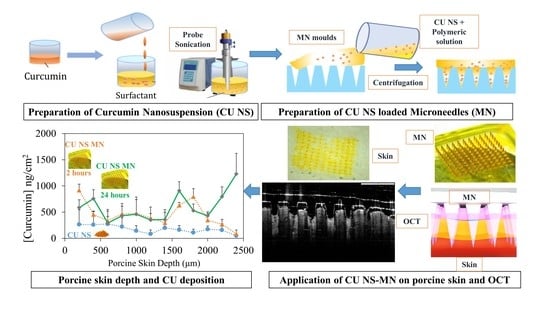
Nanosuspension-Based Dissolving Microneedle Arrays for Intradermal Delivery of Curcumin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
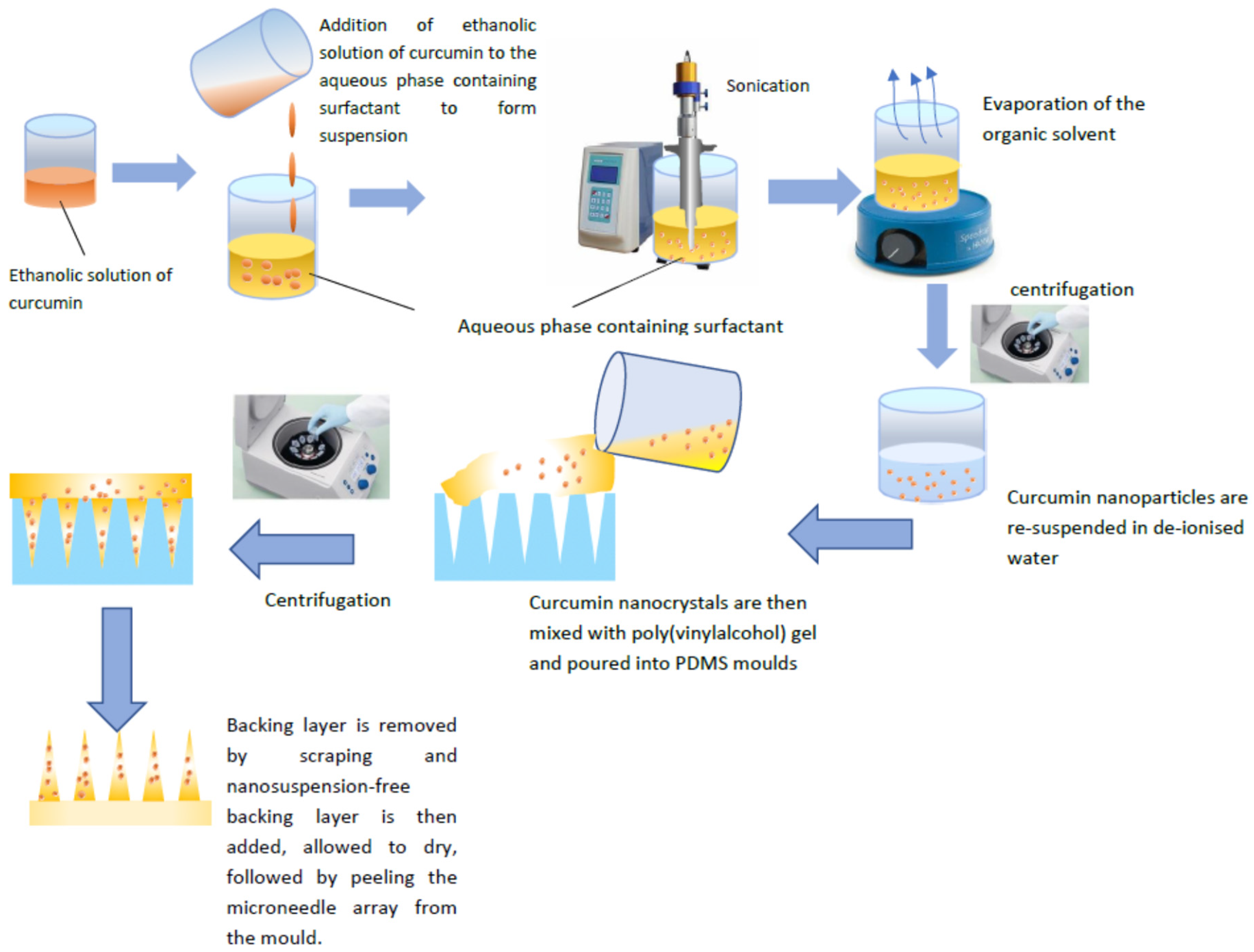
2.2. CU-NS Preparation
2.3. Microneedle Preparation
2.4. CU-NS Characterisation
2.4.1. Particle Size and Zeta Potential
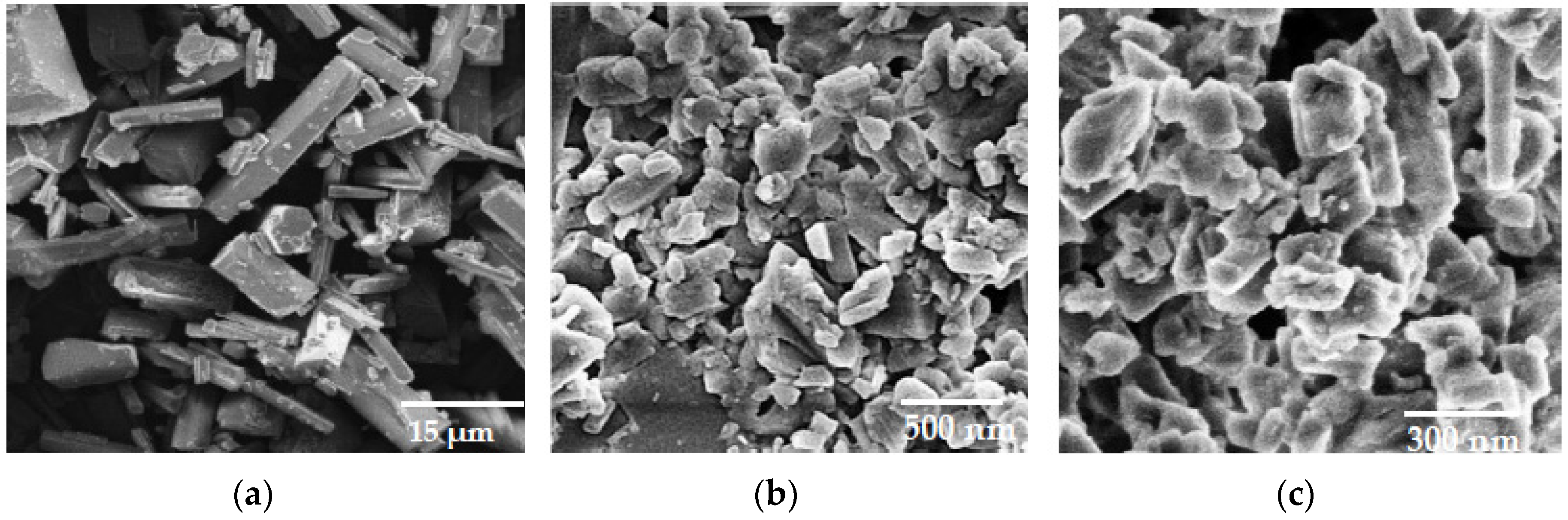
2.4.2. Scanning Electron Microscopy
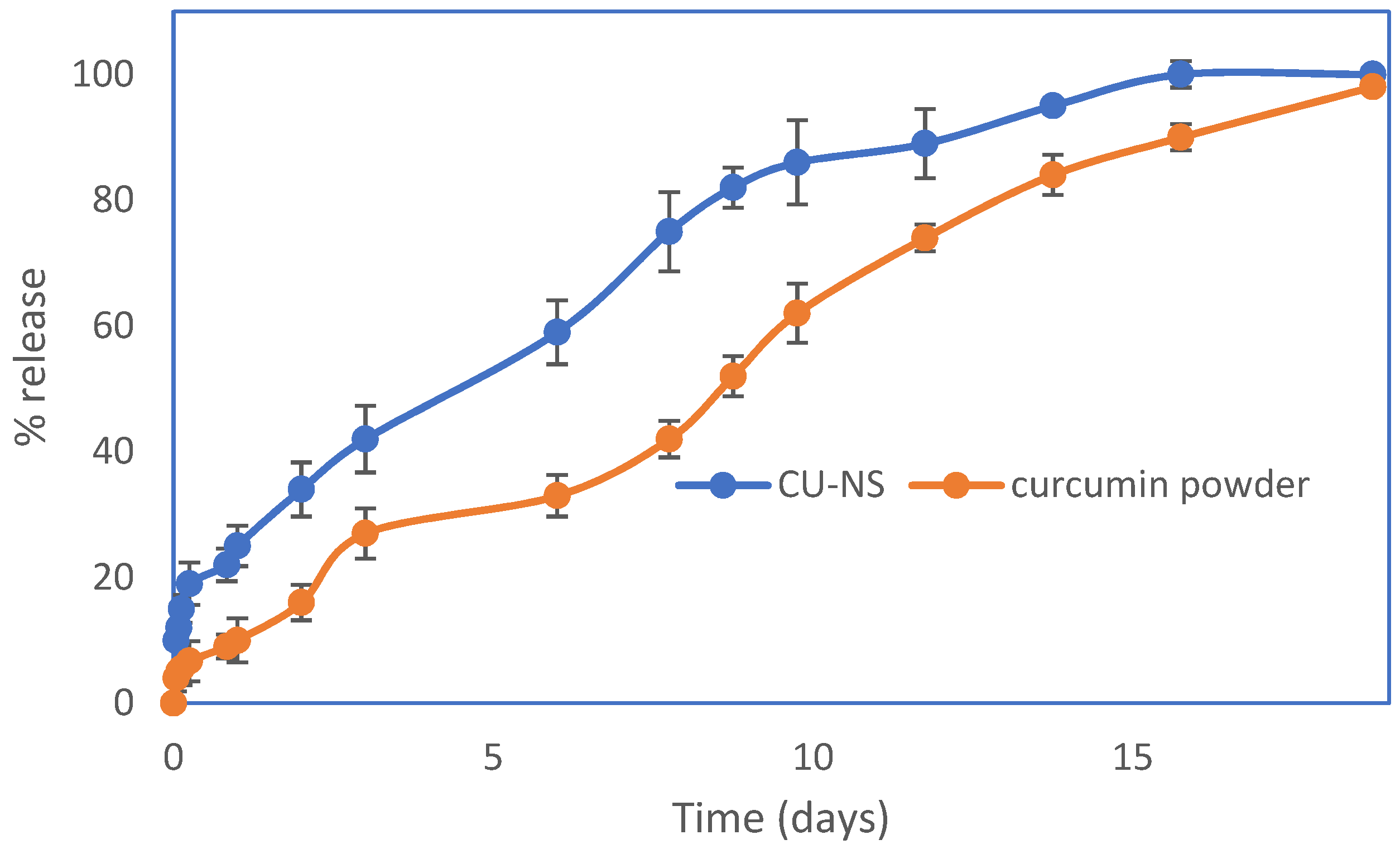
2.4.3. In Vitro Release
2.4.4. HPLC Analysis
2.5. Microneedle Characterisation
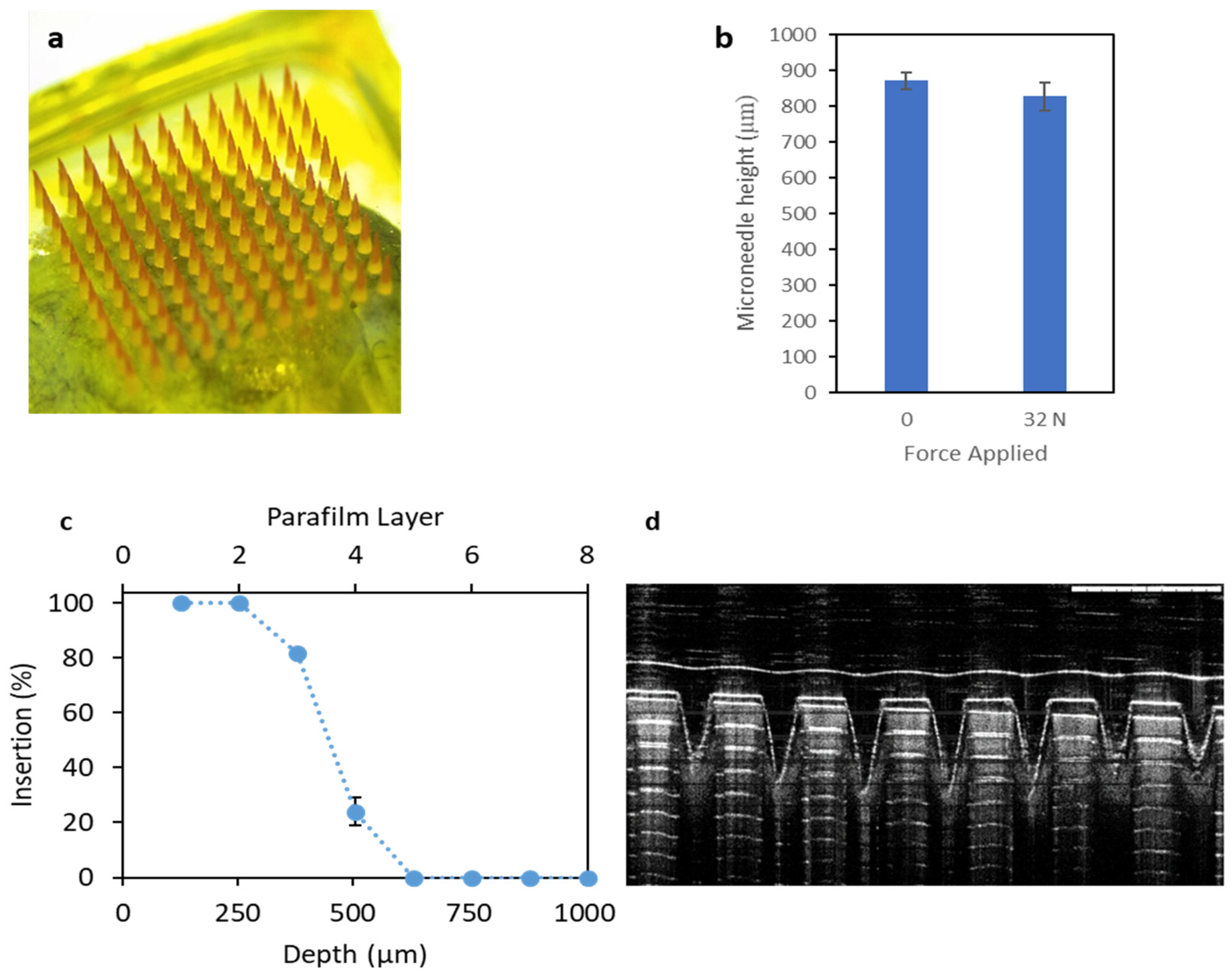
2.5.1. Microneedles Mechanical Strength
2.5.2. CU-MN Drug Content
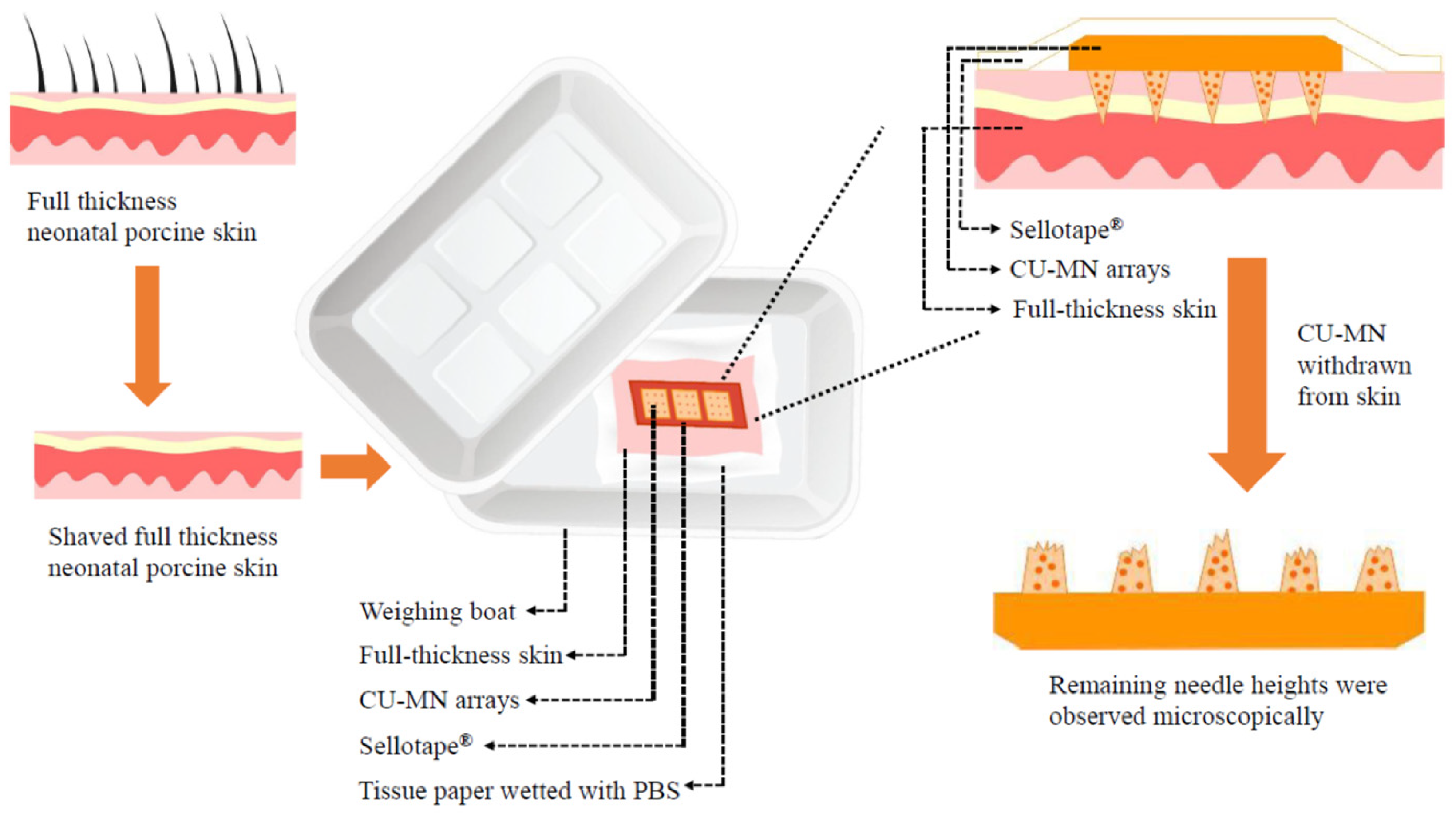
2.5.3. Microneedles Insertion Studies
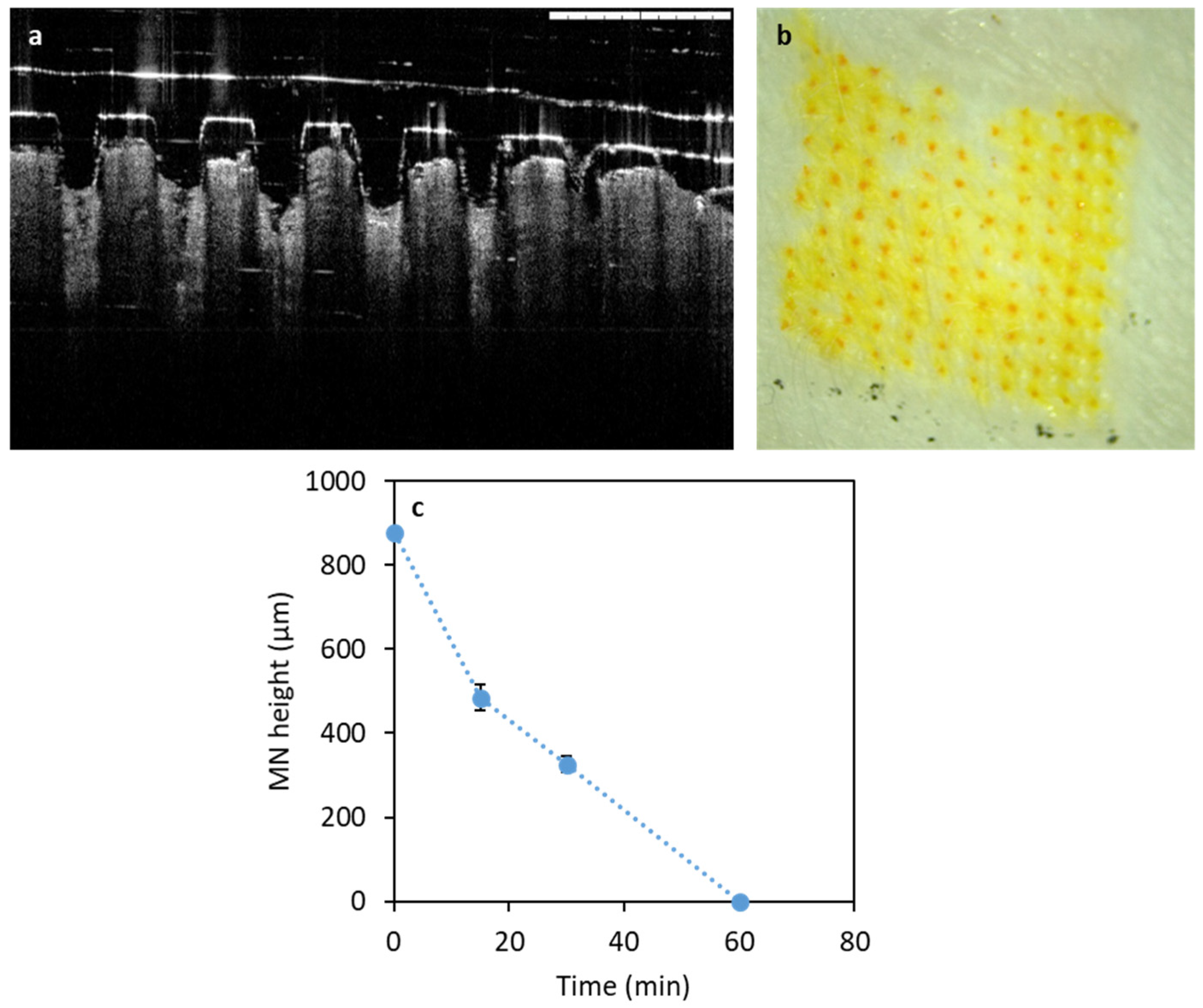
2.5.4. Microneedles Dissolution in Skin
2.5.5. Optical Coherence Tomography
2.5.6. Ex Vivo Drug Deposition in Skin
2.6. Statistical Analysis
3. Results
3.1. Nanosuspension Characterisation
3.2. Microneedle Characterisation
3.2.1. In Vitro Characterisation of Microneedles
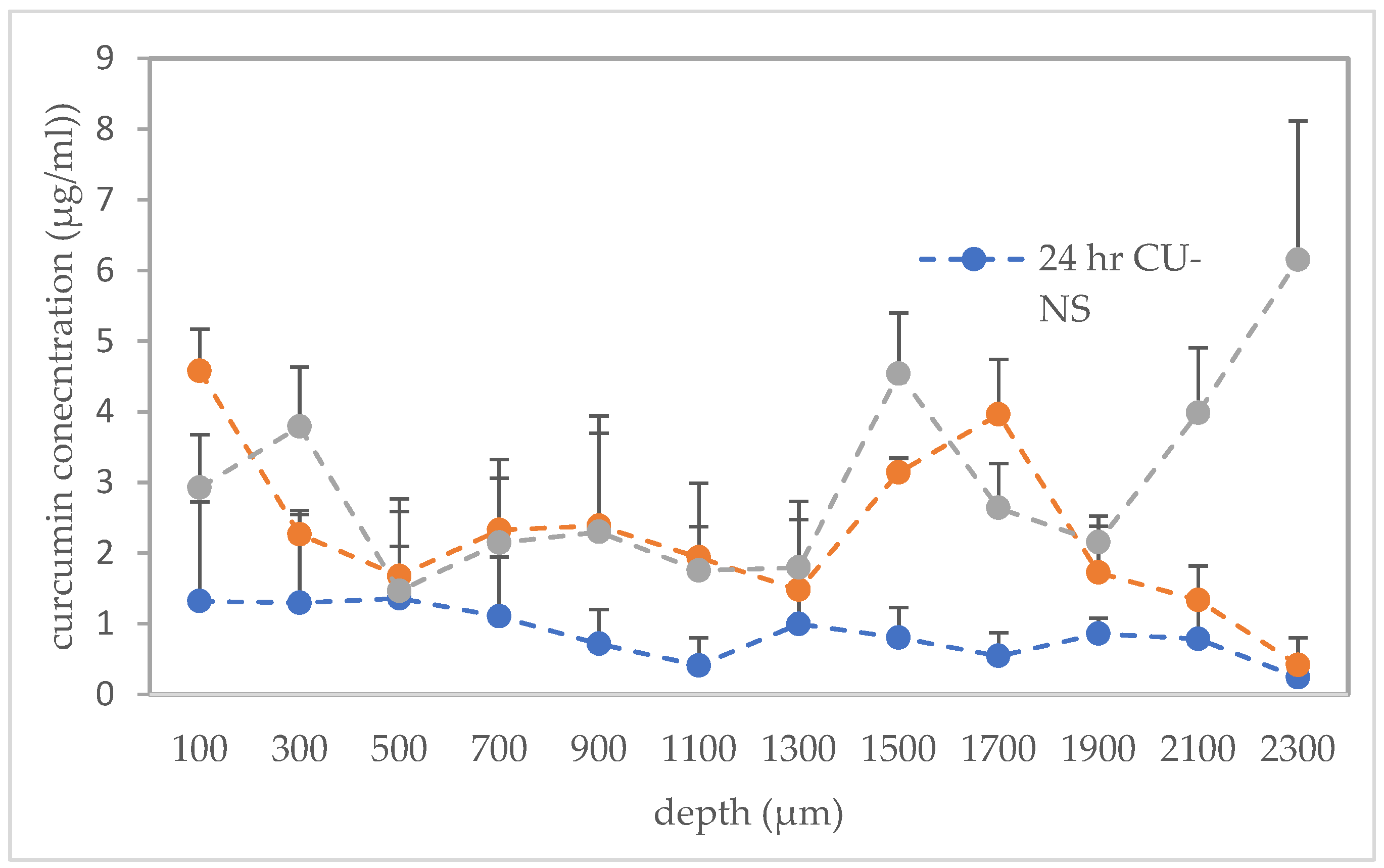
3.2.2. Performance of Microneedles in Ex Vivo Neonatal Porcine Skin
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, T.R.; Garland, M.J.; Cassidy, C.M.; Migalska, K.; Demir, Y.K.; Abdelghany, S.; Ryan, E.; Woolfson, A.D.; Donnelly, R.F. Microporation techniques for enhanced delivery of therapeutic agents. Recent Pat. Drug Deliv. Formul. 2010, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Mikszta, J.A.; Cormier, M.; Andrianov, A.K. Microneedle-based vaccines. Curr. Top. Microbiol. Immunol. 2009, 333, 369–393. [Google Scholar] [PubMed]
