Molecularly Imprinted Polymers (MIPs) as Theranostic Systems for Sunitinib Controlled Release and Self-Monitoring in Cancer Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
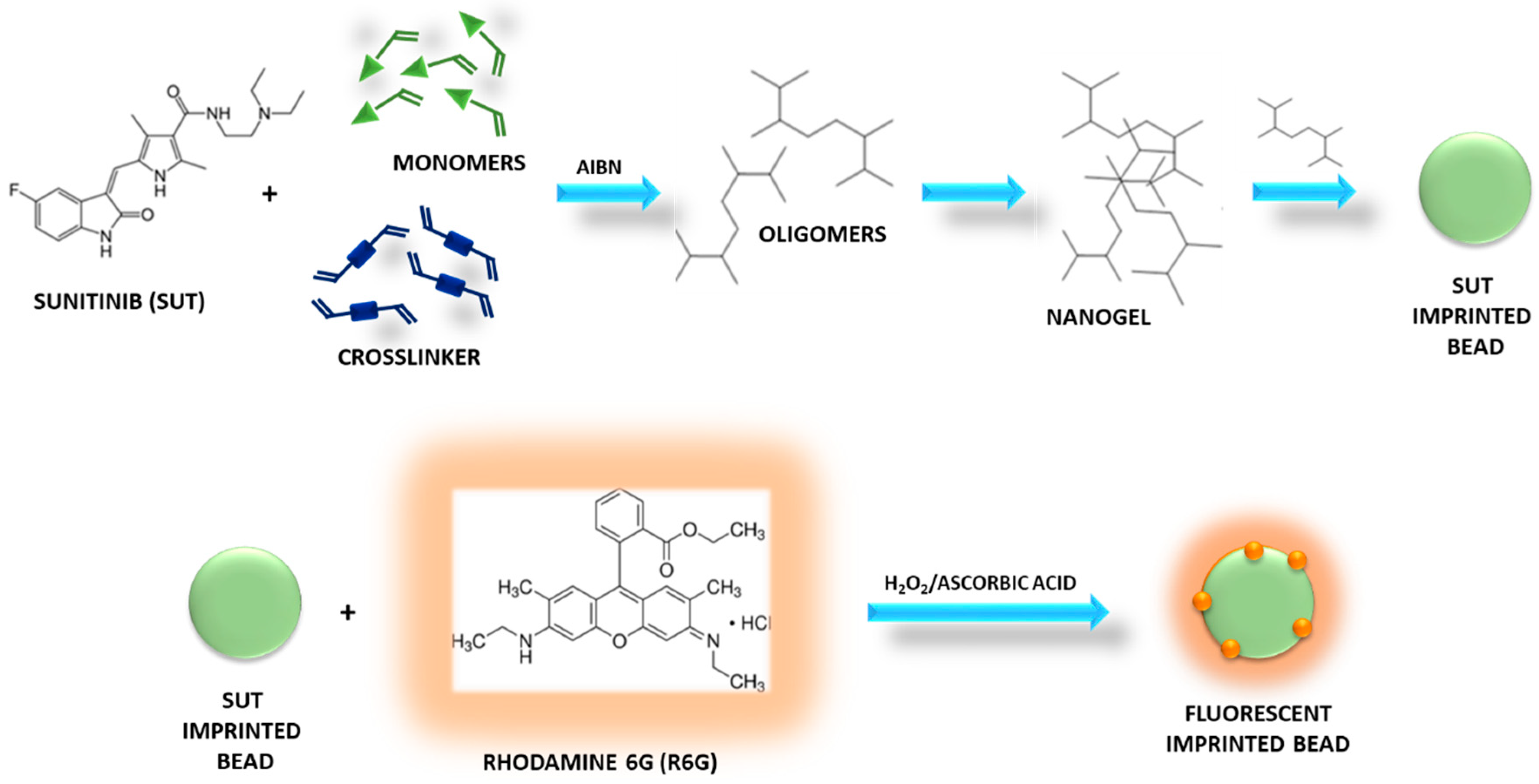
2.3. Synthesis of the Sunitinib Molecularly Imprinted Polymer (SUT-MIP)
2.4. Functionalization of Imprinted and Non-Imprinted Polymeric Particles
2.5. Binding Studies
2.5.1. Static Equilibrium Adsorption Experiments
2.5.2. Kinetic Adsorption Experiments
2.6. Protein Adsorption Measurement and Swelling Behavior
2.7. Drug Loading Procedure and In Vitro Release Studies
3. Results and Discussion
3.1. Synthesis of Sunitinib Molecularly Imprinted Polymers Functionalized with Rhodamine 6G (R6G)
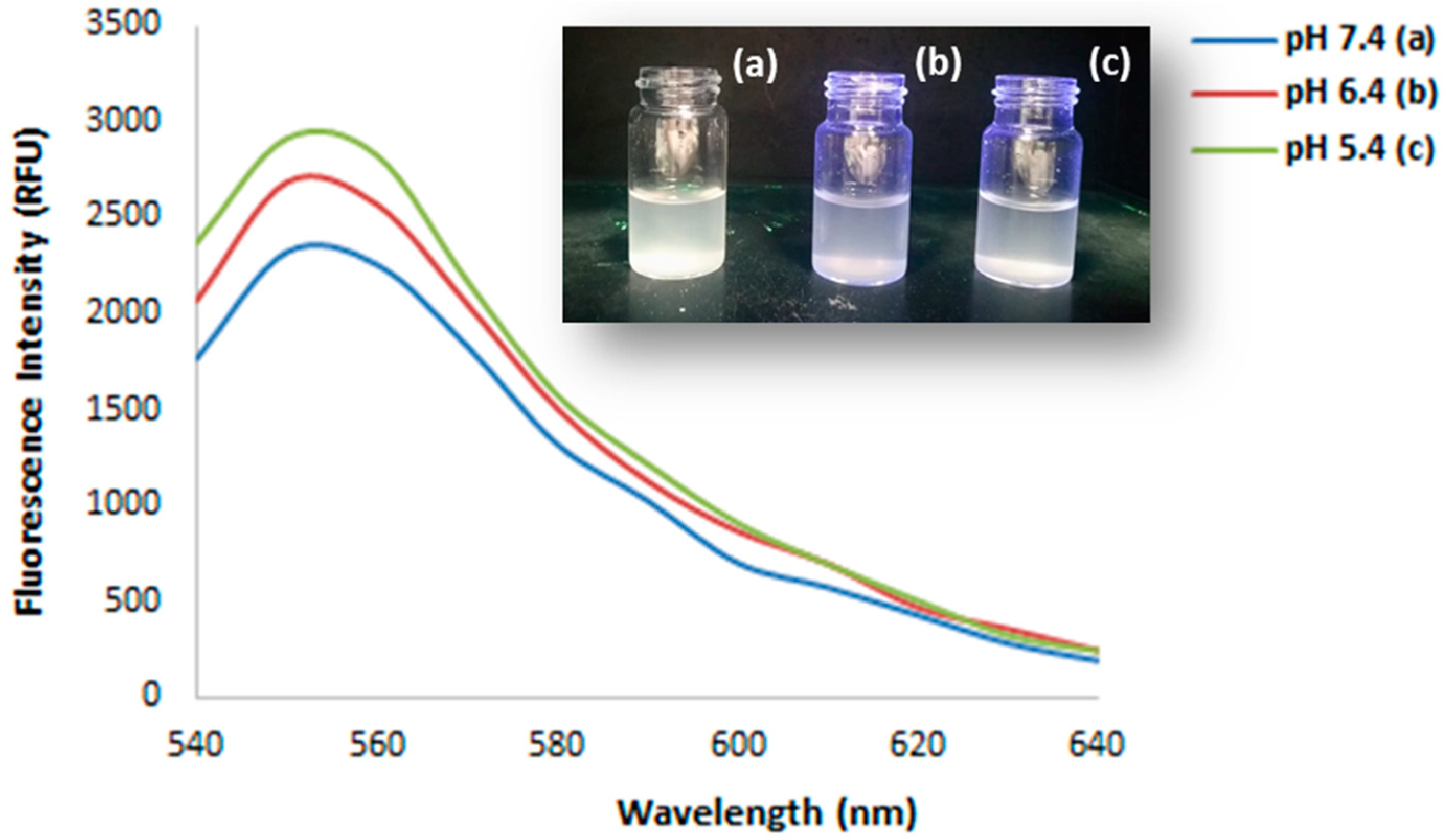
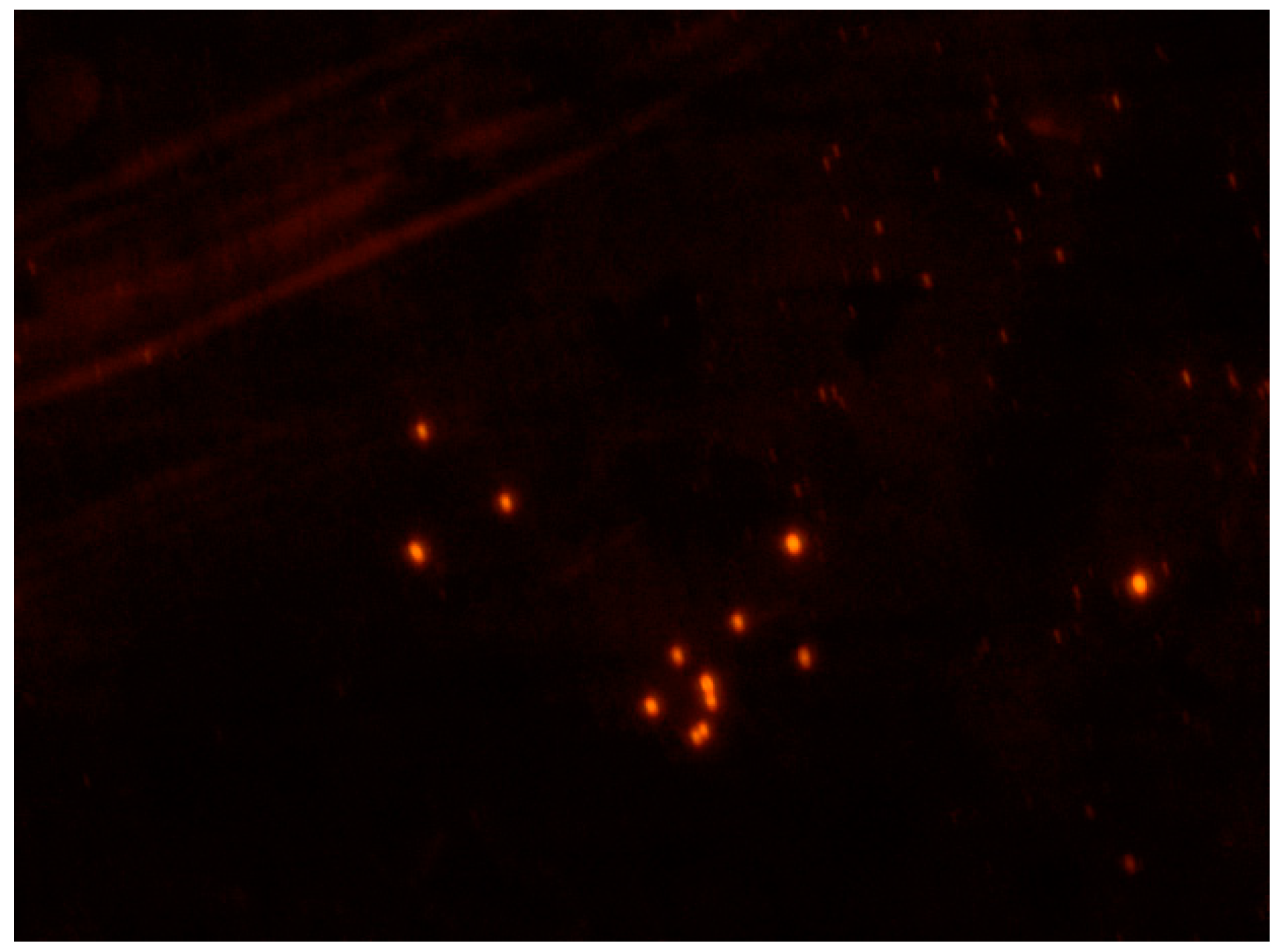
3.2. Characterization of Fluorescent Sunitinib Molecularly Imprinted Polymer (SUT-MIP)
3.3. Binding Studies
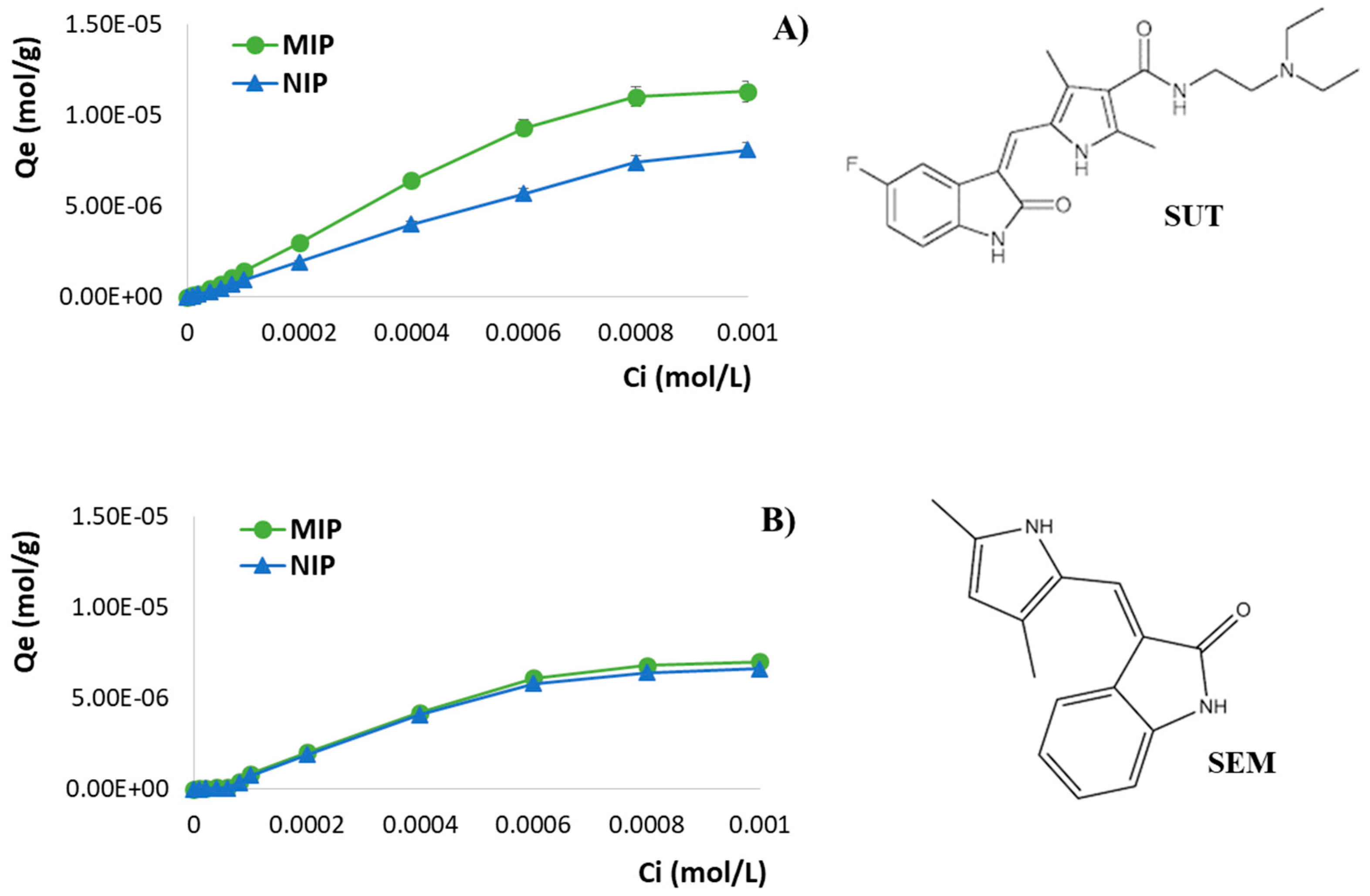
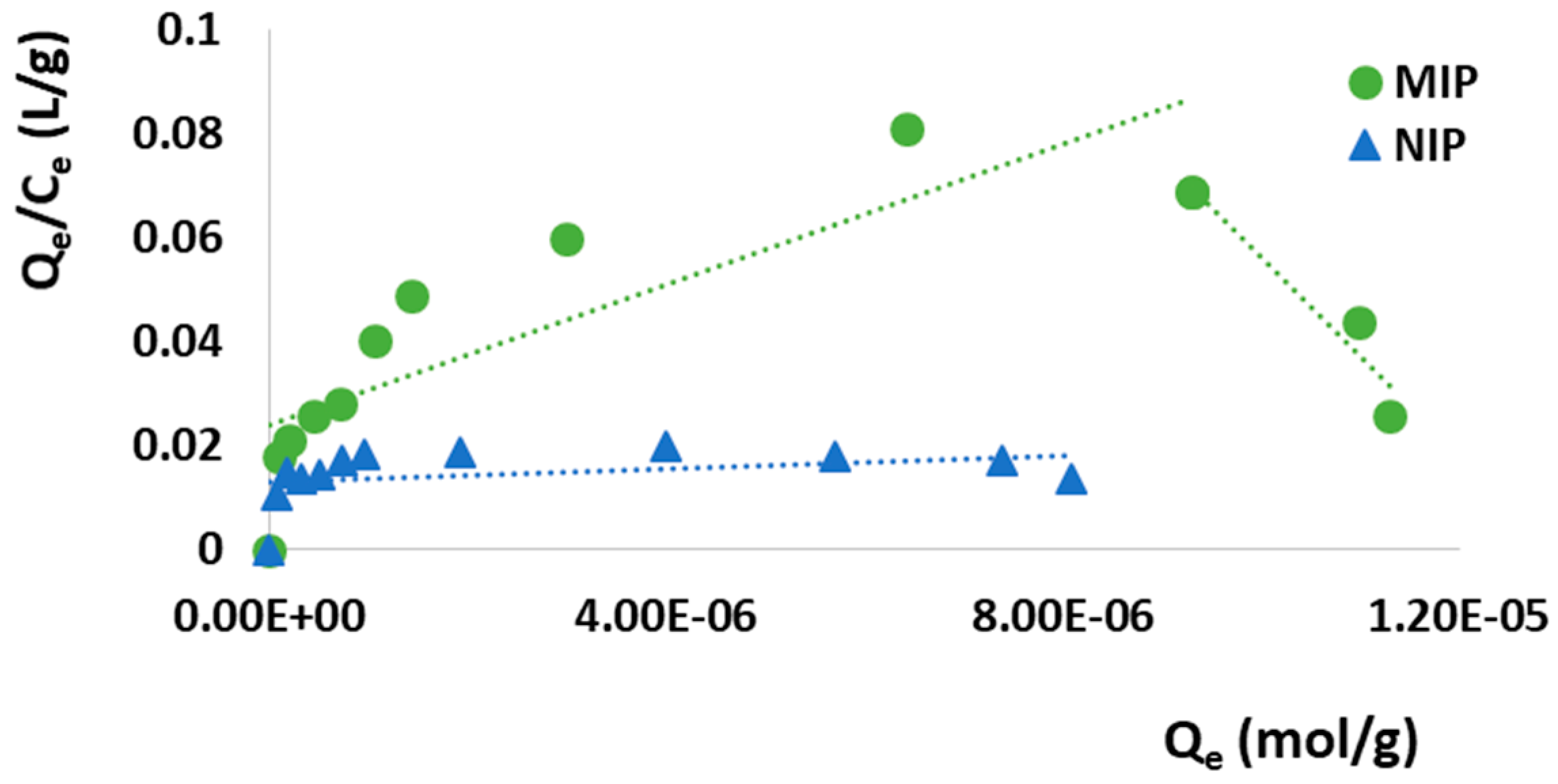
3.3.1. Imprinting Efficacy and Selectivity
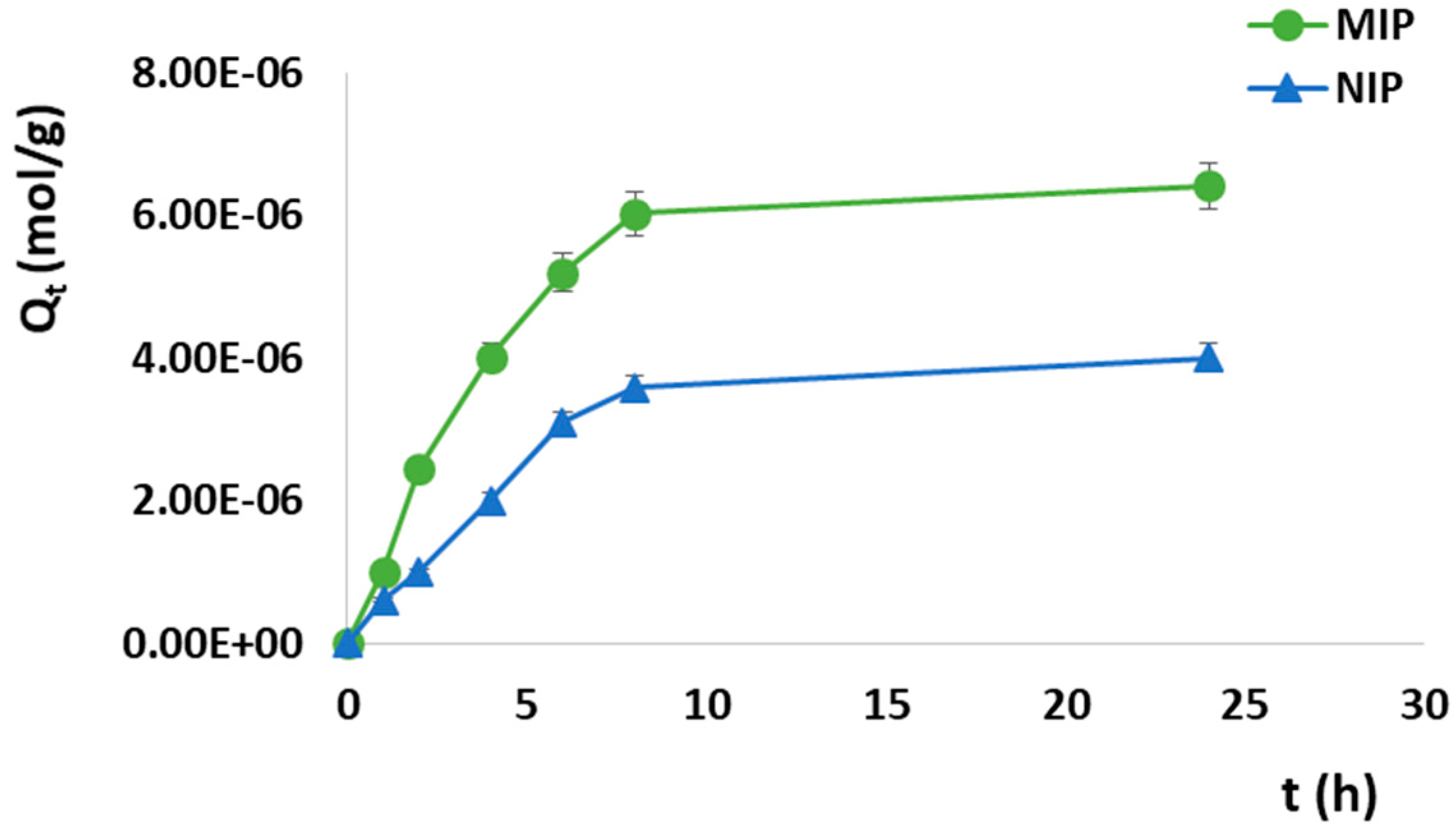
3.3.2. Adsorption Kinetics
3.4. Protein Adsorption Measurement and Swelling Behavior
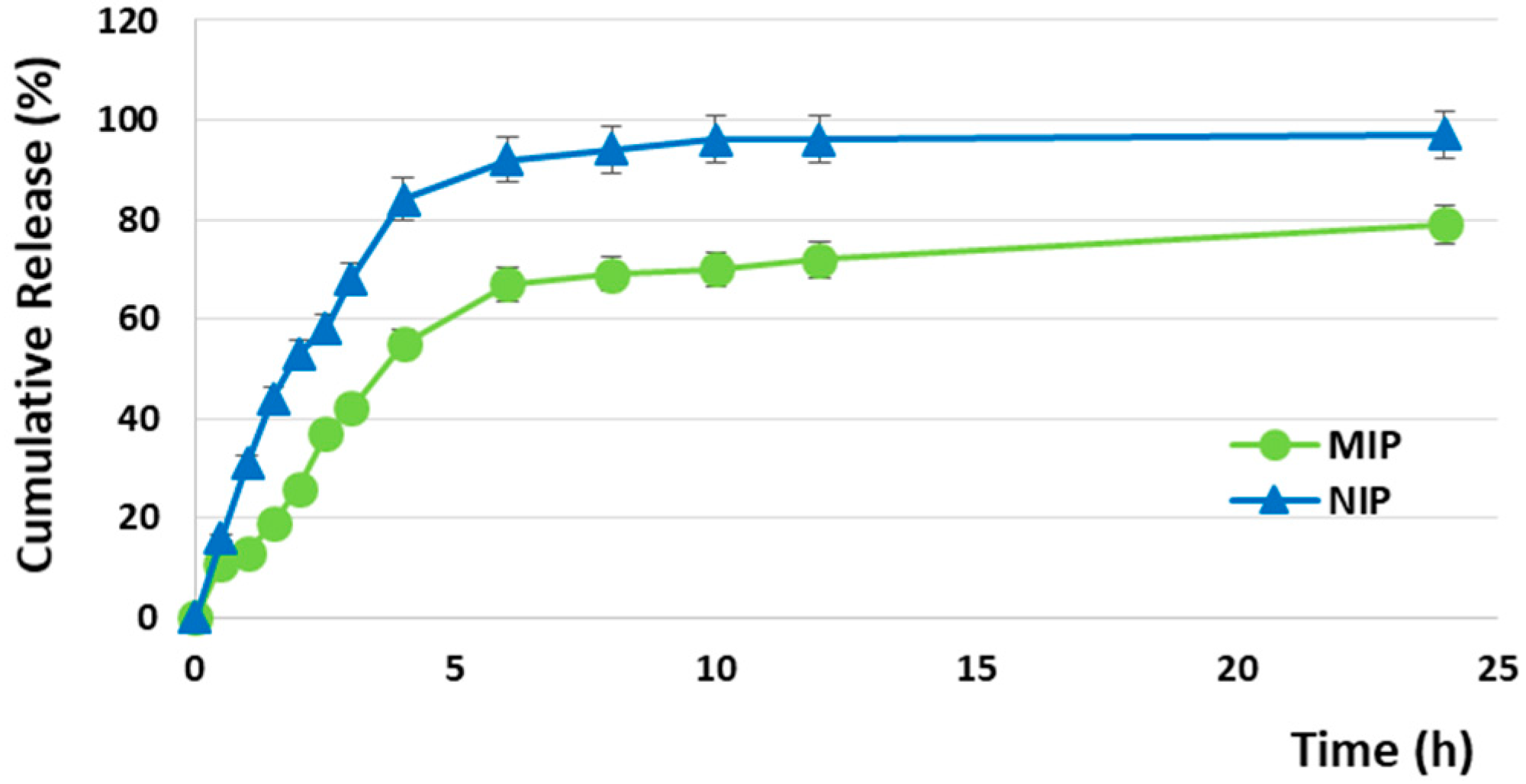
3.5. In Vitro Release Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77. [Google Scholar] [CrossRef]
- Bhojani, M.S.; Van Dort, M.; Rehemtulla, A.; Ross, B.D. Targeted imaging and therapy of brain cancer using theranostic nanoparticles. Mol. Pharm. 2010, 7, 1921–1929. [Google Scholar] [CrossRef]
- Cirillo, G.; Parisi, O.I.; Curcio, M.; Puoci, F.; Iemma, F.; Spizzirri, U.G.; Picci, N. Molecularly imprinted polymers as drug delivery systems for the sustained release of glycyrrhizic acid. J. Pharm. Pharmacol. 2010, 62, 577–582. [Google Scholar] [CrossRef]
