Dose-Dependent Pharmacokinetics of Tofacitinib in Rats: Influence of Hepatic and Intestinal First-Pass Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Estimation of the Appropriate Number of Animals
2.4. Intravenus and Oral Administration of Tofacitinib
2.5. Hepatic First-Pass Effects of Tofacitinib
2.6. Gastric and Intestinal First-Pass Effect of Tofacitinib
2.7. Tissue Distribution of Tofacitinib
2.8. Biliary Excretion of Tofacitinib
2.9. HPLC Analysis of Tofacitinib
2.10. Pharmacokinetics Analysis
2.11. Statistical Analysis
3. Results
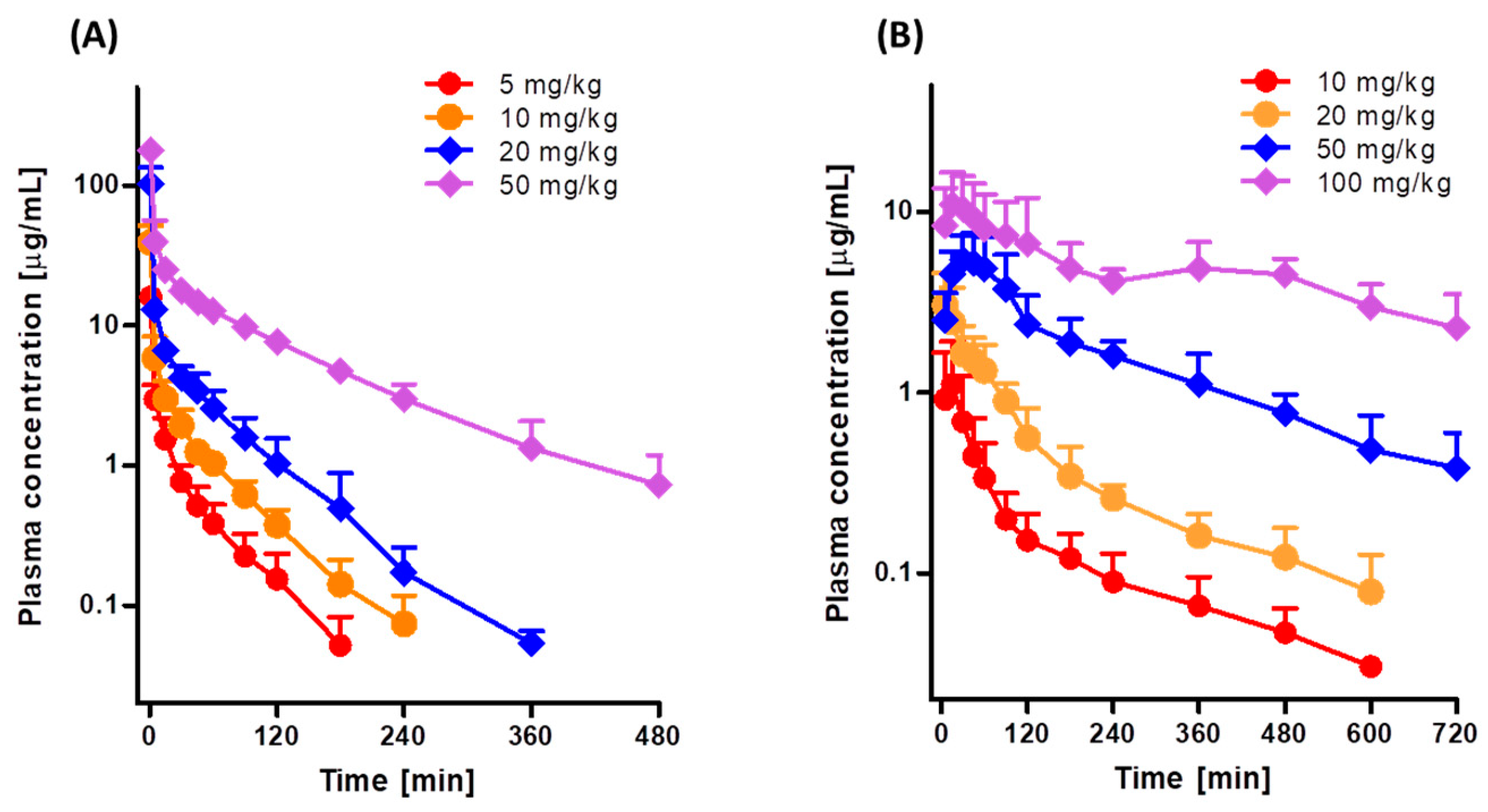
3.1. Pharmacokinetics of Tofacitinib after Intravenous and Oral Administration to Rats
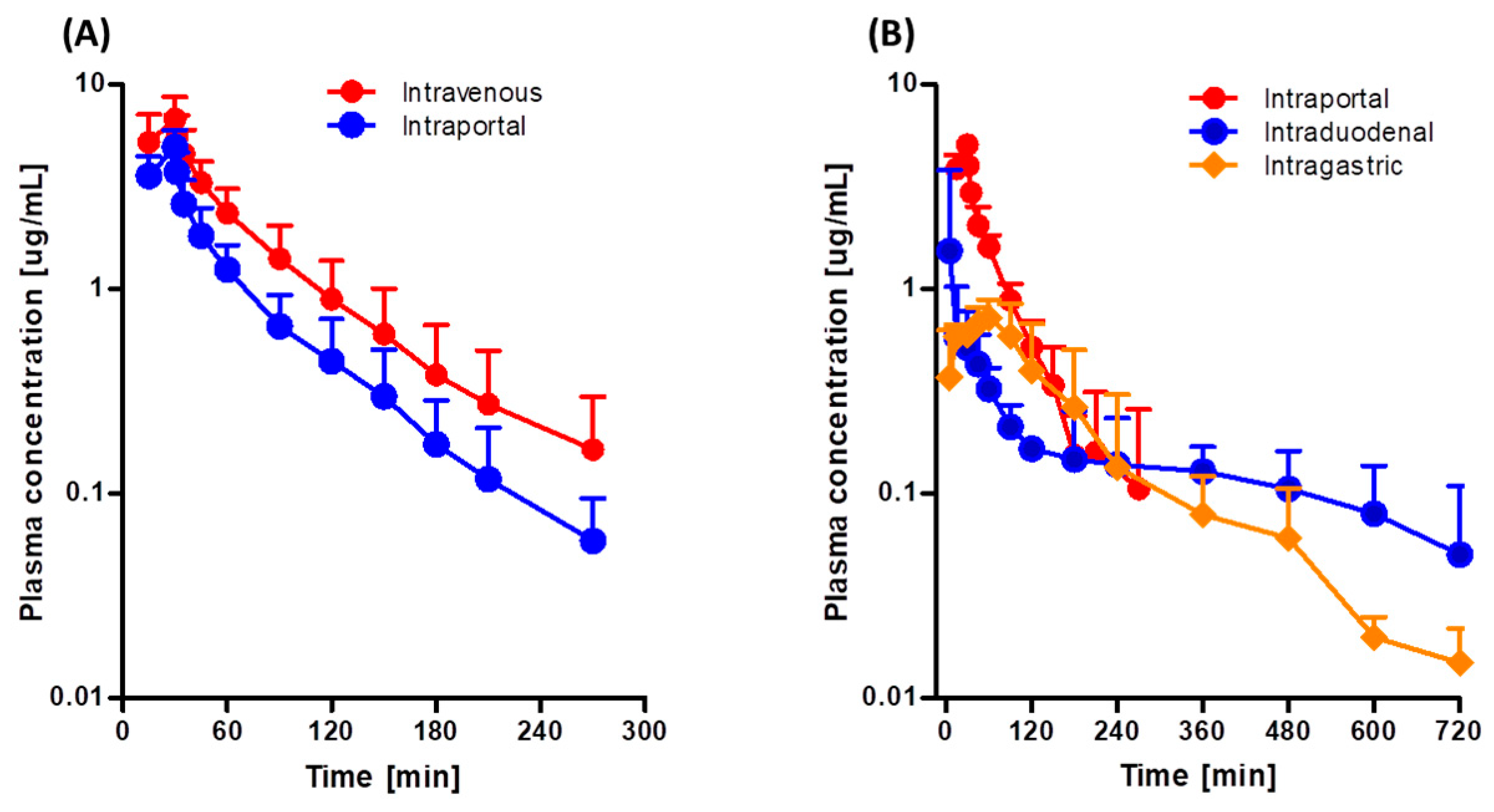
3.2. Hepatic First-Pass Effect of Tofacitinib in Rats
3.3. Gastric and Intestinal First-Pass Effects of Tofacitinib in Rats
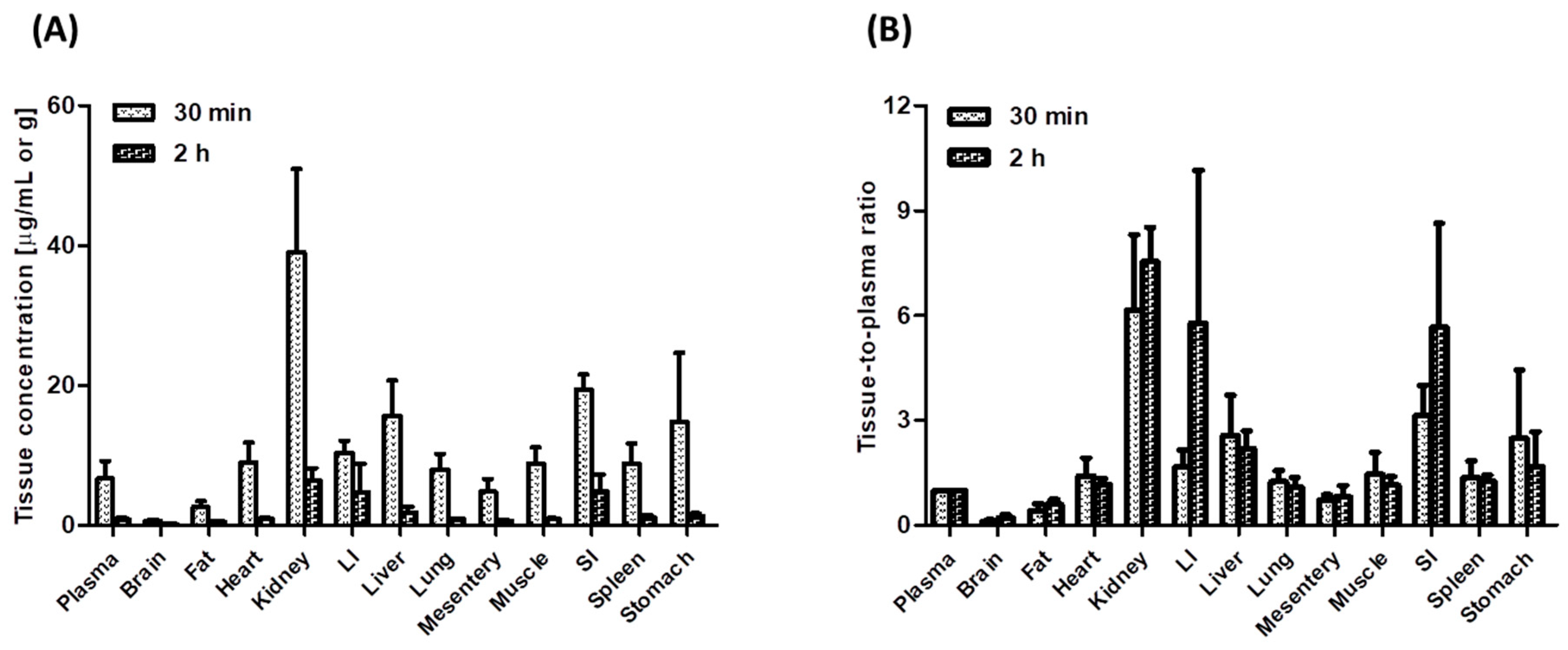
3.4. Tissue Distribution of Tofacitinib in Rats
3.5. Biliary Excretion of Tofacitinib in Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fleischmann, R.; Kremer, J.; Cush, J.; Schulze-Koops, H.; Connell, C.A.; Bradley, J.D.; Kanik, K.S. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N. Engl. J. Med. 2012, 367, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Strand, V.; van Vollenhoven, R.F.; Lee, E.B.; Fleischmann, R.; Zwillich, S.H.; Gruben, D.; Wallenstein, G. Tofacitinib or adalimumab versus placebo: Patient-reported outcomes from a phase 3 study of active rheumatoid arthritis. Rheumatology 2016, 55, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Dowty, M.E.; Lin, J.; Ryder, T.F.; Wang, W.; Walker, G.S.; Vaz, A.; Prakash, C. The pharmacokinetics, metabolism, and clearance mechanisms of tofacitinib, a janus kinase inhibitor, in humans. Drug Metab. Dispos. 2014, 42, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Claxton, L.; Taylor, M.; Soonasra, A.; Bourret, J.A.; Gerber, R.A. An economic evaluation of tofacitinib treatment in rheumatoid arthritis after methotrexate or after 1 or 2 TNF inhibitors from a U.S. payer perspective. J. Manag. Care Spec. Pharm. 