In Vitro Cytotoxicity of Oleanolic/Ursolic Acids-Loaded in PLGA Nanoparticles in Different Cell Lines
Abstract
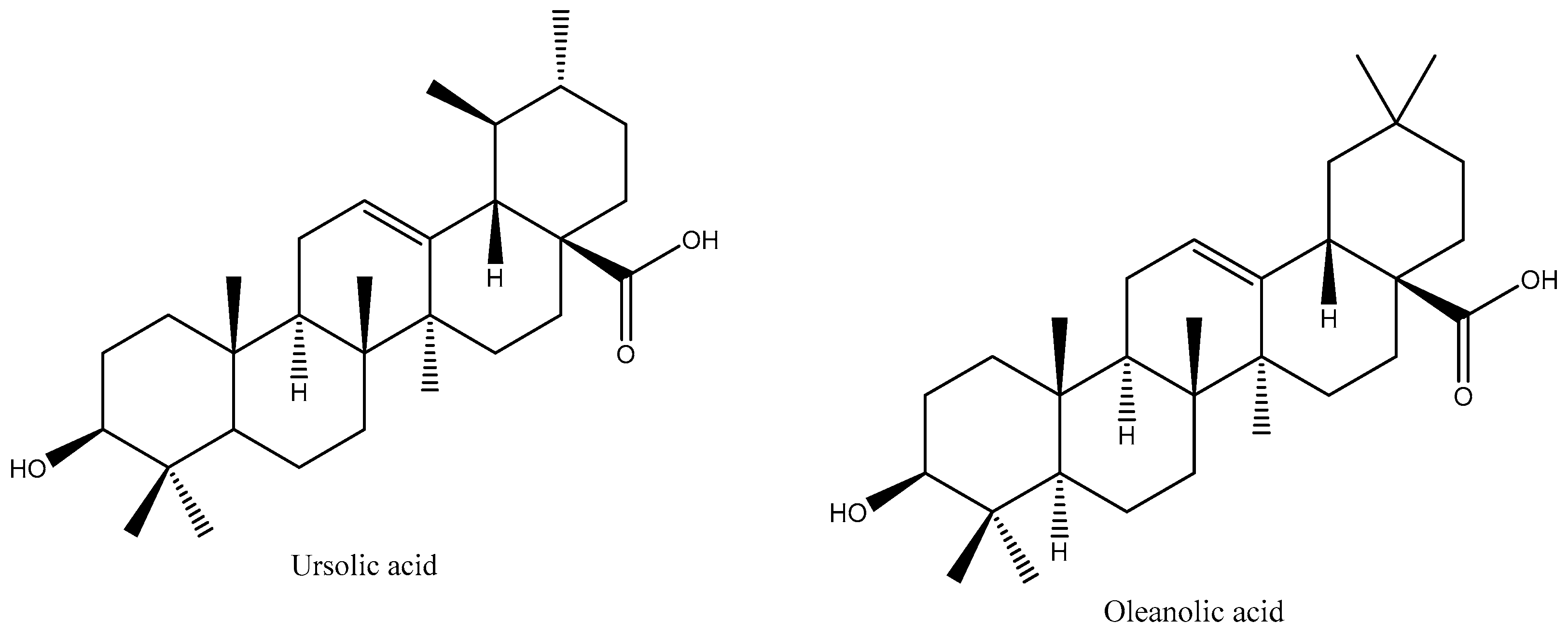
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Production of Natural (NM) and Synthetic (SM) Mixtures of Pentacyclic Triterpenes
2.3. Preparation of Nanoparticles
2.4. Cell Culture
2.5. Cytotoxicity Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Nanoparticles
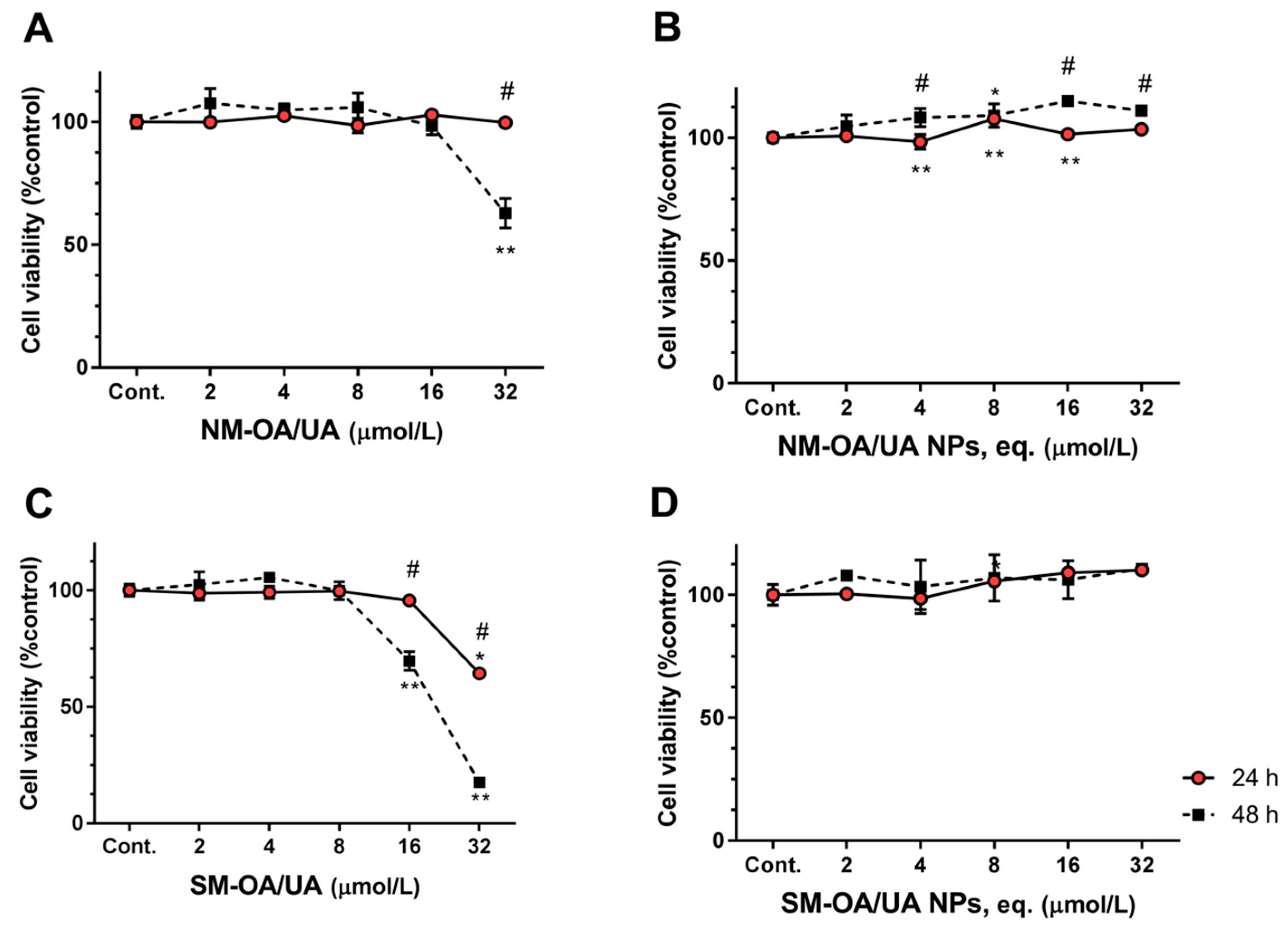
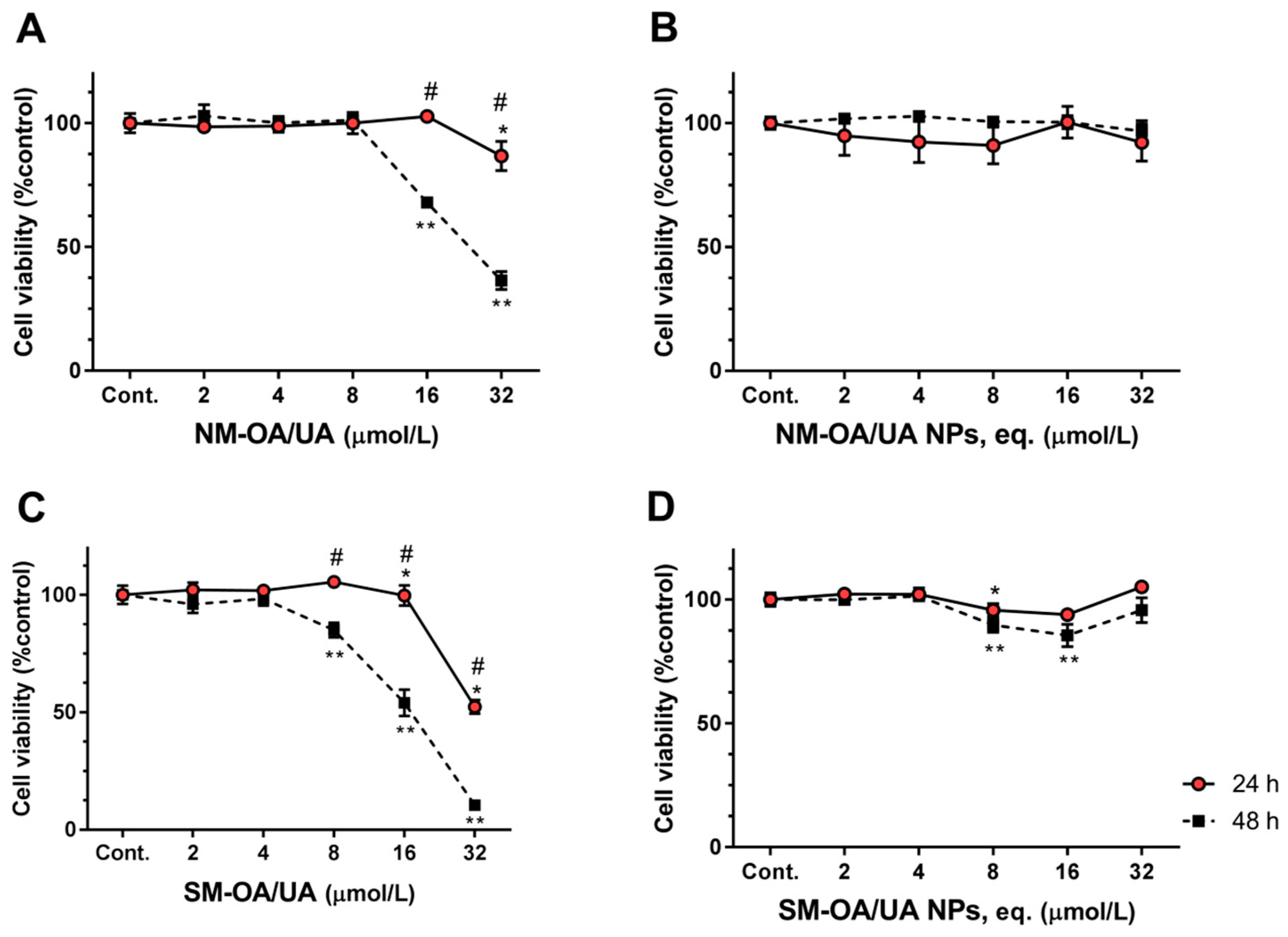
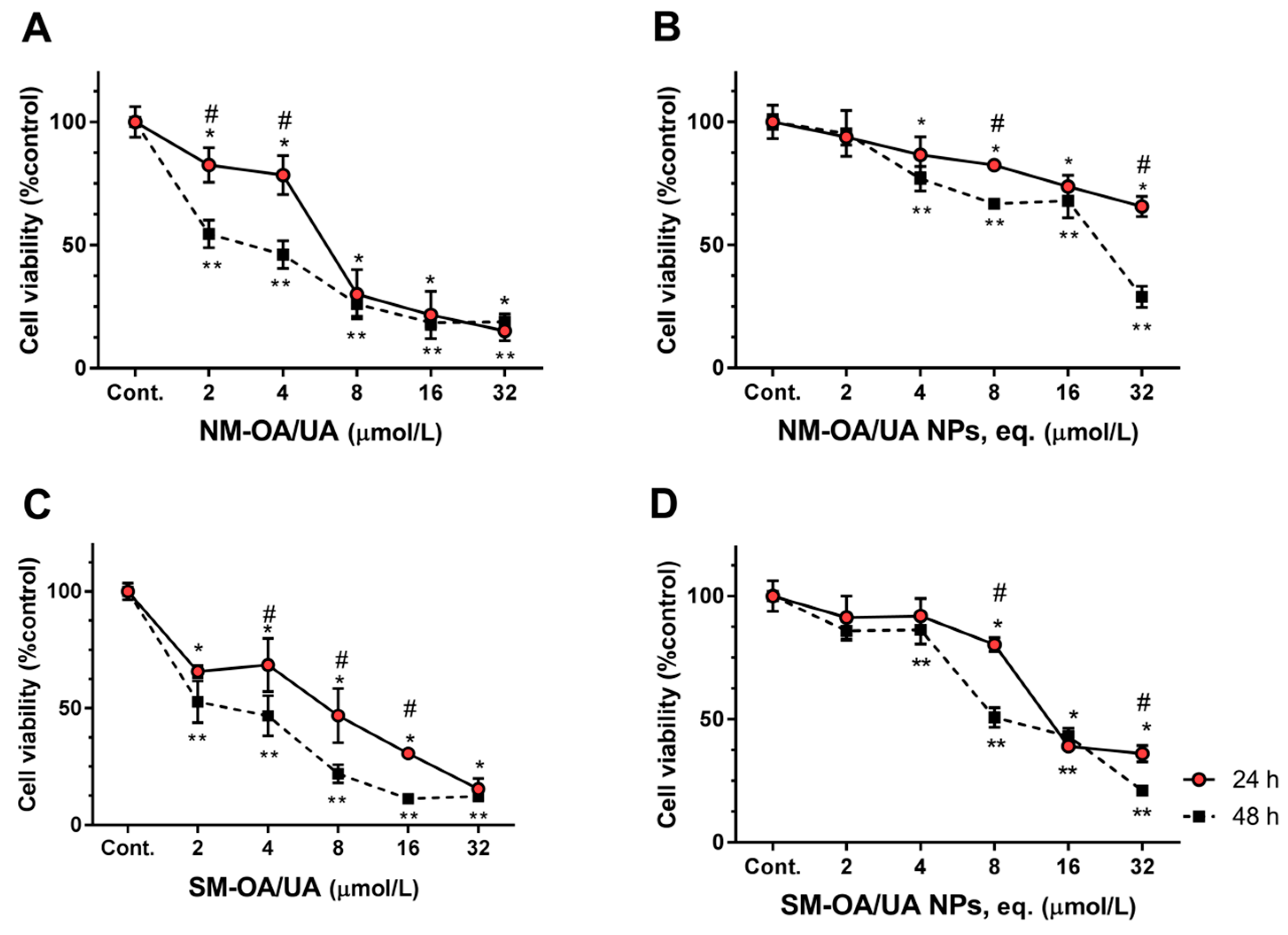
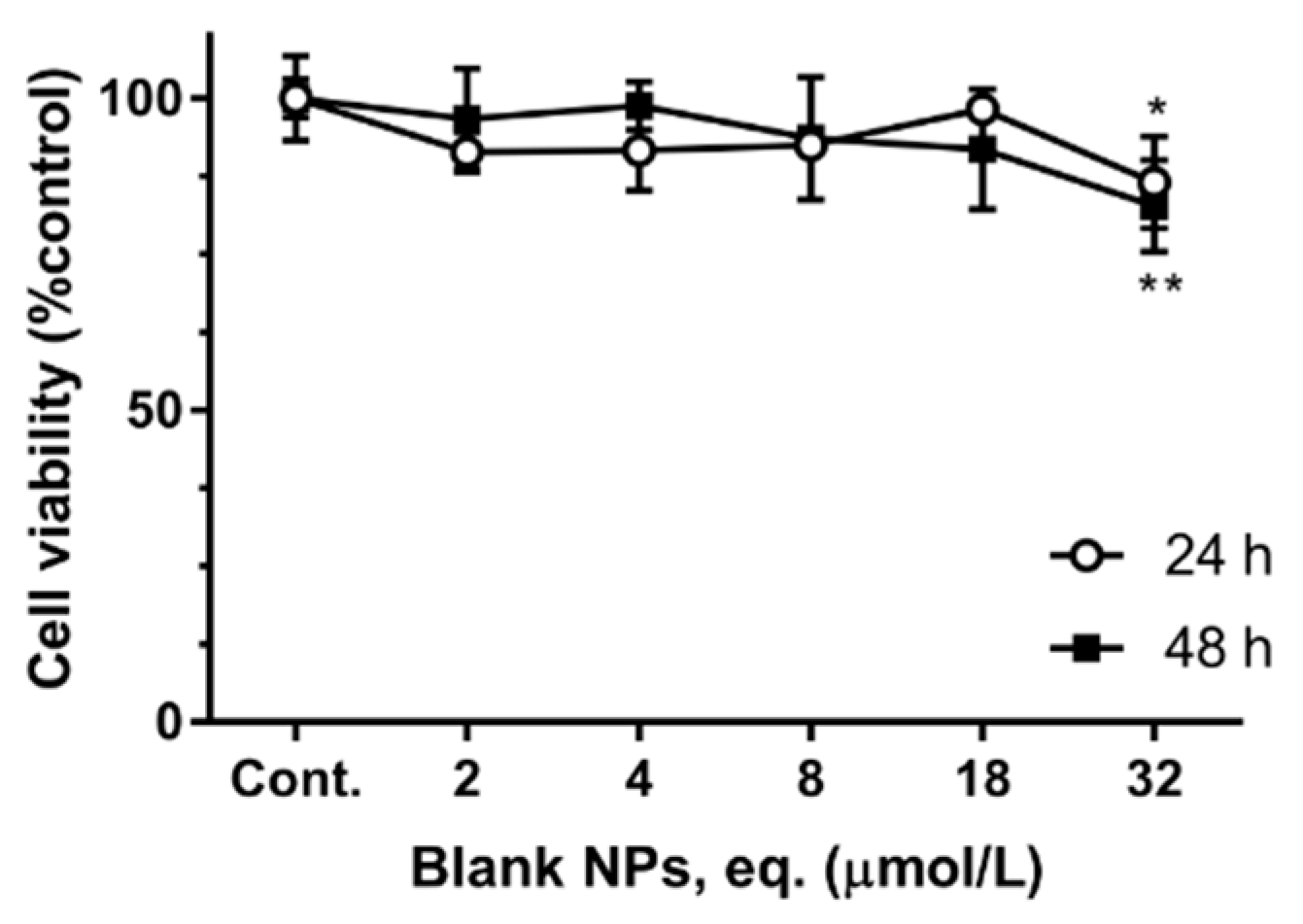
3.2. Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jesus, J.A. Antimicrobial activity of oleanolic and ursolic acids: An update. Evid. Based Complement. Altern. Med. 2015, 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Valdes, K. Potential use of nanocarriers with pentacyclic triterpenes in cancer treatments. Nanomedicine 2016, 11, 3139–3156. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [PubMed]
- Martins-Gomes, C. Chemical characterization and bioactive properties of decoctions and hydroethanolic extracts of Thymus carnosus Boiss. J. Funct. Foods 2018, 43, 154–164. [Google Scholar] [CrossRef]
- Taghouti, M. Thymus pulegioides L. as a rich source of antioxidant, anti-proliferative and neuroprotective phenolic compounds. Food Funct. 2018, 9, 3617–3629. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, J.; Heller, L.; Csuk, R. Targeting cancer cells with oleanolic and ursolic acid derived hydroxamates. Bioorg. Med. Chem. Lett. 2016, 26, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, B. Synthesis, structural studies, and cytotoxic evaluation of novel ursolic acid hybrids with capabilities to arrest breast cancer cells in mitosis. J. Asian Nat. Prod. Res. 2016, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sommerwerk, S. Urea derivates of ursolic, oleanolic and maslinic acid induce apoptosis and are selective cytotoxic for several human tumor cell lines. Eur. J. Med. Chem. 2016, 119, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Laszczyk, M.N. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Oprean, C. Anti-proliferative and antibacterial in vitro evaluation of the polyurethane nanostructures incorporating pentacyclic triterpenes. Pharm. Biol. 2016, 54, 2714–2722. [Google Scholar] [CrossRef]
- Sui, C.G. Antiproliferative activity of rosamultic acid is associated with induction of apoptosis, cell cycle arrest, inhibition of cell migration and caspase activation in human gastric cancer (SGC-7901) cells. Phytomedicine 2015, 22, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Ganbold, M. Cytotoxicity and bioavailability studies on a decoction of Oldenlandia diffusa and its fractions separated by HPLC. J. Ethnopharmacol. 2010, 131, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Yamai, H. Triterpenes augment the inhibitory effects of anticancer drugs on growth of human esophageal carcinoma cells in vitro and suppress experimental metastasis in vivo. Int. J. Cancer 2009, 125, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F. Design of cationic lipid nanoparticles for ocular delivery: Development, characterization and cytotoxicity. Int. J. Pharm. 2014, 461, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z. Induction of apoptosis in the SW620 colon carcinoma cell line by triterpene-enriched extracts from Ganoderma lucidum through activation of caspase-3. Oncol. Lett. 2011, 2, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Aguilar, M. The use of the microplate alamar blue assay (MABA) to assess the susceptibility of Mycobacterium lepraemurium to anti-leprosy and other drugs. J. Infect. Chemother. 2012, 18, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Severino, P. Solid lipid nanoparticles for hydrophilic biotech drugs: Optimization and cell viability studies (Caco-2 & HEPG-2 cell lines). Eur. J. Med. Chem. 2014, 81, 28–34. [Google Scholar] [PubMed]
- Martins-Gomes, C. Thymus carnosus extracts induce anti-proliferative activity in Caco-2 cells through mechanisms that involve cell cycle arrest and apoptosis. J. Funct. Foods 2019, 54, 128–135. [Google Scholar] [CrossRef]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers-A systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef]
