Hyaluronic Acid-Coated Nanomedicine for Targeted Cancer Therapy
Abstract
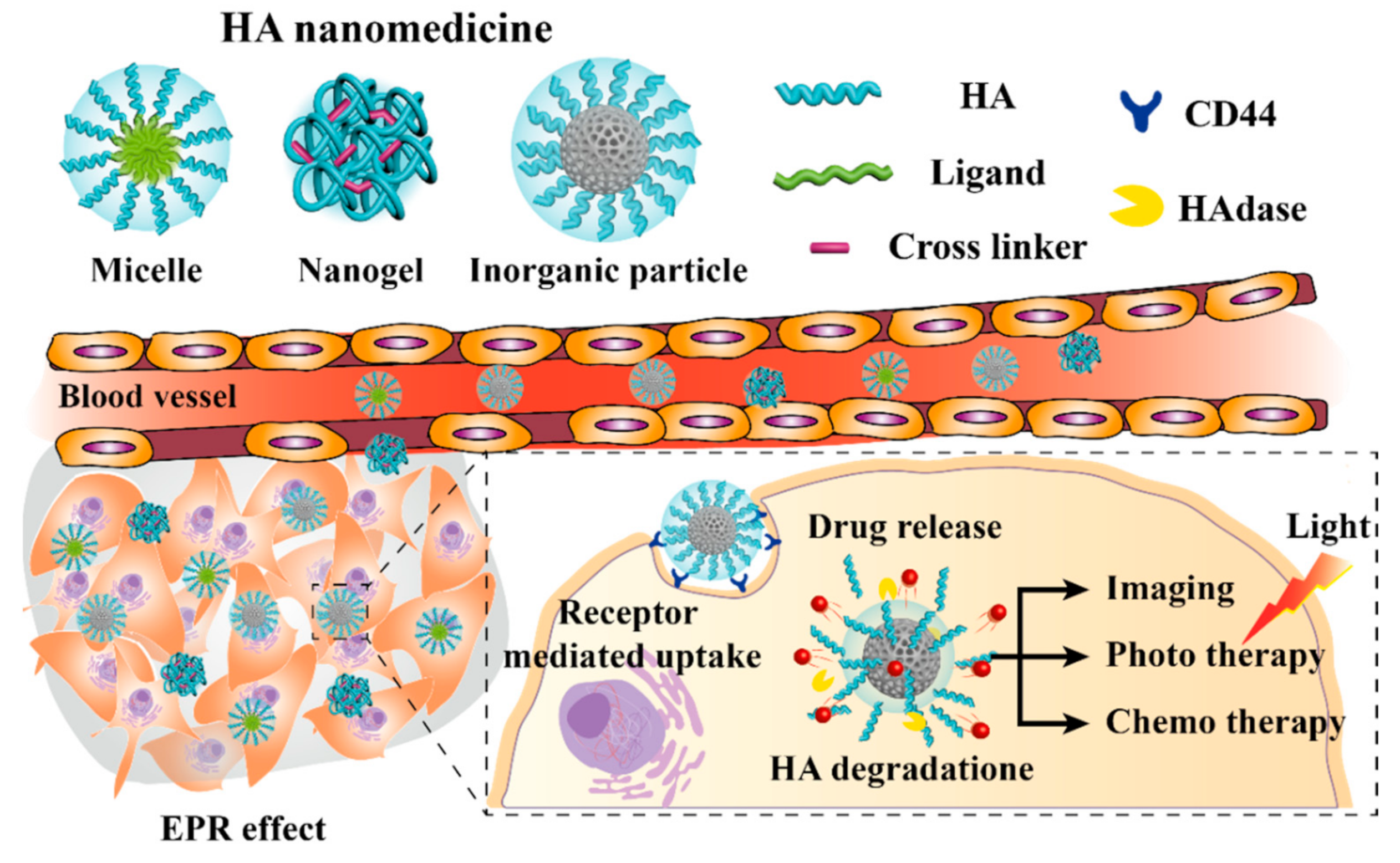