- Scrivano, L.; Parisi, O.I.; Iacopetta, D.; Ruffo, M.; Ceramella, J.; Sinicropi, M.S.; Puoci, F. Molecularly imprinted hydrogels for sustained release of sunitinib in breast cancer therapy. Polym. Adv. Technol. 2019, 30, 743–748. [Google Scholar] [CrossRef]
- Pellizzoni, E.; Tommasini, M.; Marangon, E.; Rizzolio, F.; Saito, G.; Benedetti, F.; Toffoli, G.; Resmini, M.; Berti, F. Fluorescent molecularly imprinted nanogels for the detection of anticancer drugs in human plasma. Biosens. Bioelectron. 2016, 86, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Gui, R.; Jin, H. Recent advances in synthetic methods and applications of photo-luminescent molecularly imprinted polymers. J. Photochem. Photobiol. C Photochem. Rev. 2019. [Google Scholar] [CrossRef]
- Yang, Q.; Li, J.; Wang, X.; Peng, H.; Xiong, H.; Chen, L. Strategies of molecular imprinting-based fluorescence sensors for chemical and biological analysis. Biosens. Bioelectron. 2018, 112, 54–71. [Google Scholar] [CrossRef]
- Qin, Y.-T.; Peng, H.; He, X.-W.; Li, W.-Y.; Zhang, Y. Highly Effective Drug Delivery and Cell Imaging Using Fluorescent Double-Imprinted Nanoparticles by Targeting Recognition of the Epitope of Membrane Protein. Anal. Chem. 2019, 91, 12696–12703. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, H.; Zhao, J. Risk of fatal adverse events in cancer patients treated with sunitinib. Crit. Rev. Oncol. Hematol. 2019. [Google Scholar] [CrossRef]
- Parisi, O.I.; Morelli, C.; Scrivano, L.; Sinicropi, M.S.; Cesario, M.G.; Candamano, S.; Puoci, F.; Sisci, D. Controlled release of sunitinib in targeted cancer therapy: Smart magnetically responsive hydrogels as restricted access materials. RSC Adv. 2015, 5, 65308–65315. [Google Scholar] [CrossRef]
