Hydroxyurea-Loaded Albumin Nanoparticles: Preparation, Characterization, and In Vitro Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Process Optimization and Preparation of HSA–HU-NPs
2.2.1. HSA-NPs
2.2.2. HSA–HU-NPs
2.3. Nanoparticles Size Measurement, Zeta Potential Analysis, and Surface Morphology
2.4. Yield and Encapsulation Efficiency of HSA-NPs
2.5. Determination of the In Vitro HU Drug Release
concentration of HU used) × 100.
3. Results and Discussion
3.1. Nanoparticles Optimization and Preparation
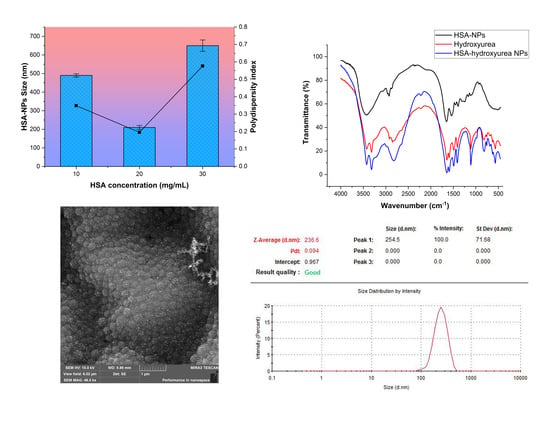
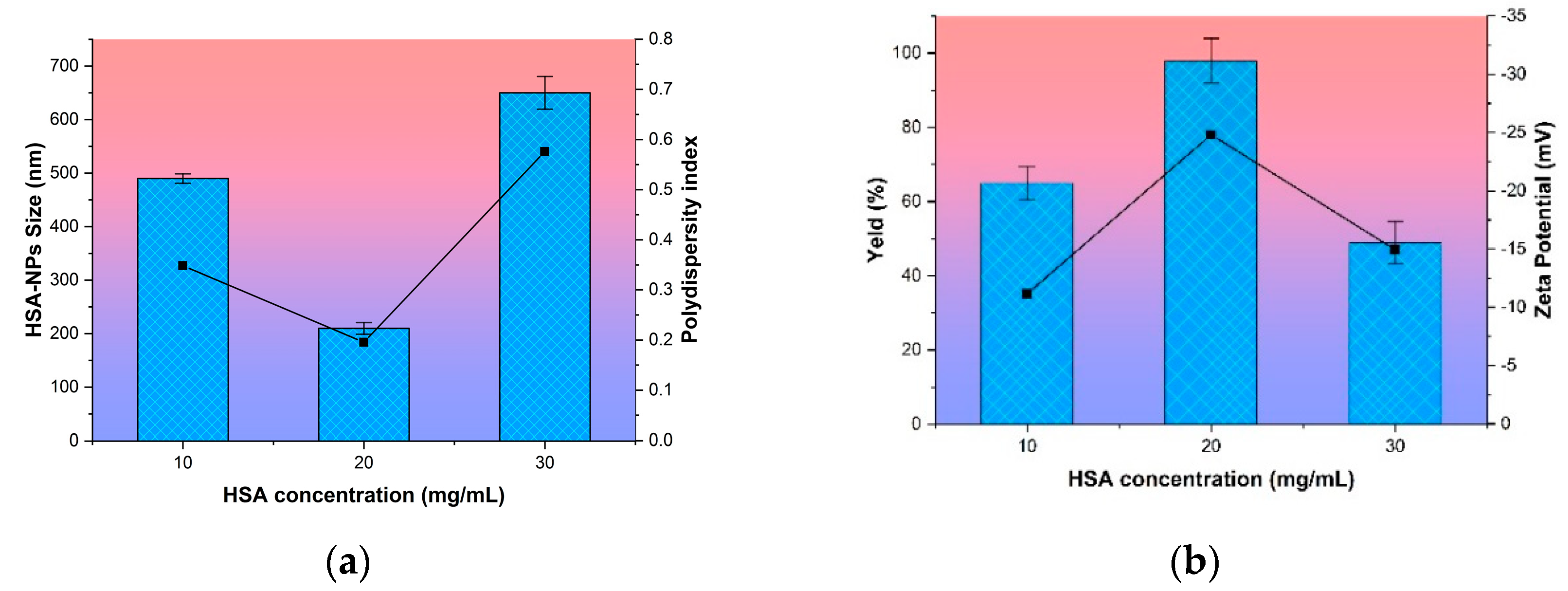
3.1.1. Effect of HSA Concentration on Nanoparticle Size
3.1.2. Effect of Urea and Cysteine Concentration on Nanoparticle Size
3.1.3. Effect of Hydroxyurea Addition in Nanoparticles
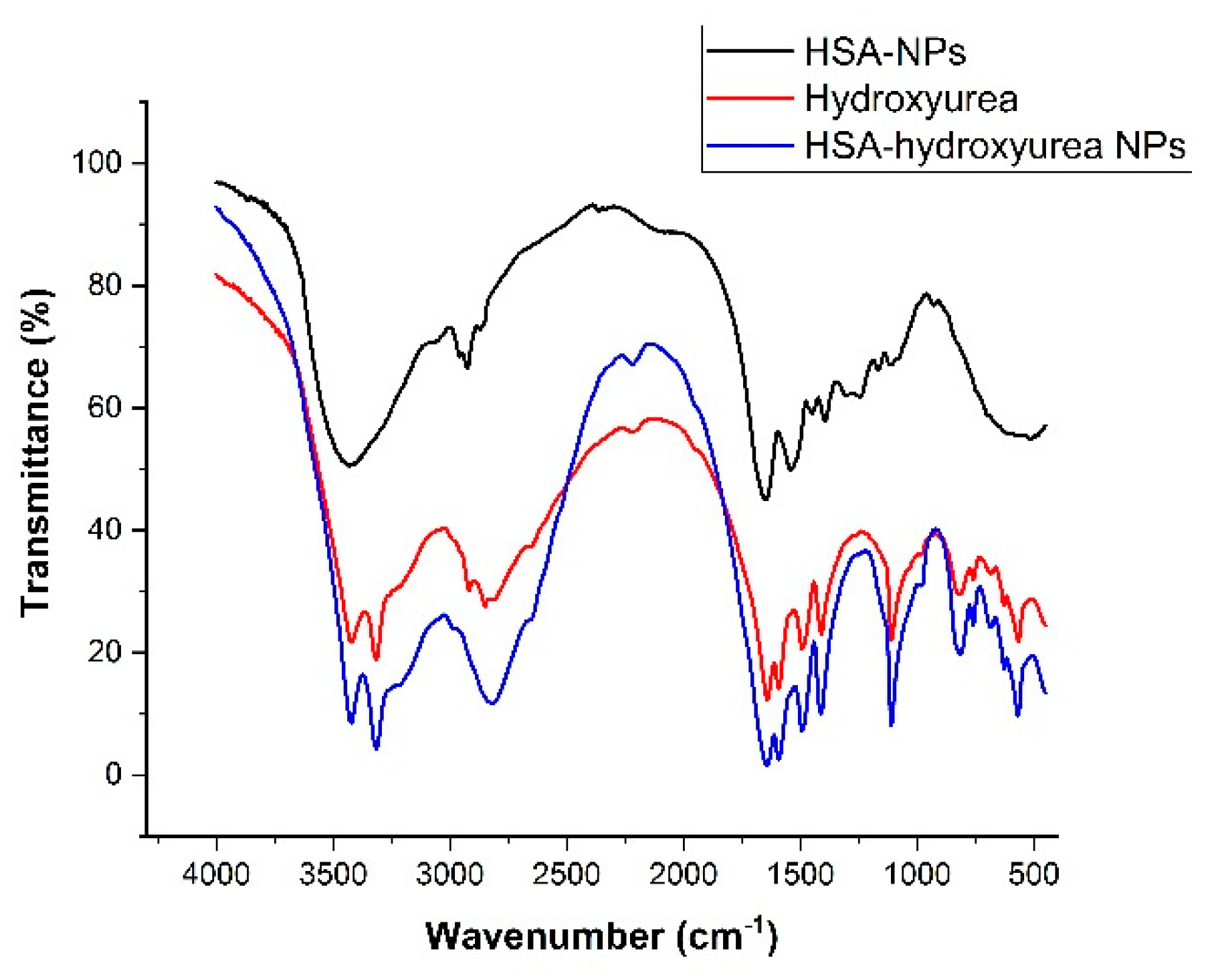
3.2. Physical-Chemical Characteristics of Nanoparticles Immobilized with HU
3.3. In Vitro Drug Release
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kreuter, J. Colloidal Drug Delivery Systems; Marcel Dekker: New York, NY, USA, 1994; p. 344. [Google Scholar]
- Marty, J.J.; Oppenheim, R.C.; Speiser, P. Nanoparticles—A new colloidal drug delivery system. Pharm. Acta Helv. 1978, 53, 17–23. [Google Scholar] [PubMed]
- Cheng, H.; Jiang, X.Y.; Zheng, R.R.; Zuo, S.J.; Zhao, L.P.; Fan, G.L.; Xie, B.R.; Yu, X.Y.; Li, S.Y.; Zhang, X.Z. A biomimetic cascade nanoreactor for tumor targeted starvation therapy-amplified chemotherapy. Biomaterials 2019, 195, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Chytil, P.; Koziolova, E.; Etrych, T.; Ulbrich, K. HPMA Copolymer-Drug Conjugates with Controlled Tumor-Specific Drug Release. Macromol. Biosci. 2018, 18, 1700209. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Ye, C.; Bai, E.H.; Zhang, L.L.; Huo, S.J.; Yu, H.H.; Xiang, S.Y.; Yu, S.Q. Co-delivery nanoparticles of doxorubicin and chloroquine for improving the anti-cancer effect in vitro. Nanotechnology 2019, 8, 085101. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Matsumura, Y. Tumoritropic and lymphotropic principles of macromolecular drugs. Crit. Rev. Ther. Drug Carr. Syst. 1989, 6, 193–210. [Google Scholar]
