Molecular Mobility and Stability Studies of Amorphous Imatinib Mesylate
Abstract
1. Introduction
2. Materials and Methods
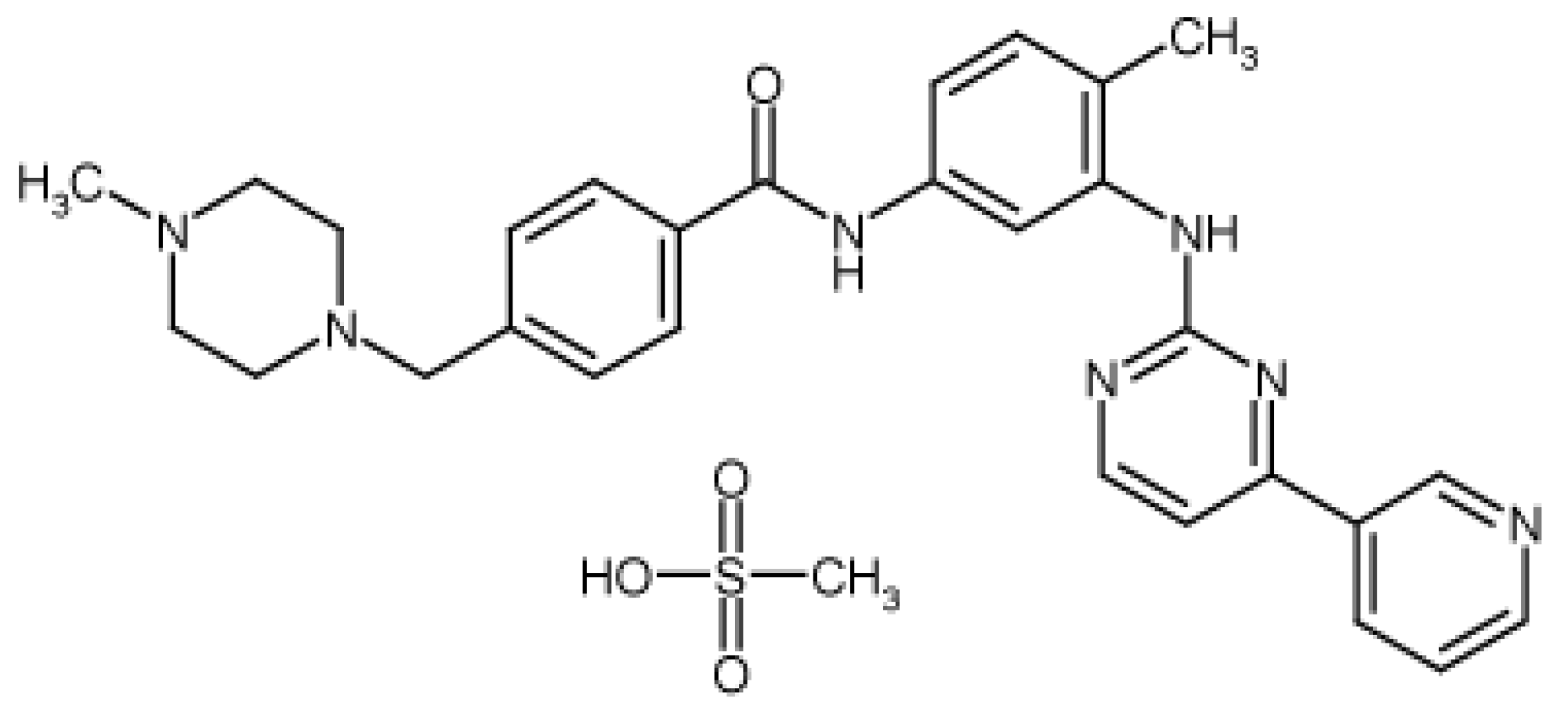
2.1. Materials
2.2. Preparation of Amorphous Sample
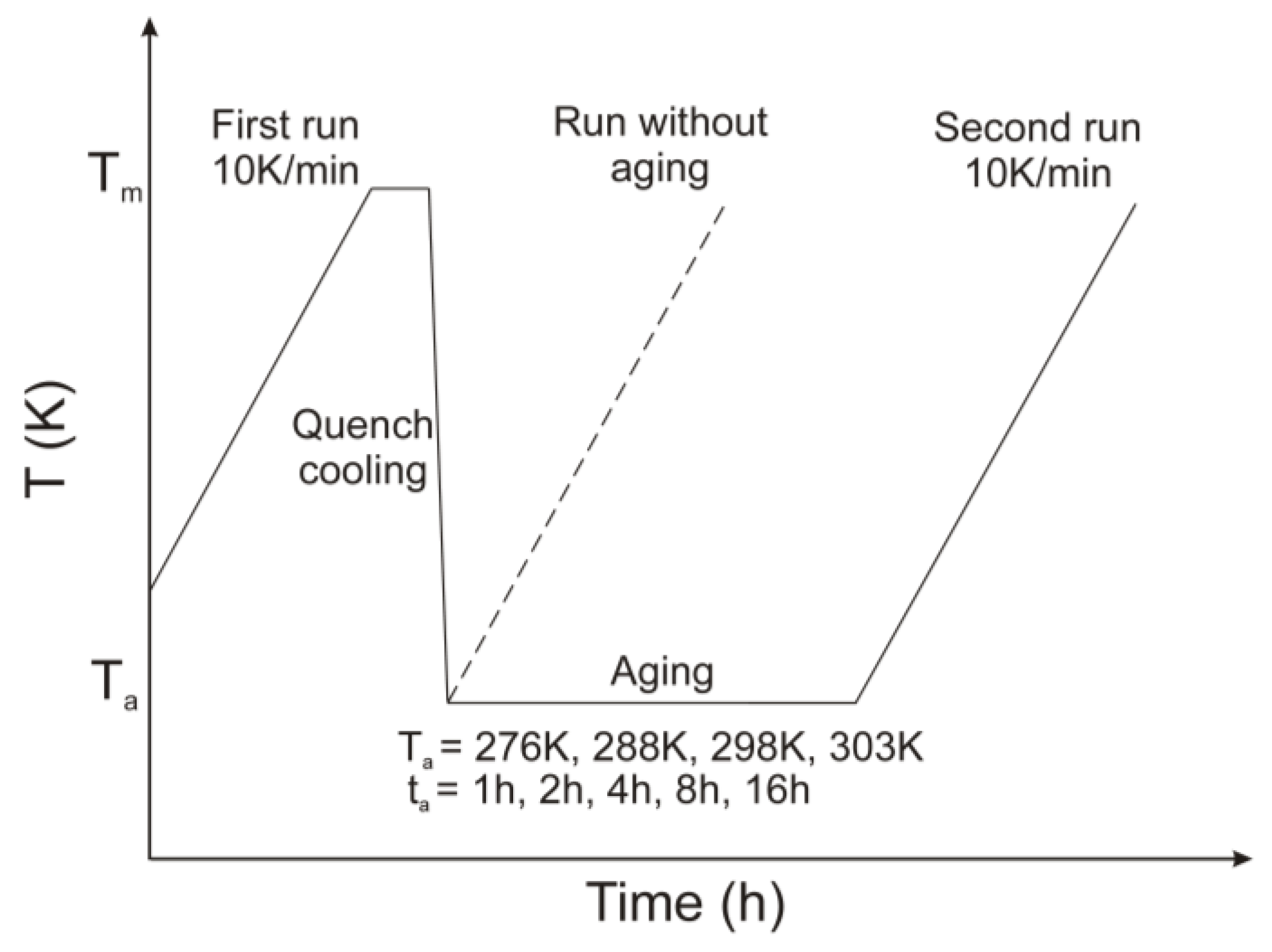
2.3. Physical Stability Studies
2.4. Differential Scanning Calorimetry (DSC)
2.5. Thermogravimetric Analysis (TGA)
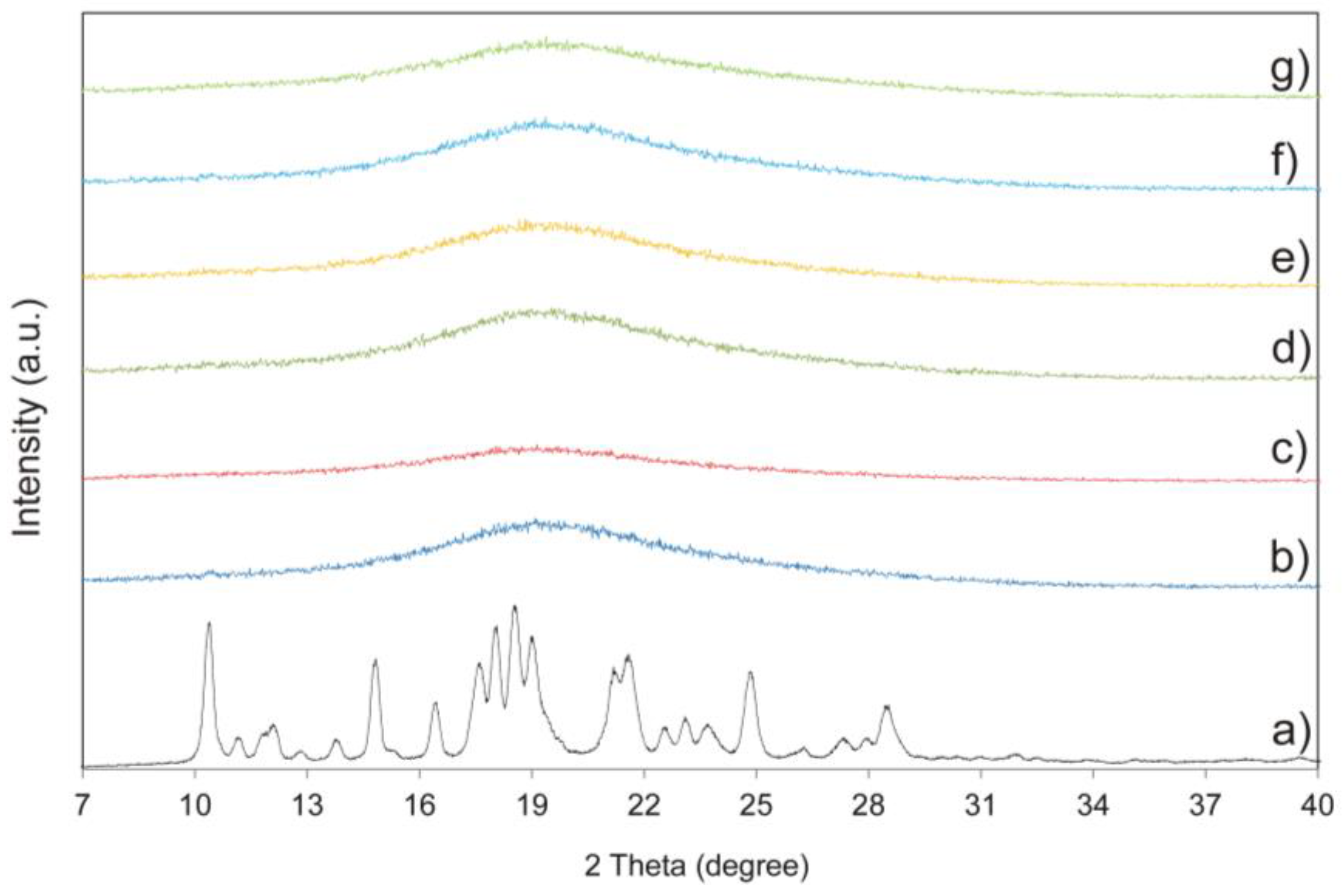
2.6. Powder X-ray Diffraction Analysis (XRPD)
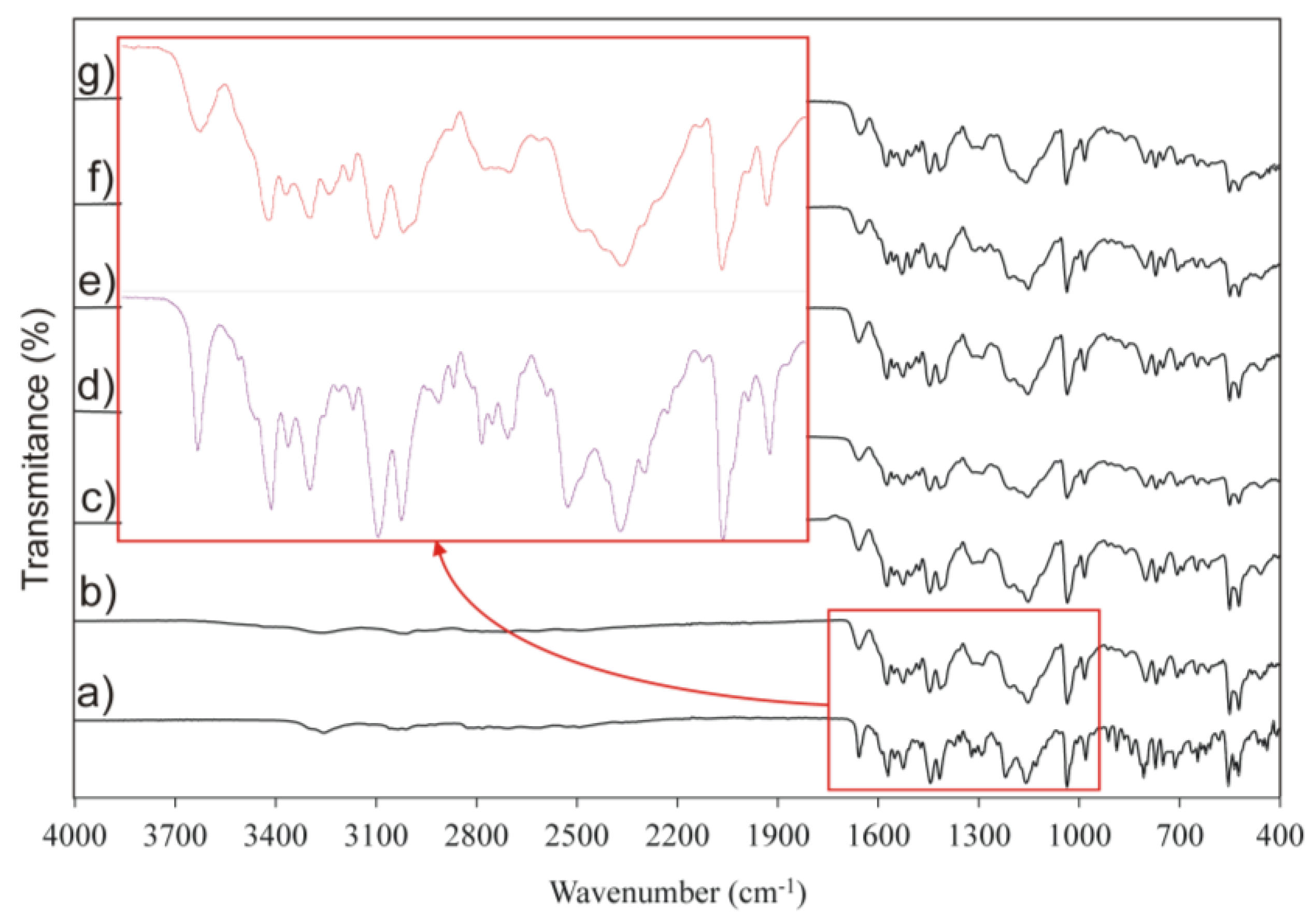
2.7. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.8. Scanning Electron Microscopy (SEM)
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yu, L. Amorphous pharmaceutical solids: Preparation, characterization and stabilization. Adv. Drug Deliv. Rev. 2001, 48, 27–42. [Google Scholar] [CrossRef]
- Hancock, B.C.; Parks, M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm. Res. 2000, 17, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.H.; Gordon, S.; Holm, R.; Selen, A.; Rades, T.; Mullertz, A. Preparation of an amorphous sodium furosemide salt improves solubility and dissolution rate and leads to a faster T-max after oral dosing to rats. Eur. J. Pharm. Biopharm. 2013, 85, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.Q.M.; Royall, P.G.; Kett, V.L.; Hopton, M.L. The relevance of the amorphous state to pharmaceutical dosage forms: Glassy drugs and freeze dried systems. Int. J. Pharm. 1999, 179, 179–207. [Google Scholar] [CrossRef]
- Alvarez-Nunez, F.A.; Leonard, M.R. Formulation of a poorly soluble drug using hot melt extrusion: The amorphous state as an alternative. Am. Pharm. Rev. 2004, 7, 88–92. [Google Scholar]
- Kim, J.-S.; Kim, M.-S.; Park, H.J.; Jin, S.-J.; Lee, S.; Hwang, S.-J. Physicochemical properties and oral bioavailability of amorphous atorvastatin hemi-calcium using spray-drying and SAS process. Int. J. Pharm. 2008, 359, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Aucamp, M.; Liebenberg, W.; Strydom, S.J.; van Tonder, E.C.; de Villiers, M.M. Physicochemical properties of amorphous roxithromycin prepared by quench cooling of the melt or desolvation of a chloroform solvate. AAPS PharmSciTech. 2012, 13, 467–476. [Google Scholar] [CrossRef][Green Version]
- Einfalt, T.; Planinšek, O.; Hrovat, K. Methods of amorphization and investigation of the amorphous state. Acta Pharm. 2013, 63, 305–334. [Google Scholar] [CrossRef]
- Karmwar, P.; Graeser, K.; Gordon, K.C.; Strachan, C.J.; Rades, T. Effect of different preparation methods on the dissolution behaviour of amorphous indomethacin. Eur. J. Pharm. Biopharm. 2012, 80, 459–464. [Google Scholar] [CrossRef]