- Baggstrom, M.Q.; Socinski, M.A.; Wang, X.F.; Gu, L.; Stinchcombe, T.E.; Edelman, M.J.; Baker, S., Jr.; Feliciano, J.; Novotny, P.; Hahn, O. Maintenance sunitinib following initial platinum-based combination chemotherapy in advanced-stage IIIB/IV non–small cell lung cancer: A randomized, double-blind, placebo-controlled phase III study—CALGB 30607 (Alliance). J. Thoracic Oncol. 2017, 12, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.H.; Liu, M.-C.; Lee, S.C.; Vanlemmens, L.; Ferrero, J.-M.; Tabei, T.; Pivot, X.; Iwata, H.; Aogi, K.; Lugo-Quintana, R. Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Res. Treat. 2010, 121, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Carrato, A.; Swieboda-Sadlej, A.; Staszewska-Skurczynska, M.; Lim, R.; Roman, L.; Shparyk, Y.; Bondarenko, I.; Jonker, D.J.; Sun, Y.; De la Cruz, J.A. Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: A randomized, phase III trial. J. Clin. Oncol. 2013, 31, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; van Oosterom, A.T.; Garrett, C.R.; Blackstein, M.E.; Shah, M.H.; Verweij, J.; McArthur, G.; Judson, I.R.; Heinrich, M.C.; Morgan, J.A. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 2006, 368, 1329–1338. [Google Scholar] [CrossRef]
- Michaelson, M.D.; Oudard, S.; Ou, Y.-C.; Sengeløv, L.; Saad, F.; Houede, N.; Ostler, P.; Stenzl, A.; Daugaard, G.; Jones, R. Randomized, placebo-controlled, phase III trial of sunitinib plus prednisone versus prednisone alone in progressive, metastatic, castration-resistant prostate cancer. J. Clin. Oncol. 2013, 32, 76–82. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.-L.; Bang, Y.-J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef]
- Ramazani, F.; Hiemstra, C.; Steendam, R.; Kazazi-Hyseni, F.; Van Nostrum, C.; Storm, G.; Kiessling, F.; Lammers, T.; Hennink, W.E.; Kok, R. Sunitinib microspheres based on [PDLLA-PEG-PDLLA]-b-PLLA multi-block copolymers for ocular drug delivery. Eur. J. Pharm. Biopharm. 2015, 95, 368–377. [Google Scholar] [CrossRef]
