ)-induced diabetic rats fed high-fat diet. J. Funct. Foods 2014, 8, 9–17. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Anti-hyperglycemic and anti-lipidemic effects of chlorogenic acid are mediated by AMPK activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: A contributor to the beneficial effects of coffee on diabetes. PLoS ONE 2012, 7, e32718. [Google Scholar] [CrossRef] [PubMed]
- Hunyadi, A.; Martins, A.; Hsieh, T.-J.; Seres, A.; Zupkó, I. Chlorogenic acid and rutin play a major role in the in vivo anti-hyperglycemic activity of Morus alba leaf extract on type II diabetic rats. PLoS ONE 2012, 7, e50619. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J. Antihyperglycemic and antioxidant properties of caffeic acid in db/db Mice. J. Pharmacol. Exp. Ther. 2006, 318, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.L.; Chen, Y.C.; Cheng, J.T. Caffeic acid as active principle from the fruit of Xanthium strumarium to lower plasma glucose in diabetic rats. Planta Med. 2000, 66, 228–230. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 1–6. [Google Scholar] [CrossRef]
- Huang, D.-W.; Shen, S.-C.; Wu, J.S.-B. Effects of Caffeic Acid and Cinnamic Acid on Glucose Uptake in Insulin-Resistant Mouse Hepatocytes. J. Agric. Food Chem. 2009, 57, 7687–7692. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Kumar, A.; Doble, M. Combination therapy: A new strategy to manage diabetes and its complications. Phytomedicine 2014, 21, 123–130. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Doble, M. Effect of natural products on commercial oral antidiabetic drugs in enhancing 2-deoxyglucose uptake by 3T3-L1 adipocytes. Ther. Adv. Endocrinol. 2011, 2, 103–114. [Google Scholar] [CrossRef]
- Amin, M.; Suksomboon, N. Pharmacotherapy of type 2 diabetes mellitus: An update on drug-drug interactions. Drug Saf. 2014, 37, 903–919. [Google Scholar] [CrossRef]
- May, M.; Schindler, C. Clinically and pharmacologically relevant interactions of antidiabetic drugs. Ther. Adv. Endocrinol. 2016, 7, 69–83. [Google Scholar] [CrossRef]
- Ríos, J.L.; Francini, F.; Schinella, G.R. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef] [PubMed]
- You, B.H.; Chin, Y.W.; Kim, H.; Choi, H.S.; Choi, Y.H. Houttuynia cordata extract increased systemic exposure and liver concentrations of metformin through OCTs and MATEs in rats. Phytother. Res. 2018, 32, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Heyneke, O.; Brown, D.; van Wyk, J.P.H.; Hamman, J.H. Impact of traditional medicinal plant extracts on antiretroviral drug absorption. J. Ethnopharmacol. 2008, 119, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Nabekura, T.; Kamiyama, S.; Kitagawa, S. Effects of dietary chemopreventive phytochemicals on P-glycoprotein function. Biochem. Biophys. Res. Commun. 2005, 327, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Juan, H.; Terhaag, B.; Cong, Z.; Bi-Kui, Z.; Rong-Hua, Z.; Feng, W.; Fen-Li, S.; Juan, S.; Jing, T.; Wen-Xing, P. Unexpected effect of concomitantly administered curcumin on the pharmacokinetics of talinolol in healthy Chinese volunteers. Eur. J. Clin. Pharmacol. 2007, 63, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.; Montori, V.M.; Wu, P.; Gallicano, K.; Clarke, M.; Guyatt, G. Interaction of St John’s wort with conventional drugs: Systematic review of clinical trials. BMJ 2004, 329, 27–30. [Google Scholar] [CrossRef]
- Rehman, S.U.; Choi, M.S.; Choe, K.; Yoo, H.H. Interactions between herbs and antidiabetics: An overview of the mechanisms, evidence, importance, and management. Arch. Pharm. Res. 2015, 38, 1281–1298. [Google Scholar] [CrossRef]
- Son, G.-H.; Lee, H.-J.; Na, Y.-G.; Lee, H.-K.; Kim, S.-J.; Huh, H.-W.; Kim, K.-T.; Kang, J.-S.; Kim, Y.-H.; Myung, C.-S.; et al. Formulation and statistical analysis of an herbal medicine tablet containing Morus alba leaf extracts. J. Pharm. Investig. 2018, 49, 625–634. [Google Scholar] [CrossRef]
- Jung, S.-H.; Han, J.-H.; Park, H.-S.; Lee, D.-H.; Kim, S.J.; Cho, H.S.; Kang, J.S.; Myung, C.-S. Effects of unaltered and bioconverted mulberry leaf extracts on cellular glucose uptake and antidiabetic action in animals. BMC Complement. Altern. Med. 2019, 19, 55. [Google Scholar] [CrossRef]
- Choi, Y.H.; Lee, D.C.; Lee, I.; Lee, M.G. Changes in metformin pharmacokinetics after intravenous and oral administration to rats with short-term and extended diabetes induced by streptozotocin. J. Pharm. Sci. 2008, 97, 5363–5375. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, S.G.; Lee, M.G. Dose-independent pharmacokinetics of metformin in rats: Hepatic and gastrointestinal first-pass effects. J. Pharm. Sci. 2006, 95, 2543–2552. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services; FDA. Bioanalytical Method Validation, Guidance for Industry. 2001. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 30 May 2018).
