Intestinal Permeability and Drug Absorption: Predictive Experimental, Computational and In Vivo Approaches
Abstract
1. Introduction
2. In Silico Gastrointestinal Absorption Predictions
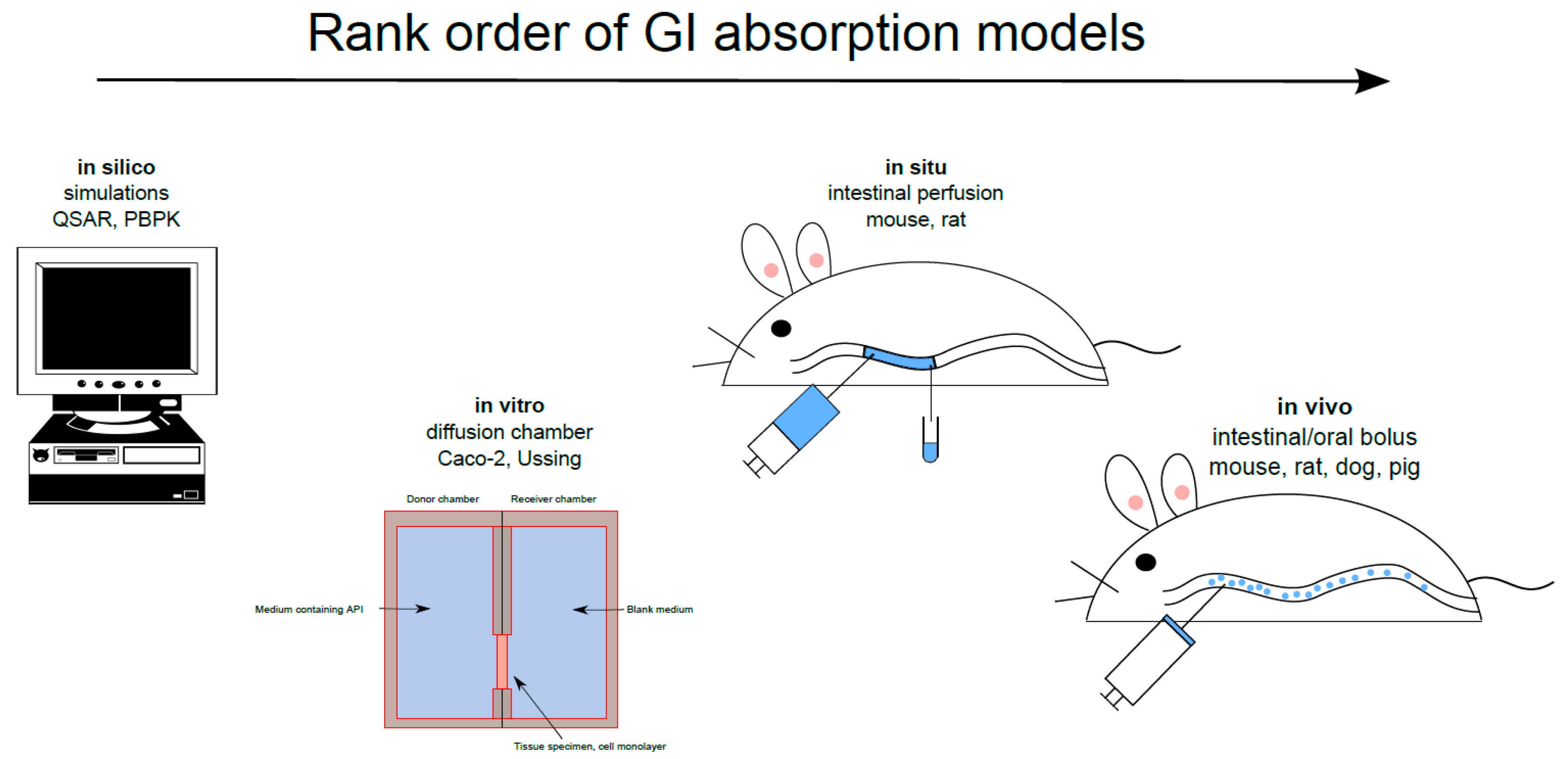
3. Gastrointestinal Experimental Absorption Models
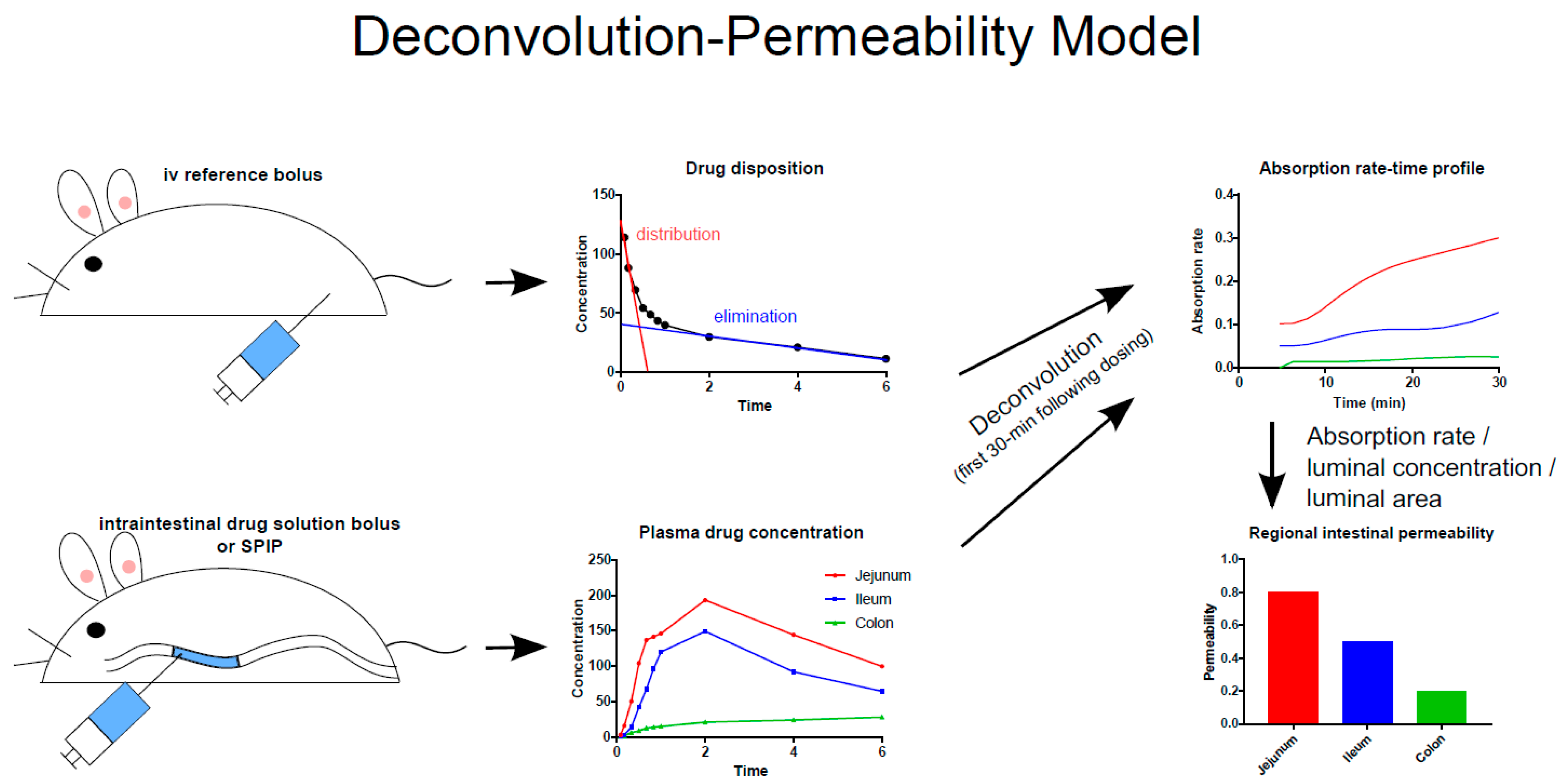
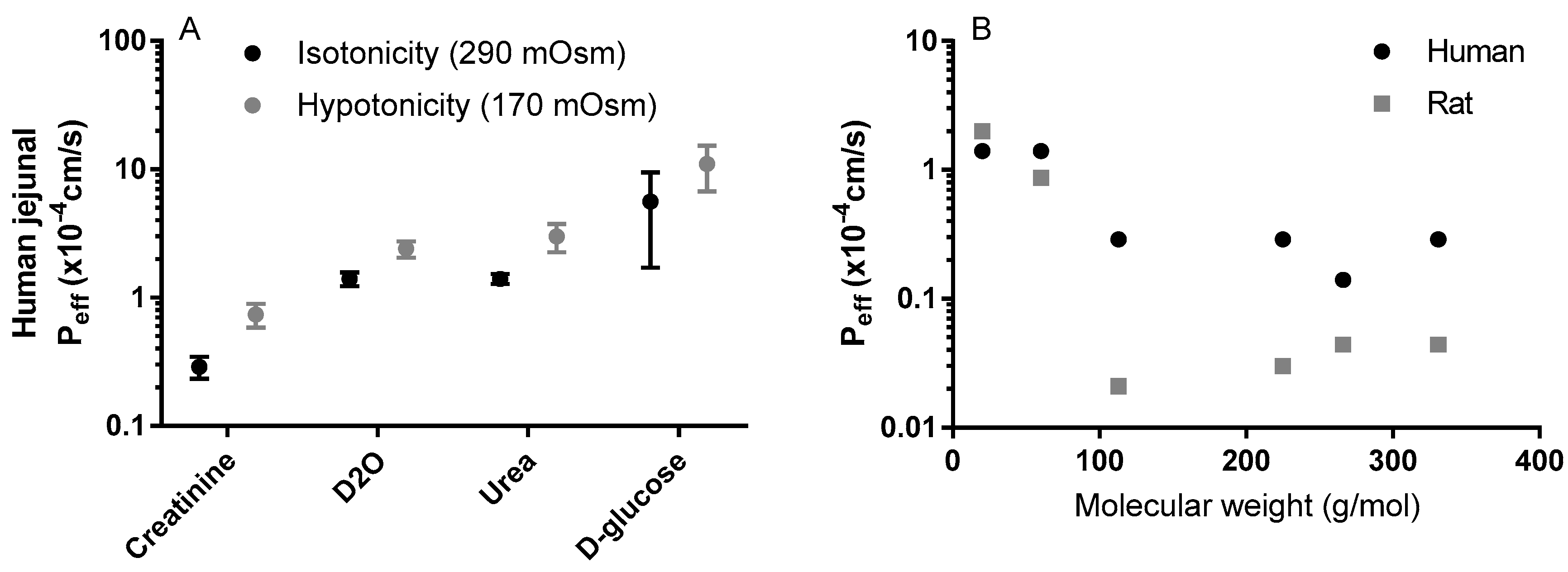
3.1. In Situ
3.2. In Vivo
3.3. In Vitro
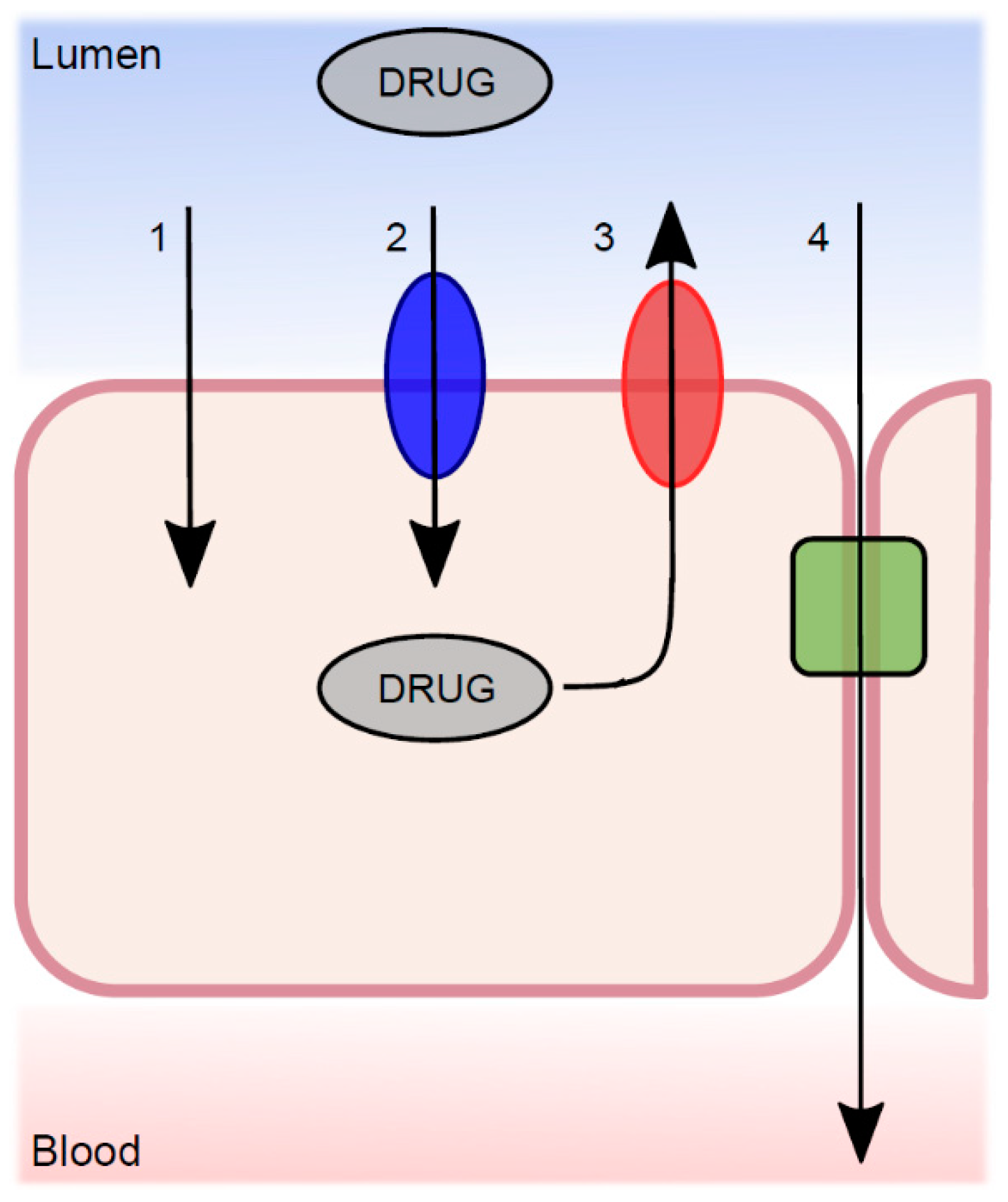
4. Intestinal Membrane Transport
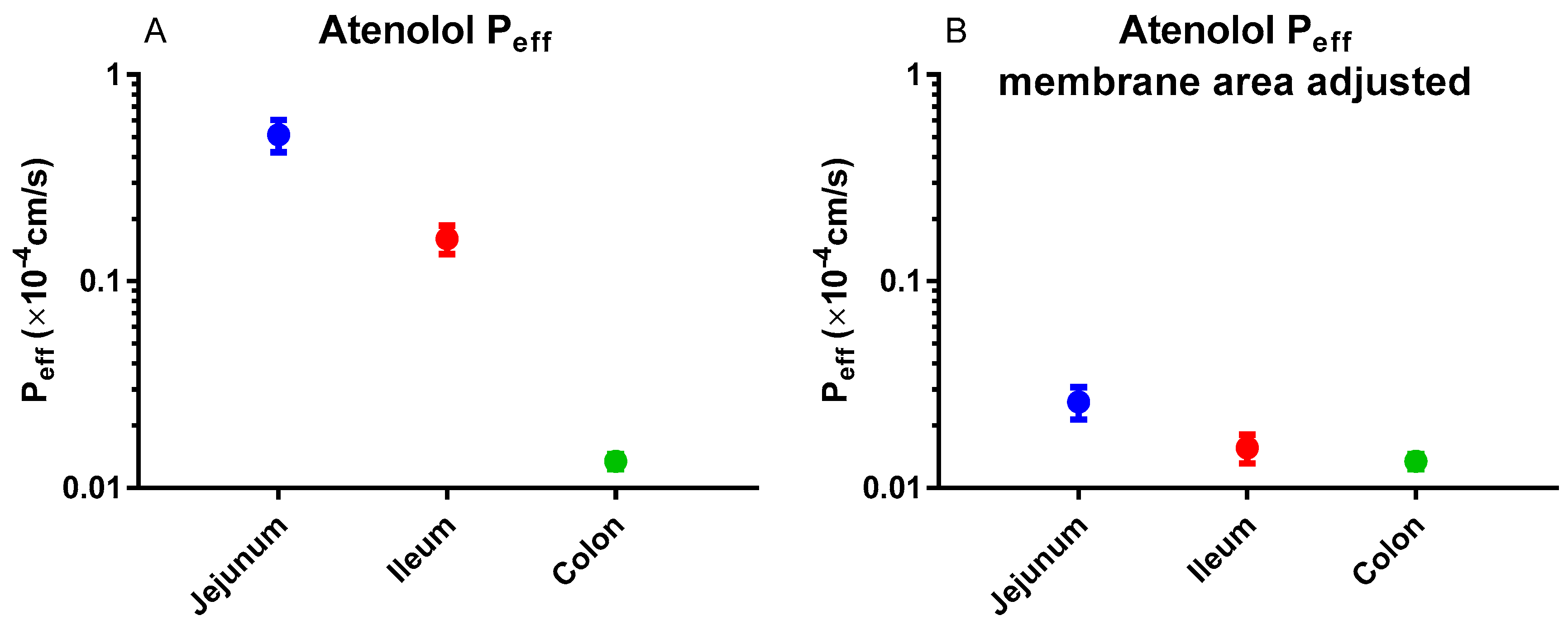
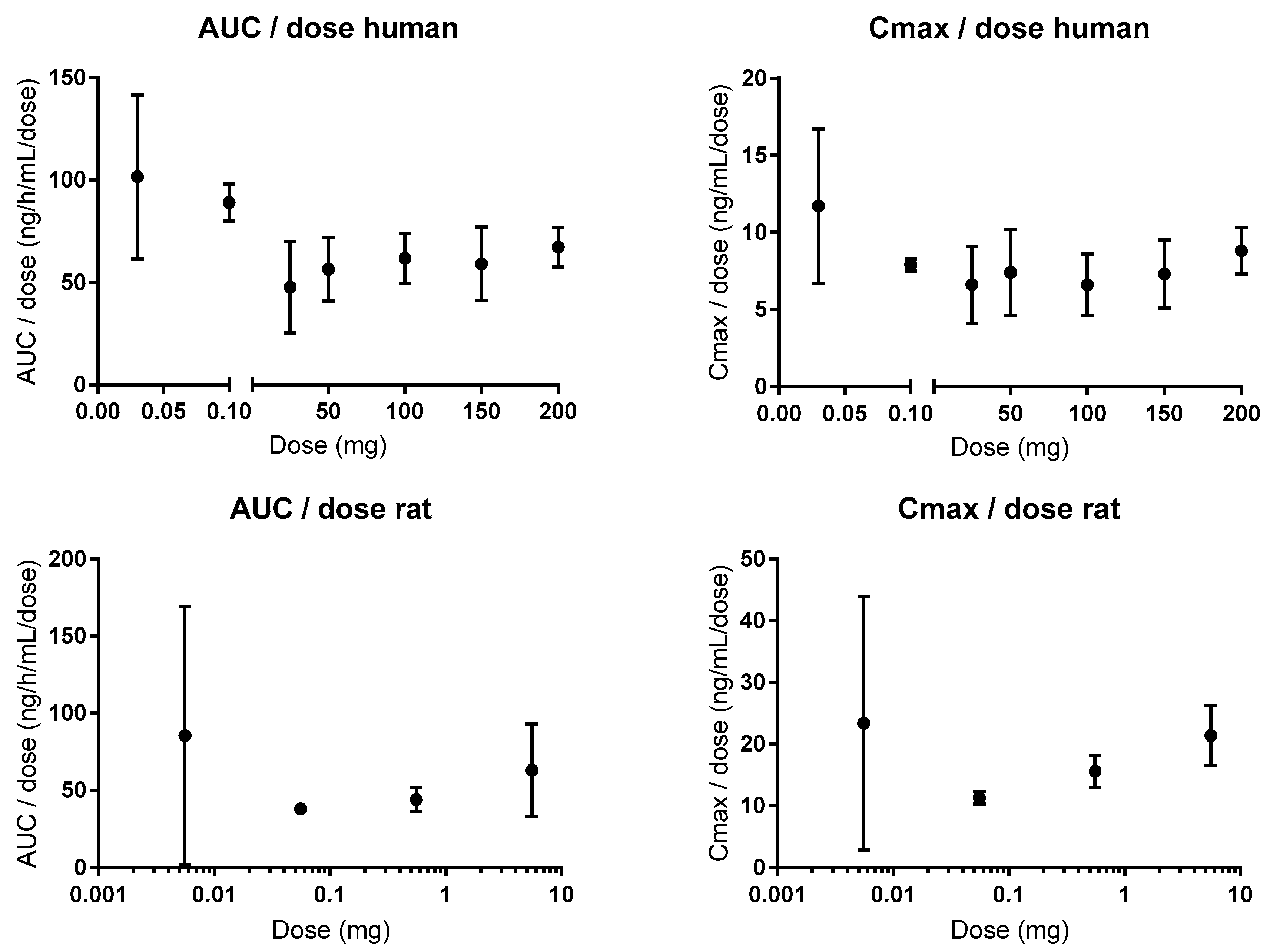
5. Atenolol
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| API | Active pharmaceutical ingredient |
| AUC | Area under the concentration-time curve |
| BBB | Blood-brain barrier |
| BCS | Biopharmaceutics classification system |
| CM | Carrier-mediated |
| Cmax | Maximum concentration |
| F | Bioavailability |
| Fabs | Fraction absorbed |
| FDA | US Food and Drug Administration |
| GI | Gastrointestinal |
| HBD | Hydrogen bond donor |
| MR | Modified-release |
| OrBiTo | Innovative Tools for Oral Biopharmaceutics |
| Peff | Effective permeability |
| PBPK | Physiologically based pharmacokinetic |
| QSAR | Quantitative structure-activity relationship |
| SPIP | Single-pass intestinal perfusion |
References
- Hellriegel, E.T.; Bjornsson, T.D. Interpatient variability in bioavailability is related to the extent of absorption: Implications for bioavailability and bioequivalence studies. Clin. Pharm. Ther. 1996, 60, 601–607. [Google Scholar] [CrossRef]
- Sjögren, E.; Abrahamsson, B.; Augustijns, P.; Becker, D.; Bolger, M.B.; Brewster, M.; Brouwers, J.; Flanagan, T.; Harwood, M.; Heinen, C.; et al. In vivo methods for drug absorption–comparative physiologies, model selection, correlations with in vitro methods (IVIVC), and applications for formulation/API/excipient characterization including food effects. Eur. J. Pharm. Sci. 2014, 57, 99–151. [Google Scholar]
- Dahlgren, D.; Roos, C.; Lundqvist, A.; Abrahamsson, B.; Tannergren, C.; Hellström, P.M.; Sjögren, E.; Lennernäs, H. Regional intestinal permeability of three model drugs in human. Mol. Pharm. 2016, 13, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Sellers, R.S.; Morton, D. The Colon From Banal to Brilliant. Toxicol. Pathol. 2013, 42, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Tannergren, C.; Bergendal, A.; Lennernäs, H.; Abrahamsson, B. Toward an increased understanding of the barriers to colonic drug absorption in humans: Implications for early controlled release candidate assessment. Mol. Pharm. 2009, 6, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lennernas, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Roos, C.; Dahlgren, D.; Tannergren, C.; Abrahamsson, B.; Sjögren, E.; Lennernas, H. Regional intestinal permeability in rats: A comparison of methods. Mol. Pharm. 2017, 14, 4252–4261. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, T.; Suarez-Sharp, S.; Kakhi, M.; Holmstock, N.; Olivares-Morales, A.; Pepin, X.; Sjögren, E.; Tsakalozou, E.; Seo, P.; Li, M. Dissolution and Translational Modeling Strategies Toward Establishing an In Vitro-In Vivo Link—A Workshop Summary Report. ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Lennernas, H.; Lindahl, A.; Van Peer, A.; Ollier, C.; Flanagan, T.; Lionberger, R.; Nordmark, A.; Yamashita, S.; Yu, L.; Amidon, G. In vivo predictive dissolution (IPD) and biopharmaceutical modeling and simulation: Future use of modern approaches and methodologies in a regulatory context. Mol. Pharm. 2017, 14, 1307–1314. [Google Scholar] [CrossRef]