1. Introduction
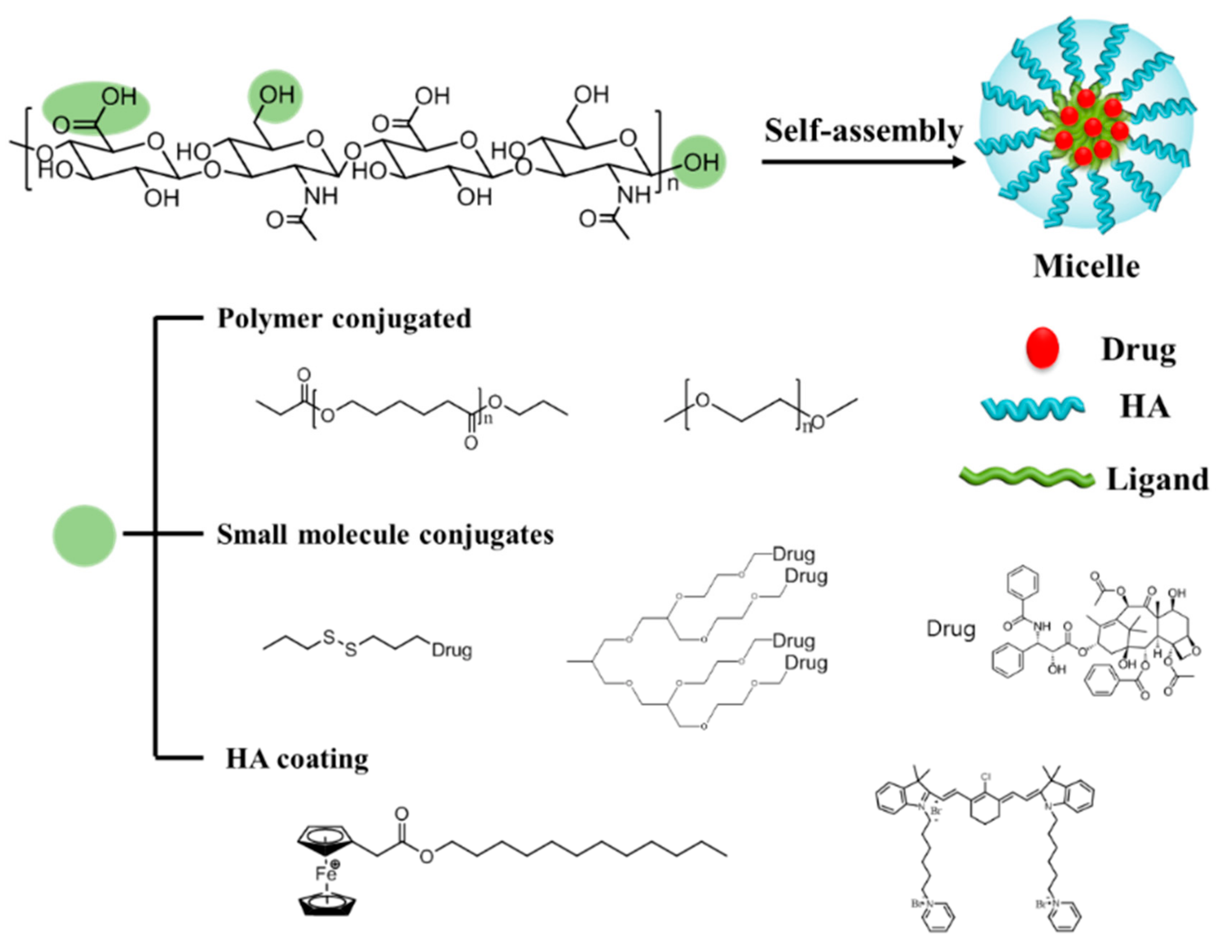
2. HA Micelle
2.1. HA Polymeric Micelles (PMs)