- Severino, P. Advances in Nanobiomaterials for Oncology Nanomedicine. In Applications of NanoBioMaterials; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 91–115. [Google Scholar]
- Swain, S. Nanoparticles for Cancer Targeting: Current and Future Directions. Curr. Drug Deliv. 2016, 13, 1290–1302. [Google Scholar] [CrossRef]
- Carbone, C. Mediterranean essential oils as precious matrix components and active ingredients of lipid nanoparticles. Int. J. Pharm. 2018, 548, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C. Clotrimazole-Loaded Mediterranean Essential Oils NLC: A Synergic Treatment of Candida Skin Infections. Pharmaceutics 2019, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.S. Nanoencapsulation of polyphenols for protective effect against colon-rectal cancer. Biotechnol. Adv. 2013, 31, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, H.L. Design and optimization of oleanolic/ursolic acid-loaded nanoplatforms for ocular anti-inflammatory applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, H.L. Development and validation of a high-performance liquid chromatography method for the quantification of ursolic/oleanic acids mixture isolated from Plumeria obtusa. J. Chromatogr. B 2015, 983–984, 111–116. [Google Scholar] [CrossRef]
- Alvarado, H.L. Nanoemulsions for dermal controlled release of oleanolic and ursolic acids: In vitro, ex vivo and in vivo characterization. Colloids Surf. B Biointerfaces 2015, 130, 40–47. [Google Scholar] [CrossRef]