- Seymour, L.W.; Miyamoto, Y.; Maeda, H.; Brereton, M.; Strohalm, J.; Ulbrich, K.; Duncan, R. Influence of molecular weight on passive tumour accumulation of a soluble macromolecular drug carrier. Eur. J. Cancer 1995, 31, 766–770. [Google Scholar] [CrossRef]
- Wunder, A.; Muller-Ladner, U.; Stelzer, E.H.; Funk, J.; Neumann, E.; Stehle, G.; Pap, T.; Sinn, H.; Gay, S.; Fiehn, C. Albumin-based drug delivery as novel therapeutic approach for rheumatoid arthritis. J. Immunol. Methods 2003, 170, 4793–4801. [Google Scholar] [CrossRef]
- Tao, C.; Chuah, Y.J.; Xu, C.J.; Wang, D.A. Albumin conjugates and assemblies as versatile bio-functional additives and carriers for biomedical applications. J. Mater. Chem. B 2019, 7, 357–367. [Google Scholar] [CrossRef]
- Anbouhi, T.S.; Esfidvajani, E.M.; Nemati, F. Albumin binding, anticancer and antibacterial properties of synthesized zero valent iron nanoparticles. Int. J. Nanomed. 2018, 14, 243–256. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Weber, C.; Kreuter, J.; Langer, K. Desolvation process and surface characteristics of HSA-nanoparticles. Int. J. Pharm. 2000, 196, 197–200. [Google Scholar] [CrossRef]
- Langer, K.; Balthasar, S.; Vogel, V.; Dinauer, N.; Von Briesen, H.; Schubert, D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int. J. Pharm. 2003, 257, 169–180. [Google Scholar] [CrossRef]
- Kufleitner, J.; Wagner, S.; Worek, F.; Briesen, H.; Kreuter, J. Adsorption of obidoxime onto human serum albumin nanoparticles: Drug loading, particle size and drug release. J. Microencapsul. 2010, 27, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Bergonzi, M.; Guccione, C.; Grossi, C.; Piazzini, V.; Torracchi, A.; Luccarini, I.; Casamenti, F.; Bilia, A. Albumin Nanoparticles for Brain Delivery: A Comparison of Chemical versus Thermal Methods and in vivo Behavior. ChemMedChem 2016, 11, 1840–1849. [Google Scholar] [CrossRef] [PubMed]