- Vranić, E. Amorphous Pharmaceutical Solids. Bosn. J. Basic Med. Sci. 2004, 4, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.C.; Carlson, G.T.; Ladipo, D.D.; Langdon, B.A.; Mullarney, M.P. Comparison of the mechanical properties of the crystalline and amorphous forms of a drug substance. Int. J. Pharm. 2002, 241, 73–85. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, G.G.; Law, D.; Grant, D.J.W.; Schmitt, E.A. Physical stability of amorphous pharmaceuticals: Importance of configurational thermodynamic quantities and molecular mobility. J. Pharm. Sci. 2002, 91, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, S.; Aso, Y. Correlations between molecular mobility and chemical stability during storage of amorphous pharmaceuticals. J. Pharm. Sci. 2007, 96, 960–981. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, G.G.; Law, D.; Grant, D.J.W.; Schmitt, E.A. Thermodynamics, molecular mobility and crystallization kinetics of amorphous griseofulvin. Mol. Pharm. 2008, 5, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Shete, G.; Puri, V.; Kumar, L.; Bansal, A.K. Solid state characterization of commercial crystalline and amorphous atorvastatin calcium samples. AAPS PharmSciTech. 2010, 11, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Grčman, M.; Vrečer, F.; Meden, A. Some physico-chemical properties of doxazosin mesylate polymorphic forms and its amorphous state. J. Therm. Anal. Calorim. 2002, 68, 373–387. [Google Scholar] [CrossRef]
- Bellur, A.E.; Karlığa, B. Quantitative determination of two polymorphic forms of imatinib mesylate in a drug substance and tablet formulation by X-ray powder diffraction, differential scanning calorimetry and attenuated total reflectance Fourier transform infrared spectroscopy. J. Pharm. Biomed. Anal. 2015, 114, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Martena, V.; Censi, R.; Hoti, E.; Malaj, L.; Di Martino, P. Physicochemical characterization of nicergoline and cabergoline in its amorphous state. J. Therm. Anal. Calorim. 2012, 108, 323–332. [Google Scholar] [CrossRef]
- Chawla, G.; Bansal, A.K. Molecular Mobility and Physical Stability of Amorphous Irbesartan. Sci Pharm. 2009, 77, 695–709. [Google Scholar] [CrossRef]
- Druker, B.J.; Talpaz, M.; Resta, D.J.; Peng, B.; Buchdunger, E.; Ford, J.M.; Lydon, N.B.; Kantarjian, H.; Capdeville, R.; Ohno-Jones, S.; et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 2001, 344, 1031–1037. [Google Scholar] [CrossRef]