- Hernández Chávez, L.I. Estudio Químico y Evaluación Farmacológica de la Actividad Antidiabética de las hojas de Plumeria Obtusa, Especie Empleada en la Medicina Tradicional Mexicana Para Los Síntomas de la Diabetes Mellitus. Ph.D. Thesis, Universidad de Colima, Colima, Mexico, 2004. [Google Scholar]
- Fessi, H. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, 1–4. [Google Scholar] [CrossRef]
- Souza, A.L. In vitro evaluation of permeation, toxicity and effect of praziquantel-loaded solid lipid nanoparticles against Schistosoma mansoni as a strategy to improve efficacy of the schistosomiasis treatment. Int. J. Pharm. 2014, 463, 31–37. [Google Scholar] [CrossRef]
- Hamid, R. Comparison of alamar blue and MTT assays for high through-put screening. Toxicol. In Vitro 2004, 18, 703–710. [Google Scholar] [CrossRef]
- Nociari, M.M. A novel one-step, highly sensitive fluorometric assay to evaluate cell-mediated cytotoxicity. J. Immunol. Methods 1998, 213, 157–167. [Google Scholar] [CrossRef]
- Reid, T.W. Characteristics of an established cell line of retinoblastoma. J. Natl. Cancer Inst. 1974, 53, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Dimaras, H. Evidence-based care for trilateral retinoblastoma. Lancet Oncol. 2014, 15, 1054–1055. [Google Scholar] [CrossRef]
- Dimaras, H. Retinoblastoma. Lancet 2012, 379, 1436–1446. [Google Scholar] [CrossRef]