- Joseph, J.J.; Sangeetha, D.; Shivashankar, M. In vitro Release and Cytotoxic Studies of Novel Alginate Nanocarrier for the Antitumor Drug: Sunitinib. Regen. Eng. Transl. Med. 2019, 5, 220–227. [Google Scholar] [CrossRef]
- Alshetaili, A.S.; Anwer, M.K.; Alshahrani, S.M.; Alalaiwe, A.; Alsulays, B.B.; Ansari, M.J.; Imam, F.; Alshehri, S. Characteristics and anticancer properties of Sunitinib malate-loaded poly-lactic-co-glycolic acid nanoparticles against human colon cancer HT-29 cells lines. Trop. J. Pharm. Res. 2018, 17, 1263–1269. [Google Scholar] [CrossRef]
- Scrivano, L.; Iacopetta, D.; Sinicropi, M.S.; Saturnino, C.; Longo, P.; Parisi, O.I.; Puoci, F. Synthesis of sericin-based conjugates by click chemistry: Enhancement of sunitinib bioavailability and cell membrane permeation. Drug Deliv. 2017, 24, 482–490. [Google Scholar] [CrossRef][Green Version]
- Provencher, S.W. CONTIN: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Parisi, O.I.; Morelli, C.; Puoci, F.; Saturnino, C.; Caruso, A.; Sisci, D.; Trombino, G.E.; Picci, N.; Sinicropi, M.S. Magnetic molecularly imprinted polymers (MMIPs) for carbazole derivative release in targeted cancer therapy. J. Mater. Chem. B 2014, 2, 6619–6625. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, H.; Cheng, X. Molecularly imprinted polymer nanocarriers for recognition and sustained release of diclofenac. Polym. Adv. Technol. 2018, 29, 1360–1371. [Google Scholar] [CrossRef]
- Pardeshi, S.; Singh, S.K. Precipitation polymerization: A versatile tool for preparing molecularly imprinted polymer beads for chromatography applications. Rsc Adv. 2016, 6, 23525–23536. [Google Scholar] [CrossRef]
- Girones Molera, J.; Alberto Mendez, J.; San Roman, J. Bioresorbable and Nonresorbable Polymers for Bone Tissue Engineering. Curr. Pharm. Des. 2012, 18, 2536–2557. [Google Scholar] [CrossRef]
