Design of Soft Nanocarriers Combining Hyaluronic Acid with Another Functional Polymer for Cancer Therapy and Other Biomedical Applications
Abstract
1. Introduction
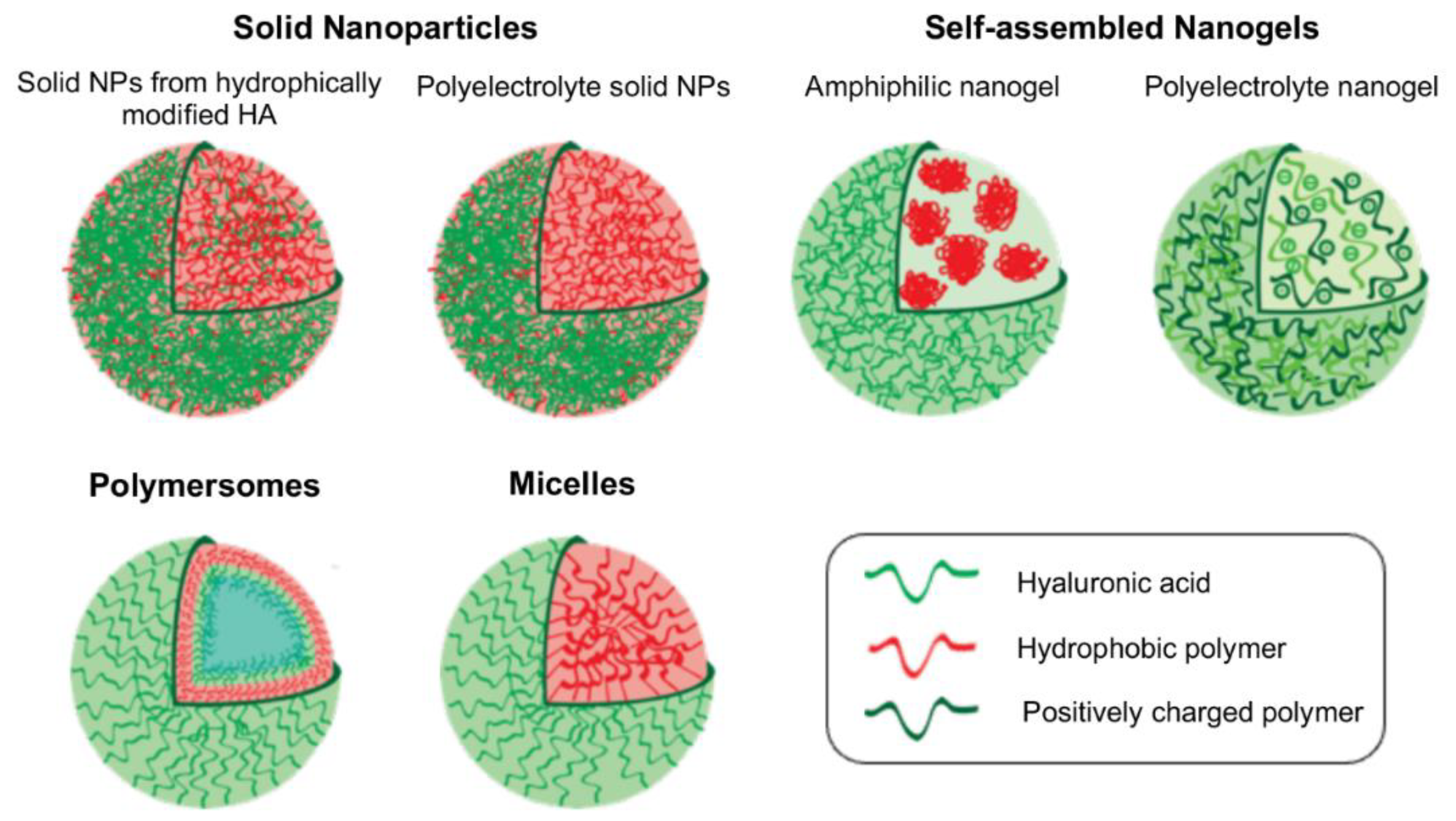
2. Types of Polymeric Nanocarrier Systems Based on HA
2.1. Solid Nanoparticles
2.2. Nanogels
2.3. Polymersomes
2.4. Micelles
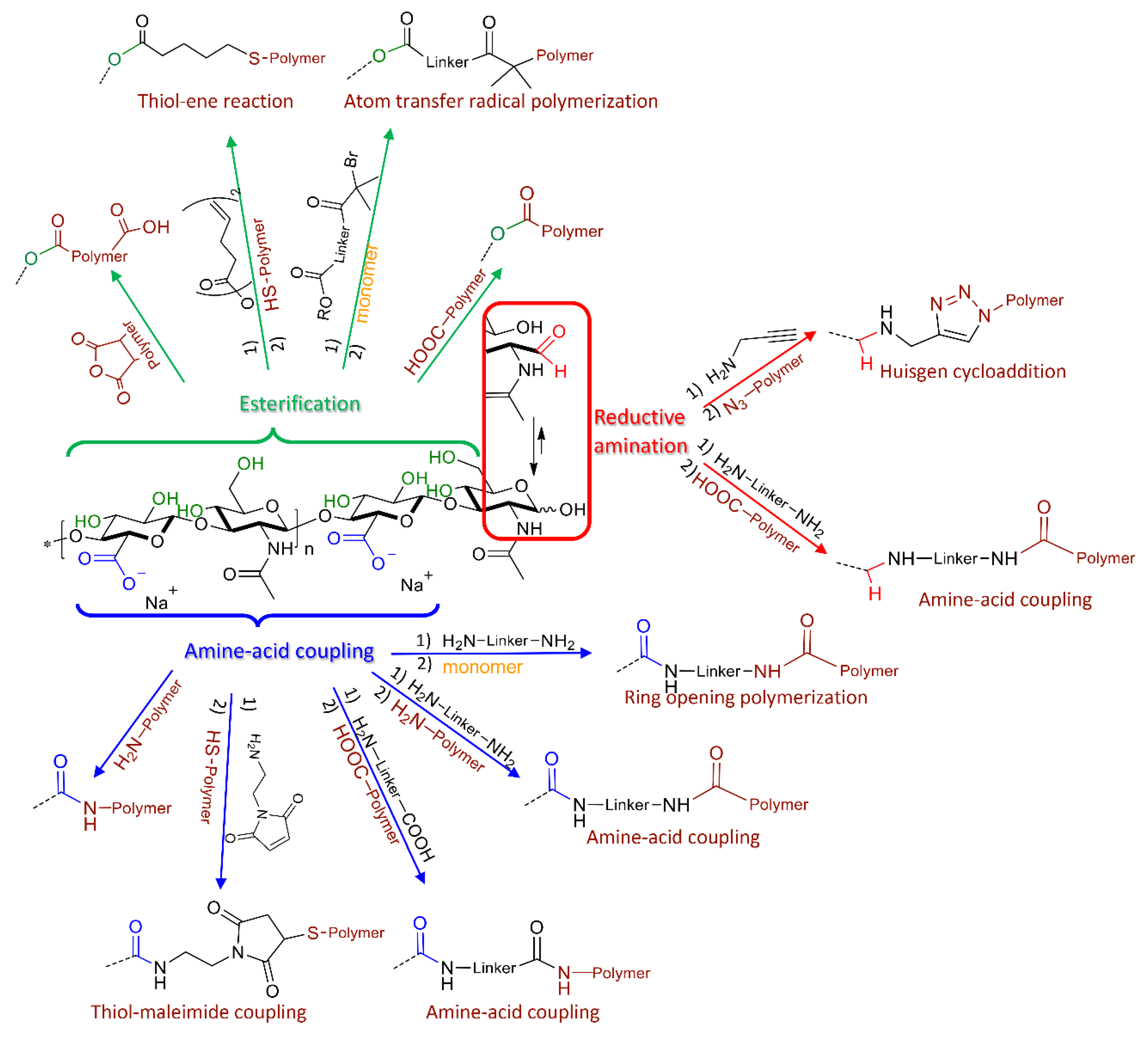
3. Synthetic Strategies for the Design of HA-Based Nanocarriers
3.1. Block Copolymers from HA
3.2. Graft Copolymers via “Grafting onto” or “Grafting from” HA
3.3. HA-Polymer Complexes
4. Nanocarriers Based on HA-Polymers Conjugates: Biomedical Applications
4.1. Biodegradable Nanocarriers
4.2. Polyelectrolyte Nanocarriers
4.3. pH-Responsive Nanocarriers
4.4. Thermoresponsive Nanocarriers
4.5. Redox-Responsive Nanocarriers
4.6. Light-Responsive Nanocarriers
5. HA-Based Polymeric Nanocarriers: Challenges and Future Prospects
6. Conclusions
Funding
Conflicts of Interest
References
- Husseini, G.A.; Pitt, W.G. Micelles and nanoparticles for ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1137–1152. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Singh, D.; Saraf, S.; Saraf, S. Nanocarriers: Promising vehicle for bioactive drugs. Biol. Pharm. Bull. 2006, 29, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Giner-Casares, J.J.; Henriksen-Lacey, M.; Coronado-Puchau, M.; Liz-Marzan, L.M. Inorganic nanoparticles for biomedicine: Where materials scientists meet medical research. Mater. Today 2016, 19, 19–28. [Google Scholar] [CrossRef]
- Karlsson, J.; Vaughan, H.J.; Green, J.J. Biodegradable Polymeric Nanoparticles for Therapeutic Cancer Treatments. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 105–127. [Google Scholar] [CrossRef]
- Kim, J.H.; Moon, M.J.; Kim, D.Y.; Heo, S.H.; Jeong, Y.Y. Hyaluronic acid-based nanomaterials for cancer therapy. Polymers 2018, 10, 1133. [Google Scholar] [CrossRef]
- Banik Brittany, L.; Fattahi, P.; Brown Justin, L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef]
- Nasir, A.; Kausar, A.; Younus, A. A Review on Preparation, Properties and Applications of Polymeric Nanoparticle-Based Materials. Polym. Plast. Technol. Eng. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Jiang, B.-P.; Zhang, L.; Zhu, Y.; Shen, X.-C.; Ji, S.-C.; Tan, X.-Y.; Cheng, L.; Liang, H. Water-soluble hyaluronic acid-hybridized polyaniline nanoparticles for effectively targeted photothermal therapy. J. Mater. Chem. B Mater. Biol. Med. 2015, 3, 3767–3776. [Google Scholar] [CrossRef]
- Gelmi, A.; Higgins, M.J.; Wallace, G.G. Attractive and Repulsive Interactions Originating from Lateral Nanometer Variations in Surface Charge/Energy of Hyaluronic Acid and Chondroitin Sulfate Doped Polypyrrole Observed Using Atomic Force Microscopy. J. Phys. Chem. B 2012, 116, 13498–13505. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, S.; Batrakova, E.; Kabanov, A. Poly(ethylene glycol)-polyethylenimine NanoGel particles: Novel drug delivery systems for antisense oligonucleotides. Colloids Surf. B Biointerfaces 1999, 16, 291–304. [Google Scholar] [CrossRef]
- Messager, L.; Portecop, N.; Hachet, E.; Lapeyre, V.; Pignot-Paintrand, I.; Catargi, B.; Auzely-Velty, R.; Ravaine, V. Photochemical crosslinking of hyaluronic acid confined in nanoemulsions: Towards nanogels with a controlled structure. J. Mater. Chem. B Mater. Biol. Med. 2013, 1, 3369–3379. [Google Scholar] [CrossRef]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Soni, G.; Yadav Khushwant, S. Nanogels as potential nanomedicine carrier for treatment of cancer: A mini review of the state of the art. Saudi Pharm. J. Spj Off. Publ. Saudi Pharm. Soc. 2016, 24, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Dorwal, D. Nanogels as novel and versatile pharmaceuticals. Int. J. Pharm. Pharm. Sci. 2012, 4, 67–74. [Google Scholar]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef]