- Cortes, J.E.; Baccarani, M.; Guilhot, F.; Druker, B.J.; Branford, S.; Kim, D.W.; Pane, F.; Pasquini, R.; Goldberg, S.L.; Kalaycio, M.; et al. Phase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: Tyrosine kinase inhibitor optimization and selectivity study. J. Clin. Oncol. 2010, 28, 424–430. [Google Scholar] [PubMed]
- Miyamura, K.; Ohnishi, K.; Ohtake, S.; Usui, N.; Nakaseko, C.; Fujita, H.; Fujisawa, S.; Sakura, T.; Okumura, H.; Iriyama, N.; et al. Randomized study of imatinib for chronic myeloid leukemia: Comparing standard dose escalation with aggressive escalation. Blood Adv. 2019, 3, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Tolomeo, M.; Dieli, F.; Gebbia, N.; Simoni, D. Tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. Anticancer Agents Med. Chem. 2009, 9, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; von Mehren, M.; Blanke, C.D.; Van den Abbeele, A.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Ben Ami, E.; Demetri, G.D. A safety evaluation of imatinib mesylate in the treatment of gastrointestinal stromal tumor. Expert Opin. Drug Saf. 2016, 15, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Lassila, M.; Allen, T.J.; Cao, Z.; Thallas, V.; Jandeleit-Dahm, K.A.; Candido, R.M.; Cooper, E. Imatinib attenuates diabetes-associated atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Nakano, K.; Funakoshi, K.; Zhao, G.; Meng, W.; Kimura, S.; Matoba, T.; Miyagawa, M.; Iwata, E.; Sunagawa, K.; et al. Imatinib mesylate-incorporated nanoparticle-eluting stent attenuates in-stent neointimal formation in porcine coronary arteries. J. Atheroscler. Thromb. 2011, 18, 1043–1053. [Google Scholar] [CrossRef]
- Ojeda-Uribe, M.; Merieau, S.; Guillon, M.; Aujoulat, O.; Hinschberger, O.; Eisenmann, J.C.; Kenizou, D.; Debliquis, A.; Veyradier, A.; Chantrel, F. Secondary thrombotic microangiopathy in two patients with Philadelphia-positive hematological malignancies treated with imatinib mesylate. J. Oncol. Pharm. Pract. 2016, 22, 361–370. [Google Scholar] [CrossRef]
- Fukada, I.; Araki, K.; Kobayashi, K.; Shibayama, T.; Hatano, M.; Takahashi, S.; Iwase, T.; Ohno, S.; Ito, Y. Imatinib could be a new strategy for pulmonary hypertension caused by pulmonary tumor thrombotic microangiopathy in metastatic breast cancer. Springer Plus. 2016, 5, 1582–1586. [Google Scholar] [CrossRef]
- Zohlnhofer, D.; Hausleiter, J.; Kastrati, A.; Mehilli, J.; Goos, C.; Schuhlen, H.; Pache, J.; Pogatsa-Murray, G.; Heemann, U.; Dirschinger, J.; et al. A randomized, double-blind, placebo-controlled trial on restenosis prevention by the receptor tyrosine kinase inhibitor imatinib. J. Am. Coll. Cardiol. 2005, 46, 1999–2003. [Google Scholar] [CrossRef][Green Version]