2018, 24, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Naganuma, M.; Kanai, T. Current new challenges in the management of ulcerative colitis. Intest. Res. 2019, 17, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, E.; Villanacci, V.; Bassotti, G. Novel oral-targeted therapies for mucosal healing in ulcerative colitis. World, J. Gastroenterol. 2018, 24, 5322–5330. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Tofacitinib: A review of its use in adult patients with rheumatoid arthritis. Drugs 2013, 73, 857–874. [Google Scholar] [CrossRef]
- Cada, D.J.; Demaris, K.; Levien, T.L.; Baker, D.E. Tofacitinib. Hosp. Pharm. 2013, 48, 413–424. [Google Scholar] [CrossRef]
- Arifin, W.N.; Zahiruddin, W.M. Sample size calculation in animal studies using resource equation approach. Malays. J. Med. Sci. 2017, 24, 101–105. [Google Scholar]
- Du, E.S.; Moon, H.S.; Lim, S.J.; Kim, S.H. Pharmacokinetics of YJC-10592, a novel chemokine receptor 2 (CCR-2) antagonist, in rats. Arch. Pharm. Res. 2016, 39, 833–842. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, Y.M.; Lee, M.G. Pharmacokinetics and pharmacodynamics of furosemide in protein-calorie malnutrition. J. Pharmacokinet. Biopharm. 1993, 21, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E. Simple Determination of Tofacitinib, A Jak Inhibitor, in Plasma, Urine and Tissue Homogenates by Hplc and Its Application to A Pharmacokinetic Study. Master’s Thesis, Ajou University, Suwon, Korea, February 2018. [Google Scholar]
- Murakami, T.; Nakanishi, M.; Yoshimori, T.; Okamura, N.; Norikura, R.; Mizojiri, K. Separate assessment of intestinal and hepatic first-pass effects using a rat model with double cannulation of the portal and jugular veins. Drug Metab. Pharmacokinet. 2003, 18, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Lee, Y.K.; Lee, M.G. Effects of 17α-ethynylestradiol-induced cholestasis on the pharmacokinetics of doxorubicin in rats: Reduced biliary excretion and hepatic metabolism of doxorubicin. Xenobiotica 2013, 43, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Gibaldi, M.; Perrier, D. Pharmacokinetics, 2nd ed.; Marcel-Dekker: New York, NY, USA, 1982. [Google Scholar]