- Busch, M. Re-characterization of established human retinoblastoma cell lines. Histochem. Cell Biol. 2015, 143, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Junco, J.J. Ursolic acid and resveratrol synergize with chloroquine to reduce melanoma cell viability. Melanoma Res. 2015, 25, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Sharma, A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, W.J.; Yang, Q.Y. Effects of ursolic acid and oleanolic acid on human colon carcinoma cell line HCT15. World J. Gastroenterol. 2002, 8, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.W. In vitro and in vivo anticancer activity evaluation of ursolic acid derivatives. Eur. J. Med. Chem. 2011, 46, 2652–2661. [Google Scholar] [CrossRef]
- Yan, S.L. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. In Vitro 2010, 24, 842–848. [Google Scholar] [CrossRef]
- Li, J.C.; Kolesnikov, A.I. The first observation of the boson peak from water vapour deposited amorphous ice. Phys. B Condens. Matter 2002, 316–317, 493–496. [Google Scholar] [CrossRef]
| IC50 (µmol/L) | |||||
|---|---|---|---|---|---|
| NM-AO/UA | SM-AO/UA | NM-AO/UA NPs | SM-AO/UA NPs | ||
| HepG2 | 24 h | >32.0 | >32.0 | >>32.0 | >>32.0 |
| 48 h | >32.0 | 24.3 ± 1.8 | >>32.0 | >>32.0 | |
| Caco-2 | 24 h | >32.0 | 32.4 ± 0.9 | >32.0 | >32.0 |
| 48 h | 24.3 ± 0.9 | 16.3 ± 0.6 | >32.0 | >32.0 | |