- Park, Y.J.; Min, S.K.; Min, S.I.; Kim, S.J.; Ha, J. Effect of imatinib mesylate and rapamycin on the preformed intimal hyperplasia in rat carotid injury model. Ann.Surg. Treat. Res. 2015, 88, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Lemm, D.; Muegge, L.O.; Hoeffken, K.; Aklan, T.; Mentzel, T.; Thorwarth, M.; Schultze-Mosgau, S. Remission with Imatinib mesylate treatment in a patient with initially unresectable dermatofibrosarcoma protuberans—A case report. Oral. Maxillofac. Surg. 2008, 12, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Van Glabbeke, M.; Rankin, C.J.; Ruka, W.; Rubin, B.P.; Debiec-Rychter, M.; Lazar, A.; Gelderblom, H.; Sciot, R.; Lopez-Terrada, D.; et al. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: Pooled analysis of two phase II clinical trials. J. Clin. Oncol. 2010, 28, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Pedeutour, F.; Negri, T.; Conca, E.; Marrari, A.; Palassini, E.; Collini, P.; Keslair, F.; Morosi, C.; Gronchi, A.; et al. Dermatofibrosarcoma protuberans-derived fibrosarcoma: Clinical history, biological profile and sensitivity to imatinib. Int. J. Cancer. 2011, 129, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Wicherts, D.A.; Coevorden, F.; Klomp, H.M.; Huizum, M.A.; Kerst, J.M.; Haas, R.L.M.; van Boven, H.H.; van der Hage, J.A. Complete resection of recurrent and initially unresectable dermatofibrosarcoma protuberans downsized by Imatinib. World J. Surg. Oncol. 2013, 11, 59–61. [Google Scholar] [CrossRef]
- Wang, C.; Luo, Z.; Chen, J.; Zheng, B.; Zhang, R.; Chen, Y.; Shi, Y. Target therapy of unresectable or metastatic dermatofibrosarcoma protuberans with imatinib mesylate: An analysis on 22 chinese patients. Medicine 2015, 94, e773. [Google Scholar] [CrossRef] [PubMed]
- Tatai, T.; Gomi, D.; Fukushima, T.; Kobayashi, T.; Sekiguchi, N.; Sakamoto, A.; Sasaki, S.; Koizumi, T.; Sano, K. Effectiveness of Imatinib Mesylate Treatment in a Patient with Dermatofibrosarcoma Protuberans with Pulmonary and Pancreatic Metastases. Intern. Med. 2016, 55, 2507–2511. [Google Scholar] [CrossRef][Green Version]
- Khunt, M.D.; Patil, N.S.; Pagire, H.S.; Pradhan, N.S.; Valgeirsson, J. Anhydrous amorphous imatinib mesylate. U.S. Patent US20090181977, 10 January 2008. [Google Scholar]
- Zimmermann, J.; Sutter, B.; Buerger, H. Crystal modification of a N-phenyl-2-pyrimidineamine derivative, processes for its manufacture and its use. U.S. Patent US20020115858A1, 22 August 2002. [Google Scholar]