- Chiou, W.L. Critical evaluation of the potential error in pharmacokinetic studies of using the linear trapezoidal rule method for the calculation of the area under the plasma level-time curve. J. Pharmacokinet. Biopharm. 1978, 6, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.L. New calculation method of mean total body clearance of drugs and its application to dosage regimens. J. Pharm. Sci. 1980, 69, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Eatman, F.B.; Colburn, W.A.; Boxenbaum, H.G.; Posmanter, H.N.; Weinfeld, R.E.; Ronfeld, R.; Kaplan, S.A. Pharmacokinetics of diazepam following multiple-dose oral administration to healthy human subjects. J. Pharmacokinet. Biopharm. 1977, 5, 481–494. [Google Scholar] [CrossRef]
- Chiou, W.L. New calculation method for mean apparent drug volume of distribution and application to rational dosage regimens. J. Pharm. Sci. 1979, 68, 1067–1069. [Google Scholar] [CrossRef]
- Davies, B.; Morris, T. Physiological parameters in laboratory animals and humans. Pharm. Res. 1993, 10, 1093–1095. [Google Scholar] [CrossRef]
- Mitruka, B.M.; Rawnsley, H.M. Clinical Biomedical and Hematological Reference Values in Normal Experimental Animals and Normal Humans, 2nd ed.; Masson Publishing USA Inc.: New York, NY, USA, 1981. [Google Scholar]
- Caporali, R.; Zavaglia, D. Real-World Experience with Tofacitinib for Treatment of Rheumatoid Arthritis. Available online: https://www.ncbi.nlm.nih.gov/pubmed/30183607 (accessed on 29 August 2018).
