RETRACTED: Retinal Pigment Epithelial Cell Line with Fast Differentiation and Improved Barrier Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
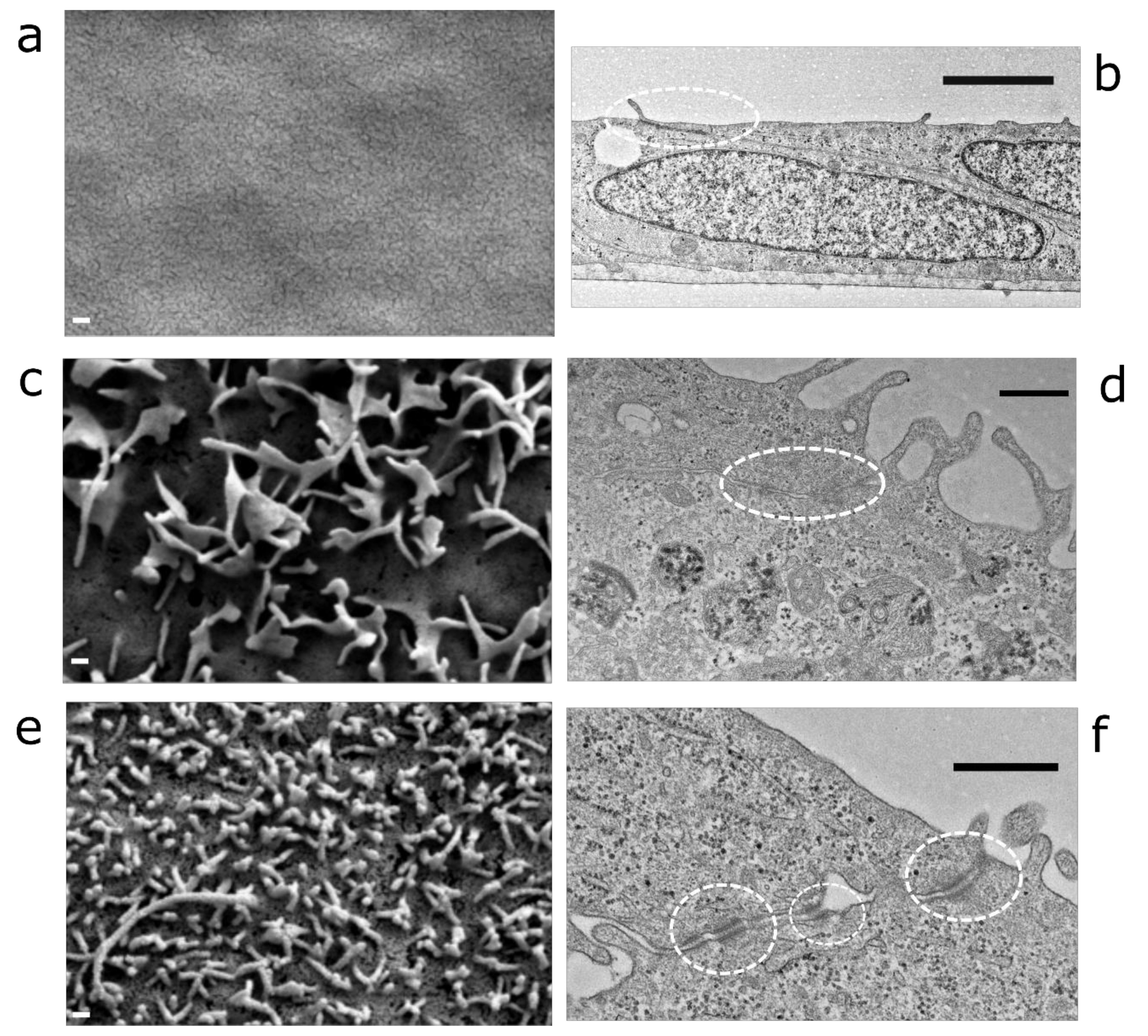
2.2. Transmission and Scanning Electron Microscopy
2.3. Immunofluorescence Staining
2.4. Phagocytosis Assay
2.5. Doubling Time
2.6. Permeability Studies
2.7. Drug Concentration Determination
3. Results
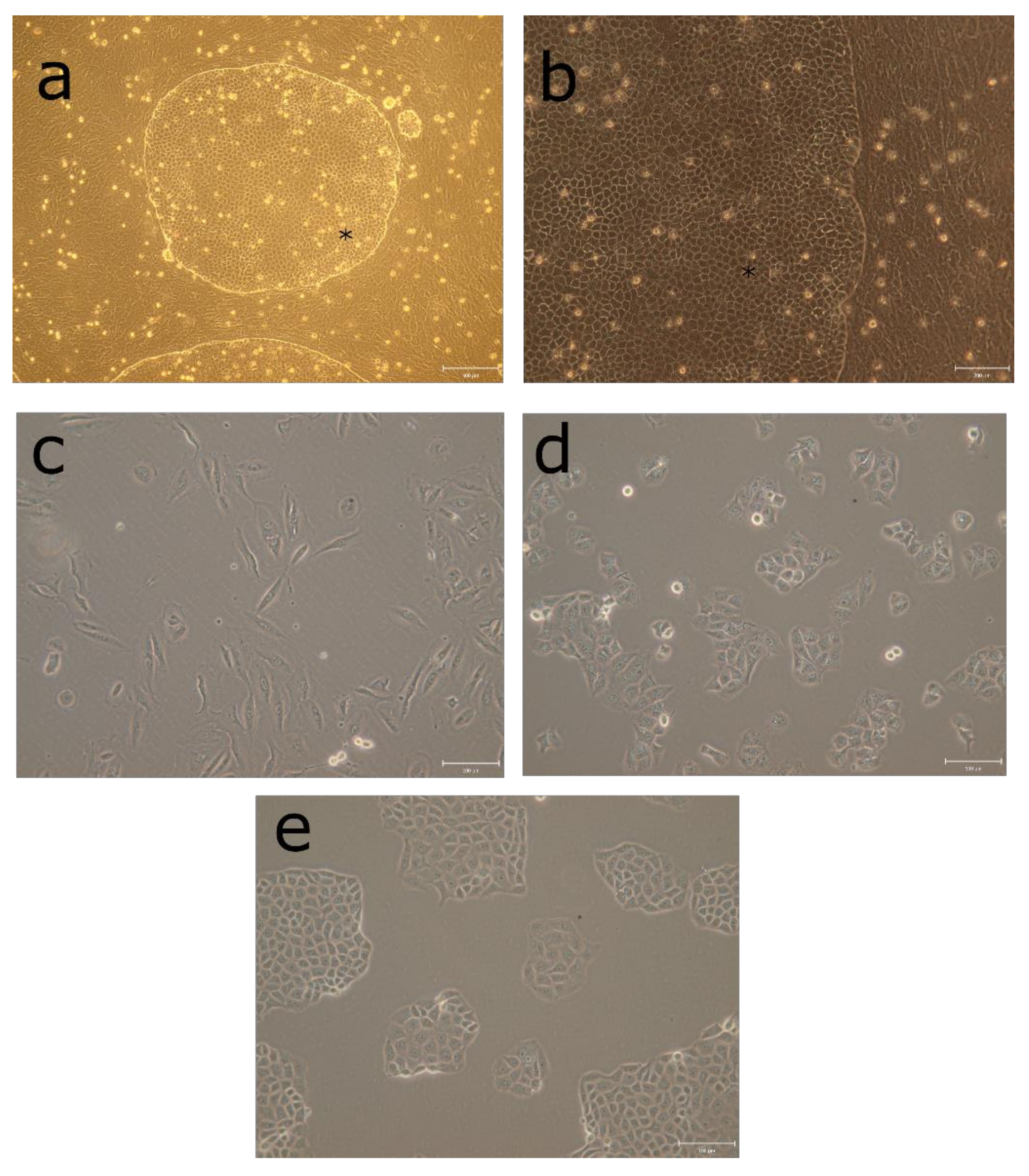
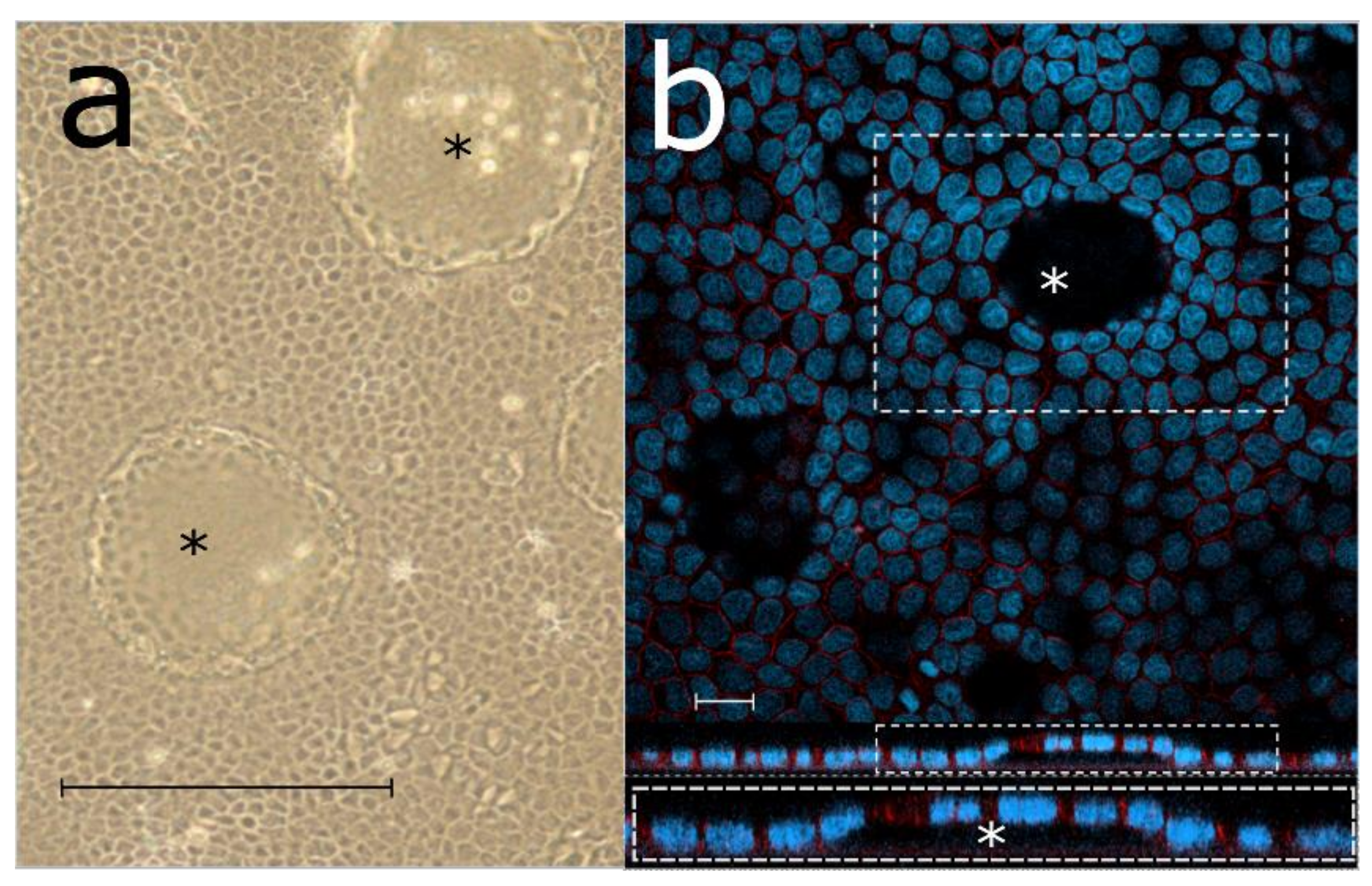
3.1. Morphology of Rapidly Growing LEPI Cells Is Similar to Primary RPE Cultures
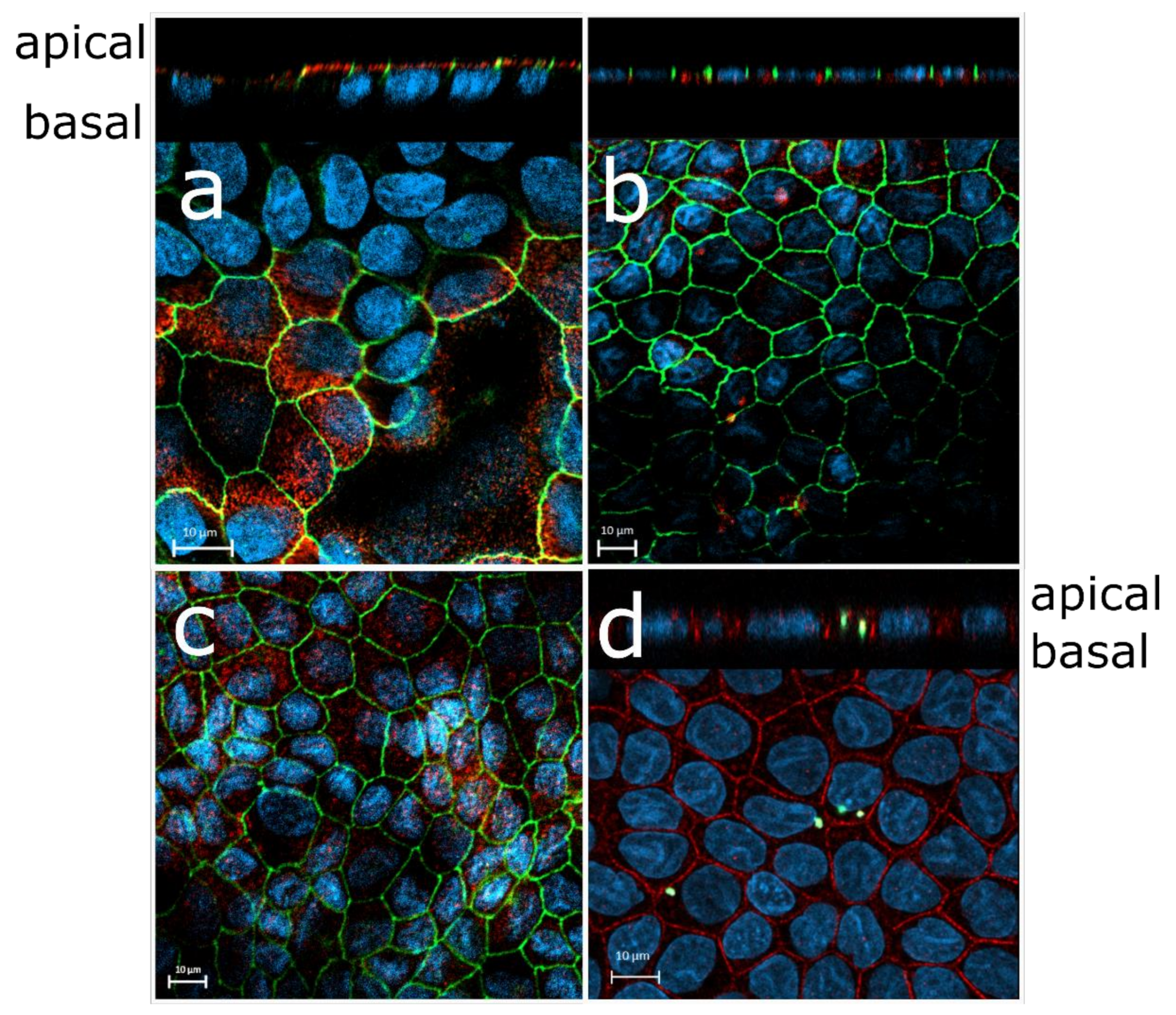
3.2. LEPI Cells Display RPE-Specific Protein Expression and Function
3.3. LEPI Cells Form an Appropriate Outer Blood–Retinal Barrier Model
4. Discussion
4.1. Morphology and RPE-Specific Protein Expression of LEPI Cells Reveal the Differentiated Phenotype
4.2. LEPI Cells Are Suitable as an Outer Blood–Retinal Barrier Model
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, E.M.; Rimpela, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic Aspects of Retinal Drug Delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef] [PubMed]
- Pavan, B.; Dalpiaz, A. Retinal Pigment Epithelial Cells as a Therapeutic Tool and Target Against Retinopathies. Drug Discov. Today 2018, 23, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjelmeland, L.M. ARPE-19, a Human Retinal Pigment Epithelial Cell