Amphiphilic Polypeptides for VEGF siRNA Delivery into Retinal Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis and Characterization of Polypeptides
2.2.2. Preparation and Characterization of Polypeptide Particles
2.2.3. Encapsulation and Release of RNA, Duplex Oligo-dT-dA and siRNA
2.2.4. Cytotoxicity of Particles
2.2.5. Cellular Uptake
2.2.6. VEGF Gene Silencing
2.2.7. Total RNA Isolation, Reverse Transcription and Quantitative Real-Time PCR Analysis
2.2.8. Western Blotting
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Polypeptides
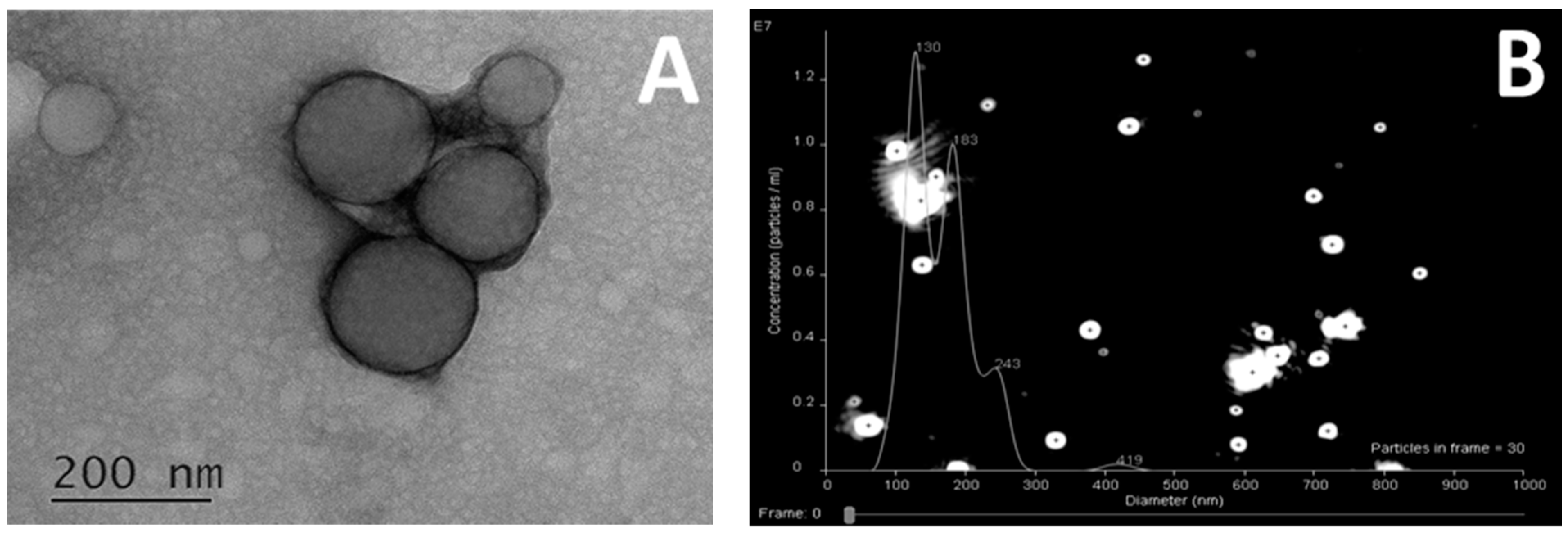
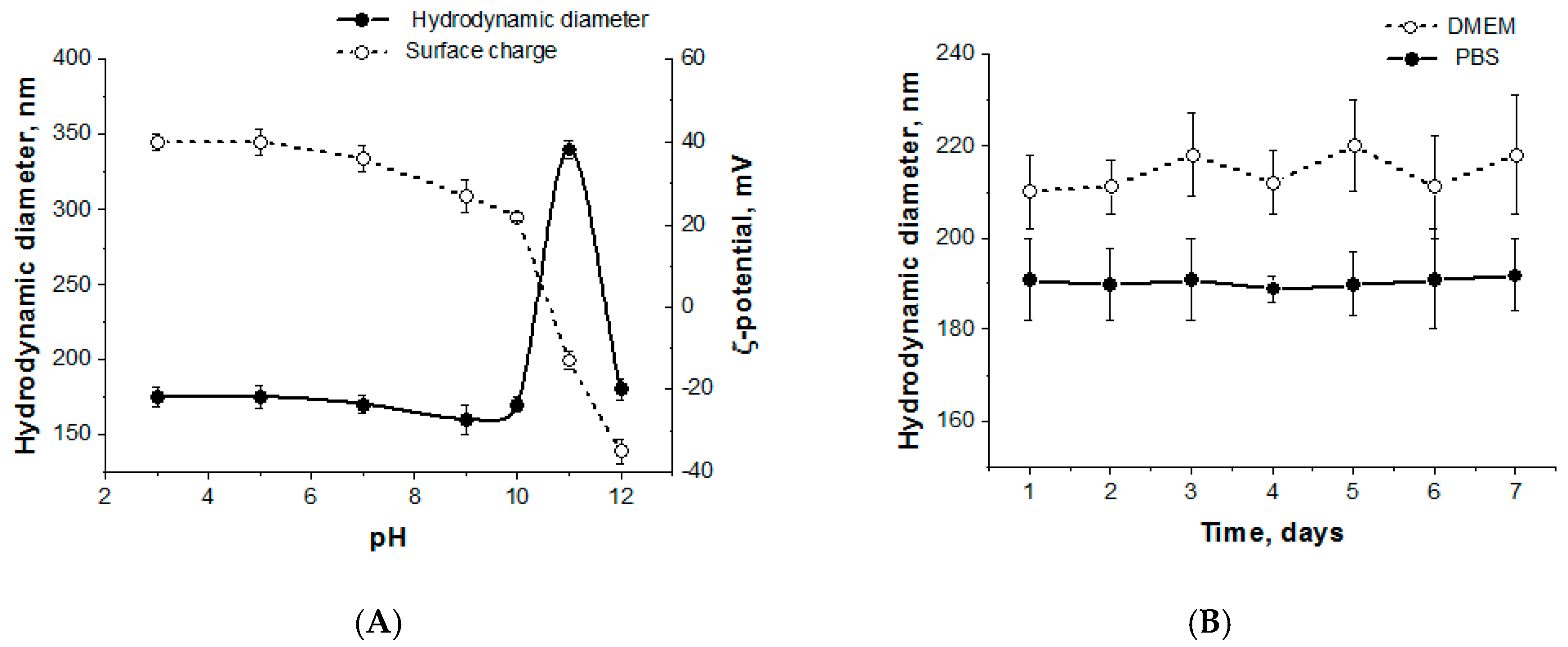
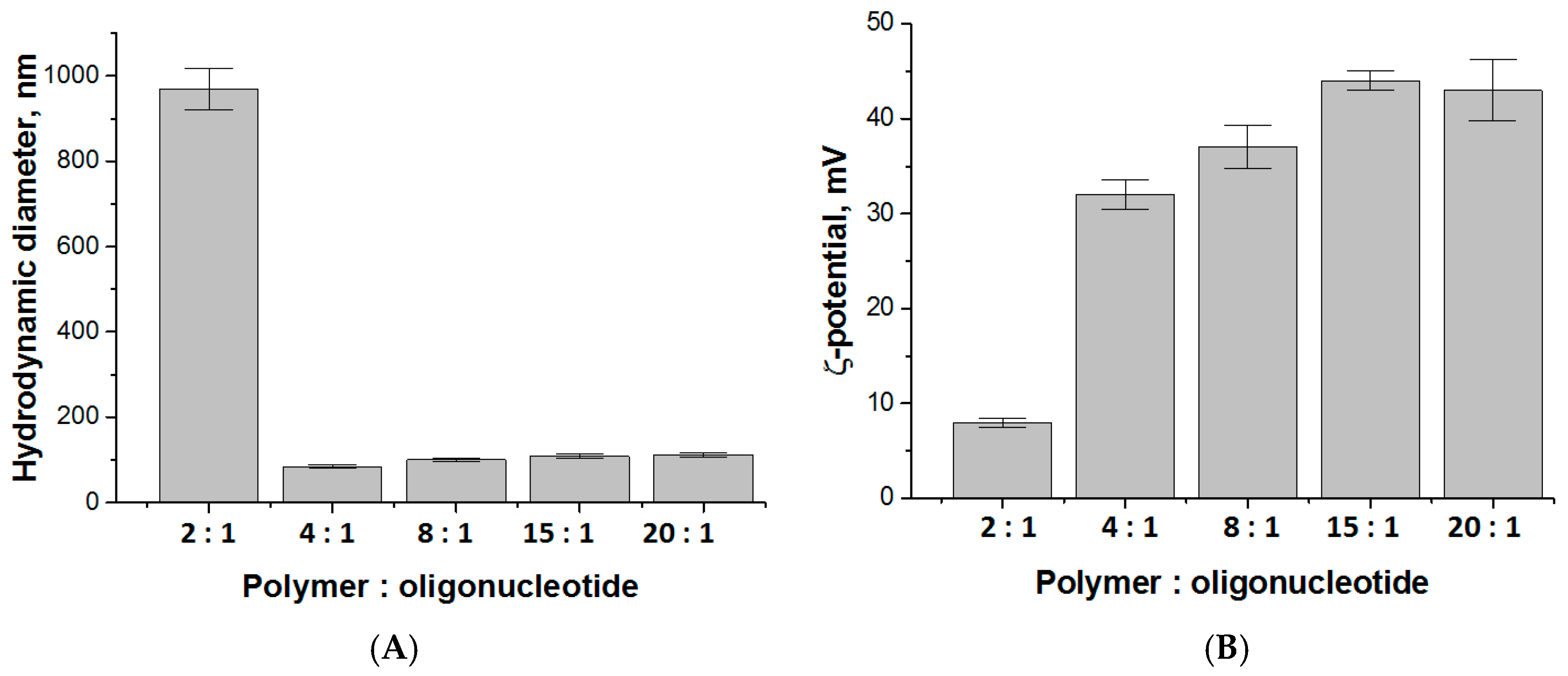
3.2. Preparation and Characterization of Polypeptide Particles
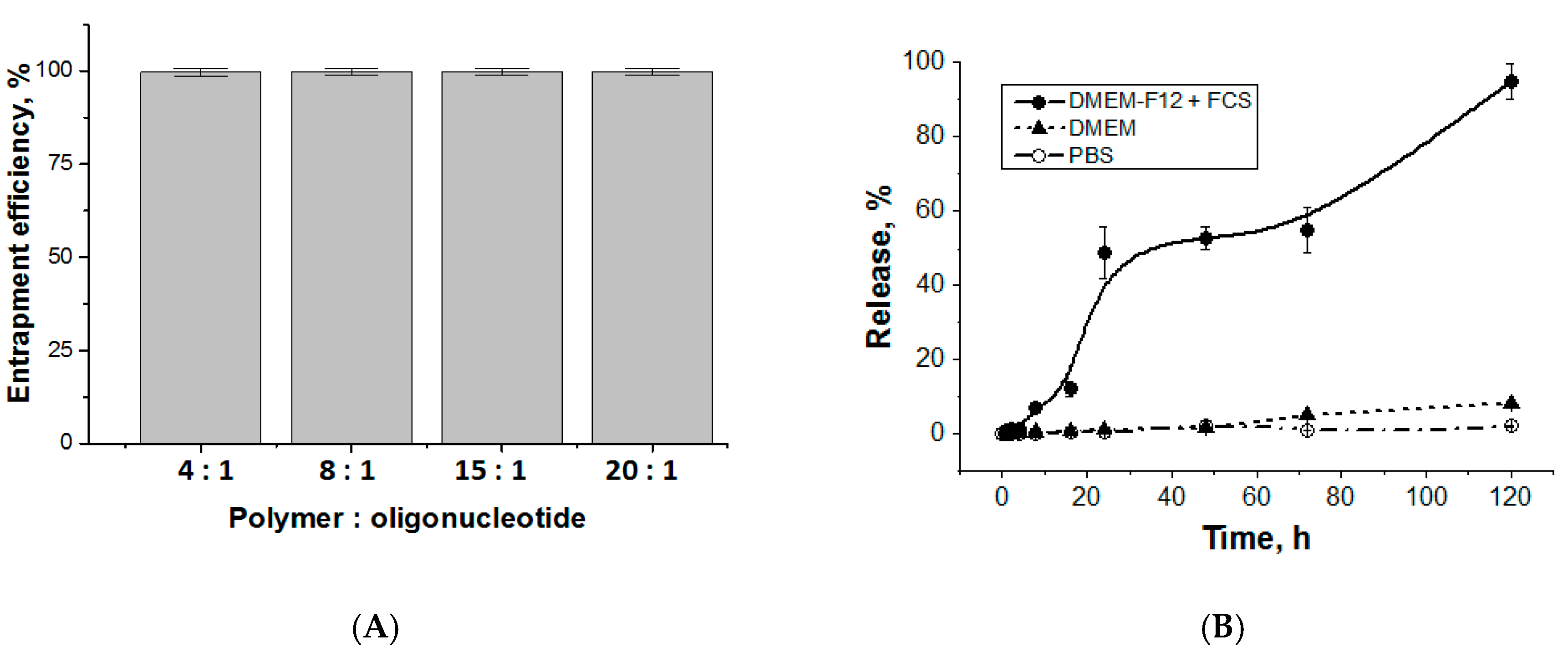
3.3. Entrapment and Release of Duplex Oligo-dT-dA
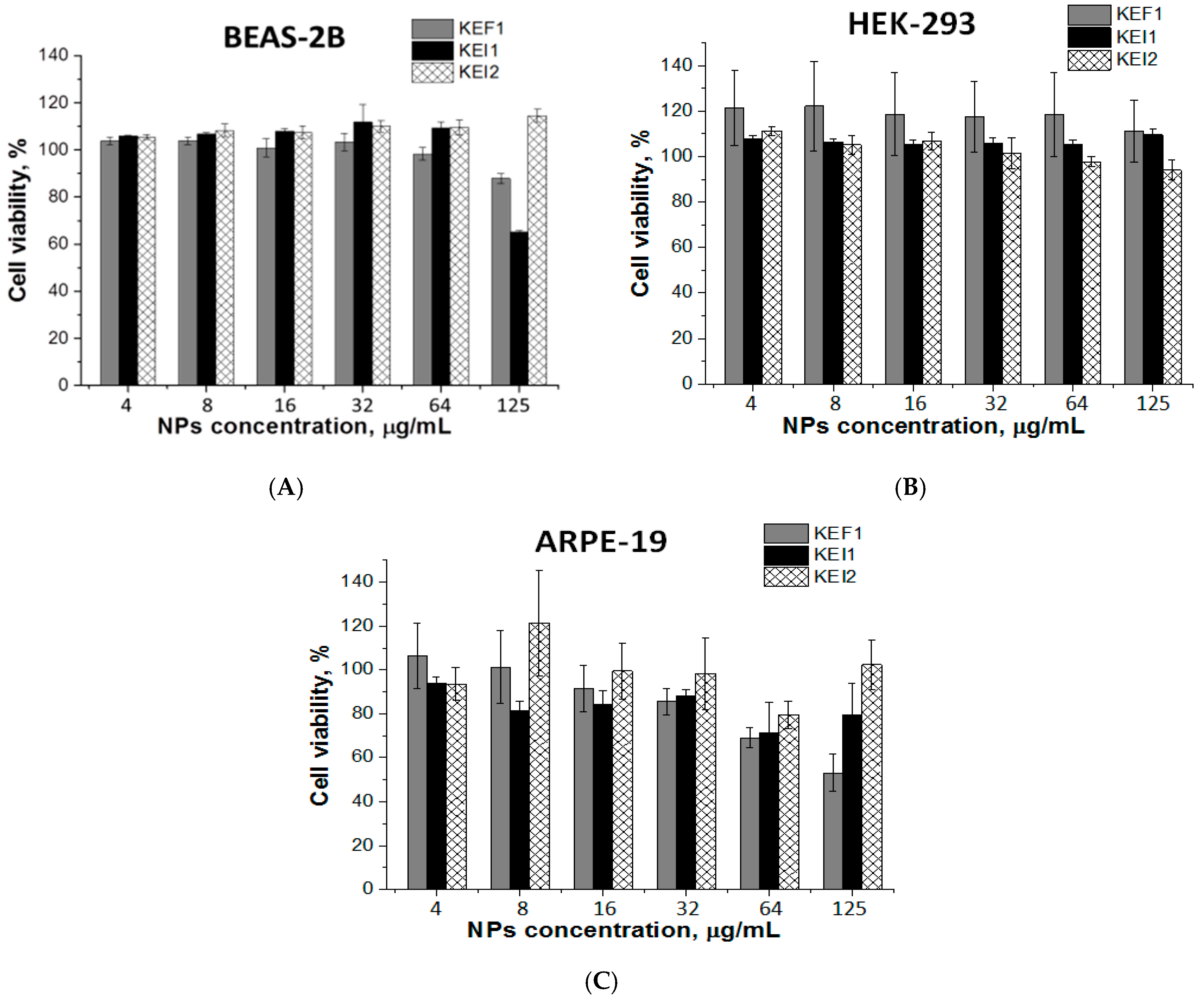
3.4. Cytotoxicity
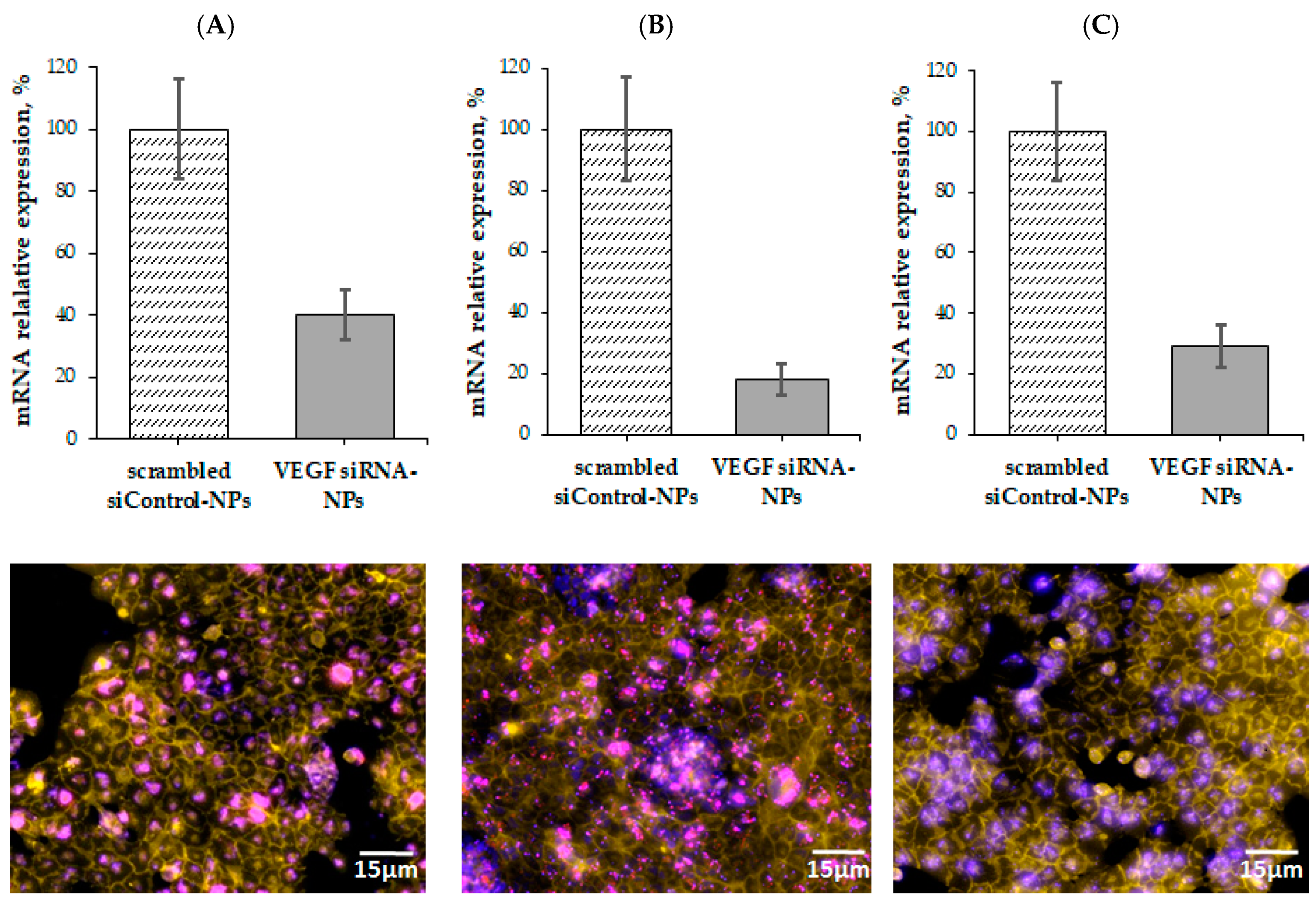
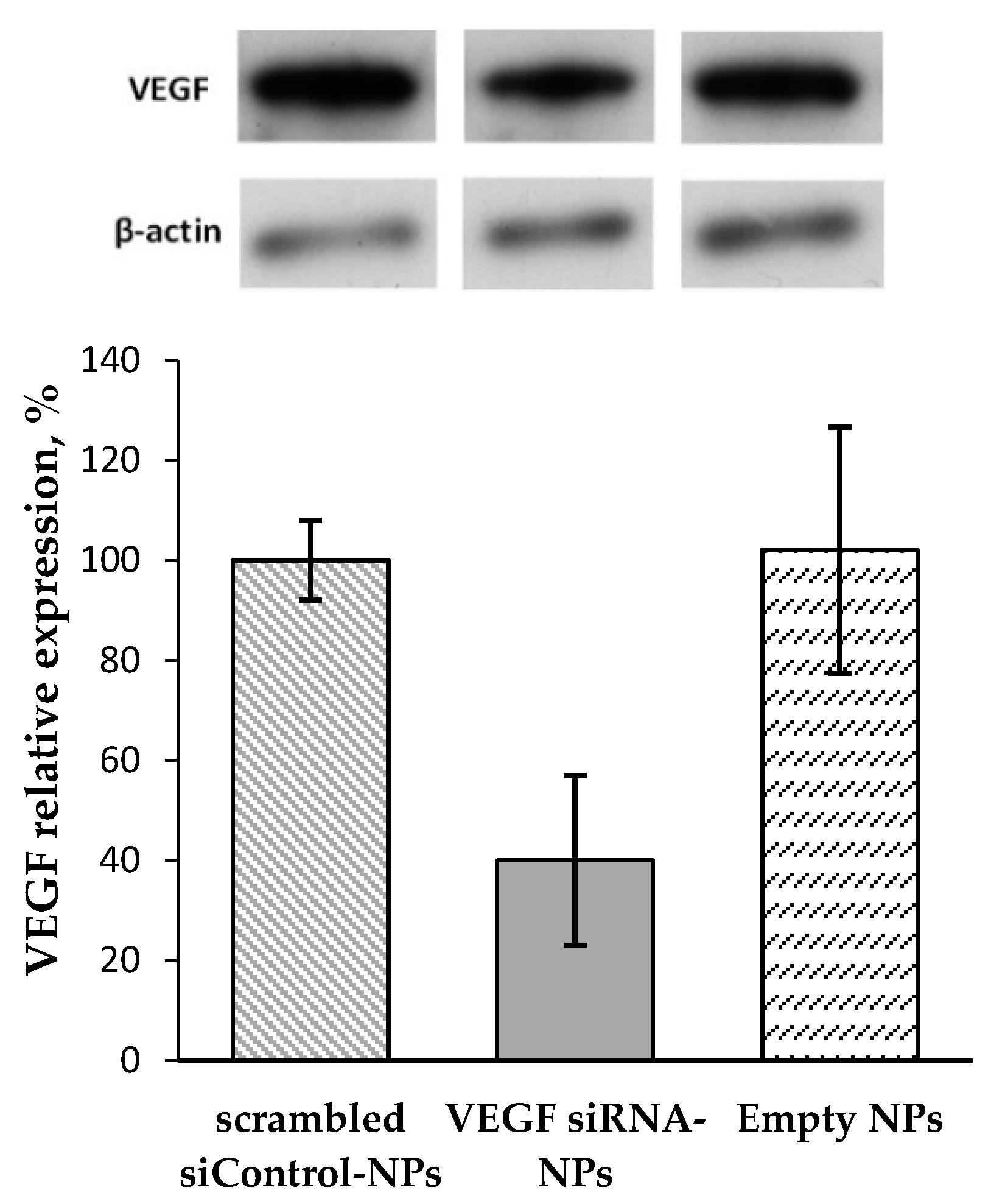
3.5. VEGF Gene Silencing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheung, N.; Wong, I.Y.; Wong, T.Y. Ocular anti-VEGF therapy for diabetic retinopathy: Overview of clinical efficacy and evolving applications. Diabetes Care 2014, 37, 900–905. [Google Scholar] [CrossRef]
- Amoaku, W.M.; Chakravarthy, U.; Gale, R.; Gavin, M.; Ghanchi, F.; Gibson, J.; Harding, S.; Johnston, R.L.; Kelly, S.; Lotery, A.; et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye 2015, 29, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. VEGF and Intraocular Neovascularization: From Discovery to Therapy. Transl. Vis. Sci. Technol. 