| Y-79 | 24 h | 6.4 ± 0.9 | 6.5 ± 0.9 | 82.0 ± 10.2 | 16.6 ± 1.3 |
| 48 h | 2.6 ± 0.4 | 2.9 ± 0.3 | 19.1 ± 2.2 | 11.1 ± 0.8 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.M.; Alvarado, H.L.; Abrego, G.; Martins-Gomes, C.; Garduño-Ramirez, M.L.; García, M.L.; Calpena, A.C.; Souto, E.B. In Vitro Cytotoxicity of Oleanolic/Ursolic Acids-Loaded in PLGA Nanoparticles in Different Cell Lines. Pharmaceutics 2019, 11, 362. https://doi.org/10.3390/pharmaceutics11080362
Silva AM, Alvarado HL, Abrego G, Martins-Gomes C, Garduño-Ramirez ML, García ML, Calpena AC, Souto EB. In Vitro Cytotoxicity of Oleanolic/Ursolic Acids-Loaded in PLGA Nanoparticles in Different Cell Lines. Pharmaceutics. 2019; 11(8):362. https://doi.org/10.3390/pharmaceutics11080362
Chicago/Turabian StyleSilva, Amélia M., Helen L. Alvarado, Guadalupe Abrego, Carlos Martins-Gomes, Maria L. Garduño-Ramirez, María L. García, Ana C. Calpena, and Eliana B. Souto. 2019. "In Vitro Cytotoxicity of Oleanolic/Ursolic Acids-Loaded in PLGA Nanoparticles in Different Cell Lines" Pharmaceutics 11, no. 8: 362. https://doi.org/10.3390/pharmaceutics11080362
APA StyleSilva, A. M., Alvarado, H. L., Abrego, G., Martins-Gomes, C., Garduño-Ramirez, M. L., García, M. L., Calpena, A. C., & Souto, E. B. (2019). In Vitro Cytotoxicity of Oleanolic/Ursolic Acids-Loaded in PLGA Nanoparticles in Different Cell Lines. Pharmaceutics, 11(8), 362. https://doi.org/10.3390/pharmaceutics11080362