- Bandi, P.R.; Kura, R.R.; Rapolu, R.R.; Dasari, M.R.; Kesireddy, S.C.R. Novel polymorphs of imatinib mesylate. WO2004/106326A1, 9 December 2004. [Google Scholar]
- Patel, H.V.; Jani, R.J.; Thennati, R. Imatinib mesylate crystal form and process for preparation thereof. WO2004/WO2006048890A1, 11 May 2006. [Google Scholar]
- Haas, P.D.; Koc, F.; Karliga, B.; Atici, E.B.; Sivasligil, R. Polymorphs of imatinib. Eur. Patent EP2604596A1, 16 December 2011. [Google Scholar]
- Pathi, S.L.; Puppala, R.; Kankan, R.N.; Rao, D.R. Stable crystal form of imatinib mesylate and process for the preparation thereof. U.S. Patent USOO8269003B2, 18 September 2012. [Google Scholar]
- Lin, M.; Wu, Y.; Rohani, S. A kinetic study of crystallization process of imatinib mesylate with polymorphic transformation phenomenon. J. Cryst. Growth. 2019, 507, 146–153. [Google Scholar] [CrossRef]
- Grillo, D.; Polla, G.; Vega, D. Conformational polymorphism on imatinib mesylate: Grinding effects. J. Pharm. Sci. 2012, 101, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Joshi, B.D.; Tandon, P.; Ayala, A.P.; Bansal, A.K.; Grillo, D. Study of polymorphism in imatinib mesylate: A quantum chemical approach using electronic and vibrational spectra. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 103, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Bhardwajand, S.P.; Suryanarayanan, R. Molecular mobility as an effective predictor of the physical stability of amorphous trehalose. Mol. Pharmaceutics 2012, 9, 3209–3217. [Google Scholar] [CrossRef] [PubMed]
- Schammé, B.; Couvrat, N.; Malpeli, P.; Delbreilh, L.; Dupray, V.; Dargent, É.; Coquerel, G. Crystallization kinetics and molecular mobility of an amorphous active pharmaceutical ingredient: A case study with Biclotymol. Int. J. Pharm. 2015, 490, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Reszke, E.; Łojkowski, W.; Łapczyński, M.R.; Niklewicz, P.G. Microwave reactorfor chemical reactions. PL Patent 395891, 8 August 2011. [Google Scholar]
- Mucha, I.; Baranowski, P.; Owczarek, A.; Gajda, M.; Pluta, J.; Górniak, A.; Niklewicz, P.; Karolewicz, B. Thermal stability and decompositions kinetics under non-isothermalconditions of imatinib mesylate α form. J. Pharm. Biom. Anal. 2016, 129, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Guideline for Industry Q1A (R2) Stability Testing of New Drug Substances and Products, International Conference on Harmonisation. 2003. Available online: https://www.fda.gov/media/71707/download (accessed on 24 June 2019).