2016, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Tolentino, M. Systemic and Ocular Safety of Intravitreal Anti-VEGF Therapies for Ocular Neovascular Disease. Surv. Ophthalmol. 2011, 56, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Amadio, M.; Govoni, S.; Pascale, A. Targeting VEGF in eye neovascularization: What’s new?: A comprehensive review on current therapies and oligonucleotide-based interventions under development. Pharmacol. Res. 2016, 103, 253–269. [Google Scholar] [CrossRef]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef]
- Bordet, T.; Behar-Cohen, F. Ocular gene therapies in clinical practice: Viral vectors and nonviral alternative. Drug Discov. Today 2019, 24, 1685–1693. [Google Scholar] [CrossRef]
- Cavallaro, G.; Sardo, C.; Craparo, E.F.; Porsio, B.; Giammona, G. Polymeric nanoparticles for siRNA delivery: Production and applications. Int. J. Pharm. 2017, 525, 313–333. [Google Scholar] [CrossRef]
- Dominska, M.; Dykxhoorn, D.M. Breaking down the barriers: siRNA delivery and endosome escape. J. Cell Sci. 2010, 123, 1183–1189. [Google Scholar] [CrossRef]
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.Y.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010, 28, 172–176. [Google Scholar] [CrossRef]
- Schroeder, A.; Levins, C.G.; Cortez, C.; Langer, R.; Anderson, D.G. Lipid-based nanotherapeutics for siRNA delivery. J. Intern. Med. 2010, 267, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dong, S.; Li, X.; Yu, K.; Sun, F.; Lee, R.J.; Li, Y.; Teng, L. Delivery of siRNA using folate receptor-targeted pH-sensitive polymeric nanoparticles for rheumatoid arthritis therapy. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102017. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Meng, Y.; Ye, J.; Xia, X.; Wang, H.; Li, L.; Dong, W.; Jin, D.; Liu, Y. Sequential delivery of VEGF siRNA and paclitaxel for PVN destruction, anti-angiogenesis, and tumor cell apoptosis procedurally via a multi-functional polymer micelle. J. Control. Release 2018, 287, 103–120. [Google Scholar] [CrossRef]
- Egorova, A.; Petrosyan, M.; Maretina, M.; Balashova, N.; Polyanskih, L.; Baranov, V.; Kiselev, A. Anti-angiogenic treatment of endometriosis via anti-VEGFA siRNA delivery by means of peptide-based carrier in a rat subcutaneous model. Gene Ther. 2018, 25, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Egorova, A.; Shubina, A.; Sokolov, D.; Selkov, S.; Baranov, V.; Kiselev, A. CXCR4-targeted modular peptide carriers for efficient anti-VEGF siRNA delivery. Int. J. Pharm. 2016, 515, 431–440. [Google Scholar] [CrossRef]
- Imperiale, J.C.; Acosta, G.B.; Sosnik, A. Polymer-based carriers for ophthalmic drug delivery. J. Control. Release 2018, 285, 106–141. [Google Scholar] [CrossRef]
- Helmschrodt, C.; Höbel, S.; Schöniger, S.; Bauer, A.; Bonicelli, J.; Gringmuth, M.; Fietz, S.A.; Aigner, A.; Richter, A.; Richter, F. Polyethylenimine Nanoparticle-Mediated siRNA Delivery to Reduce α-Synuclein Expression in a Model of Parkinson’s Disease. Mol. Ther. Nucleic Acids 2017, 9, 57–68. [Google Scholar] [CrossRef]