- Yadav, H.; Al Halabi, N.; Alsalloum, G. Nanogels as Novel Drug Delivery Systems—A Review. J. Pharm. Pharm. Res. 2017, 1, 5. [Google Scholar]
- Garcia, F.P.; Rippe, M.; Companhoni, M.V.P.; Stefanello, T.F.; Louage, B.; Van Herck, S.; Sancey, L.; Coll, J.-L.; De Geest, B.G.; Vataru Nakamura, C.; et al. A versatile method for the selective core-crosslinking of hyaluronic acid nanogels via ketone-hydrazide chemistry: From chemical characterization to in vivo biodistribution. Biomater. Sci. 2018, 6, 1754–1763. [Google Scholar] [CrossRef]
- Jha, A.K.; Hule, R.A.; Jiao, T.; Teller, S.S.; Clifton, R.J.; Duncan, R.L.; Pochan, D.J.; Jia, X. Structural Analysis and Mechanical Characterization of Hyaluronic Acid-Based Doubly Cross-Linked Networks. Macromolecules 2009, 42, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef] [PubMed]
- Novoa-Carballal, R.; Pergushov, D.V.; Mueller, A.H.E. Interpolyelectrolyte complexes based on hyaluronic acid-block-poly(ethylene glycol) and poly-l-lysine. Soft Matter 2013, 9, 4297–4303. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, J.E.; Cho, M.Y.; Hong, J.H.; Cho, S.H.; Lim, Y.T. Hyaluronic Acid/Poly(β-Amino Ester) Polymer Nanogels for Cancer-Cell-Specific NIR Fluorescence Switch. Macromol. Rapid Commun. 2012, 33, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Anajafi, T.; Mallik, S. Polymersome-based drug-delivery strategies for cancer therapeutics. Ther. Deliv. 2015, 6, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, Y.; Xie, Z.; Jing, X.; Bellotti, A.; Gu, Z. Stimuli-Responsive Polymersomes for Biomedical Applications. Biomacromolecules 2017, 18, 649–673. [Google Scholar] [CrossRef] [PubMed]
- Schatz, C.; Lecommandoux, S. Polysaccharide-containing block copolymers: Synthesis, properties and applications of an emerging family of glycoconjugates. Macromol. Rapid Commun. 2010, 31, 1664–1684. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, K.K.; Bhatt, A.N.; Castro, E.; Mishra, A.K.; Chuttani, K.; Dwarakanath, B.S.; Schatz, C.; Le Meins, J.-F.; Misra, A.; Lecommandoux, S. In vitro and In vivo Evaluation of Docetaxel Loaded Biodegradable Polymersomes. Macromol. Biosci. 2010, 10, 503–512. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Bhatt, A.N.; Mishra, A.K.; Dwarakanath, B.S.; Jain, S.; Schatz, C.; Le Meins, J.-F.; Farooque, A.; Chandraiah, G.; Jain, A.K.; et al. The intracellular drug delivery and anti tumor activity of doxorubicin loaded poly(γ-benzyl -glutamate)-b-hyaluronan polymersomes. Biomaterials 2010, 31, 2882–2892. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, K.K.; Mishra, A.K.; Chuttani, K.; Kaul, A.; Schatz, C.; Le Meins, J.-F.; Misra, A.; Lecommandoux, S. The in vivo behavior and antitumor activity of doxorubicin-loaded poly(γ-benzyl L-glutamate)-block-hyaluronan polymersomes in Ehrlich ascites tumor-bearing BalB/c mice. Nanomedicine 2012, 8, 71–80. [Google Scholar] [CrossRef]
- Haas, S.; Hain, N.; Raoufi, M.; Handschuh-Wang, S.; Wang, T.; Jiang, X.; Schoenherr, H. Enzyme Degradable Polymersomes from Hyaluronic Acid-block-poly(ε-caprolactone) Copolymers for the Detection of Enzymes of Pathogenic Bacteria. Biomacromolecules 2015, 16, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Pramod, K.; Malviya, R. Utilization of Polymeric Nanoparticle in Cancer Treatment: A Review. J. Pharm. Care Health Syst. 2017, 4, 172. [Google Scholar] [CrossRef]