- Graeser, K.A.; Patterson, J.E.; Zeitler, J.A.; Gordon, K.C.; Rades, T. Correlating thermodynamic and kinetic parameters with amorphous stability. Eur. J. Pharm. Sci. 2009, 37, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Veverka, M.; Šimon, P.; Lokaj, J.; Veverková, E. Crystal habit modifications of imatinib mesylate under various precipitation condition. Monatsh. Chem. 2012, 143, 65–71. [Google Scholar]
- Chadha, R.; Kashid, N.; Jain, D.V.S. Characterization and quantification of amorphous content in same selected parenteral cephalosporins by calorimetric method. J. Therm. Anal. Calorim. 2005, 81, 277–284. [Google Scholar] [CrossRef]
- Alhalaweh, A.; Alzghoul, A.; Mahlin, D.; Bergström, C.A.S. Physical stability of drugs after storage above and below the glass transition temperature: Relationship to glass-forming ability. Int. J. Pharm. 2015, 495, 312–317. [Google Scholar] [CrossRef] [PubMed]
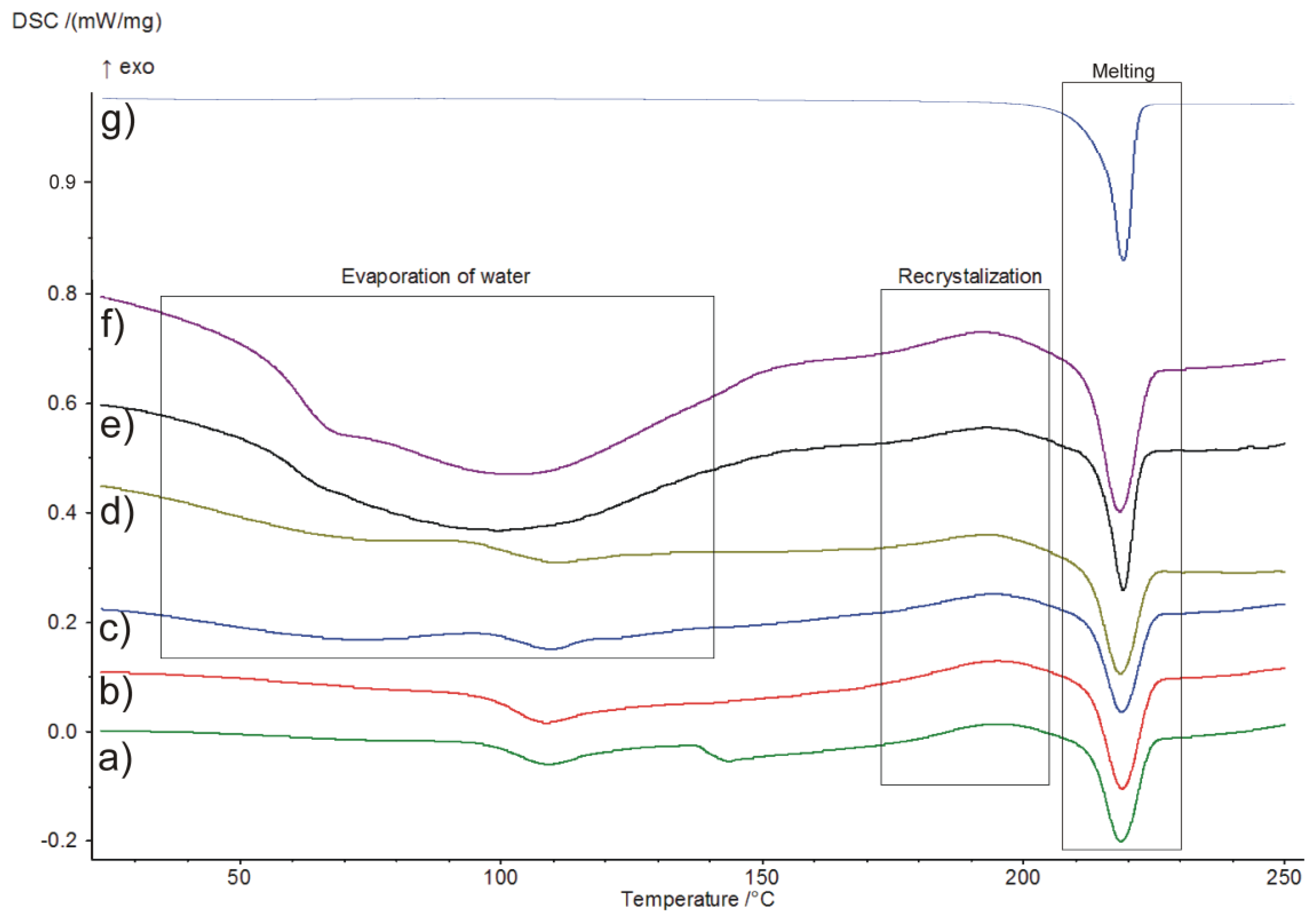


| Time | Tp [oC] | TOnset [oC] | TEndset [oC] | ΔHf [J g−1] | Tg [°C] | ΔCp [J g−1] |
|---|---|---|---|---|---|---|
| 0 day | 218.7 | 212.4 | 224.5 | 10.73 | 100.9 | 0.168 |
| 2 weeks | 218.9 | 212.3 | 224.5 | 11.42 | 101.0 | 0.238 |
| 1 month | 218.8 | 212.1 | 224.8 | 10.52 | 99.0 | 0.140 |
| 3 months | 218.5 | 212.0 | 224.4 | 11.37 | 100.0 | 0.208 |
| 6 months | 219.1 | 213.8 | 222.6 | 10.70 | 105.2 | 0.301 |
| 15 months | 218.3 | 211.9 | 224.3 | 11.76 | 106.4 | 0.302 |
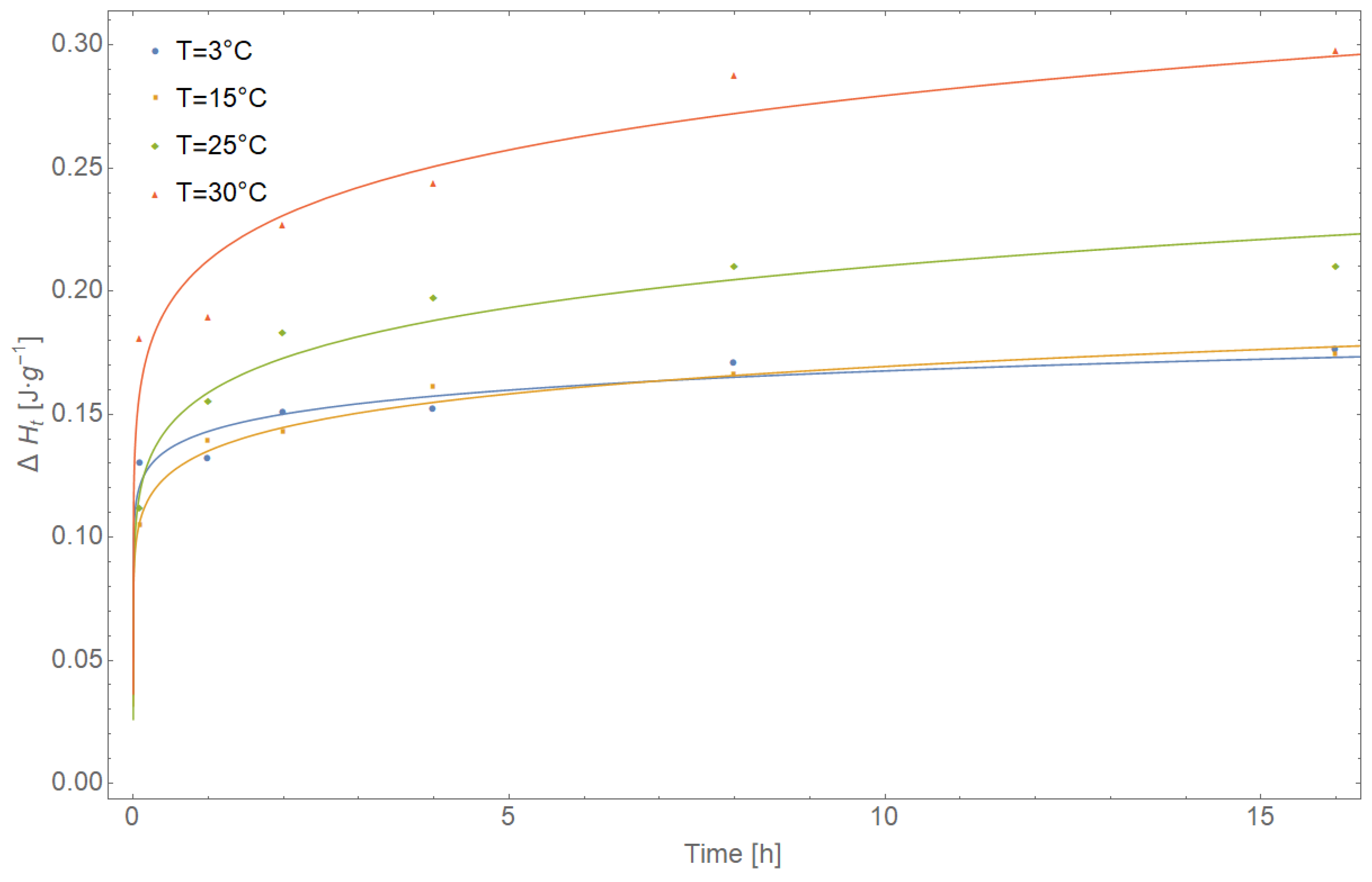
| T [°C] | Maximum Enthalpy ∆H∞ [J∙g−1] | Estimated Parameters | R-Value | t50% [h] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean molecular Relaxation Time Constant τ [s] | Relaxation Time Distribution Parameter β | ||||||||||
| Estimate τ | Standard Error SE | Confidence Interval −95% CI/+95% CI | P-Value | Estimate β | Standard Error SE | Confidence Interval −95% CI/+95% CI | P-Value | ||||
| 3 | 43.4 | 3.6 × 1039 | 2.8 × 1035 | 2.80 × 1035/ 1.72 × 1036 | 0.0161 | 0.0689 | 0.000267 | 0.0682/ 0.0697 | 1.29 × 10−9 | 0.998 | 4.92 × 1033 |