- Lennernas, H. Intestinal permeability and its relevance for absorption and elimination. Xenobiotica 2007, 37, 1015–1051. [Google Scholar] [CrossRef]
- Roffey, S.J.; Obach, R.S.; Gedge, J.I.; Smith, D.A. What is the objective of the mass balance study? A retrospective analysis of data in animal and human excretion studies employing radiolabeled drugs. Drug Metab. Rev. 2007, 39, 17–43. [Google Scholar] [CrossRef]
- Food, Drug and Administration. Guidance for Industry: Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System; Food Drug Administrain: Rockville, MD, USA, 2000. [Google Scholar]
- Gozalbes, R.; Jacewicz, M.; Annand, R.; Tsaioun, K.; Pineda-Lucena, A. QSAR-based permeability model for drug-like compounds. Bioorg. Med. Chem. 2011, 19, 2615–2624. [Google Scholar] [CrossRef]
- Scior, T.; Medina-Franco, J.; Do, Q.T.; Martínez-Mayorga, K.; Yunes Rojas, J.; Bernard, P. How to recognize and workaround pitfalls in QSAR studies: A critical review. Curr. Med. Chem. 2009, 16, 4297–4313. [Google Scholar] [CrossRef]
- Awoonor-Williams, E.; Rowley, C.N. Molecular simulation of nonfacilitated membrane permeation. Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 1672–1687. [Google Scholar] [CrossRef]
- Lee, C.T.; Comer, J.; Herndon, C.; Leung, N.; Pavlova, A.; Swift, R.V.; Tung, C.; Rowley, C.N.; Amaro, R.E.; Chipot, C. Simulation-based approaches for determining membrane permeability of small compounds. J. Chem. Inf. Model. 2016, 56, 721–733. [Google Scholar] [CrossRef]
- Mathiowetz, A.M. Design Principles for Intestinal Permeability of Cyclic Peptides; Cyclic Peptide Design, Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–15. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Kostewicz, E.S.; Aarons, L.; Bergstrand, M.; Bolger, M.B.; Galetin, A.; Hatley, O.; Jamei, M.; Lloyd, R.; Pepin, X.; Rostami-Hodjegan, A.; et al. PBPK models for the prediction of in vivo performance of oral dosage forms. Eur. J. Pharm. Sci. 2013, 57, 300–321. [Google Scholar] [CrossRef]
- Sugano, K. Introduction to computational oral absorption simulation. Expert Opin. Drug Metab. Toxicol. 2009, 5, 259–293. [Google Scholar] [CrossRef]
- Sjögren, E.; Thörn, H.; Tannergren, C. In silico modeling of gastrointestinal drug absorption: Predictive performance of three physiologically based absorption models. Mol. Pharm. 2016, 13, 1763–1778. [Google Scholar] [CrossRef]
- Jones, H.; Chen, Y.; Gibson, C.; Heimbach, T.; Parrott, N.; Peters, S.; Snoeys, J.; Upreti, V.; Zheng, M.; Hall, S. Physiologically based pharmacokinetic modeling in drug discovery and development: A pharmaceutical industry perspective. Clin. Pharm. Ther. 2015, 97, 247–262. [Google Scholar] [CrossRef]
- Flanagan, T.; Van Peer, A.; Lindahl, A. Use of physiologically relevant biopharmaceutics tools within the pharmaceutical industry and in regulatory sciences: Where are we now and what are the gaps? Eur. J. Pharm. Sci. 2016, 91, 84–90. [Google Scholar] [CrossRef]
- Margolskee, A.; Darwich, A.S.; Pepin, X.; Aarons, L.; Galetin, A.; Rostami-Hodjegan, A.; Carlert, S.; Hammarberg, M.; Hilgendorf, C.; Johansson, P. IMI–oral biopharmaceutics tools project–evaluation of bottom-up PBPK prediction success part 2: An introduction to the simulation exercise and overview of results. Eur. J. Pharm. Sci. 2017, 96, 610–625. [Google Scholar] [CrossRef]