- Stoner, C.L.; Cleton, A.; Johnson, K.; Oh, D.M.; Hallak, H.; Brodfuehrer, J.; Surendran, N.; Han, H.K. Integrated oral bioavailability projection using in vitro screening data as a selection tool in drug discovery. Int. J. Pharm. 2004, 269, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Gulati, N.; Gupta, H. Parenteral drug delivery: A review. Recent Pat. Drug Deliv. Formul. 2011, 5, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Agac, E.; Gunes, U.Y. Effect on pain of changing the needle prior to administering medicine intramuscularly: A randomized controlled trial. J. Adv. Nurs. 2011, 67, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Orellana, I. Strategies to improve oral drug bioavailability. Expert Opin. Drug Deliv. 2005, 2, 419–433. [Google Scholar] [CrossRef]
- Ita, K. Transdermal Delivery of Drugs with Microneedles—Potential and Challenges. Pharmaceutics 2015, 7, 90–105. [Google Scholar] [CrossRef]
- Sullivan, S.P.; Murthy, N.; Prausnitz, M.R. Minimally invasive protein delivery with rapidly dissolving polymer microneedles. Adv. Mater. 2008, 20, 933–938. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Majithiya, R.; Singh, T.R.; Morrow, D.I.; Garland, M.J.; Demir, Y.K.; Migalska, K.; Ryan, E.; Gillen, D.; Scott, C.J.; et al. Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharm. Res. 2011, 28, 41–57. [Google Scholar] [CrossRef]