- Lee, M.G.; Chiou, W.L. Evaluation of potential causes for the incomplete bioavailability of furosemide: Gastric first-pass metabolism. J. Pharmacokinet. Biopharm. 1983, 11, 623–640. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, M.G. Pharmacokinetics of ipriflavone, an isoflavone derivative, after intravenous and oral administration to rats hepatic and intestinal first-pass effects. Life Sci. 2002, 70, 1299–1315. [Google Scholar] [CrossRef]
- Bae, S.K.; Kim, J.W.; Kim, Y.H.; Kim, Y.G.; Kim, S.G.; Lee, M.G. Hepatic and intestinal first-pass effects of oltipraz in rats. Biopharm. Drug Dispos. 2005, 26, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Bae, S.K.; Lee, M.G. Pharmacokinetics of sildenafil after intravenous and oral administration in rats: Hepatic and intestinal first-pass effects. Int. J. Pharm. 2006, 320, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Thummel, K.E.; O’shea, D.; Paine, M.F.; Shen, D.D.; Kunze, K.L.; Perkins, J.D.; Wilkinson, G.R. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin. Pharmacol. Ther. 1996, 59, 491–502. [Google Scholar] [CrossRef]
- Hurst, S.; Loi, C.M.; Brodfuehrer, J.; El-Kattan, A. Impact of physiological, physicochemical and biopharmaceutical factors in absorption and metabolism mechanisms on the drug oral bioavailability of rats and humans. Expert Opin. Drug Metab. Toxicol. 2007, 3, 469–489. [Google Scholar] [CrossRef] [PubMed]
- Lindell, M.; Lang, M.; Lennernas, H. Expression of genes encoding for drug metabolizing cytochrome P450 enzymes and P-glycoprotein the rat small intestine: Comparison to the liver. Eur. J. Drug Metab. Pharmacokinet. 2003, 28, 41–48. [Google Scholar] [CrossRef]
- Lewis, D.F.V. P450 Substrate Specificity and Metabolism. Cytochromes 450. In Structure, Function and Mechanism; Taylor & Francis Inc.: Philadelphia, PA, USA, 1996; pp. 102–116. [Google Scholar]
- Park, M.Y. Effects of Cytochrome P450 (CYP) Inducers and Inhibitors on Tofacitinib Pharmacokinetics in Rats. Master’s Thesis, Ajou University, Suwon, Korea, February 2018. [Google Scholar]