- Margolskee, A.; Darwich, A.S.; Pepin, X.; Pathak, S.M.; Bolger, M.B.; Aarons, L.; Rostami-Hodjegan, A.; Angstenberger, J.; Graf, F.; Laplanche, L. IMI–oral biopharmaceutics tools project–evaluation of bottom-up PBPK prediction success part 1: Characterisation of the OrBiTo database of compounds. Eur. J. Pharm. Sci. 2017, 96, 598–609. [Google Scholar] [CrossRef]
- Darwich, A.S.; Margolskee, A.; Pepin, X.; Aarons, L.; Galetin, A.; Rostami-Hodjegan, A.; Carlert, S.; Hammarberg, M.; Hilgendorf, C.; Johansson, P. IMI–Oral biopharmaceutics tools project–Evaluation of bottom-up PBPK prediction success part 3: Identifying gaps in system parameters by analysing In Silico performance across different compound classes. Eur. J. Pharm. Sci. 2017, 96, 626–642. [Google Scholar] [CrossRef]
- Wagner, C.; Zhao, P.; Pan, Y.; Hsu, V.; Grillo, J.; Huang, S.; Sinha, V. Application of physiologically based pharmacokinetic (PBPK) modeling to support dose selection: Report of an FDA public workshop on PBPK. CPT Pharmacomet. Syst. Pharmacol. 2015, 4, 226–230. [Google Scholar] [CrossRef]
- Grønlund, D.; Poulsen, J.; Sandberg, T.; Olesen, A.; Madzak, A.; Krogh, K.; Frøkjaer, J.; Drewes, A. Established and emerging methods for assessment of small and large intestinal motility. Neurogastroenterol. Motil. 2017, 29, e13008. [Google Scholar] [CrossRef]
- Amoako-Tuffour, Y.; Jones, M.L.; Shalabi, N.; Labbé, A.; Vengallatore, S.; Prakash, S. Ingestible gastrointestinal sampling devices: State-of-the-art and future directions. Crit. Rev. ™ Biomed. Eng. 2014, 42, 1–15. [Google Scholar] [CrossRef]
- Lozoya-Agullo, I.; Gonzalez-Alvarez, I.; Zur, M.; Fine-Shamir, N.; Cohen, Y.; Markovic, M.; Garrigues, T.M.; Dahan, A.; Gonzalez-Alvarez, M.; Merino-Sanjuán, M. Closed-Loop Doluisio (Colon, Small Intestine) and Single-Pass Intestinal Perfusion (Colon, Jejunum) in Rat—Biophysical Model and Predictions Based on Caco-2. Pharm. Res. 2018, 35, 2. [Google Scholar] [CrossRef]
- Dahlgren, D.; Roos, C.; Lundqvist, A.; Langguth, P.; Tannergren, C.; Sjöblom, M.; Sjögren, E.; Lennernas, H. Preclinical effect of absorption modifying excipients on rat intestinal transport of five model compounds and the intestinal barrier marker 51Cr-EDTA. Mol. Pharm. 2017, 14, 4243–4251. [Google Scholar] [CrossRef]
- Sedin, J.; Sjöblom, M.; Nylander, O. The selective cyclooxygenase-2 inhibitor parecoxib markedly improves the ability of the duodenum to regulate luminal hypertonicity in anaesthetized rats. Acta Physiol. 2012, 205, 433–451. [Google Scholar] [CrossRef]
- Dahlgren, D.; Roos, C.; Lundqvist, A.; Tannergren, C.; Sjöblom, M.; Sjögren, E.; Lennernas, H. Effect of absorption-modifying excipients, hypotonicity, and enteric neural activity in an in vivo model for small intestinal transport. Int. J. Pharm. 2018, 549, 239–248. [Google Scholar] [CrossRef]
- Nylander, O. The impact of cyclooxygenase inhibition on duodenal motility and mucosal alkaline secretion in anaesthetized rats. Acta Physiol. 2011, 201, 179–192. [Google Scholar] [CrossRef]
- Dahlgren, D.; Roos, C.; Peters, K.; Lundqvist, A.; Tannergren, C.; Sjögren, E.; Sjöblom, M.; Lennernäs, H. Evaluation of drug permeability calculation based on luminal disappearance and plasma appearance in the rat single-pass intestinal perfusion model. Eur. J. Pharm. Biopharm. 2019, 142, 31–37. [Google Scholar] [CrossRef]
- Sjögren, E.; Dahlgren, D.; Roos, C.; Lennernas, H. Human in vivo regional intestinal permeability: Quantitation using site-specific drug absorption data. Mol. Pharm. 2015, 12, 2026–2039. [Google Scholar] [CrossRef]