- Luppi, B.; Bigucci, F.; Corace, G.; Delucca, A.; Cerchiara, T.; Sorrenti, M.; Catenacci, L.; Di Pietra, A.; Zecchi, V. Albumin nanoparticles carrying cyclodextrins for nasal delivery of the anti-Alzheimer drug tacrine. Eur. J. Pharm. Sci. 2011, 44, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Sebak, S.; Mirzael, M.; Malhotra, M.; Kulamarva, A.; Prakash, S. Human serum albumin nanoparticles as an efficient noscapine drug delivery system for potential use in breast cancer: Preparation and in vitro analysis. Int. J. Nanomed. 2010, 5, 525–532. [Google Scholar]
- Cui, W.; Wang, A.; Zhao, J.; Yang, X.; Cai, P.; Li, J. Layer by layer assembly of albumin nanoparticles with selective recognition of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J. Colloid Interface Sci. 2016, 465, 11–17. [Google Scholar] [CrossRef]
- Qu, N.; Lee, J.R.; Sun, Y. Cabazitaxel-loaded human serum albumin nanoparticles as a therapeutic agent against prostate cancer. Int. J. Nanomed. 2016, 11, 3451–3459. [Google Scholar]
- Lomis, N.; Westfall, S.; Farahdel, L. Human Serum Albumin Nanoparticles for Use in Cancer Drug Delivery: Process Optimization and in vitro Characterization. Nanomaterials 2016, 6, 116. [Google Scholar] [CrossRef]
- Burkeev, M.Z.; Kreuter, J.; Tazhbayev, Y.M.; Zhaparova, L.Z.; Zhumalieva, T.S. Preparation, characterization and investigation of in vitro release of anti-tuberculosis drug p-amino salicylic acid based on human serum albumin. Bull. Karaganda Univ. 2017, 87, 38–44. [Google Scholar] [CrossRef]
- Gun’ko, V.; Savina, I.; Mikhalovsky, S. Cryogels: Morphological, structural and adsorption characterisation. Adv. Colloid Interface Sci. 2013, 187, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I.; Okay, O. Basic principles of cryotropic gelation. Adv. Polym. Sci. 2014, 263, 49–102. [Google Scholar]