- Park, H.-K.; Lee, S.J.; Oh, J.-S.; Lee, S.-G.; Jeong, Y.-I.; Lee, H.C. Smart Nanoparticles Based on Hyaluronic Acid for Redox-Responsive and CD44 Receptor-Mediated Targeting of Tumor. Nanoscale Res. Lett. 2015, 10, 288. [Google Scholar] [CrossRef]
- Han, H.S.; Thambi, T.; Choi, K.Y.; Son, S.; Ko, H.; Lee, M.C.; Jo, D.-G.; Chae, Y.S.; Kang, Y.M.; Lee, J.Y.; et al. Bioreducible Shell-Cross-Linked Hyaluronic Acid Nanoparticles for Tumor-Targeted Drug Delivery. Biomacromolecules 2015, 16, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, V.; Mazzaferro, S.; Lavaud, J.; Vanwonterghem, L.; Henry, M.; Arboleas, M.; Vollaire, J.; Josserand, V.; Coll, J.-L.; Lecommandoux, S.; et al. Targeting CD44 receptor-positive lung tumors using polysaccharide-based nanocarriers: Influence of nanoparticle size and administration route. Nanomedicine 2016, 12, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, K.; Sun, H.; Zhang, J.; Yuan, J.; Zhong, Z. Hyaluronic Acid-Shelled Disulfide-Cross-Linked Nanopolymersomes for Ultrahigh-Efficiency Reactive Encapsulation and CD44-Targeted Delivery of Mertansine Toxin. Acs Appl. Mater. Interfaces 2018, 10, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Choi, K.Y.; Ko, H.; Jeon, J.; Saravanakumar, G.; Suh, Y.D.; Lee, D.S.; Park, J.H. Bioreducible core-crosslinked hyaluronic acid micelle for targeted cancer therapy. J. Control. Release 2015, 200, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Tizzotti, M.; Charlot, A.; Fleury, E.; Stenzel, M.; Bernard, J. Modification of Polysaccharides Through Controlled/Living Radical Polymerization Grafting-Towards the Generation of High Performance Hybrids. Macromol. Rapid Commun. 2010, 31, 1751–1772. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Mishra, P.; Jain, S.; Mishra, P.; Mishra, A.K.; Agrawal, G.P. Preparation and characterization of HA-PEG-PCL intelligent core-corona nanoparticles for delivery of doxorubicin. J. Drug Target. 2008, 16, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, F.S.; Bavuso Volpe, A.; Bongiovi, F.; Pitarresi, G.; Giammona, G. A New Hyaluronic Acid Derivative Obtained from Atom Transfer Radical Polymerization as a siRNA Vector for CD44 Receptor Tumor Targeting. Macromol. Biosci. 2015, 15, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Park, K.; Kim, K.S.; Kim, J.; Yang, J.-A.; Kong, J.-H.; Lee, M.Y.; Hoffman, A.S.; Hahn, S.K. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J. Control. Release 2010, 141, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Alaimo, D.; De Vlieghere, E.; Jerome, C.; De Wever, O.; De Geest, B.G.; Auzely-Velty, R. Tunable self-assembled nanogels composed of well-defined thermoresponsive hyaluronic acid-polymer conjugates. J. Mater. Chem. B Mater. Biol. Med. 2013, 1, 3883–3887. [Google Scholar] [CrossRef]
- Stefanello, T.F.; Couturaud, B.; Szarpak-Jankowska, A.; Fournier, D.; Louage, B.; Garcia, F.P.; Nakamura, C.V.; De Geest, B.G.; Woisel, P.; van der Sanden, B.; et al. Coumarin-containing thermoresponsive hyaluronic acid-based nanogels as delivery systems for anticancer chemotherapy. Nanoscale 2017, 9, 12150–12162. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Zhou, H.; Liu, Y.; Liu, Z.; Liu, J.; Tang, J.; Li, J.; Zhang, J.; Sheng, W.; Zhao, Y.; et al. Hyaluronic acid functional amphipathic and redox-responsive polymer particles for the co-delivery of doxorubicin and cyclopamine to eradicate breast cancer cells and cancer stem cells. Nanoscale 2015, 7, 8607–8618. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Chen, S.-H.; Chiang, W.-H.; Huang, C.-W.; Lo, C.-L.; Chern, C.-S.; Chiu, H.-C. Tumor Microenvironment-Responsive Nanoparticle Delivery of Chemotherapy for Enhanced Selective Cellular Uptake and Transportation within Tumor. Biomacromolecules 2016, 17, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Agarwal, A.; Rai, G.; Mishra, P.; Jain, S.; Mishra, A.K.; Agrawal, H.; Agrawal, G.P. Development and characterization of hyaluronic acid decorated PLGA nanoparticles for delivery of 5-fluorouracil. Drug Deliv. 2010, 17, 561–572. [Google Scholar] [CrossRef]