- Vora, L.K.; Vavia, P.R.; Larraneta, E.; Bell, S.E.J.; Donnelly, R.F. Novel nanosuspension-based dissolving microneedle arrays for transdermal delivery of a hydrophobic drug. J. Interdiscip. Nanomed. 2018, 3, 89–101. [Google Scholar] [CrossRef]
- Chogale, M.M.; Ghodake, V.N.; Patravale, V.B. Performance Parameters and Characterizations of Nanocrystals: A Brief Review. Pharmaceutics 2016, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives–A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Friedman, A.J. Curcumin: A novel treatment for skin-related disorders. J. Drugs Dermatol. JDD 2013, 12, 1131–1137. [Google Scholar] [PubMed]
- Sonavane, K.; Phillips, J.; Ekshyyan, O.; Moore-Medlin, T.; Roberts Gill, J.; Rong, X.; Lakshmaiah, R.R.; Abreo, F.; Boudreaux, D.; Clifford, J.L.; et al. Topical Curcumin-Based Cream Is Equivalent to Dietary Curcumin in a Skin Cancer Model. J. Skin Cancer 2012, 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- Moorthi, C.; Kathiresan, K. Fabrication of highly stable sonication assisted curcumin nanocrystals by nanoprecipitation method. Drug Invent. Today 2013, 5, 66–69. [Google Scholar] [CrossRef]