- Ragelle, H.; Vanvarenberg, K.; Vandermeulen, G.; Préat, V. Chitosan Nanoparticles for SiRNA Delivery In Vitro. In SiRNA Delivery Methods; Humana Press: New York, NY, USA, 2016; pp. 143–150. [Google Scholar]
- Kodama, Y.; Kuramoto, H.; Mieda, Y.; Muro, T.; Nakagawa, H.; Kurosaki, T.; Sakaguchi, M.; Nakamura, T.; Kitahara, T.; Sasaki, H. Application of biodegradable dendrigraft poly-l-lysine to a small interfering RNA delivery system. J. Drug Target. 2017, 25, 49–57. [Google Scholar] [CrossRef]
- Hennig, R.; Goepferich, A. Nanoparticles for the treatment of ocular neovascularizations. Eur. J. Pharm. Biopharm. 2015, 95, 294–306. [Google Scholar] [CrossRef]
- Tatiparti, K.; Sau, S.; Kashaw, S.K.; Iyer, A.K. siRNA Delivery Strategies: A Comprehensive Review of Recent Developments. Nanomaterials 2017, 7, 77. [Google Scholar] [CrossRef]
- Auguste, D.; Furman, K.; Wong, A.; Fuller, J.; Armes, S.; Deming, T.; Langer, R. Triggered release of siRNA from poly(ethylene glycol)-protected, pH-dependent liposomes. J. Control. Release 2008, 130, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Tian, J.; Chen, X. Effect of surface properties on liposomal siRNA delivery. Biomaterials 2016, 79, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Raik, S.V.; Andranovitš, S.; Petrova, V.A.; Xu, Y.; Lam, J.K.