- Schante, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Fernandes Stefanello, T.; Szarpak-Jankowska, A.; Appaix, F.; Louage, B.; Hamard, L.; De Geest, B.G.; van der Sanden, B.; Nakamura, C.V.; Auzely-Velty, R. Thermoresponsive hyaluronic acid nanogels as hydrophobic drug carrier to macrophages. Acta Biomater. 2014, 10, 4750–4758. [Google Scholar] [CrossRef]
- Rippe, M.; Stefanello, T.F.; Kaplum, V.; Britta, E.A.; Garcia, F.P.; Poirot, R.; Companhoni, M.V.P.; Nakamura, C.V.; Szarpak-Jankowska, A.; Auzely-Velty, R. Heparosan as a potential alternative to hyaluronic acid for the design of biopolymer-based nanovectors for anticancer therapy. Biomater. Sci. 2019, 7, 2850–2860. [Google Scholar] [CrossRef]
- Pitarresi, G.; Palumbo, F.S.; Albanese, A.; Fiorica, C.; Picone, P.; Giammona, G. Self-assembled amphiphilic hyaluronic acid graft copolymers for targeted release of antitumoral drug. J. Drug Target. 2010, 18, 264–276. [Google Scholar] [CrossRef]
- Son, G.M.; Kim, H.Y.; Ryu, J.H.; Chu, C.W.; Kang, D.H.; Park, S.B.; Jeong, Y.-I. Self-assembled polymeric micelles based on hyaluronic acid-g-poly(d,l-lactide-co-glycolide) copolymer for tumor targeting. Int. J. Mol. Sci. 2014, 15, 16057–16068. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Mishra, P.; Mishra, A.K.; Mishra, P.; Jain, S.; Agrawal, G.P. Development and characterization of hyaluronic acid-anchored PLGA nanoparticulate carriers of doxorubicin. Nanomedicine 2007, 3, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Banerjee, S.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Hyaluronic Acid Engineered Nanomicelles Loaded with 3,4-Difluorobenzylidene Curcumin for Targeted Killing of CD44+ Stem-Like Pancreatic Cancer Cells. Biomacromolecules 2015, 16, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Iyer, A.K.; Morrissey, D.V.; Amiji, M.M. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials 2013, 34, 3489–3502. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Iyer, A.K.; Gattacceca, F.; Morrissey, D.V.; Amiji, M.M. In vivo biodistribution of siRNA and cisplatin administered using CD44-targeted hyaluronic acid nanoparticles. J. Control. Release 2013, 172, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Wang, L.; Yin, L.; Zhou, J.; Huo, M. Co-delivery of hydrophobic paclitaxel and hydrophilic AURKA specific siRNA by redox-sensitive micelles for effective treatment of breast cancer. Biomaterials 2015, 61, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Qiao, M.; Long, M.; Wang, M.; Zhang, X.; Li, Z.; Tian, C.; Chen, D. Self-assembled pH-responsive hyaluronic acid-g-poly((L)-histidine) copolymer micelles for targeted intracellular delivery of doxorubicin. Acta Biomater. 2014, 10, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Qiao, M.; Chen, Q.; Tian, C.; Long, M.; Wang, M.; Li, Z.; Hu, W.; Li, G.; Cheng, L.; et al. Enhanced effect of pH-sensitive mixed copolymer micelles for overcoming multidrug resistance of doxorubicin. Biomaterials 2014, 35, 9877–9887. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Long, M.; Qiu, L.; Zhu, M.; Li, Z.; Qiao, M.; Hu, H.; Zhao, X.; Chen, D. Decoration of pH-sensitive copolymer micelles with tumor-specific peptide for enhanced cellular uptake of doxorubicin. Int. J. Nanomed. 2016, 11, 5415–5427. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Na, K. Photochemically triggered cytosolic drug delivery using pH-responsive hyaluronic acid nanoparticles for light-induced cancer therapy. Biomacromolecules 2014, 15, 4228–4238. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef] [PubMed]