- Dahlgren, D.; Roos, C.; Johansson, P.; Tannergren, C.; Lundqvist, A.; Langguth, P.; Sjöblom, M.; Sjögren, E.; Lennernas, H. The effects of three absorption-modifying critical excipients on the in vivo intestinal absorption of six model compounds in rats and dogs. Int. J. Pharm. 2018, 547, 158–168. [Google Scholar] [CrossRef]
- Dahlgren, D.; Roos, C.; Johansson, P.; Lundqvist, A.; Tannergren, C.; Abrahamsson, B.; Sjögren, E.; Lennernäs, H. Regional intestinal permeability in dogs: Biopharmaceutical aspects for development of oral modified-release dosage forms. Mol. Pharm. 2016, 13, 3022–3033. [Google Scholar] [CrossRef]
- Baker, J.R.; Dickens, J.R.; Koenigsknecht, M.; Frances, A.; Lee, A.A.; Shedden, K.A.; Brasseur, J.G.; Amidon, G.L.; Sun, D.; Hasler, W.L. Propagation Characteristics of Fasting Duodeno-Jejunal Contractions in Healthy Controls Measured by Clustered Closely-spaced Manometric Sensors. J. Neurogastroenterol. Motil. 2019, 25, 100. [Google Scholar] [CrossRef]
- Akamatsu, M.; Fujikawa, M.; Nakao, K.; Shimizu, R. In silico prediction of human oral absorption based on QSAR analyses of PAMPA permeability. Chem. Biodivers. 2009, 6, 1845–1866. [Google Scholar] [CrossRef]
- Sjöberg, Å.; Lutz, M.; Tannergren, C.; Wingolf, C.; Borde, A.; Ungell, A.L. Comprehensive study on regional human intestinal permeability and prediction of fraction absorbed of drugs using the Ussing chamber technique. Eur. J. Pharm. Sci. 2013, 48, 166–180. [Google Scholar] [CrossRef]
- Artursson, P. Epithelial transport of drugs in cell culture. I: A model for studying the passive diffusion of drugs over intestinal absorbtive (Caco-2) cells. J. Pharm. Sci. 1990, 79, 476–482. [Google Scholar] [CrossRef]
- Nielsen, C.U.; Andersen, R.; Brodin, B.; Frokjaer, S.; Taub, M.E.; Steffansen, B. Dipeptide model prodrugs for the intestinal oligopeptide transporter. Affinity for and transport via hPepT1 in the human intestinal Caco-2 cell line. J. Control. Release 2001, 76, 129–138. [Google Scholar] [CrossRef]
- Neuhoff, S.; Ungell, A.L.; Zamora, I.; Artursson, P. pH-dependent bidirectional transport of weakly basic drugs across Caco-2 monolayers: Implications for drug–drug interactions. Pharm. Res. 2003, 20, 1141–1148. [Google Scholar] [CrossRef]
- Larregieu, C.A.; Benet, L.Z. Drug Discovery and Regulatory Considerations for Improving In Silico and In Vitro Predictions that Use Caco-2 as a Surrogate for Human Intestinal Permeability Measurements. AAPS J. 2013, 15, 483–497. [Google Scholar] [CrossRef]
- Mochel, J.P.; Jergens, A.E.; Kingsbury, D.; Kim, H.J.; Martín, M.G.; Allenspach, K. Intestinal stem cells to advance drug development, precision, and regenerative medicine: A paradigm shift in translational research. AAPS J. 2018, 20, 17. [Google Scholar] [CrossRef]
- Chandra, L.; Borcherding, D.C.; Kingsbury, D.; Atherly, T.; Ambrosini, Y.M.; Bourgois-Mochel, A.; Yuan, W.; Kimber, M.; Qi, Y.; Wang, Q. Derivation of adult canine intestinal organoids for translational research in gastroenterology. BMC Biol. 2019, 17, 33. [Google Scholar] [CrossRef]
- Fujii, M.; Matano, M.; Toshimitsu, K.; Takano, A.; Mikami, Y.; Nishikori, S.; Sugimoto, S.; Sato, T. Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell. 2018, 23, 787–793. [Google Scholar] [CrossRef]
- Sato, T.; Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: Mechanism and applications. Science 2013, 340, 1190–1194. [Google Scholar] [CrossRef]