Line with Differentiated Properties. Exp. Eye Res. 1996, 62, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Shadforth, A.M.A.; Suzuki, S.; Theodoropoulos, C.; Richardson, N.A.; Chirila, T.V.; Harkin, D.G. A Bruch’s Membrane Substitute Fabricated from Silk Fibroin Supports the Function of Retinal Pigment Epithelial Cells in Vitro. J. Tissue Eng. Regen. Med. 2017, 11, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Mannermaa, E.; Reinisalo, M.; Ranta, V.P.; Vellonen, K.S.; Kokki, H.; Saarikko, A.; Kaarniranta, K.; Urtti, A. Filter-Cultured ARPE-19 Cells as Outer Blood-Retinal Barrier Model. Eur. J. Pharm. Sci. 2010, 40, 289–296. [Google Scholar] [CrossRef]
- Pitkanen, L.; Ranta, V.P.; Moilanen, H.; Urtti, A. Permeability of Retinal Pigment Epithelium: Effects of Permeant Molecular Weight and Lipophilicity. Invest. Ophthalmol. Vis. Sci. 2005, 46, 641–646. [Google Scholar] [CrossRef]
- Pelkonen, L.; Sato, K.; Reinisalo, M.; Kidron, H.; Tachikawa, M.; Watanabe, M.; Uchida, Y.; Urtti, A.; Terasaki, T. LC-MS/MS Based Quantitation of ABC and SLC Transporter Proteins in Plasma Membranes of Cultured Primary Human Retinal Pigment Epithelium Cells and Immortalized ARPE19 Cell Line. Mol. Pharm. 2017, 14, 605–613. [Google Scholar] [CrossRef]
- Rizzolo, L.J. Barrier Properties of Cultured Retinal Pigment Epithelium. Exp. Eye Res. 2014, 126, 16–26. [Google Scholar] [CrossRef]