- Kost, J.; Langer, R. Responsive polymeric delivery systems. Adv. Drug Deliv. Rev. 2001, 46, 125–148. [Google Scholar] [CrossRef]
- Coury, A.J.; Levy, R.J.; Ratner, B.D.; Schoen, F.J.; Williams, D.F.; Williams, R.L. Degradation of materials in the biological environment. In Biomaterial Science An Introduction to Materials in Medicine, 2nd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2004; pp. 411–454. [Google Scholar]
- Li, Y.; Gao, G.H.; Lee, D.S. Stimulus-Sensitive Polymeric Nanoparticles and Their Applications as Drug and Gene Carriers. Adv. Healthc. Mater. 2013, 2, 388–417. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Chen, D.; Zhang, X.; Zeng, J.; Hu, H.; Zhao, X.; Qiao, M. Reversing multidrug resistance by intracellular delivery of Pluronic® P85 unimers. Biomaterials 2013, 34, 9602–9614. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Cao, J.; Huang, S.; Chen, Y.; Li, S.; Li, X.; Deng, D.; Qian, Z.; Tang, L.; Gu, Y. Near-infrared light-triggered micelles for fast controlled drug release in deep tissue. Biomaterials 2013, 34, 6272–6283. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Allard, J.-F.; Morris, D.; Dory, Y.L.; Lepage, M.; Zhao, Y. Near-infrared light sensitive polypeptide block copolymer micelles for drug delivery. J. Mater. Chem. 2012, 22, 7252–7257. [Google Scholar] [CrossRef]
- Zhao, P.; Zheng, M.; Luo, Z.; Gong, P.; Gao, G.; Sheng, Z.; Zheng, C.; Ma, Y.; Cai, L. NIR-driven Smart Theranostic Nanomedicine for On-demand Drug Release and Synergistic Antitumour Therapy. Sci. Rep. 2015, 5, 14258. [Google Scholar] [CrossRef]
- Harris, E.N.; Weigel, P.H. The ligand-binding profile of HARE: Hyaluronan and chondroitin sulfates A, C, and D bind to overlapping sites distinct from the sites for heparin, acetylated low-density lipoprotein, dermatan sulfate, and CS-E. Glycobiology 2008, 18, 638–648. [Google Scholar] [CrossRef]
- Choi, K.Y.; Min, K.H.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Choi, K.; Jeong, S.Y. PEGylation of hyaluronic acid nanoparticles improves tumor targetability in vivo. Biomaterials 2011, 32, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Yoon, H.Y.; Kim, J.-H.; Bae, S.M.; Park, R.-W.; Kang, Y.M.; Kim, I.-S.; Kwon, I.C.; Choi, K.; Jeong, S.Y.; et al. Smart Nanocarrier Based on PEGylated Hyaluronic Acid for Cancer Therapy. Acs Nano 2011, 5, 8591–8599. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Lee, J.; Kim, H.R.; Chae, S.Y.; Kim, M.; Saravanakumar, G.; Yoon, H.Y.; You, D.G.; Ko, H.; Kim, K.; et al. Robust PEGylated hyaluronic acid nanoparticles as the carrier of doxorubicin: Mineralization and its effect on tumor targetability in vivo. J. Control. Release 2013, 168, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Garay, R.P.; El-Gewely, R.; Armstrong, J.K.; Garratty, G.; Richette, P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin. Drug Deliv. 2012, 9, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, J.J.F.; Carpenter, J.F.; Anchordoquy, T.J.; Schellekens, H. Potential induction of anti-PEG antibodies and complement activation toward PEGylated therapeutics. Drug Discov. Today 2014, 19, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lai, S.K. Anti-PEG immunity: Emergence, characteristics, and unaddressed questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 655–677. [Google Scholar] [CrossRef]