- James, H.P.; John, R.; Alex, A.; Anoop, K. Smart polymers for the controlled delivery of drugs–a concise overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef]
- Traitel, T.; Goldbart, R.; Kost, J. Smart polymers for responsive drug-delivery systems. J. Biomater. Sci. Polym. Ed. 2008, 19, 755–767. [Google Scholar] [CrossRef]
- Xinming, L.; Yingde, C.; Lloyd, A.W.; Mikhalovsky, S.V.; Sandeman, S.R.; Howel, C.A.; Liewen, L. Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems: A review. Contact Lens Anterior Eye 2008, 31, 57–64. [Google Scholar] [CrossRef]
- Spizzirri, U.; Altimari, I.; Puoci, F.; Parisi, O.; Iemma, F.; Picci, N. Innovative antioxidant thermo-responsive hydrogels by radical grafting of catechin on inulin chain. Carbohydr. Polym. 2011, 84, 517–523. [Google Scholar] [CrossRef]
- Liu, J.; Pu, H.; Chen, C.; Liu, Y.; Bai, R.; Kan, J.; Jin, C. Reaction mechanisms and structural and physicochemical properties of caffeic acid grafted chitosan synthesized in ascorbic acid and hydroxyl peroxide redox system. J. Agric. Food Chem. 2017, 66, 279–289. [Google Scholar] [CrossRef]
- Pei, M.; Jia, X.; Li, G.; Liu, P. Versatile Polymeric Microspheres with Tumor Microenvironment Bioreducible Degradation, pH-Activated Surface Charge Reversal, pH-Triggered “off–on” Fluorescence and Drug Release as Theranostic Nanoplatforms. Mol. Pharm. 2018, 16, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhao, X.; Tian, K.; Zhou, T.; Li, J.; Zhang, R.; Liu, P. Novel fluorescent pH/reduction dual stimuli-responsive polymeric nanoparticles for intracellular triggered anticancer drug release. Chem. Eng. J. 2016, 295, 468–476. [Google Scholar] [CrossRef]