- Kell, D.B.; Dobson, P.D.; Bilsland, E.; Oliver, S.G. The promiscuous binding of pharmaceutical drugs and their transporter-mediated uptake into cells: What we (need to) know and how we can do so. Drug Discov. Today 2013, 18, 218–239. [Google Scholar] [CrossRef]
- Kell, D.B.; Dobson, P.D.; Oliver, S.G. Pharmaceutical drug transport: The issues and the implications that it is essentially carrier-mediated only. Drug Discov. Today. 2011, 16, 704–714. [Google Scholar] [CrossRef]
- Sugano, K.; Kansy, M.; Artursson, P.; Avdeef, A.; Bendels, S.; Di, L.; Ecker, G.F.; Faller, B.; Fischer, H.; Gerebtzoff, G.; et al. Coexistence of passive and carrier-mediated processes in drug transport. Nat. Rev. Drug Discov. 2010, 9, 597–614. [Google Scholar] [CrossRef]
- Smith, D.; Artursson, P.; Avdeef, A.; Di, L.; Ecker, G.F.; Faller, B.; Houston, J.B.; Kansy, M.; Kerns, E.H.; Krämer, S.D. Passive lipoidal diffusion and carrier-mediated cell uptake are both important mechanisms of membrane permeation in drug disposition. Mol. Pharm. 2014, 11, 1727–1738. [Google Scholar] [CrossRef]
- Thomae, A.V.; Wunderli-Allenspach, H.; Krämer, S.D. Permeation of aromatic carboxylic acids across lipid bilayers: The pH-partition hypothesis revisited. Biophys. J. 2005, 89, 1802–1811. [Google Scholar] [CrossRef]
- Palm, K.; Luthman, K.; Ros, J.; Gråsjö, J.; Artursson, P. Effect of molecular charge on intestinal epithelial drug transport: pH-dependent transport of cationic drugs. J. Pharm. Exp. Ther. 1999, 291, 435–443. [Google Scholar]
- Zheng, Y.; Benet, L.Z.; Okochi, H.; Chen, X. pH dependent but not P-gp dependent bidirectional transport study of S-propranolol: The importance of passive diffusion. Pharm. Res. 2015, 32, 2516–2526. [Google Scholar] [CrossRef]
- Karasov, W.H. Integrative physiology of transcellular and paracellular intestinal absorption. J. Exp. Biol. 2017, 220, 2495–2501. [Google Scholar] [CrossRef]
- Fagerholm, U.; Nilsson, D.; Knutson, L.; Lennernäs, H. Jejunal permeability in humans in vivo and rats in situ: Investigation of molecular size selectivity and solvent drag. Acta Physiol. Scand. 1999, 165, 315–324. [Google Scholar] [CrossRef]
- Lingaraju, A.; Long, T.M.; Wang, Y.; Austin II, J.R.; Turner, J.R. Conceptual barriers to understanding physical barriers. Semin. Cell Dev. Biol. 2015, 42, 13–21. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Fanning, A.S.; Anderson, J.M. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am. J. Physiol. Ren. Physiol. 2003, 285, F1078–F1084. [Google Scholar] [CrossRef]
- Price, E.R.; Brun, A.; Gontero-Fourcade, M.; Fernández-Marinone, G.; Cruz-Neto, A.P.; Karasov, W.H.; Caviedes-Vidal, E. Intestinal water absorption varies with expected dietary water load among bats but does not drive paracellular nutrient absorption. Physiol. Biochem. Zool. 2015, 88, 680–684. [Google Scholar] [CrossRef]
- Günzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Avdeef, A.; Tam, K.Y. How Well Can the Caco-2/Madin− Darby Canine Kidney Models Predict Effective Human Jejunal Permeability? J. Med. Chem. 2010, 53, 3566–3584. [Google Scholar] [CrossRef]
- Wang, Y.T.; Mohammed, S.D.; Farmer, A.D.; Wang, D.; Zarate, N.; Hobson, A.R.; Hellström, P.M.; Semler, J.R.; Kuo, B.; Rao, S.S. Regional gastrointestinal transit and pH studied in 215 healthy volunteers using the wireless motility capsule: Influence of age, gender, study country and testing protocol. Aliment. Pharm. Ther. 2015, 42, 761–772. [Google Scholar] [CrossRef]