- Choi, K.Y.; Han, H.S.; Lee, E.S.; Shin, J.M.; Almquist, B.D.; Lee, D.S.; Park, J.H. Hyaluronic acid-based activatable nanomaterials for stimuli-responsive imaging and therapeutics: Beyond CD44-mediated drug delivery. Adv. Mater. 2019. Ahead of Print. [Google Scholar] [CrossRef]
- Naor, D.; Nedvetzki, S. CD44 in rheumatoid arthritis. Arthritis Res. Ther. 2003, 5, 105–115. [Google Scholar] [CrossRef]
- Saba, G.-E.; Abdullah, M.A. Polymeric nanoparticle mediated targeted drug delivery to cancer cells. In Biotechnology and Bioinformatics; Apple Academic Press: Waretown, NJ, USA, 2015; pp. 1–34. [Google Scholar]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Rao, N.V.; Yoon, H.Y.; Han, H.S.; Ko, H.; Son, S.; Lee, M.; Lee, H.; Jo, D.-G.; Kang, Y.M.; Park, J.H. Recent developments in hyaluronic acid-based nanomedicine for targeted cancer treatment. Expert Opin. Drug Deliv. 2016, 13, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Chawla, S.; Gadeval, A.; Reddy, G.; Maheshwari, R.; Kalia, K.; Tekade, R.K. Nanostructured Hyaluronic Acid-based Materials for the Delivery of siRNA. Curr. Pharm. Des. 2018, 24, 2678–2691. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Fu, J.; Li, R.; Zhang, F.; Ling, G.; Zhang, P. A potential carrier for anti-tumor targeted delivery-hyaluronic acid nanoparticles. Carbohydr. Polym. 2019, 208, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef] [PubMed]
| Mw of HA kg/mol | Polymer Structure a | Size (nm) b | DL (EE) % | In Vitro and/or in Vivo Biological Studies a | Ref |
|---|---|---|---|---|---|
| 5 | 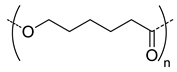 PCL, biodegradable | - c | - | Detection of pathogenic bacteria “Staphylococcus aureus” and drug release of after enzymatic degradation. | [31] |
| 2100 | ~200 d | ||||
| 12 | 198 e,f | 7 (74) f | In vitro: cytotoxicity and intracellular drug release of DOX in SCC7 cell line. In vivo: biodistribution and antitumor efficacy in SCC7 cells-bearing mice. | [34] | |
| 162 g,h | 9 (90) g | ||||
| 7.5 | 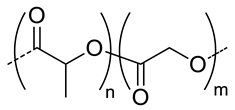 PLGA, biodegradable | <200 h | 8 | In vitro: cytotoxicity in MDA-MB231 and NIH3T3 cell lines and release behavior of DOX. In vivo: biodistribution and antitumor efficacy in mice bearing MDA-MB231 or NIH3T3 cells grafted. | [33] |
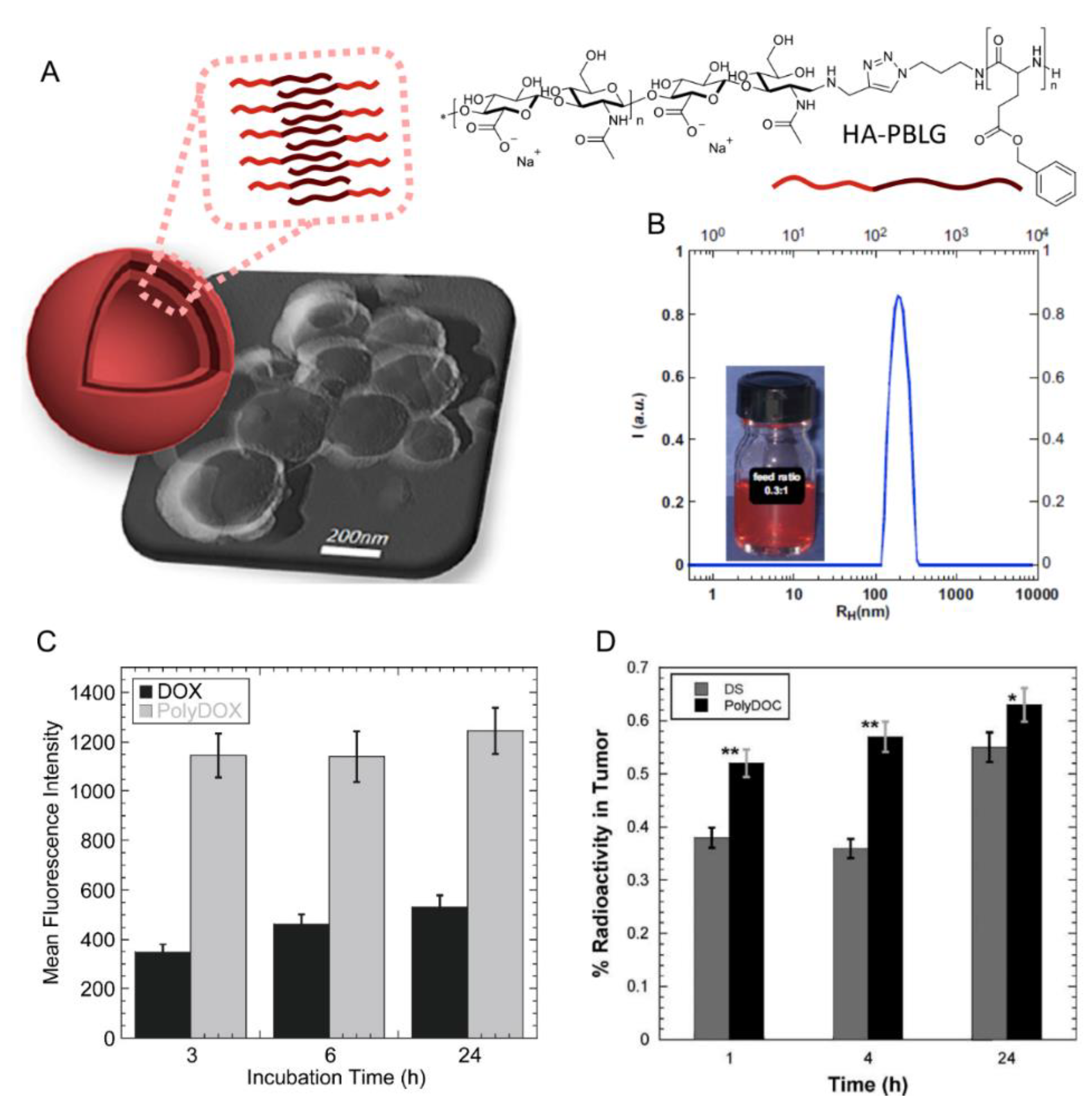
| 5 | 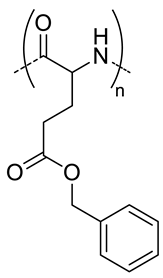 PBLG, biodegradable | 220 d | 12 (40) | In vitro: cytotoxicity, cell uptake of DOX containing nanocarriers in MCF-7 and U87 cells lines. In vivo: antitumor efficacy in mice with DMBA induced tumor. | [29] |
| 135 d | 9.8 (49) | In vitro: cytotoxicity in MCF-7 and U87 cells lines and release behavior of DOC. In vivo: biodistribution in EAT–bearing mice. | [28] | ||
| - i | - i | In vivo: stability, biodistribution, pharmacokinetics and antitumor activity of DOX containing nanocarriers in EAT-bearing mice. | [30] | ||
| 30 and 300 d | - | In vitro and in vivo lung tumor cells targeting effect of the size. | [35] | ||
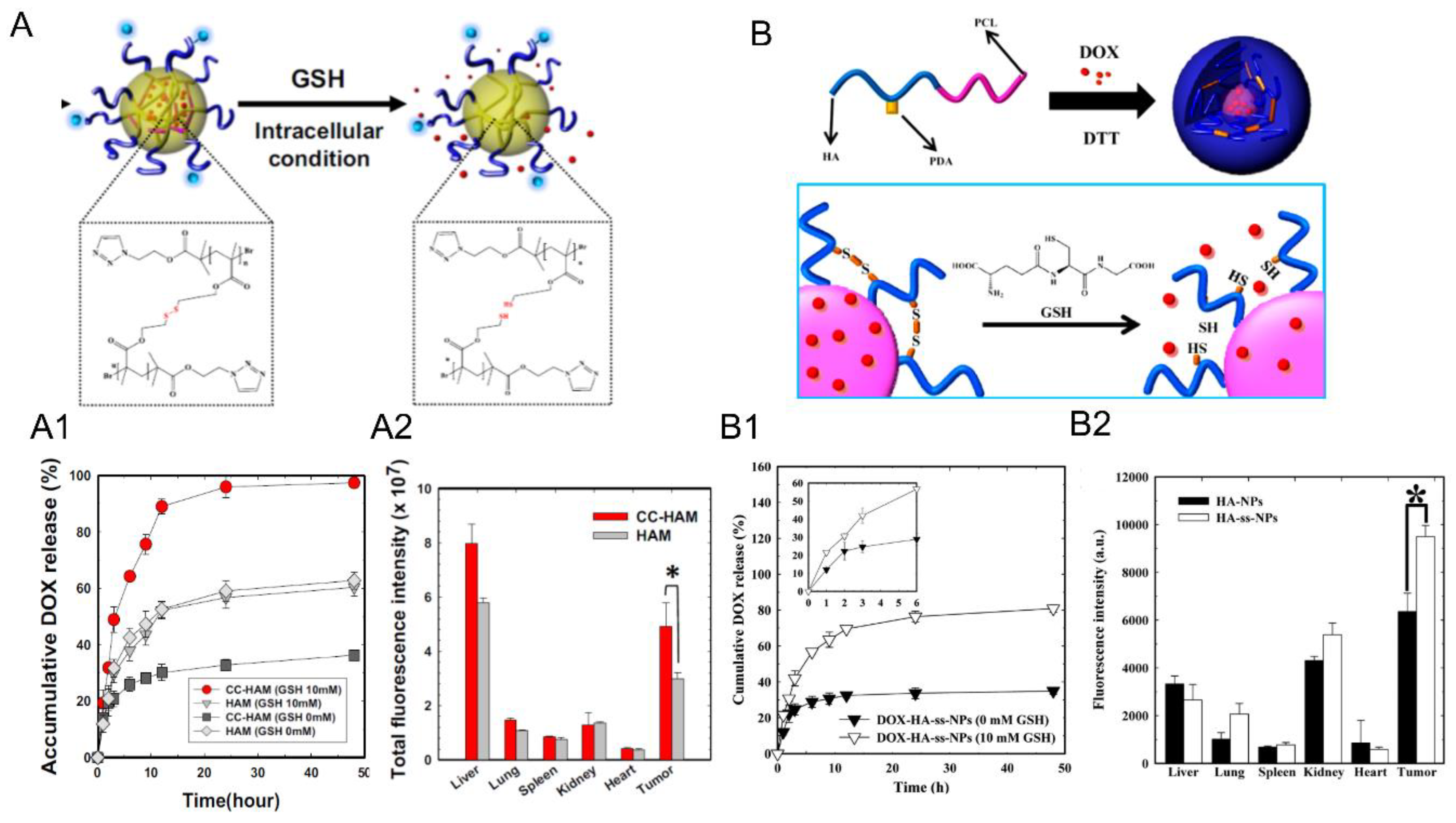
| 8 | 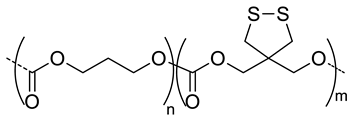 P(TMC-co-DTC), biodegradable and redox-responsive | 103 d,g | 17 (99) | In vitro: cytotoxicity in MDA-MB-231 cell line and release behavior of DM1. In vivo: pharmacokinetics and anticancer efficacy in MDA-MB-231 cells-bearing mice. | [36] |
| 7.4 | 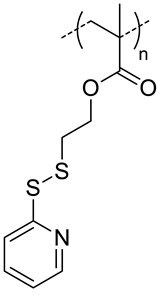 PDSMA, redox-responsive | 215 f,h 187 g,h | 8 (80) f 9 (87) g | In vitro: drug release of DOX. In vivo: biodistribution, pharmacokinetics, tumor accumulation profiles and antitumor efficacy in SCC7 cells-bearing mice. | [37] |