- Correia, M.A.; Appendix, B. Rat and human liver cytochromes P450. In Substrate and Inhibitor Specificities and Functional Markers, 2nd ed.; Ortiz de Montellano, P.R., Ed.; Plenum Press: New York, NY, USA; London, UK, 1995; pp. 607–630. [Google Scholar]
- Ogiso, T.; Iwaki, M.; Tanaka, H.; Kobayashi, E.; Tanino, T.; Sawada, A.; Uno, S. Pharmacokinetic drug interactions between ampiroxicam and sulfaphenazole in rats. Biol. Pharm. Bull. 1999, 22, 191–196. [Google Scholar] [CrossRef]
- De Martin, S.; Gabbia, D.; Albertin, G.; Sfriso, M.M.; Mescoli, C.; Albertoni, L.; Palatini, P. Differential effect of liver cirrhosis on the pregnane X receptor-mediated induction of CYP3A1 and 3A2 in the rat. Drug Metab. Dispos. 2014, 42, 1617–1626. [Google Scholar] [CrossRef]
- Dixit, V.; Moore, A.; Tsao, H.; Hariparsad, N. Application of micropatterned cocultured hepatocytes to evaluate the inductive potential and degradation rate of major xenobiotic metabolizing enzymes. Drug Metab. Dispos. 2016, 44, 250–261. [Google Scholar] [CrossRef]
- Papp, K.A.; Menter, M.A.; Abe, M.; Elewski, B.; Feldman, S.R.; Gottlieb, A.B.; Gupta, P. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: Results from two, randomized, placebo-controlled, Phase 3 trials. Br. J. Dermatol. 2015, 173, 949–961. [Google Scholar] [CrossRef]
- Bachelez, H.; Van de Kerkhof, P.C.; Strohal, R.; Kubanov, A.; Valenzuela, F.; Lee, J.H.; Gupta, P. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: A phase 3 randomized non-inferiority trial. Lancet 2015, 386, 552–561. [Google Scholar] [CrossRef]
- Crispin, M.K.; Ko, J.M.; Craiglow, B.G.; Li, S.; Shankar, G.; Urban, J.R.; Marinkovich, M.P. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight 2016, 1, e89776. [Google Scholar]
- Levy, L.L.; Urban, J.; King, B.A. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J. Am. Acad. Dermatol. 2015, 73, 395–399. [Google Scholar] [CrossRef]
- Tahir, H. Therapies in ankylosing spondylitis-from clinical trials to clinical practice. Rheumatology 2018, 57 (Suppl. 6), vi23–vi28. [Google Scholar] [CrossRef]