- Rodionov, I.A.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.; Lozinsky, V.I. Cryostructuring of Polymer Systems. Proteinaceous Wide-Pore Cryogels Generated by the Action of Denaturant/Reductant Mixtures on Bovine Serum Albumin in Moderately-Frozen Aqueous Media. Soft Matter 2015, 11, 4921–4931. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhang, M.; Li, G. Effect of cyclic freeze–thawing process on the structure and properties of collagen. Int. J. Biol. Macromol. 2015, 80, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Liu, L.; Lu, Y.; Zhang, Y.; He, X.; Chen, X.; Zhang, Y.; Chen, Q.; Guo, Q.; Jiang, C.; et al. Substance P-modified human serum albumin nanoparticles loaded with paclitaxel for targeted therapy of glioma. Acta Pharm. Sin. B 2018, 8, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Lopes, E.D.; Cardoso, A.V. Effect of hydroxyurea (HU) on gelatinization mechanism of type I collagen suspensions. Materia (Rio de Janeiro) 2018, 23, 112–125. [Google Scholar]
- Veverka, M.; Simon, P.; Gallovic, J.; Jorik, V.; Veverkova, E.; Dubaj, T. Imatinib mesylate cocrystals: Synthesis, screening, and preliminary characterization. Mon. Fur Chem. 2012, 143, 1405–1415. [Google Scholar] [CrossRef]
- Bronze-Uhle, E.S.; Costa, B.C.; Ximenes, V.F.; Lisboa-Filho, P.N. Synthetic nanoparticles of bovine serum albumin with entrapped salicylic acid. Nanotechnol. Sci. Appl. 2017, 10, 11–21. [Google Scholar] [CrossRef]
- Juliana, K.; Débora, F.V.; Jociani, A.; Rubiana, M. Nanoencapsulation of Apocynin in Bovine Serum Albumin Nanoparticles: Physicochemical Characterization. Nanosci. Nanotechnol. Asia 2018, 8, 90–99. [Google Scholar]




| Precursor Concentration | Particle Characteristics | ||||||
|---|---|---|---|---|---|---|---|
| Immediately after Purification | After 2 Days | After 4 Days | |||||
| Average Diameter NPs, nm | PI | Average Diameter NPs, nm | PI | Average Diameter NPs, nm | PI | ||
| Urea, mg/mL | Cysteine, mg/mL | ||||||
| I | |||||||
| 40 | 0.001 | 308 ± 40 | 0.350 | 112 ± 6 | 0.572 | - | - |