- Sugano, K.; Nabuchi, Y.; Machida, M.; Asoh, Y. Permeation characteristics of a hydrophilic basic compound across a bio-mimetic artificial membrane. Int. J. Pharm. 2004, 275, 271–278. [Google Scholar] [CrossRef]
- Fischer, H.; Kansy, M.; Avdeef, A.; Senner, F. Permeation of permanently positive charged molecules through artificial membranes—Influence of physico-chemical properties. Eur. J. Pharm. Sci. 2007, 31, 32–42. [Google Scholar] [CrossRef]
- Krämer, S.D.; Aschmann, H.E.; Hatibovic, M.; Hermann, K.F.; Neuhaus, C.S.; Brunner, C.; Belli, S. When barriers ignore the “rule-of-five”. Adv. Drug Deliv. Rev. 2016, 101, 62–74. [Google Scholar] [CrossRef]
- Bemporad, D.; Luttmann, C.; Essex, J. Behaviour of small solutes and large drugs in a lipid bilayer from computer simulations. Biochim. Biophys. Acta (BBA) Biomembr. 2005, 1718, 1–21. [Google Scholar] [CrossRef]
- Vorobyov, I.; Olson, T.E.; Kim, J.H.; Koeppe Ii, R.E.; Andersen, O.S.; Allen, T.W. Ion-induced defect permeation of lipid membranes. Biophys. J. 2014, 106, 586–597. [Google Scholar] [CrossRef]
- Van Hell, A.J.; Melo, M.N.; Van Blitterswijk, W.J.; Gueth, D.M.; Braumuller, T.M.; Pedrosa, L.R.; Song, J.Y.; Marrink, S.J.; Koning, G.A.; Jonkers, J. Defined lipid analogues induce transient channels to facilitate drug-membrane traversal and circumvent cancer therapy resistance. Sci. Rep. 2013, 3, 1949. [Google Scholar] [CrossRef]
- Xiang, T.X.; Anderson, B. Influence of a transmembrane protein on the permeability of small molecules across lipid membranes. J. Membr. Biol. 2000, 173, 187–201. [Google Scholar] [CrossRef]
- Miller, J.M.; Dahan, A.; Gupta, D.; Varghese, S.; Amidon, G.L. Enabling the intestinal absorption of highly polar antiviral agents: Ion-pair facilitated membrane permeation of zanamivir heptyl ester and guanidino oseltamivir. Mol. Pharm. 2010, 7, 1223–1234. [Google Scholar] [CrossRef]
- Neubert, R. Ion pair transport across membranes. Pharm. Res. 1989, 6, 743–747. [Google Scholar] [CrossRef]
- Khavrutskii, I.V.; Gorfe, A.A.; Lu, B.; McCammon, J.A. Free energy for the permeation of Na+ and Cl− ions and their ion-pair through a zwitterionic dimyristoyl phosphatidylcholine lipid bilayer by umbrella integration with harmonic fourier beads. J. Am. Chem. Soc. 2009, 131, 1706–1716. [Google Scholar] [CrossRef][Green Version]
- Bickerton, G.R.; Paolini, G.V.; Besnard, J.; Muresan, S.; Hopkins, A.L. Quantifying the chemical beauty of drugs. Nat. Chem. 2012, 4, 90. [Google Scholar] [CrossRef]
- Alex, A.; Millan, D.S.; Perez, M.; Wakenhut, F.; Whitlock, G.A. Intramolecular hydrogen bonding to improve membrane permeability and absorption in beyond rule of five chemical space. Med. Chem. Comm. 2011, 2, 669–674. [Google Scholar] [CrossRef]
- Kuhn, B.; Mohr, P.; Stahl, M. Intramolecular hydrogen bonding in medicinal chemistry. J. Med. Chem. 2010, 53, 2601–2611. [Google Scholar] [CrossRef]
- Clegg, W.; Teat, S.J. Tetracycline hydrochloride: A synchrotron microcrystal study. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2000, 56, 1343–1345. [Google Scholar] [CrossRef]
- Bennion, B.J.; Be, N.A.; McNerney, M.W.; Lao, V.; Carlson, E.M.; Valdez, C.A.; Malfatti, M.A.; Enright, H.A.; Nguyen, T.H.; Lightstone, F.C. Predicting a drug’s membrane permeability: A computational model validated with in vitro permeability assay data. J. Phys. Chem. B. 2017, 121, 5228–5237. [Google Scholar] [CrossRef]
- Orsi, M.; Essex, J.W. Permeability of drugs and hormones through a lipid bilayer: Insights from dual-resolution molecular dynamics. Soft Matter 2010, 6, 3797–3808. [Google Scholar] [CrossRef]