- Lawendy, N.; Lamba, M.; Chan, G.; Wang, R.; Alvey, C.W.; Krishnaswami, S. The effect of mild and moderate hepatic impairment on the pharmacokinetics of tofacitinib, an orally active Janus kinase inhibitor. Clin. Pharmacol. Drug Dev. 2014, 3, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswami, S.; Chow, V.; Boy, M.; Wang, C.; Chan, G. Pharmacokinetics of tofacitinib, a janus kinase inhibitor, in patients with impaired renal function and end-stage renal disease. J. Clin. Pharmacol. 2014, 54, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Xie, R.; Strober, B.; Langley, R.; Ito, K.; Krishnaswami, S.; Wolk, R.; Valdez, H.; Rottinghaus, S.; Tallman, A.; et al. Pharmacokinetic characteristics of tofacitinib in adult patients with moderate to severe chronic plaque psoriasis. Clin. Pharmacol. Drug Dev. 2018, 7, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Battat, R.; Jairath, V.; Vande Casteele, N. Advances in therapeutic drug monitoring for small-molecule and biologic therapies in inflammatory bowel disease. Curr. Treat. Options Gastroenterol. 2019, 17, 127–145. [Google Scholar] [CrossRef]
- Hussa, D.A. 2013 new drug update: What do new approvals hold for the elderly? Consult. Pharm. 2014, 29, 224–238. [Google Scholar] [CrossRef]
- Gupta, P.; Chow, V.; Wang, R.; Kaplan, I.; Chan, G.; Alvey, C.; Ni, G.; Ndongo, M.N.; LaBadie, R.R.; Krishnaswami, S. Evaluation of the effect of fluconazole and ketoconazole on the pharmacokinetics of tofacitinib in healthy adult subjects. Clin. Pharmacol. Drug Dev. 2014, 3, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Zwillich, S.H.; Chow, V.; Labadie, R.R.; Wilkinson, B. Co-administration of the JAK inhibitor CP-690,550 and methotrexate is well tolerated in patients with rheumatoid arthritis without need for dose adjustment. Br. J. Clin. Pharmacol. 2010, 69, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Riese, R.; Wang, R.; Alvey, C.; Shi, H.; Petit, W.; Krishnaswami, S. Evaluation of the effect of tofacitinib on the pharmacokinetics of oral contraceptive steroids in healthy female volunteers. Clin. Pharmacol. Drug Dev. 2016, 5, 336–342. [Google Scholar] [CrossRef] [PubMed]