| 15 | 38.3 | 2.95 ×∙1028 | 8.62 × 1023 | 5.99 × 1024/ 1.04 × 1025 | 0.000216 | 0.098 | 0.000206 | 0.0979/ 0.099 | 1.15 × 10−10 | 0.999 | 1.98 × 1023 |
| 25 | 34.1 | 4.13 ×∙1022 | 2.04 × 1018 | 6.23 × 1018/ 1.67 × 1019 | 0.00245 | 0.122 | 0.000570 | 0.121/ 0.124 | 2.84 × 10−9 | 0.997 | 5.73 × 1017 |
| 30 | 32.0 | 6.39 ×∙1021 | 3.93 × 1017 | 7.66 × 1017/ 2.79 × 1018 | 0.006289 | 0.119 | 0.000724 | 0.117/ 0.121 | 8.17 × 10−9 | 0.997 | 8.22 × 1016 |
| T [°C] | Time [h] | Observed Value | Predicted Value | Residual | Standard Error | Confidence Interval | Residual Sum of Squares RRS | Shapiro Wilk W/p | |
|---|---|---|---|---|---|---|---|---|---|
| −95% CIi | +95% CIi | ||||||||
| 3 °C | 0.1 | 0.130 | 0.122 | 0.00791 | 0.0027 | 0.115 | 0.130 | 0.0002599 | W = 0.9426/ p = 0.6808 |
| 1.0 | 0.132 | 0.143 | −0.01145 | 0.0031 | 0.134 | 0.152 | |||
| 2.0 | 0.151 | 0.150 | 0.00056 | 0.0033 | 0.141 | 0.159 | |||
| 4.0 | 0.152 | 0.157 | −0.00527 | 0.0034 | 0.148 | 0.167 | |||
| 8.0 | 0.170 | 0.165 | 0.00535 | 0.0035 | 0.155 | 0.175 | |||
| 16.0 | 0.176 | 0.173 | 0.00309 | 0.0037 | 0.163 | 0.183 | |||
| 15 °C | 0.1 | 0.105 | 0.108 | −0.00315 | 0.0013 | 0.104 | 0.111 | 0.0000712 | W = 0.8655/ p = 0.20908 |
| 1.0 | 0.139 | 0.135 | 0.00349 | 0.0016 | 0.131 | 0.140 | |||
| 2.0 | 0.142 | 0.145 | −0.00254 | 0.0017 | 0.140 | 0.149 | |||
| 4.0 | 0.160 | 0.155 | 0.00557 | 0.0018 | 0.150 | 0.160 | |||
| 8.0 | 0.166 | 0.166 | −0.00014 | 0.0018 | 0.161 | 0.171 | |||
| 16.0 | 0.174 | 0.177 | −0.00341 | 0.0020 | 0.172 | 0.183 | |||
| 25 °C | 0.1 | 0.112 | 0.120 | −0.00821 | 0.0032 | 0.111 | 0.129 | 0.0004607 | W = 0.9089/ p = 0.42970 |
| 1.0 | 0.155 | 0.159 | −0.00383 | 0.0040 | 0.148 | 0.170 | |||
| 2.0 | 0.183 | 0.173 | 0.0101 | 0.0042 | 0.161 | 0.185 | |||
| 4.0 | 0.197 | 0.188 | 0.00890 | 0.0046 | 0.176 | 0.201 | |||
| 8.0 | 0.210 | 0.205 | 0.00531 | 0.0049 | 0.191 | 0.218 | |||
| 16.0 | 0.210 | 0.223 | −0.0131 | 0.0052 | 0.208 | 0.237 | |||