- Larraneta, E.; Imizcoz, M.; Toh, J.X.; Irwin, N.J.; Ripolin, A.; Perminova, A.; Dominguez-Robles, J.; Rodriguez, A.; Donnelly, R.F. Synthesis and Characterization of Lignin Hydrogels for Potential Applications as Drug Eluting Antimicrobial Coatings for Medical Materials. ACS Sustain. Chem. Eng. 2018, 6, 9037–9046. [Google Scholar] [CrossRef] [PubMed]
- Larraneta, E.; Barturen, L.; Ervine, M.; Donnelly, R.F. Hydrogels based on poly(methyl vinyl ether-co-maleic acid) and Tween 85 for sustained delivery of hydrophobic drugs. Int. J. Pharm. 2018, 538, 147–158. [Google Scholar] [CrossRef]
- Eltayib, E.; Brady, A.J.; Caffarel-Salvador, E.; Gonzalez-Vazquez, P.; Zaid Alkilani, A.; McCarthy, H.O.; McElnay, J.C.; Donnelly, R.F. Hydrogel-forming microneedle arrays: Potential for use in minimally-invasive lithium monitoring. Eur. J. Pharm. Biopharm. 2016, 102, 123–131. [Google Scholar] [CrossRef]
- Larraneta, E.; Moore, J.; Vicente-Perez, E.M.; Gonzalez-Vazquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef]
- Summerfield, A.; Meurens, F.; Ricklin, M.E. The immunology of the porcine skin and its value as a model for human skin. Mol. Immunol. 2015, 66, 14–21. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Garland, M.J.; Morrow, D.I.J.; Migalska, K.; Singh, T.R.R.; Majithiya, R.; Woolfson, A.D. Optical coherence tomography is a valuable tool in the study of the effects of microneedle geometry on skin penetration characteristics and in-skin dissolution. J. Control. Release 2010, 147, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-M.; Yoon, G.H.; Lee, H.C.; Jung, M.H.; Yu, S.I.; Yeon, S.J.; Min, S.K.; Kwon, Y.S.; Hwang, J.H.; Shin, H.S. Thermodynamic Insights and Conceptual Design of Skin-Sensitive Chitosan Coated Ceramide/PLGA Nanodrug for Regeneration of Stratum Corneum on Atopic Dermatitis. Sci. Rep. 2015, 5, 18089. [Google Scholar] [CrossRef] [PubMed]
- Md, S.C.M.; Kit, B.; Jagdish, S.; David, J.P.D.; Pandey, M.; Alok Chatterjee, L. Development and in vitro evaluation of a zerumbone loaded nanosuspension drug delivery system. Crystals 2018, 8, 286. [Google Scholar] [CrossRef]
- Elham, G.; Mahsa, P.; Vahid, R. Spray drying of nanoparticles to form fast dissolving glipizide. Asian J. Pharm. 2015, 9, 213–218. [Google Scholar]
- Bhawana, B.R.K.; Buttar, H.S.; Jain, V.K.; Jain, N. Curcumin Nanoparticles: Preparation, Characterization, and Antimicrobial Study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R. Studies on Dissolution Behaviour of Nanoparticulate Curcumin Formulation. Adv. Nanopart. 2013, 2, 9. [Google Scholar] [CrossRef]
- Muselik, J.; Franc, A.; Dolezel, P.; Gonec, R.; Krondlova, A.; Lukasova, I. Influence of process parameters on content uniformity of a low dose active pharmaceutical ingredient in a tablet formulation according to GMP. Acta Pharm. 2014, 64, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Microneedles: A New Frontier in Nanomedicine Delivery. Pharm. Res. 2016, 33, 1055–1073. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Moffatt, K.; Alkilani, A.Z.; Vicente-Perez, E.M.; Barry, J.; McCrudden, M.T.; Woolfson, A.D. Hydrogel-forming microneedle arrays can be effectively inserted in skin by self-application: A pilot study centred on pharmacist intervention and a patient information leaflet. Pharm. Res. 2014, 31, 1989–1999. [Google Scholar] [CrossRef]
- Vora, L.K.; Donnelly, R.F.; Larraneta, E.; Gonzalez-Vazquez, P.; Thakur, R.R.S.; Vavia, P.R. Novel bilayer dissolving microneedle arrays with concentrated PLGA nano-microparticles for targeted intradermal delivery: Proof of concept. J. Control. Release 2017, 265, 93–101. [Google Scholar] [CrossRef]
- DeMuth, P.C.; Garcia-Beltran, W.F.; Ai-Ling, M.L.; Hammond, P.T.; Irvine, D.J. Composite dissolving microneedles for coordinated control of antigen and adjuvant delivery kinetics in transcutaneous vaccination. Adv. Funct. Mater. 2013, 23, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vazquez, P.; Larraneta, E.; McCrudden, M.T.C.; Jarrahian, C.; Rein-Weston, A.; Quintanar-Solares, M.; Zehrung, D.; McCarthy, H.; Courtenay, A.J.; Donnelly, R.F. Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis. J. Control. Release 2017, 265, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Derm.-Endocrinol. 