| Parameters. | 5 mg/kg | 10 mg/kg | 20 mg/kg | 50 mg/kg |
|---|---|---|---|---|
| (n = 9) | (n = 8) | (n = 7) | (n = 7) | |
| Body weight (g) | 337 ± 39.2 | 347 ± 11.0 | 317 ± 15.9 | 356 ± 22.0 |
| AUC (μg∙min/mL) a | 141 ± 23.0 | 342 ± 33.1 | 807 ± 146 | 3526 ± 572 |
| Dose-normalized AUC (μg∙min/mL) a | 282 ± 46.0 | 342 ± 33.1 | 404 ± 73.0 | 705 ± 114 |
| Terminal half-life (min) b | 38.2 ± 11.7 | 41.6 ± 7.86 | 48.1 ± 5.56 | 97.8 ± 19.2 |
| MRT (min) b | 35.2 ± 11.4 | 41.1 ± 20.2 | 35.5 ± 7.50 | 105 ± 21.4 |
| CL (mL/min/kg) c | 36.3 ± 6.02 | 29.5 ± 2.99 | 25.4 ± 4.26 | 14.5 ± 2.33 |
| CLR (mL/min/kg) | 2.69 ± 1.70 | 2.80 ± 1.69 | 2.80 ± 1.17 | 0.824 ± 0.651 |
| CLNR (mL/min/kg) d | 32.9 ± 7.72 | 26.3 ± 2.16 | 22.6 ± 3.90 | 13.7 ± 2.12 |
| Vss (mL/kg) | 1258 ± 460 | 1208 ± 579 | 900 ± 218 | 1489 ± 134 |
| Ae0–24 h (% of dose) | 7.62 ± 5.08 | 9.40 ± 5.06 | 11.0 ± 3.72 | 5.55 ± 3.91 |
| Parameters | 10 mg/kg | 20 mg/kg | 50 mg/kg | 100 mg/kg |
|---|---|---|---|---|
| (n = 7) | (n = 8) | (n = 9) | (n = 7) | |
| Body weight (g) | 312 ± 19.8 | 308 ± 24.5 | 284 ± 22.3 | 286 ± 17.7 |
| AUC (μg∙min/mL) a | 99.4 ± 35.5 | 269 ± 53.7 | 1192 ± 280 | 4073 ± 1787 |
| Dose-normalized AUC (μg∙min/mL) a | 99.4 ± 35.5 | 135 ± 26.9 | 238 ± 56.0 | 407 ± 179 |
| Cmax (μg/mL) | 1.13 ± 0.774 | 3.13 ± 1.27 | 5.62 ± 2.18 | 13.1 ± 5.48 |
| Tmax (min) b | 15.7 ± 7.32 | 15.7 ± 15.9 | 34.4 ± 26.7 | 170 ± 192 |
| CLR (mL/min/kg) c | 11.6 ± 2.87 | 6.97 ± 3.95 | 7.61 ± 2.82 | 3.37 ± 2.52 |
| Ae0–24 h (% of dose) | 11.3 ± 4.60 | 10.0 ± 6.51 | 17.2 ± 3.98 | 13.0± 9.47 |
| GI24 h (% of dose) | 3.16 ± 5.12 | 3.13 ± 3.27 | 2.77 ± 3.96 | 0.772± 0.905 |
| F (%) | 29.1 | 39.3 | 69.7 | 119 |
| Parameter | Intravenous | Intraportal |
|---|---|---|
| (n = 7) | (n = 7) | |
| Body weight (g) | 292 ± 18.0 | 299 ± 28.6 |