- Gong, L.; Goswami, S.; Giacomini, K.M.; Altman, R.B.; Klein, T.E. Metformin pathways: Pharmacokinetics and pharmacodynamics. Pharmacogenet. Genom. 2012, 22, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Z.; Xu, H.D.; Kim, K.H.; Ahn, T.H.; Bae, J.S.; Lee, J.Y.; Woo, S.J.; Yoo, H.J.; Lee, H.K.; Kim, K.H.; et al. Reference data of the main physiological parameters in control Sprague-Dawley rats from pre-clinical toxicity studies. Lab. Anim. Res. 2010, 26, 153–164. [Google Scholar] [CrossRef]
- Andallu, B.; Suryakantham, V.; Srikanthi, B.L.; Reddy, G.K. Effect of mulberry (Morus indica L.) therapy on plasma and erythrocyte membrane lipids in patients with type 2 diabetes. Clin. Chim. Acta 2001, 314, 47–53. [Google Scholar] [CrossRef]
- Park, M.-Y.; Lee, K.-S.; Sung, M.-K. Effects of dietary mulberry, Korean red ginseng, and banaba on glucose homeostasis in relation to PPAR-α, PPAR-γ, and LPL mRNA expressions. Life Sci. 2005, 77, 3344–3354. [Google Scholar] [CrossRef] [PubMed]
- Andallu, B.; Varadacharyulu, N.C. Gluconeogenic substrates and hepatic gluconeogenic enzymes in streptozotocin-diabetic rats: Effect of mulberry (Morus indica L.) Leaves. J. Med. Food 2007, 10, 41–48. [Google Scholar] [CrossRef]
- Miyahara, C.; Miyazawa, M.; Satoh, S.; Sakai, A.; Mizusaki, S. Inhibitory effects of mulberry leaf extract on postprandial hyperglycemia in normal rats. J. Nutr. Sci. Vitaminol. 2004, 50, 161–164. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Kanto, K.; Ito, H.; Noso, S.; Babaya, N.; Hiromine, Y.; Taketomo, Y.; Toma, J.; Niwano, F.; Yasutake, S.; Kawabata, Y.; et al. Effects of dosage and dosing frequency on the efficacy and safety of high-dose metformin in Japanese patients with type 2 diabetes mellitus. J. Diabetes. Investig. 2018, 9, 587–593. [Google Scholar] [CrossRef]
- Holstein, A.; Beil, W.; Kovacs, P. CYP2C metabolism of oral antidiabetic drugs-impact on pharmacokinetics, drug interactions and pharmacogenetic aspects. Expert Opin. Drug Metab. Toxicol. 2012, 8, 1549–1563. [Google Scholar] [CrossRef]
- Shu, Y.; Sheardown, S.A.; Brown, C.; Owen, R.P.; Zhang, S.; Castro, R.A.; Ianculescu, A.G.; Yue, L.; Lo, J.C.; Burchard, E.G.; et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J. Clin. Investig. 2007, 117, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Kudolo, G.B.; Wang, W.; Javors, M.; Blodgett, J. The effect of the ingestion of Ginkgo biloba extract (EGb 761) on the pharmacokinetics of metformin in non-diabetic and type 2 diabetic subjects—A double blind placebo-controlled, crossover study. Clin. Nutr. 2006, 25, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Geng, T.; Si, H.; Kang, D.; Li, Y.; Huang, W.; Ding, G.; Wang, Z.; Bi, Y.; Zhang, H.; Xiao, W. Influences of Re Du Ning Injection, a traditional Chinese medicine injection, on the CYP450 activities in rats using a cocktail method. J. Ethnopharmacol. 