| 0.01 | 237 ± 1.5 | 0.094 | 240 ± 4 | 0.062 | 264 ± 5 | 0.084 | |
| 0.1 | 285 ± 2 | 0.073 | 304 ± 2 | 0.053 | 299 ± 8 | 0.188 | |
| 0.3 | 321 ± 2.5 | 0.114 | 409 ± 6 | 0.085 | 429 ± 12 | 0.066 | |
| 0.5 | 331 ± 3 | 0.116 | 355 ± 2 | 0.158 | 386 ± 6 | 0.057 | |
| II | |||||||
| 5 | 0.5 | 378 ± 15 | 0.115 | 389 ± 12 | 0.304 | 375 ± 14 | 0.540 |
| 10 | 310 ± 5 | 0.085 | 320 ± 7 | 0.058 | 317 ± 5 | 0.092 | |
| 20 | 389 ± 7 | 0.114 | 392 ± 8 | 0.176 | 388 ± 9 | 0.165 | |
| 40 | 398 ± 10 | 0.128 | 385 ± 9 | 0.125 | 399 ± 12 | 0.105 | |
| The Concentration of HU mg/mL | Particle Size (nm) | Encapsulation Efficiency | PI | Zeta Potential (mV) | Size Distribution |
|---|---|---|---|---|---|
| 2 | 288 ± 10 | 40 ± 2 | 0.15 | −17.4 ± 0.5 | 100% (210–454) |
| 4 | 336 ± 2.5 | 22 ± 0.5 | 0.24 | −16.6 ± 0.4 | 100% (205–556) |
| 6 | 277 ± 1.5 | 68 ± 2 | 0.20 | −22.5 ± 0.7 | 100% (170–425) |
| 8 | 312 ± 2 | 77 ± 4 | 0.59 | −3.3 ± 0.2 | 45% (720–1265); 29% (177–550); 26% (32–90). |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tazhbayev, Y.; Mukashev, O.; Burkeev, M.; Kreuter, J. Hydroxyurea-Loaded Albumin Nanoparticles: Preparation, Characterization, and In Vitro Studies. Pharmaceutics 2019, 11, 410. https://doi.org/10.3390/pharmaceutics11080410
Tazhbayev Y, Mukashev O, Burkeev M, Kreuter J. Hydroxyurea-Loaded Albumin Nanoparticles: Preparation, Characterization, and In Vitro Studies. Pharmaceutics. 2019; 11(8):410. https://doi.org/10.3390/pharmaceutics11080410
Chicago/Turabian StyleTazhbayev, Yerkeblan, Olzhas Mukashev, Meiram Burkeev, and Jörg Kreuter. 2019. "Hydroxyurea-Loaded Albumin Nanoparticles: Preparation, Characterization, and In Vitro Studies" Pharmaceutics 11, no. 8: 410. https://doi.org/10.3390/pharmaceutics11080410
APA StyleTazhbayev, Y., Mukashev, O., Burkeev, M., & Kreuter, J. (2019). Hydroxyurea-Loaded Albumin Nanoparticles: Preparation, Characterization, and In Vitro Studies. Pharmaceutics, 11(8), 410. https://doi.org/10.3390/pharmaceutics11080410