- Parisi, O.; Ruffo, M.; Scrivano, L.; Malivindi, R.; Vassallo, A.; Puoci, F. Smart Bandage Based on Molecularly Imprinted Polymers (MIPs) for Diclofenac Controlled Release. Pharmaceuticals 2018, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, L.; Zhu, H.; Jin, R.; Xing, J. Comparative study of capsaicin molecularly imprinted polymers prepared by different polymerization methods. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 157–164. [Google Scholar] [CrossRef]
- Viveiros, R.; Lopes, M.I.; Heggie, W.; Casimiro, T. Green approach on the development of lock-and-key polymers for API purification. Chem. Eng. J. 2017, 308, 229–239. [Google Scholar] [CrossRef]
- Naowanat, N.; Thouchprasitchai, N.; Pongstabodee, S. Adsorption of emulsified oil from metalworking fluid on activated bleaching earth-chitosan-SDS composites: Optimization, kinetics, isotherms. J. Environ. Manag. 2016, 169, 103–115. [Google Scholar] [CrossRef]
- Arici, M.; Topbas, O.; Karavana, S.Y.; Ertan, G.; Sariisik, M.; Ozturk, C. Preparation of naproxen–ethyl cellulose microparticles by spray-drying technique and their application to textile materials. J. Microencapsul. 2014, 31, 654–666. [Google Scholar] [CrossRef]
- Rinaki, E.; Valsami, G.; Macheras, P. The power law can describe the ‘entire’drug release curve from HPMC-based matrix tablets: A hypothesis. Int. J. Pharm. 2003, 255, 199–207. [Google Scholar] [CrossRef]
- Tang, L.; Zhao, C.-Y.; Wang, X.-H.; Li, R.-S.; Yang, J.-R.; Huang, Y.-P.; Liu, Z.-S. Macromolecular crowding of molecular imprinting: A facile pathway to produce drug delivery devices for zero-order sustained release. Int. J. Pharm. 2015, 496, 822–833. [Google Scholar] [CrossRef]
- Chidambaram, N.; Porter, W.; Flood, K.; Qiu, Y. Formulation and characterization of new layered diffusional matrices for zero-order sustained release. J. Control. Release 1998, 52, 149–158. [Google Scholar] [CrossRef]
| Parameter | MIP | NIP |
|---|---|---|