2009, 1, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Mc Crudden, M.T.C.; Larrañeta, E.; Clark, A.; Jarrahian, C.; Rein-Weston, A.; Lachau-Durand, S.; Niemeijer, N.; Williams, P.; Haeck, C.; McCarthy, H.O.; et al. Design, formulation and evaluation of novel dissolving microarray patches containing a long-acting rilpivirine nanosuspension. J. Control. Release 2018, 292, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Richter, H.; Teichmann, A.; Otberg, N.; Blume-Peytavi, U.; Luengo, J.; Weiss, B.; Schaefer, U.F.; Lehr, C.M.; Wepf, R.; et al. Nanoparticles—An efficient carrier for drug delivery into the hair follicles. Eur. J. Pharm. Biopharm. 2007, 66, 159–164. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, J.; Zhu, Q.; Zhang, M.; Ding, X.; Wang, X.; Hou, X.; Fan, W.; Ding, B.; Wu, X.; et al. Penetration and distribution of PLGA nanoparticles in the human skin treated with microneedles. Int. J. Pharm. 2010, 402, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Larrañeta, E. Microarray patches: potentially useful delivery systems for long-acting nanosuspensions. Drug Discov. Today 2018, 23, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Vicente-Perez, E.M.; Larrañet, E.; McCrudden, M.T.C.; Kissenpfennig, A.; Hegarty, S.; McCarthy, H.O.; Donnelly, R.F. Repeat application of microneedles does not alter skin appearance or barrier function and causes no measurable disturbance of serum biomarkers of infection, inflammation or immunity in mice in vivo. Eur. J. Pharm. Biopharm. 2017, 117, 400–407. [Google Scholar] [CrossRef]