-W.; Morris, G.A.; Brodskaia, A.V.; Casettari, L.; Kritchenkov, A.S.; Skorik, Y.A. Comparative Study of Diethylaminoethyl-Chitosan and Methylglycol-Chitosan as Potential Non-Viral Vectors for Gene Therapy. Polymers 2018, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Pilipenko, I.; Korzhikov-Vlakh, V.; Sharoyko, V.; Zhang, N.; Schäfer-Korting, M.; Rühl, E.; Zoschke, C.; Tennikova, T. pH-Sensitive Chitosan–Heparin Nanoparticles for Effective Delivery of Genetic Drugs into Epithelial Cells. Pharmaceutics 2019, 11, 317. [Google Scholar] [CrossRef]
- Wilder, R.; Mobashery, S. The use of triphosgene in preparation of N-carboxy-alpha-amino acid anhydrides. J. Org. Chem. 1992, 57, 2755–2756. [Google Scholar] [CrossRef]
- Schmuck, C.; Hernandez-Folgado, L. Synthesis of a new artificial host for the binding of dipeptides in water. Org. Biomol. Chem. 2007, 5, 2390–2394. [Google Scholar] [CrossRef]
- Abcam. ab228550 Hoechst 33258 Staining Dye Solution, 2018. Available online: https://www.abcam.com/ps/products/228/ab228550/documents/ab228550%20Hoechst%2033258%20Staining%20Dye%20Solution%20v1a%20(website).pdf (accessed on 13 November 2019).
- Chen, C.W.; Yeh, M.K.; Shiau, C.Y.; Chiang, C.H.; Lu, D.W. Efficient downregulation of VEGF in Retinal pigment epithelial cells by integrin ligand-labeled liposome-mediated siRNA delivery. Int. J. Nanomed. 2013, 8, 2613–2627. [Google Scholar]
- Murata, M.; Takanami, T.; Shimizu, S.; Kubota, Y.; Horiuchi, S.; Habano, W.; Ma, J.X.; Sato, S. Inhibition of ocular angiogenesis by diced small interfering RNAs (siRNAs) specific to vascular endothelial growth factor (VEGF). Curr. Eye Res. 2006, 31, 171–180. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor: New York, NY, USA, 2001. [Google Scholar]