- Samuel, W.; Jaworski, C.; Postnikova, O.A.; Kutty, R.K.; Duncan, T.; Tan, L.X.; Poliakov, E.; Lakkaraju, A.; Redmond, T.M. Appropriately Differentiated ARPE-19 Cells Regain Phenotype and Gene Expression Profiles Similar to those of Native RPE Cells. Mol. Vis. 2017, 23, 60–89. [Google Scholar]
- Philp, N.J.; Wang, D.; Yoon, H.; Hjelmeland, L.M. Polarized Expression of Monocarboxylate Transporters in Human Retinal Pigment Epithelium and ARPE-19 Cells. Invest. Ophthalmol. Vis. Sci. 2003, 44, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Strunnikova, N.V.; Maminishkis, A.; Barb, J.J.; Wang, F.; Zhi, C.; Sergeev, Y.; Chen, W.; Edwards, A.O.; Stambolian, D.; Abecasis, G.; et al. Transcriptome Analysis and Molecular Signature of Human Retinal Pigment Epithelium. Hum. Mol. Genet. 2010, 19, 2468–2486. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ishibashi, K.; Honda, S.; Boylan, S.A.; Hjelmeland, L.M.; Handa, J.T. The Expression of Native and Cultured Human Retinal Pigment Epithelial Cells Grown in Different Culture Conditions. Br. J. Ophthalmol. 2005, 89, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Capes-Davis, A.; Reid, Y.A.; Kline, M.C.; Storts, D.R.; Strauss, E.; Dirks, W.G.; Drexler, H.G.; MacLeod, R.A.; Sykes, G.; Kohara, A.; et al. Match Criteria for Human Cell Line Authentication: Where do we Draw the Line? Int. J. Cancer 2013, 132, 2510–2519. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, E.; Ruponen, M.; Picardat, T.; Tengvall, U.; Tuomainen, M.; Auriola, S.; Toropainen, E.; Urtti, A.; Del Amo, E.M. Impact of Chemical Structure on Conjunctival Drug Permeability: Adopting Porcine Conjunctiva and Cassette Dosing for Construction of in Silico Model. J. Pharm. Sci. 2017, 106, 2463–2471. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, L.; Tengvall-Unadike, U.; Ruponen, M.; Kidron, H.; Del Amo, E.M.; Reinisalo, M.; Urtti, A. Melanin Binding Study of Clinical Drugs with Cassette Dosing and Rapid Equilibrium Dialysis Inserts. Eur. J. Pharm. Sci. 2017, 109, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Adijanto, J.; Philp, N.J. Cultured Primary Human Fetal Retinal Pigment Epithelium (hfRPE) as a Model for Evaluating RPE Metabolism. Exp. Eye Res. 2014, 126, 77–84. [Google Scholar] [CrossRef]
- Oganesian, A.; Bueno, E.; Yan, Q.; Spee, C.; Black, J.; Rao, N.A.; Lopez, P.F. Scanning and Transmission Electron Microscopic Findings during RPE Wound Healing in Vivo. Int. Ophthalmol. 1997, 21, 165–175. [Google Scholar] [CrossRef]
- Tamiya, S.; Liu, L.; Kaplan, H.J. Epithelial-Mesenchymal Transition and Proliferation of Retinal Pigment Epithelial Cells Initiated upon Loss of Cell-Cell Contact. Invest. Ophthalmol. Vis. Sci. 2010, 51, 2755–2763. [Google Scholar] [CrossRef]