| Hydrodynamic mean diameter (nm) | 714.6 ± 9.7 | 709.2 ± 9.9 |
| Polydispersity Index (PI) | 0.305 | 0.297 |
| ζ-Potential (mV) | −23.8 ± 0.3 | −29.3 ± 0.4 |
| Ci (mol/L) | Bound SUT (%) | Bound SEM (%) | α SUT | α SEM | ɛ | ||
|---|---|---|---|---|---|---|---|
| MIP | NIP | MIP | NIP | ||||
| 0.00001 | 47.2 ± 0.6 | 34.5 ± 0.5 | 12.5 ± 0.6 | 11.5 ± 0.4 | 1.37 | 1.09 | 3.77 |
| 0.00002 | 51.5 ± 0.7 | 42.5 ± 0.7 | 14.1 ± 0.6 | 11.8 ± 0.7 | 1.21 | 1.19 | 3.66 |
| 0.00004 | 56.3 ± 0.4 | 40.3 ± 0.3 | 11.8 ± 0.5 | 10.1 ± 0.6 | 1.40 | 1.16 | 4.79 |
| 0.00006 | 58.3 ± 0.5 | 41.7 ± 0.5 | 7.9 ± 0.8 | 6.6 ± 0.4 | 1.40 | 1.20 | 7.37 |
| 0.00008 | 66.9 ± 0.6 | 46.3 ± 0.4 | 26.9 ± 0.4 | 22.5 ± 0.8 | 1.45 | 1.19 | 2.49 |
| 0.0001 | 71.0 ± 0.7 | 47.9 ± 0.6 | 42.1 ± 0.5 | 37.9 ± 0.5 | 1.48 | 1.11 | 1.69 |
| 0.0002 | 75.0 ± 0.4 | 48.3 ± 0.6 | 50.2 ± 0.7 | 47.3 ± 0.5 | 1.55 | 1.06 | 1.49 |
| 0.0004 | 80.3 ± 0.8 | 50.0 ± 0.3 | 52.8 ± 0.5 | 51.3 ± 0.7 | 1.61 | 1.03 | 1.52 |
| 0.0006 | 77.5 ± 0.5 | 47.5 ± 0.8 | 50.8 ± 0.6 | 48.3 ± 0.3 | 1.63 | 1.05 | 1.52 |
| 0.0008 | 68.8 ± 0.3 | 46.3 ± 0.5 | 42.5 ± 0.4 | 40.1 ± 0.7 | 1.49 | 1.06 | 1.62 |
| Polymer | High Affinity Sites | Low Affinity Sites | ||||
|---|---|---|---|---|---|---|
| Ka (M−1) | Bmax (mM/g) | R2 | Ka (M−1) | Bmax (mM/g) | R2 | |
| MIP | 19,132 | 0.01 | 0.92 | 6,760 | 3.54 × 10−3 | 0.70 |
| NIP | - | - | - | 629.13 | 0.02 | 0.12 |
| Polymer | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| KL (L/mol) | Qmax (mmol/g) | R2 | KF | m | R2 | |
| MIP | 10.29 | 1.74 × 10−3 | 0.98 | 0.25 | 1.19 | 0.93 |
| NIP | 4.40 | 2.51 × 10−3 | 0.98 | 0.03 | 1.07 | 0.99 |
| Polymer | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|
| K1 | Qe | R2 | K2 | Qe | R2 | |
| MIP | 0.36 | 8.61 × 10−6 | 0.97 | 4.27 × 103 | 1.70 × 105 | 0.77 |
| NIP | 0.30 | 5.39 × 10−6 | 0.97 | 1.58 × 103 | 1.78 × 105 | 0.63 |