- Kim, B.; Tang, Q.; Biswas, P.S.; Xu, J.; Schiffelers, R.M.; Xie, F.Y.; Ansari, A.M.; Scaria, P.V.; Woodle, M.C.; Lu, P.; et al. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: Therapeutic strategy for herpetic stromal keratitis. Am. J. Pathol. 2004, 165, 2177–2185. [Google Scholar] [CrossRef]
- Cheng, J.; Deming, T.J. Synthesis of Polypeptides by Ring-Opening Polymerization of α-Amino Acid N-Carboxyanhydrides. In Peptide-Based Materials; Springer: Berlin, Germany, 2011; pp. 1–26. [Google Scholar]
- Nesměrák, K.; Němcová, I. Determination of Critical Micelle Concentration by Electrochemical Means. Anal. Lett. 2006, 39, 1023–1040. [Google Scholar] [CrossRef]
- Imai, S.; Hirai, Y.; Nagao, C.; Sawamoto, M.; Terashima, T. Programmed Self-Assembly Systems of Amphiphilic Random Copolymers into Size-Controlled and Thermoresponsive Micelles in Water. Macromolecules 2018, 51, 398–409. [Google Scholar] [CrossRef]
- Pegg, J.C.; Czajka, A.; Hill, C.; James, C.; Peach, J.; Rogers, S.E.; Eastoe, J. Alternative Route to Nanoscale Aggregates with a pH-Responsive Random Copolymer. Langmuir 2017, 33, 2628–2638. [Google Scholar] [CrossRef] [PubMed]