- Zhou, J.; He, S.; Zhang, N.; Spee, C.; Zhou, P.; Ryan, S.J.; Kannan, R.; Hinton, D.R. Neutrophils Compromise Retinal Pigment Epithelial Barrier Integrity. J. Biomed. Biotechnol. 2010, 2010, 289360. [Google Scholar] [CrossRef]
- Westerhout, J.; Van de Steeg, E.; Grossouw, D.; Zeijdner, E.E.; Krul, C.A.; Verwei, M.; Wortelboer, H.M. A New Approach to Predict Human Intestinal Absorption using Porcine Intestinal Tissue and Biorelevant Matrices. Eur. J. Pharm. Sci. 2014, 63, 167–177. [Google Scholar] [CrossRef] [PubMed]

| Primary Antibody | Cat n:o and Vendor | Dilution | Secondary Antibody | Cat n:o and Vendor | Dilution |
|---|---|---|---|---|---|
| ezrin | sc-58758, Santa Cruz Biotechnology | 1:100 | goat anti-mouse AF594 | A-11032 Thermo Fischer Scientific | 1:1000 |
| occludin | 71-1500, Thermo Fischer Scientific | 1:100 | goat anti-rabbit AF488 | A-11034 Thermo Fischer Scientific | 1:1000 |
| RPE65 | NB100-355, Novus Biologicals | 1:100 | goat anti-mouse AF594 | A-11032 Thermo Fischer Scientific | 1:1000 |
| MRP1 | ab3368, Abcam | 1:100 | goat anti-Rat AF568 | A-11029 Thermo Fischer Scientific | 1:1000 |
| Compound | logD7.4 (Predicted, ACDLabs) 1 | Papp across LEPI Cells ± SD (×10−6cm/s, Apical-to-Basolateral) | Papp across Bovine RPE-Choroid 2 ± SD (×10−6 cm/s, Apical-to-Basolateral) | Fold Differences in Papp (RPE-Choroid/LEPI) |
|---|---|---|---|---|
| atenolol | −1.85 | 0.39 ± 0.15 * | 2.00 ± 0.47 | 5.1 |