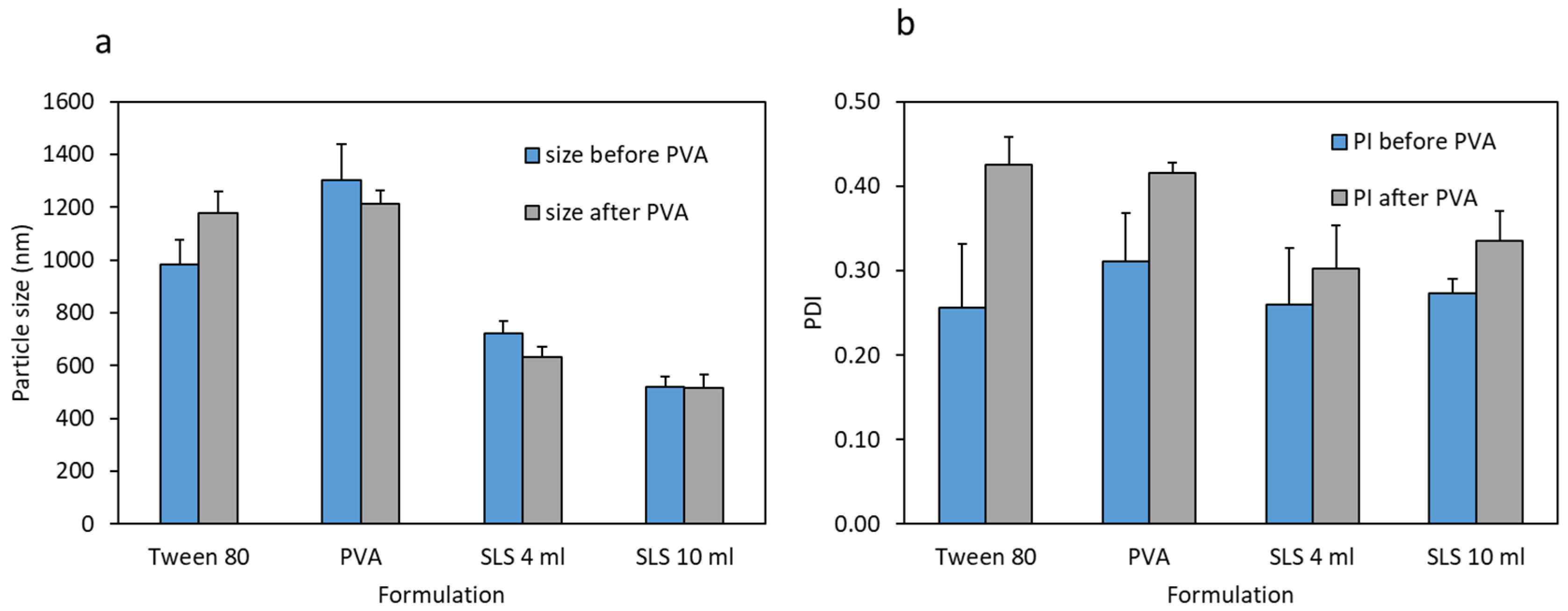
| Formula | Zeta Potential (Before Mixing with PVA Solution) | Zeta Potential (After Mixing with PVA Solution) |
|---|---|---|
| 4 mL SLS | −3.1 ± 2.1 | −1.9 ± 2.4 |
| 10 mL SLS | −5.4 ± 3.7 | −2.3 ± 1.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelghany, S.; Tekko, I.A.; Vora, L.; Larrañeta, E.; Permana, A.D.; Donnelly, R.F. Nanosuspension-Based Dissolving Microneedle Arrays for Intradermal Delivery of Curcumin. Pharmaceutics 2019, 11, 308. https://doi.org/10.3390/pharmaceutics11070308
Abdelghany S, Tekko IA, Vora L, Larrañeta E, Permana AD, Donnelly RF. Nanosuspension-Based Dissolving Microneedle Arrays for Intradermal Delivery of Curcumin. Pharmaceutics. 2019; 11(7):308. https://doi.org/10.3390/pharmaceutics11070308
Chicago/Turabian StyleAbdelghany, Sharif, Ismaiel A. Tekko, Lalitkumar Vora, Eneko Larrañeta, Andi Dian Permana, and Ryan F. Donnelly. 2019. "Nanosuspension-Based Dissolving Microneedle Arrays for Intradermal Delivery of Curcumin" Pharmaceutics 11, no. 7: 308. https://doi.org/10.3390/pharmaceutics11070308
APA StyleAbdelghany, S., Tekko, I. A., Vora, L., Larrañeta, E., Permana, A. D., & Donnelly, R. F. (2019). Nanosuspension-Based Dissolving Microneedle Arrays for Intradermal Delivery of Curcumin. Pharmaceutics, 11(7), 308. https://doi.org/10.3390/pharmaceutics11070308