- Zashikhina, N.; Sharoyko, V.; Antipchik, M.; Tarasenko, I.; Anufrikov, Y.; Lavrentieva, A.; Tennikova, T.; Korzhikova-Vlakh, E. Novel Formulations of C-Peptide with Long-Acting Therapeutic Potential for Treatment of Diabetic Complications. Pharmaceutics 2019, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Jung, S.; Luo, S.; Meng, F.; Zhu, X.; Park, T.G.; Zhong, Z. Co-delivery of siRNA and paclitaxel into cancer cells by biodegradable cationic micelles based on PDMAEMA-PCL-PDMAEMA triblock copolymers. Biomaterials 2010, 31, 2408–2416. [Google Scholar] [CrossRef]
- Wei, W.; Lv, P.P.; Chen, X.M.; Yue, Z.G.; Fu, Q.; Liu, S.Y.; Yue, H.; Ma, G.H. Codelivery of mTERT siRNA and paclitaxel by chitosan-based nanoparticles promoted synergistic tumor suppression. Biomaterials 2013, 34, 3912–3923. [Google Scholar] [CrossRef]
| Sample | Polymer Characteristics | |||
|---|---|---|---|---|
| SEC | SLS | |||
| Mn | Mw | Ð | Mw | |
| P(Lys(Z)n-co-Glu(OBzl)m-co-Phek) | ||||
| KEF1 | 20,170 | 23,600 | 1.17 | 23,000 |
| KEF2 | 9400 | 17,000 | 1.80 | 17,500 |
| KEF3 | 10,000 | 13,200 | 1.32 | 17,400 |
| P(Lys(Z)n-co-Glu(OBzl)m-co-Ilek) | ||||
| KEI1 | 21,350 | 28,400 | 1.33 | 18,800 |
| KEI2 | 16,090 | 21,240 | 1.32 | 17,100 |
| KEI3 | 17,560 | 22,300 | 1.27 | - |
| Sample | Determined Polymer Composition (mol%) | ||||
|---|---|---|---|---|---|
| HPLC | 1HNMR | ||||
| Lys | Glu | Phe/Ile | Lys + Glu | Phe/Ile | |
| P(Lysn-co-Glum-co-Phek) | |||||
| KEF1 | 55 | 25 | 20 | 76 | 24 |
| KEF2 | 75 | 9 | 16 | 75 | 25 |
| KEF3 | 21 | 54 | 25 | 64 | 36 |
| P(Lysn-co-Glum-co-Ilek) | |||||
| KEI1 | 66 | 16 | 18 | 85 | 15 |
| KEI2 | 57 | 31 | 12 | 88 | 12 |
| KEI3 | 54 | 36 | 10 | - | - |
| Sample | Particle Characteristics | ||