| 30 °C | 0.1 | 0.181 | 0.162 | 0.0188 | 0.0052 | 0.147 | 0.176 | 0.0012054 | W = 0.9593/ p = 0.81450 |
| 1.0 | 0.189 | 0.212 | −0.0234 | 0.0065 | 0.195 | 0.231 | |||
| 2.0 | 0.227 | 0.231 | −0.00406 | 0.0069 | 0.212 | 0.250 | |||
| 4.0 | 0.243 | 0.251 | −0.00738 | 0.0074 | 0.230 | 0.271 | |||
| 8.0 | 0.287 | 0.272 | 0.0151 | 0.0078 | 0.250 | 0.294 | |||
| 16.0 | 0.298 | 0.296 | 0.00206 | 0.0084 | 0.272 | 0.319 | |||
| Time | Tm [oC] | Rate of Mass Loss [% min−1] | TOnset [oC] | TEndset [oC] | Res [%] | T0.5 wt.% [oC] | T1.0 wt.% [oC] | Moisture Content [%] |
|---|---|---|---|---|---|---|---|---|
| 0 day | 373.9 | 5.72 | 326.2 | 419.6 | 39.71 | 271.5 | 287.6 | - |
| 2 weeks | 373.6 | 5.44 | 327.7 | 419.4 | 42.37 | 269.3 | 287.2 | - |
| 1 month | 379.7 | 5.36 | 327.8 | 419.3 | 42.48 | 275.5 | 291.5 | 1.16 |
| 3 months | 373.9 | 5.58 | 326.3 | 419.8 | 38.92 | 275.7 | 289.5 | 2.51 |
| 6 months | 365.7 | 5.05 | 322.6 | 411.9 | 43.75 | 274.6 | 280.3 | 2.54 |
| 15 months | 362.6 | 5.35 | 319.9 | 410.4 | 41.55 | 278.4 | 288.1 | 3.95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karolewicz, B.; Górniak, A.; Marciniak, D.M.; Mucha, I. Molecular Mobility and Stability Studies of Amorphous Imatinib Mesylate. Pharmaceutics 2019, 11, 304. https://doi.org/10.3390/pharmaceutics11070304
Karolewicz B, Górniak A, Marciniak DM, Mucha I. Molecular Mobility and Stability Studies of Amorphous Imatinib Mesylate. Pharmaceutics. 2019; 11(7):304. https://doi.org/10.3390/pharmaceutics11070304
Chicago/Turabian StyleKarolewicz, Bożena, Agata Górniak, Dominik M. Marciniak, and Igor Mucha. 2019. "Molecular Mobility and Stability Studies of Amorphous Imatinib Mesylate" Pharmaceutics 11, no. 7: 304. https://doi.org/10.3390/pharmaceutics11070304
APA StyleKarolewicz, B., Górniak, A., Marciniak, D. M., & Mucha, I. (2019). Molecular Mobility and Stability Studies of Amorphous Imatinib Mesylate. Pharmaceutics, 11(7), 304. https://doi.org/10.3390/pharmaceutics11070304