- Mahajan, R.; Parvez, A.; Gupta, K. Microdosing vs. Therapeutic dosing for evaluation of pharmacokinetic data: A comparative study. J. Young Pharm. 2009, 1, 290. [Google Scholar] [CrossRef]
- Wakelkamp, M.; Alván, G.; Paintaud, G.; Hedman, A. Dose proportional absorption of 25–150 mg atenolol. Eur. J. Clin. Pharmacol. 1993, 44, 305–306. [Google Scholar] [CrossRef]
- Fitzgerald, J.; Ruffin, R.; Smedstad, K.; Roberts, R.; McAinsh, J. Studies on the pharmacokinetics and pharmacodynamics of atenolol in man. Eur. J. Clin. Pharm. 1978, 13, 81–89. [Google Scholar] [CrossRef]
- Ieiri, I.; Maeda, K.; Sasaki, T.; Kimura, M.; Hirota, T.; Chiyoda, T.; Miyagawa, M.; Irie, S.; Iwasaki, K.; Sugiyama, Y. Microdosing clinical study: Pharmacokinetic, pharmacogenomic (SLCO2B1), and interaction (grapefruit juice) profiles of celiprolol following the oral microdose and therapeutic dose. J. Clin. Pharm. 2012, 52, 1078–1089. [Google Scholar] [CrossRef]
- Kato, Y.; Miyazaki, T.; Kano, T.; Sugiura, T.; Kubo, Y.; Tsuji, A. Involvement of influx and efflux transport systems in gastrointestinal absorption of celiprolol. J. Pharm. Sci. 2009, 98, 2529–2539. [Google Scholar] [CrossRef]
- Reeves, P.R.; Barnfield, D.J.; Longshaw, S.; McIntosh, D.A.; Winrow, M.J. Disposition and metabolism of atenolol in animals. Xenobiotica 1978, 8, 305–311. [Google Scholar] [CrossRef]
- Reeves, P.R.; McAinsh, J.; McIntosh, D.A.; Winrow, M.J. Metabolism of atenolol in man. Xenobiotica 1978, 8, 313–320. [Google Scholar] [CrossRef]
- Mason, W.; Winer, N.; Kochak, G.; Cohen, I.; Bell, R. Kinetics and absolute bioavailability of atenolol. Clin. Pharm. Ther. 1979, 25, 408–415. [Google Scholar] [CrossRef]
- Yin, J.; Duan, H.; Shirasaka, Y.; Prasad, B.; Wang, J. Atenolol Renal Secretion Is Mediated by Human Organic Cation Transporter 2 and Multidrug and Toxin Extrusion Proteins. Drug Metab. Dispos. 2015, 43, 1872–1881. [Google Scholar] [CrossRef]
- Jeon, H.; Jang, I.J.; Lee, S.; Ohashi, K.; Kotegawa, T.; Ieiri, I.; Cho, J.Y.; Yoon, S.H.; Shin, S.G.; Yu, K.S.; et al. Apple juice greatly reduces systemic exposure to atenolol. Br. J. Clin. Pharm. 2013, 75, 172–179. [Google Scholar] [CrossRef]
- Lilja, J.; Raaska, K.; Neuvonen, P. Effects of orange juice on the pharmacokinetics of atenolol. Eur. J. Clin. Pharm. 2005, 61, 337–340. [Google Scholar] [CrossRef]
- Bailey, D.G. Fruit juice inhibition of uptake transport: A new type of food–drug interaction. Br. J. Clin. Pharm. 2010, 70, 645–655. [Google Scholar] [CrossRef]
- Riley, S.; Kim, M.; Sutcliffe, F.; Kapas, M.; Rowland, M.; Turnberg, L. Effects of a non-absorbable osmotic load on drug absorption in healthy volunteers. Br. J. Clin. Pharm. 1992, 34, 40–46. [Google Scholar] [CrossRef]
- Augustijns, P.; Mols, R. HPLC with programmed wavelength fluorescence detection for the simultaneous determination of marker compounds of integrity and P-gp functionality in the Caco-2 intestinal absorption model. J. Pharm. Biomed. Anal. 2004, 34, 971–978. [Google Scholar] [CrossRef]
- Saaby, L.; Helms, H.C.C.; Brodin, B. IPEC-J2 MDR1, a novel high-resistance cell line with functional expression of human P-glycoprotein (ABCB1) for drug screening studies. Mol. Pharm. 2016, 13, 640–652. [Google Scholar] [CrossRef]