| Drug Loading Content (DLC) | Drug Loading Efficiency (DLE) | ||
|---|---|---|---|
| MIP | NIP | MIP | NIP |
| 8.8 ± 0.1% | 7.5 ± 0.3% | 87.2 ± 0.2% | 71.5 ± 0.4% |
| Polymer | Zero-Order Kinetic Model | First-Order Kinetic Model | Higuchi Kinetic Model | Ritger-Peppas Kinetic Model | |||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | K0 | R2 | K1 | R2 | KH | R2 | Kp | n | |
| MIP | 0.6328 | 0.0297 | 0.7597 | 0.0659 | 0.8276 | 0.1884 | 0.9396 | 0.1589 | 0.8312 |
| NIP | 0.5062 | 0.0301 | 0.7196 | 0.1572 | 0.7283 | 0.2001 | 0.9865 | 0.2961 | 0.8178 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisi, O.I.; Ruffo, M.; Malivindi, R.; Vattimo, A.F.; Pezzi, V.; Puoci, F. Molecularly Imprinted Polymers (MIPs) as Theranostic Systems for Sunitinib Controlled Release and Self-Monitoring in Cancer Therapy. Pharmaceutics 2020, 12, 41. https://doi.org/10.3390/pharmaceutics12010041
Parisi OI, Ruffo M, Malivindi R, Vattimo AF, Pezzi V, Puoci F. Molecularly Imprinted Polymers (MIPs) as Theranostic Systems for Sunitinib Controlled Release and Self-Monitoring in Cancer Therapy. Pharmaceutics. 2020; 12(1):41. https://doi.org/10.3390/pharmaceutics12010041
Chicago/Turabian StyleParisi, Ortensia Ilaria, Mariarosa Ruffo, Rocco Malivindi, Anna Francesca Vattimo, Vincenzo Pezzi, and Francesco Puoci. 2020. "Molecularly Imprinted Polymers (MIPs) as Theranostic Systems for Sunitinib Controlled Release and Self-Monitoring in Cancer Therapy" Pharmaceutics 12, no. 1: 41. https://doi.org/10.3390/pharmaceutics12010041
APA StyleParisi, O. I., Ruffo, M., Malivindi, R., Vattimo, A. F., Pezzi, V., & Puoci, F. (2020). Molecularly Imprinted Polymers (MIPs) as Theranostic Systems for Sunitinib Controlled Release and Self-Monitoring in Cancer Therapy. Pharmaceutics, 12(1), 41. https://doi.org/10.3390/pharmaceutics12010041