| Mw of HA kg/mol | Polymer Structure a | Size (nm) b | DL (EE) % a | In Vitro and/or in Vivo Biological Studies a | Ref |
|---|---|---|---|---|---|
| 5.7 | 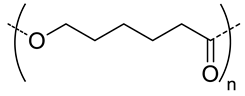 PCL, biodegradable | 95 c | (96) | In vitro: hemolytic toxicity and stability of DOX containing nanocarriers. In vivo: biodistribution and tumor inhibition in EAT-bearing mice. | [39] |
| 1500 | 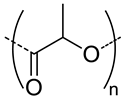 PLA, biodegradable | 30 c | 5 (10) | In vitro: cytotoxicity and uptake in HCT-166-cells and release behavior of DOX. | [50] |
| 8.3 | 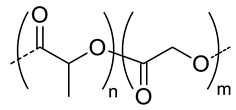 PLGA, biodegradable | 103 c | 8 | In vitro: cell viability and uptake in Hep G2 cells and CT26 cell lines and release behavior of DOX. | [51] |
| 15 | - d | 16 (87) | In vitro: cytotoxicity and uptake in Raw 264.7-cells and release behavior of SN38 In vivo biodistribution, and tumor inhibition in tramp-C1 cells–bearing mice. | [45] | |
| 5.7 | 152 e | (80) | In vitro: cytotoxicity in EAT-cells and release behavior of 5-FU In vivo: biodistribution, and tumor inhibition in EAT–bearing mice. | [46] | |
| 6.4 | 245 c | (71) DOX (58) CYC | In vitro: cell viability and uptake in MCF-7 and MDA-MB-231 cell lines and release behavior of DOX and CYC. In vivo: synergic antitumor efficacy of DOX and CYC in MDA-MB231 cell-bearing mice. | [44] | |
| 5.7 | 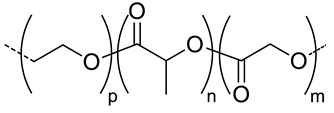 PEG-co-PLGA, biodegradable | - d | (~90) | In vitro: drug release of DOX. In vivo: biodistribution, and tumor inhibition in EAT–bearing mice. | [52] |
| 10 | 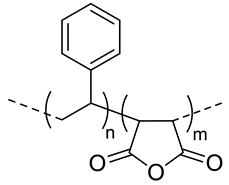 SMA, pH-responsive after hydrolysis of anhydrides | - d | (16) | In vitro: cell viability and uptake of CDF containing nanocarriers in MiaPaCa-2 and AsPC-1; activity on CD44+ and CD44- pancreatic cells. | [53] |
| 20 | 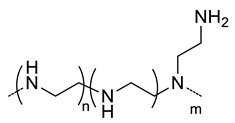 Branched PEI, positive charged at pH 7.4 | ~200 c,f | - | In vitro: siRNA release, cell uptake and gene silencing in MDA-MB468 cell line. In vivo: tumor uptake and gene-silencing in A549 cells-bearing mice. | [54] |
| ~100 c,g | - | In vivo: biodistribution and quantification of siRNA in mice bearing A549, A549DDP, H69 or H69Ar cells grafted. | [55] | ||
| ~200 c,f | 33 (86) | In vitro: cytotoxicity, cell uptake and gene silencing of siRNA and PTX-containing nanocarriers in MDA-MB-231 cell line. In vivo: biodistribution and antitumor efficacy in MDA-MB-231 cells-bearing mice. | [56] | ||
| - g | 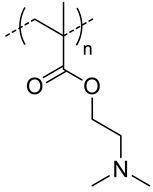 pDEAEMA, positive charged at pH 7.4 | 155 c | - | In vitro: cytotoxicity in HCT 116 cells and uptake of siRNA. | [40] |
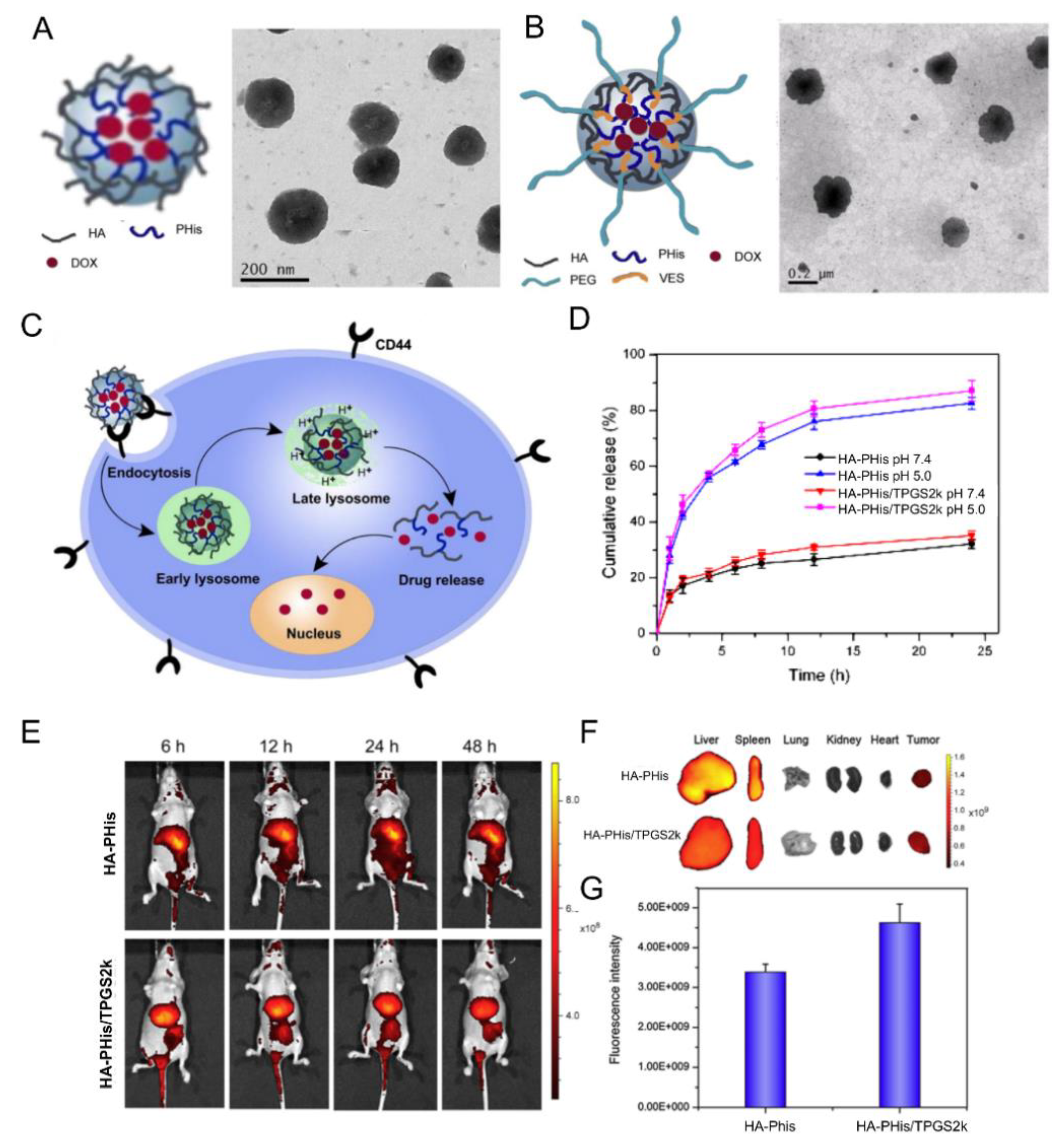
| 11 | 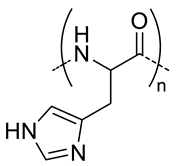 pHis, pH-responsive | ~400 c (pH = 7.4) | 7 (90) | In vitro: cell viability and uptake in MCF-7 cell line; endocytosis inhibition. pH release behavior of DOX containing nanocarriers. | [57] |
| 10 (92) | In vitro: cell viability and uptake in MCF-7 and MCF-7/ADR cell lines; endocytosis inhibition. pH release behavior of DOX and tocopheryl-PEG containing nanocarriers. In vivo: biodistribution in MCF-7/ADR cells–bearing mice. | [58] | |||
| - d | 10 (93) | In vitro: cytotoxicity and cellular uptake in MDA-MB-231 cell lines. pH release behavior of DOX and Her2 peptide-tocopheryl-PEG containing nanocarriers. In vivo: biodistribution and antitumor efficacy in MDA-MB-231 cell-bearing mice. | [59] | ||
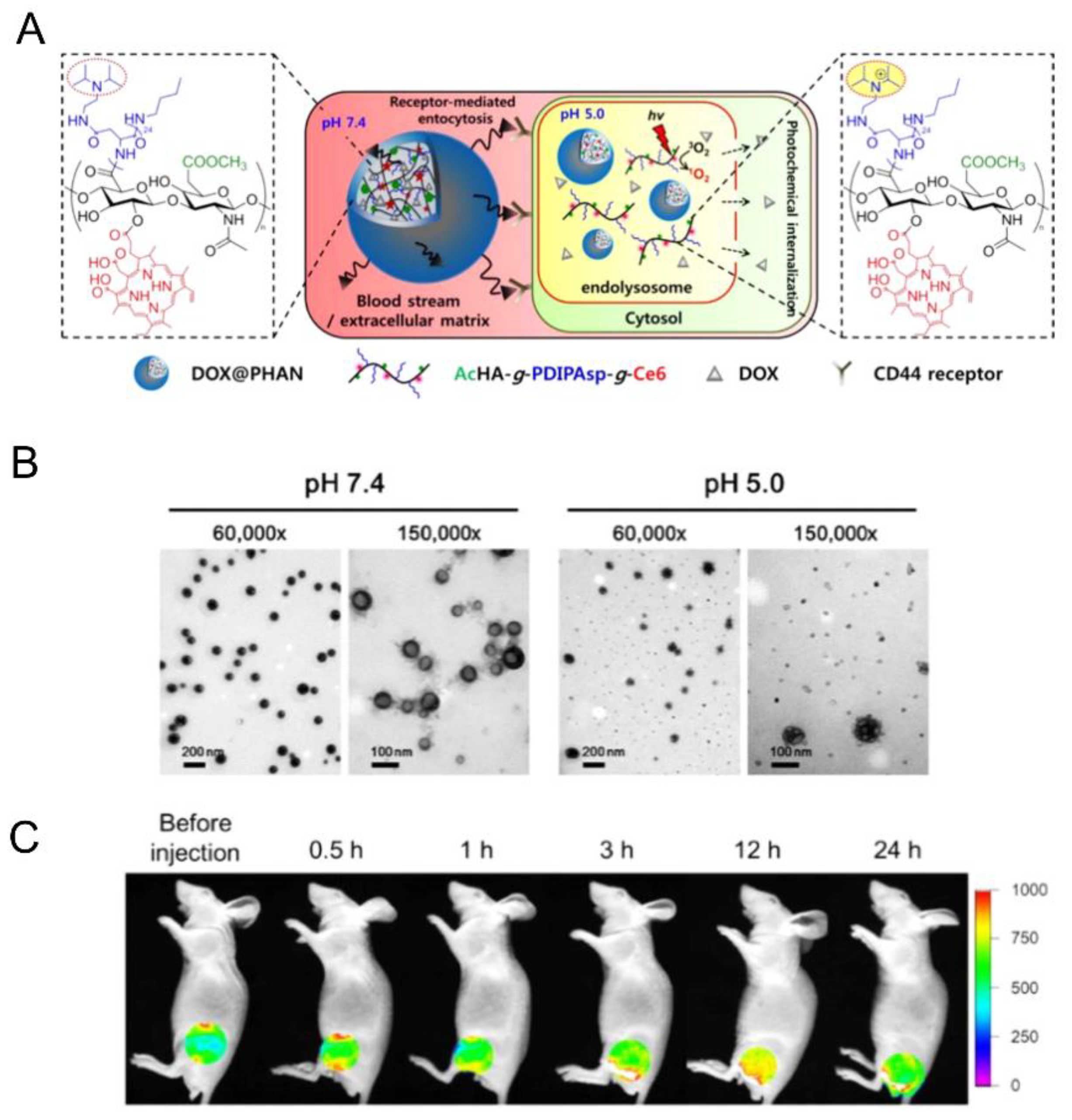
| 5.8 | 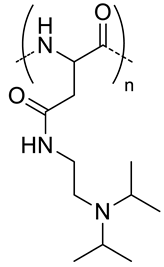 PDIPASP, pH-responsive | - d | 14 | In vitro: cell viability and uptake in CT-26 cell line; photosensible endosome scape; pH release behavior of DOX. In vivo: biodistribution and antitumor efficacy in CT-26 cell-bearing mice. | [60] |