- Hayeshi, R.; Hilgendorf, C.; Artursson, P.; Augustijns, P.; Brodin, B.; Dehertogh, P.; Fisher, K.; Fossati, L.; Hovenkamp, E.; Korjamo, T. Comparison of drug transporter gene expression and functionality in Caco-2 cells from 10 different laboratories. Eur J. Pharm. Sci. 2008, 35, 383–396. [Google Scholar] [CrossRef]
- Tronde, A.; Nordén, B.; Jeppsson, A.B.; Brunmark, P.; Nilsson, E.; Lennernäs, H.; Bengtsson, U.H. Drug absorption from the isolated perfused rat lung–correlations with drug physicochemical properties and epithelial permeability. J. Drug Target. 2003, 11, 61–74. [Google Scholar] [CrossRef]
- Mols, R.; Brouwers, J.; Schinkel, A.H.; Annaert, P.; Augustijns, P. Intestinal perfusion with mesenteric blood sampling in wild-type and knockout mice evaluation of a novel tool in biopharmaceutical drug profiling. Drug Metab. Dispos. 2009, 37, 1334–1337. [Google Scholar] [CrossRef]
- Brouwers, J.; Mols, R.; Annaert, P.; Augustijns, P. Validation of a differential in situ perfusion method with mesenteric blood sampling in rats for intestinal drug interaction profiling. Biopharm. Drug Dispos. 2010, 31, 278–285. [Google Scholar] [CrossRef]
- Terao, T.; Hisanaga, E.; Sai, Y.; Tamai, I.; Tsuji, A. Active secretion of drugs from the small intestinal epithelium in rats by P-glycoprotein functioning as an absorption barrier. J. Pharm. Pharm. 1996, 48, 1083–1089. [Google Scholar] [CrossRef]
- Lilja, J.J.; Backman, J.T.; Neuvonen, P.J. Effect of itraconazole on the pharmacokinetics of atenolol. Basic Clin. Pharm. Toxicol. 2005, 97, 395–398. [Google Scholar] [CrossRef]
- Ni, J.; Ouyang, H.; Aiello, M.; Seto, C.; Borbridge, L.; Sakuma, T.; Ellis, R.; Welty, D.; Acheampong, A. Microdosing assessment to evaluate pharmacokinetics and drug metabolism in rats using liquid chromatography-tandem mass spectrometry. Pharm. Res. 2008, 25, 1572–1582. [Google Scholar] [CrossRef]
- Helander, H.F.; Fändriks, L. Surface area of the digestive tract-revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef]
- Van Bree, J.B.; Baljet, A.V.; van Geyt, A.; de Boer, A.G.; Danhof, M.; Breimer, D.D. The unit impulse response procedure for the pharmacokinetic evaluation of drug entry into the central nervous system. J. Pharm. Biopharm. 1989, 17, 441–462. [Google Scholar] [CrossRef]
- Nakagawa, S.; Deli, M.A.; Kawaguchi, H.; Shimizudani, T.; Shimono, T.; Kittel, A.; Tanaka, K.; Niwa, M. A new blood–brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem. Int. 2009, 54, 253–263. [Google Scholar] [CrossRef]
- Chen, X.; Slättengren, T.; Lange, E.C.; Smith, D.E.; Hammarlund-Udenaes, M. Revisiting atenolol as a low passive permeability marker. Fluids Barriers CNS 2017, 14, 30. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahlgren, D.; Lennernäs, H. Intestinal Permeability and Drug Absorption: Predictive Experimental, Computational and In Vivo Approaches. Pharmaceutics 2019, 11, 411. https://doi.org/10.3390/pharmaceutics11080411
Dahlgren D, Lennernäs H. Intestinal Permeability and Drug Absorption: Predictive Experimental, Computational and In Vivo Approaches. Pharmaceutics. 2019; 11(8):411. https://doi.org/10.3390/pharmaceutics11080411
Chicago/Turabian StyleDahlgren, David, and Hans Lennernäs. 2019. "Intestinal Permeability and Drug Absorption: Predictive Experimental, Computational and In Vivo Approaches" Pharmaceutics 11, no. 8: 411. https://doi.org/10.3390/pharmaceutics11080411
APA StyleDahlgren, D., & Lennernäs, H. (2019). Intestinal Permeability and Drug Absorption: Predictive Experimental, Computational and In Vivo Approaches. Pharmaceutics, 11(8), 411. https://doi.org/10.3390/pharmaceutics11080411