|---|---|---|---|
| DH, nm | PDI | ζ-Potential, mV | |
| P(Lysn-co-Glum-co-Phek) | |||
| KEF1 | 200 ± 8 | 0.22 | +12 ± 2 |
| KEF2 | 550 ± 14 | 0.31 | +18 ± 5 |
| KEF3 | 900 ± 38 | 0.39 | −6 ± 3 |
| P(Lysn-co-Glum-co-Ilek) | |||
| KEI1 | 232 ± 11 | 0.14 | +49 ± 2 |
| KEI2 | 180 ± 19 | 0.19 | +45 ± 3 |
| KEI3 | 440 ± 31 | 0.30 | +31 ± 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osipova, O.; Sharoyko, V.; Zashikhina, N.; Zakharova, N.; Tennikova, T.; Urtti, A.; Korzhikova-Vlakh, E. Amphiphilic Polypeptides for VEGF siRNA Delivery into Retinal Epithelial Cells. Pharmaceutics 2020, 12, 39. https://doi.org/10.3390/pharmaceutics12010039
Osipova O, Sharoyko V, Zashikhina N, Zakharova N, Tennikova T, Urtti A, Korzhikova-Vlakh E. Amphiphilic Polypeptides for VEGF siRNA Delivery into Retinal Epithelial Cells. Pharmaceutics. 2020; 12(1):39. https://doi.org/10.3390/pharmaceutics12010039
Chicago/Turabian StyleOsipova, Olga, Vladimir Sharoyko, Natalia Zashikhina, Natalya Zakharova, Tatiana Tennikova, Arto Urtti, and Evgenia Korzhikova-Vlakh. 2020. "Amphiphilic Polypeptides for VEGF siRNA Delivery into Retinal Epithelial Cells" Pharmaceutics 12, no. 1: 39. https://doi.org/10.3390/pharmaceutics12010039
APA StyleOsipova, O., Sharoyko, V., Zashikhina, N., Zakharova, N., Tennikova, T., Urtti, A., & Korzhikova-Vlakh, E. (2020). Amphiphilic Polypeptides for VEGF siRNA Delivery into Retinal Epithelial Cells. Pharmaceutics, 12(1), 39. https://doi.org/10.3390/pharmaceutics12010039