| AUC (μg∙min/mL) | 417 ± 136 | 242 ± 66.0 ** |
| Terminal half-life (min) | 46.2 ± 12.5 | 42.4 ± 10.4 |
| MRT (min) | 67.5 ± 26.8 | 59.6 ± 13.9 |
| CL (mL/min/kg) | 26.3 ± 8.85 | 43.8 ± 11.0 ** |
| CLR (mL/min/kg) | 2.34 ± 2.90 | 5.00 ± 4.69 |
| CLNR (mL/min/kg) | 24.0 ± 6.46 | 38.5 ± 10.4 ** |
| Vss (mL/kg) | 1695 ± 737 | 2549 ± 720 * |
| Ae0–24 h (% of dose) | 12.5 ± 5.20 | 10.7 ± 9.42 |
| Parameter | Intraportal | Intraduodenal | Intragastric |
|---|---|---|---|
| (n = 6) | (n = 5) | (n = 5) | |
| Body weight (g) | 287 ± 10.6 | 282 ± 6.30 | 285 ± 4.97 |
| AUC (μg∙min/mL) a | 272 ± 62.7 | 138 ± 33.2 | 134 ± 44.6 |
| Terminal half-life (min) | 44.8 ± 14.2 | ||
| Cmax (μg/mL) b | 5.18 ± 0.586 | 1.55 ± 1.93 | 0.716 ± 0.255 |
| Tmax (min) | 27.5 ± 6.12 | 38.8 ± 38.2 | 63.8 ± 18.9 |
| MRT (min) | 53.0 ± 22.3 | ||
| CL (mL/min/kg) | 38.4 ± 8.47 | ||
| CLR (mL/min/kg) | 2.44 ± 1.23 | 5.72 ± 2.62 | 7.08 ± 2.91 |
| CLNR (mL/min/kg) | 38.3 ± 6.16 | ||
| Vss (mL/kg) | 1900 ± 369 | ||
| Ae0–24 h (% of dose) | 5.82 ± 2.32 | 8.30 ± 5.10 | 8.90 ± 3.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.S.; Kim, S.H. Dose-Dependent Pharmacokinetics of Tofacitinib in Rats: Influence of Hepatic and Intestinal First-Pass Metabolism. Pharmaceutics 2019, 11, 318. https://doi.org/10.3390/pharmaceutics11070318
Lee JS, Kim SH. Dose-Dependent Pharmacokinetics of Tofacitinib in Rats: Influence of Hepatic and Intestinal First-Pass Metabolism. Pharmaceutics. 2019; 11(7):318. https://doi.org/10.3390/pharmaceutics11070318
Chicago/Turabian StyleLee, Ji Sang, and So Hee Kim. 2019. "Dose-Dependent Pharmacokinetics of Tofacitinib in Rats: Influence of Hepatic and Intestinal First-Pass Metabolism" Pharmaceutics 11, no. 7: 318. https://doi.org/10.3390/pharmaceutics11070318
APA StyleLee, J. S., & Kim, S. H. (2019). Dose-Dependent Pharmacokinetics of Tofacitinib in Rats: Influence of Hepatic and Intestinal First-Pass Metabolism. Pharmaceutics, 11(7), 318. https://doi.org/10.3390/pharmaceutics11070318