2015, 174, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Geng, T.; Zhang, S.; Kang, D.; Li, Y.; Herbal, G.D.C. Inhibition of Re Du Ning Injection on enzyme activities of rat liver microsomes using cocktail method. Chin. Herb. Med. 2016, 8, 231–241. [Google Scholar] [CrossRef]

| Parameters | Unit | Saline 3 Weeks + Met | MLE 3 Weeks + Met |
|---|---|---|---|
| T1/2λz | h | 3.36 ± 1.11 | 4.4 ± 1.27 |
| Tmax | h | 1.7 ± 1.3 | 1.9 ± 2.3 |
| Cmax | μg/mL | 10.85 ± 9.66 | 12.17 ± 1.06 |
| AUC0–24 | h μg/mL | 42.74 ± 33.29 | 73.75 ± 15.23 |
| V/F | mL/kg | 6324.98 ± 2915.05 | 4143.72 ± 1291.5 |
| Cl/F | mL/h/kg | 1381.29 ± 545.57 | 657.74 ± 150.25 ** |
| Condition | QC | Intra-Day (n = 6) | Inter-Day (n = 5) | ||
|---|---|---|---|---|---|
| RSD% | RE% | RSD% | RE% | ||
| Met in rat plasma | LQC | 9.29 | 7.65 | 7.17 | 9.87 |
| MQC | 8.47 | 8.48 | 9.6 | 8.91 | |
| HQC | 3.01 | 2.17 | 5.03 | 3.77 | |
| Met in rat plasma spiked with MLE | LQC | 9.2 | 9.63 | 6.35 | 6.01 |
| MQC | 8.11 | 7.82 | 8.72 | 8.62 | |
| HQC | 2.49 | 2.03 | 4.13 | 3.14 | |
| Parameters | Units | Groups | |
|---|---|---|---|
| Control Group | MLE Group | ||
| ALP | U/L | 142.5 ± 10.2 | 145.3 ± 20.8 |
| ALT | U/L | 40.4 ± 10.3 | 42.0 ± 9.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huh, H.W.; Na, Y.-G.; Bang, K.-H.; Kim, S.-J.; Kim, M.; Kim, K.-T.; Kang, J.-S.; Kim, Y.-H.; Baek, J.-S.; Lee, H.-K.; et al. Extended Intake of Mulberry Leaf Extract Delayed Metformin Elimination via Inhibiting the Organic Cation Transporter 2. Pharmaceutics 2020, 12, 49. https://doi.org/10.3390/pharmaceutics12010049
Huh HW, Na Y-G, Bang K-H, Kim S-J, Kim M, Kim K-T, Kang J-S, Kim Y-H, Baek J-S, Lee H-K, et al. Extended Intake of Mulberry Leaf Extract Delayed Metformin Elimination via Inhibiting the Organic Cation Transporter 2. Pharmaceutics. 2020; 12(1):49. https://doi.org/10.3390/pharmaceutics12010049
Chicago/Turabian StyleHuh, Hyun Wook, Young-Guk Na, Ki-Hyun Bang, Sung-Jin Kim, Minki Kim, Kyung-Tae Kim, Jong-Seong Kang, Young-Ho Kim, Jong-Suep Baek, Hong-Ki Lee, and et al. 2020. "Extended Intake of Mulberry Leaf Extract Delayed Metformin Elimination via Inhibiting the Organic Cation Transporter 2" Pharmaceutics 12, no. 1: 49. https://doi.org/10.3390/pharmaceutics12010049
APA StyleHuh, H. W., Na, Y.-G., Bang, K.-H., Kim, S.-J., Kim, M., Kim, K.-T., Kang, J.-S., Kim, Y.-H., Baek, J.-S., Lee, H.-K., & Cho, C.-W. (2020). Extended Intake of Mulberry Leaf Extract Delayed Metformin Elimination via Inhibiting the Organic Cation Transporter 2. Pharmaceutics, 12(1), 49. https://doi.org/10.3390/pharmaceutics12010049