| 120 | 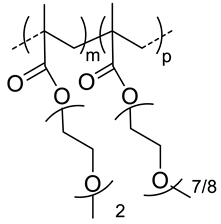 p(DEGMA-co-OEGMA), thermosensible (CAT of HA-copolymer: 34 °C) | 150 c (40 °C) | ~3 (70) | In vitro: cellular uptake by RAW264.7 macrophages of DSB containing nanocarriers. In vivo: biodistribution, and macrophage uptake in mice. | [48] |
| 300 | 211 c (40 °C) | - | - | ||
| 200 | 95 c (40 °C) | (80) | In vitro: cell viability of PTX containing nanocarriers in HCT-8/E11 and SKOV-3 cell lines. | [42] | |
| 40 | 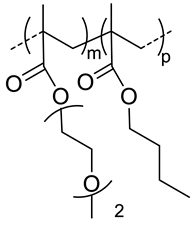 p(DEGMA-co-BMA), thermosensible | 108 c,i (37 °C) 151 c,j (37 °C) | - | In vitro: vero cells viability. In vivo: biodistribution in EAC cells-bearing mice. | [49] |
| 40 | 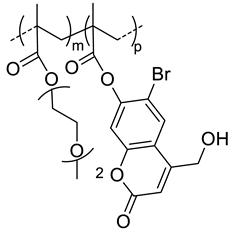 p(DEGMA-co-CMA), photo and thermosensible | ~100 c (37 °C) | 1.5 (52) | In vitro: cell viability in Vero cells and uptake in HeLa cells of PTX containing nanocarriers. In vivo: biodistribution in mice bearing HeLa subcutaneous grafts. | [43] |
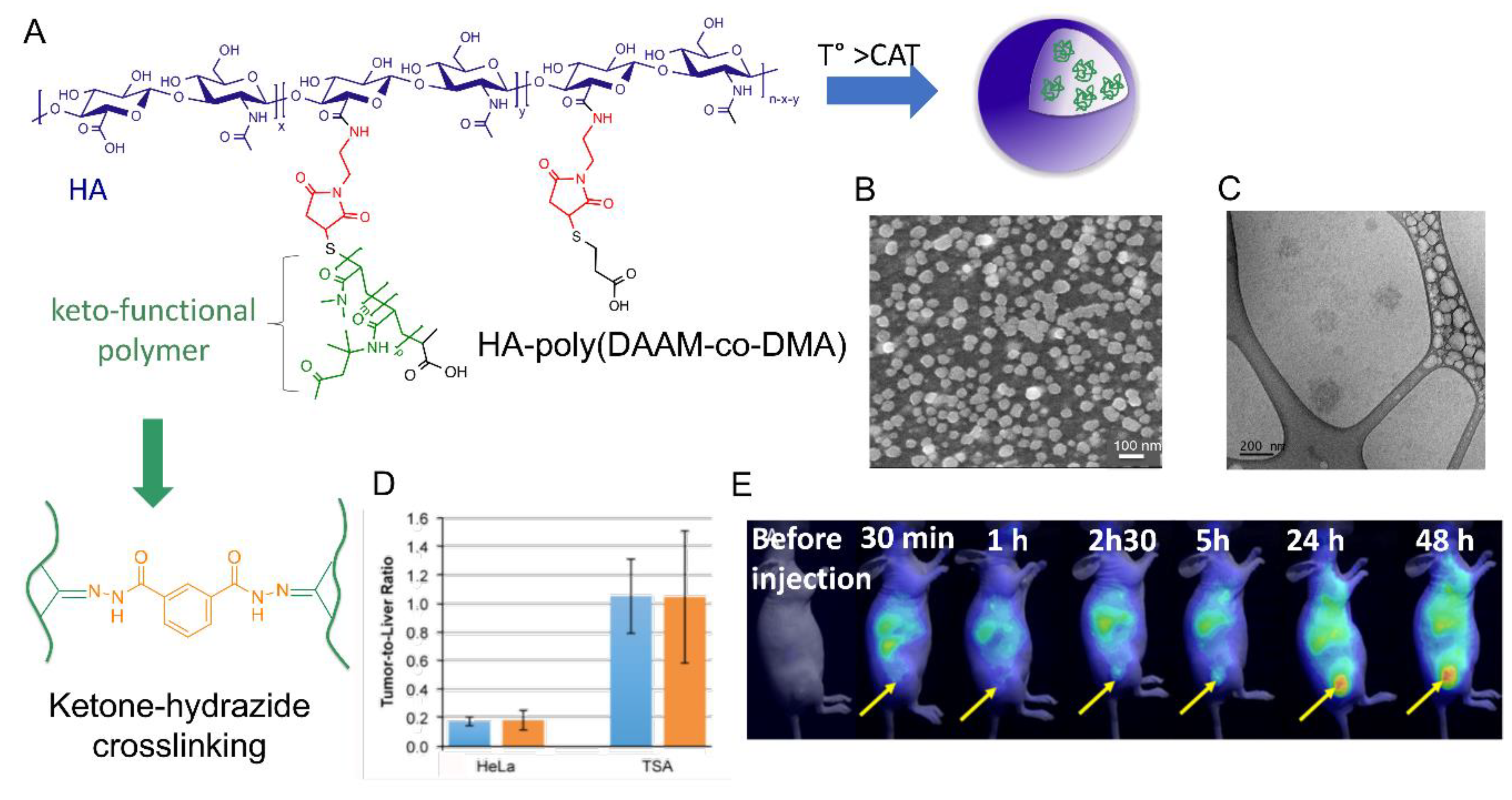
| 40 | 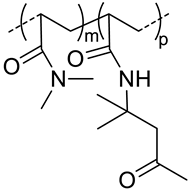 p(DAAM-co-DMA), thermoresponsive | 144 c,i (40 °C) 150 c,j (40 °C) | - | In vitro: cell viability and uptake in HeLa and TS/A-pc cell lines. In vivo: biodistribution in mice bearing HeLa or TS/A-pc subcutaneous grafts. | [20] |
| Mw of HA kg/mol | Polymer Structure | Size a in nm (T, °C) | DL (EE) % | In Vitro and/or in Vivo Biological Studies a | Ref |
|---|---|---|---|---|---|
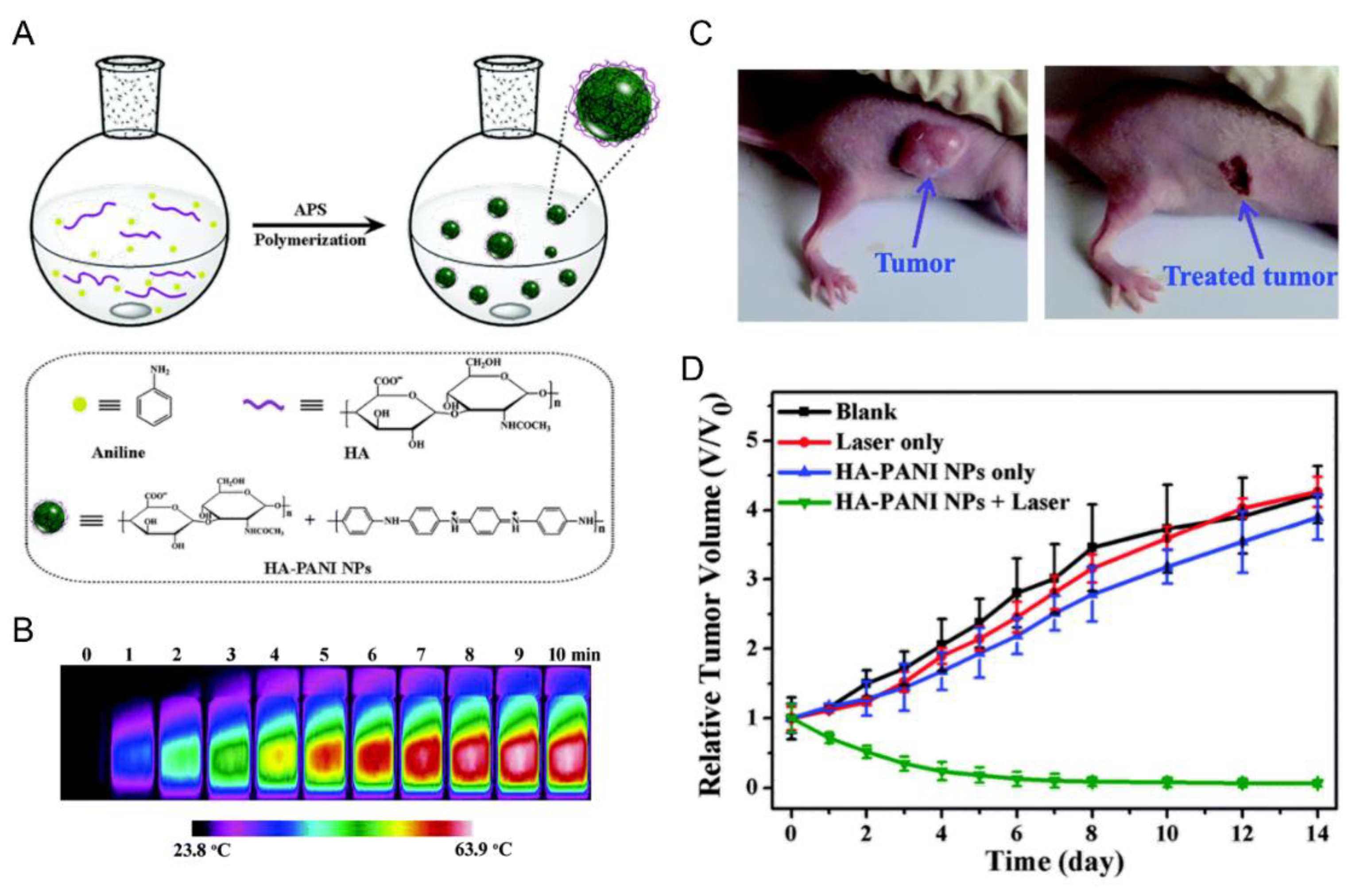
| - b | 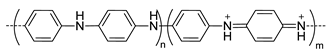 Polyaniline, esmeraldine salt, positive charged, photothermal | 100 c | - d | In vitro: cytotoxicity and photothermal therapy effect in HFF, HCT-116 and HeLa cell lines. In vivo: photothermal therapy in HeLa tumor-bearing mice. | [10] |
| 170 |  Chitosan hydrochloride salt, biodegradable, positive charged | 189 c,e | (48, DOX) (91, miR-34a) | In vitro: cytotoxicity and uptake of DOX and miR-34a containing nanocarriers in MDA-MB-231 cell line. In vivo tumor inhibition in MDA-MB-231 cells-bearing mice. | [22] |
| 1300-1800 | 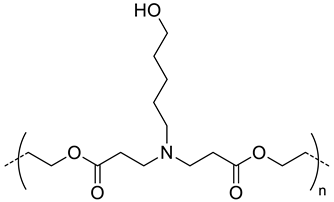 Poly(β-amino ester), biodegradable, partially positive charged at pH 7.4 | - f | - b | In vitro: cell viability and uptake in MDA-MB-231 cells. | [24] |
| 54.3 g | 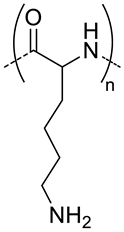 Polyd -lysine, biodegradable positive charged at pH 7.4 | 122 c,h 145 c,i | - d | - | [23] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rippe, M.; Cosenza, V.; Auzély-Velty, R. Design of Soft Nanocarriers Combining Hyaluronic Acid with Another Functional Polymer for Cancer Therapy and Other Biomedical Applications. Pharmaceutics 2019, 11, 338. https://doi.org/10.3390/pharmaceutics11070338
Rippe M, Cosenza V, Auzély-Velty R. Design of Soft Nanocarriers Combining Hyaluronic Acid with Another Functional Polymer for Cancer Therapy and Other Biomedical Applications. Pharmaceutics. 2019; 11(7):338. https://doi.org/10.3390/pharmaceutics11070338
Chicago/Turabian StyleRippe, Marlène, Vanina Cosenza, and Rachel Auzély-Velty. 2019. "Design of Soft Nanocarriers Combining Hyaluronic Acid with Another Functional Polymer for Cancer Therapy and Other Biomedical Applications" Pharmaceutics 11, no. 7: 338. https://doi.org/10.3390/pharmaceutics11070338
APA StyleRippe, M., Cosenza, V., & Auzély-Velty, R. (2019). Design of Soft Nanocarriers Combining Hyaluronic Acid with Another Functional Polymer for Cancer Therapy and Other Biomedical Applications. Pharmaceutics, 11(7), 338. https://doi.org/10.3390/pharmaceutics11070338