| nadolol | −0.86 | 0.37 ± 0.17 * | 2.03 ± 0.46 | 5.5 |
| timolol | −0.35 | 0.78 ± 0.34 | 8.41 ± 2.7 | 10.8 |
| pindolol | −0.32 | 1.8 ± 0.57 | 3.48 ± 1.7 | 1.9 |
| metoprolol | −0.25 | 2.3 ± 0.68 | 10.6 ± 3.2 | 4.6 |
| betaxolol | 0.76 | 1.7 ± 0.68 | 10.3 ± 3.7 | 6.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hellinen, L.; Pirskanen, L.; Tengvall-Unadike, U.; Urtti, A.; Reinisalo, M. RETRACTED: Retinal Pigment Epithelial Cell Line with Fast Differentiation and Improved Barrier Properties. Pharmaceutics 2019, 11, 412. https://doi.org/10.3390/pharmaceutics11080412
Hellinen L, Pirskanen L, Tengvall-Unadike U, Urtti A, Reinisalo M. RETRACTED: Retinal Pigment Epithelial Cell Line with Fast Differentiation and Improved Barrier Properties. Pharmaceutics. 2019; 11(8):412. https://doi.org/10.3390/pharmaceutics11080412
Chicago/Turabian StyleHellinen, Laura, Lea Pirskanen, Unni Tengvall-Unadike, Arto Urtti, and Mika Reinisalo. 2019. "RETRACTED: Retinal Pigment Epithelial Cell Line with Fast Differentiation and Improved Barrier Properties" Pharmaceutics 11, no. 8: 412. https://doi.org/10.3390/pharmaceutics11080412
APA StyleHellinen, L., Pirskanen, L., Tengvall-Unadike, U., Urtti, A., & Reinisalo, M. (2019). RETRACTED: Retinal Pigment Epithelial Cell Line with Fast Differentiation and Improved Barrier Properties. Pharmaceutics, 11(8), 412. https://doi.org/10.3390/pharmaceutics11080412