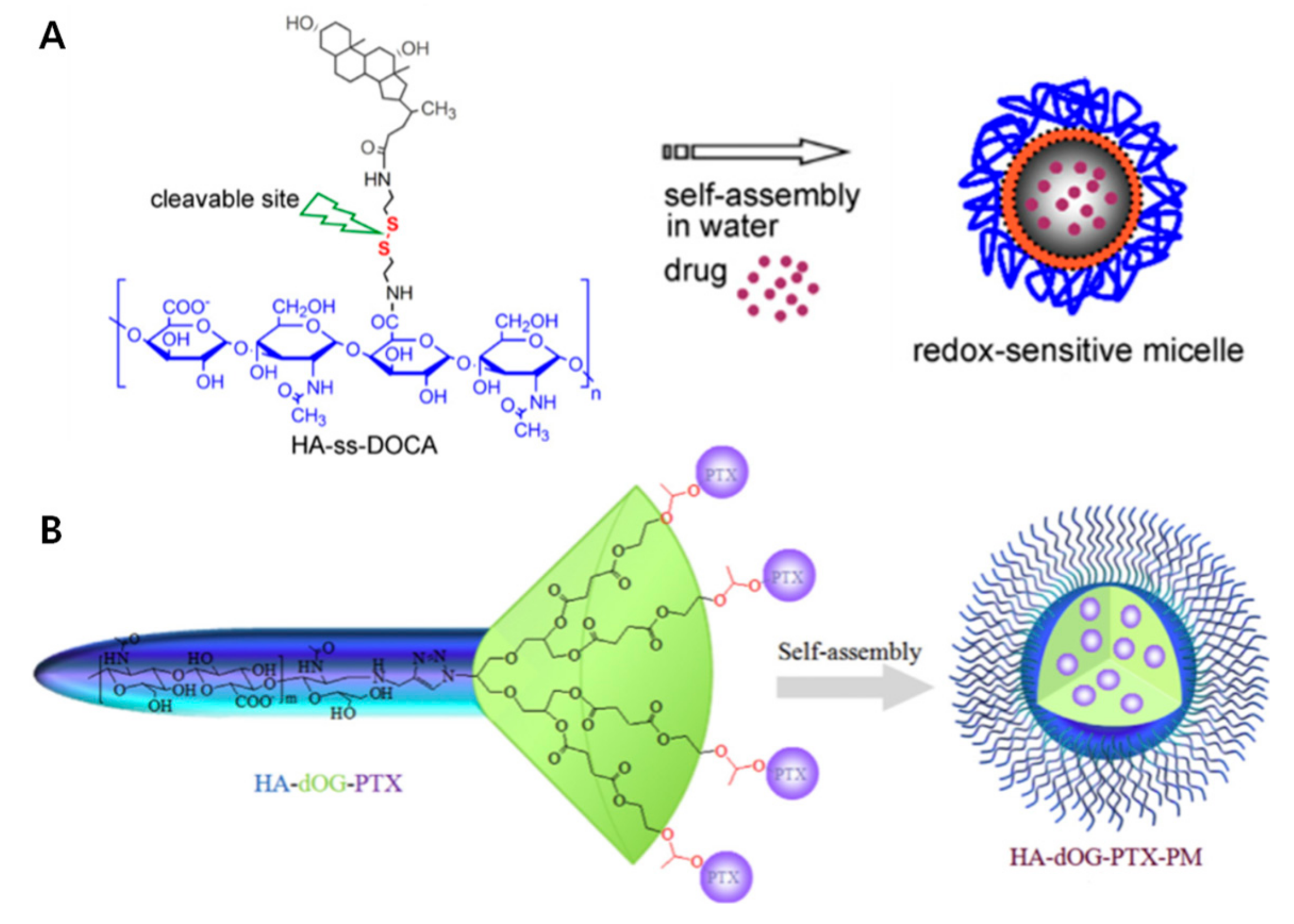
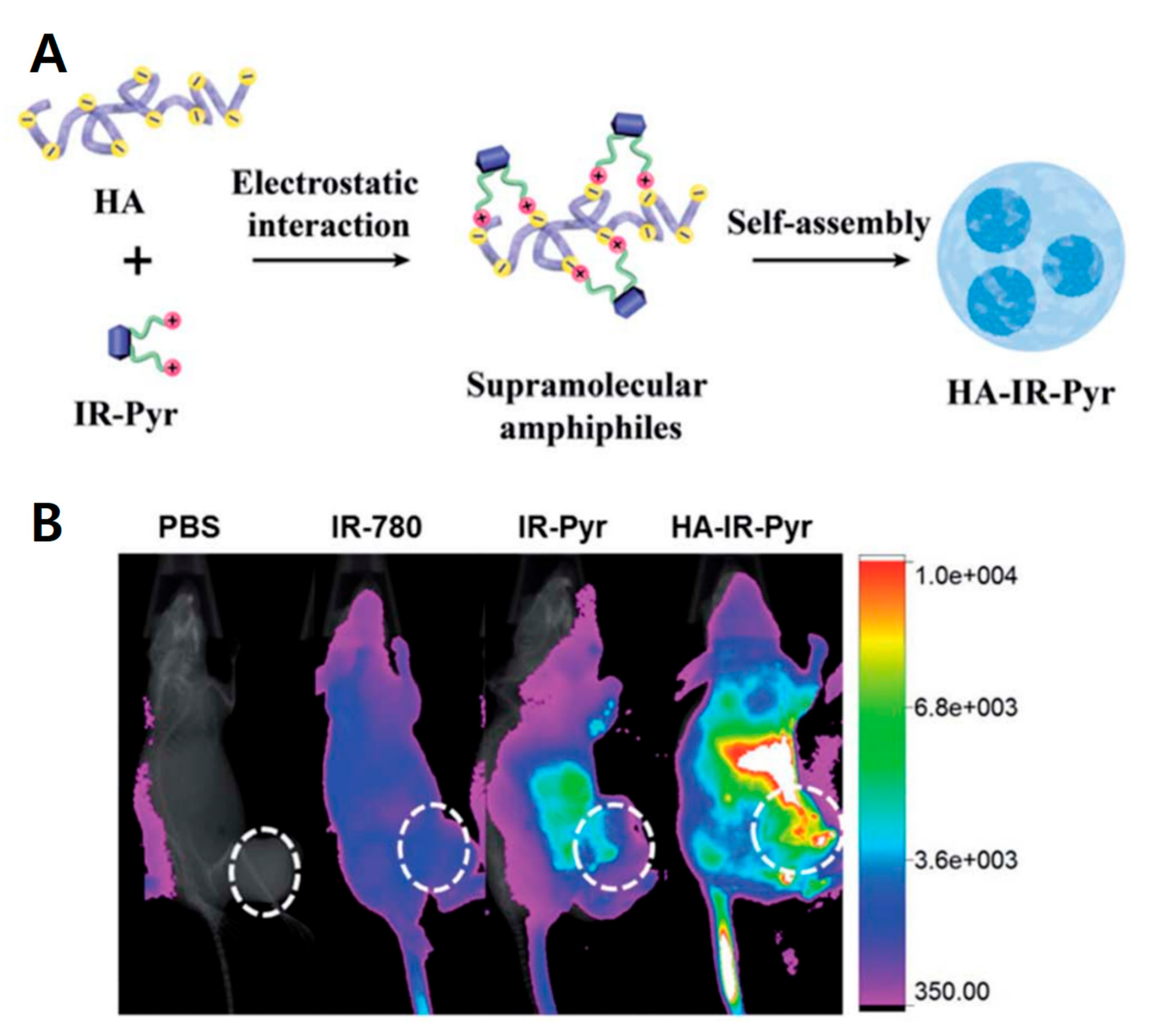
2.2. Small Molecules Conjugated to HA Micelles
2.3. HA Coating Micelle
2.4. Conclusion of HA Micelles
3. HA Nanogels
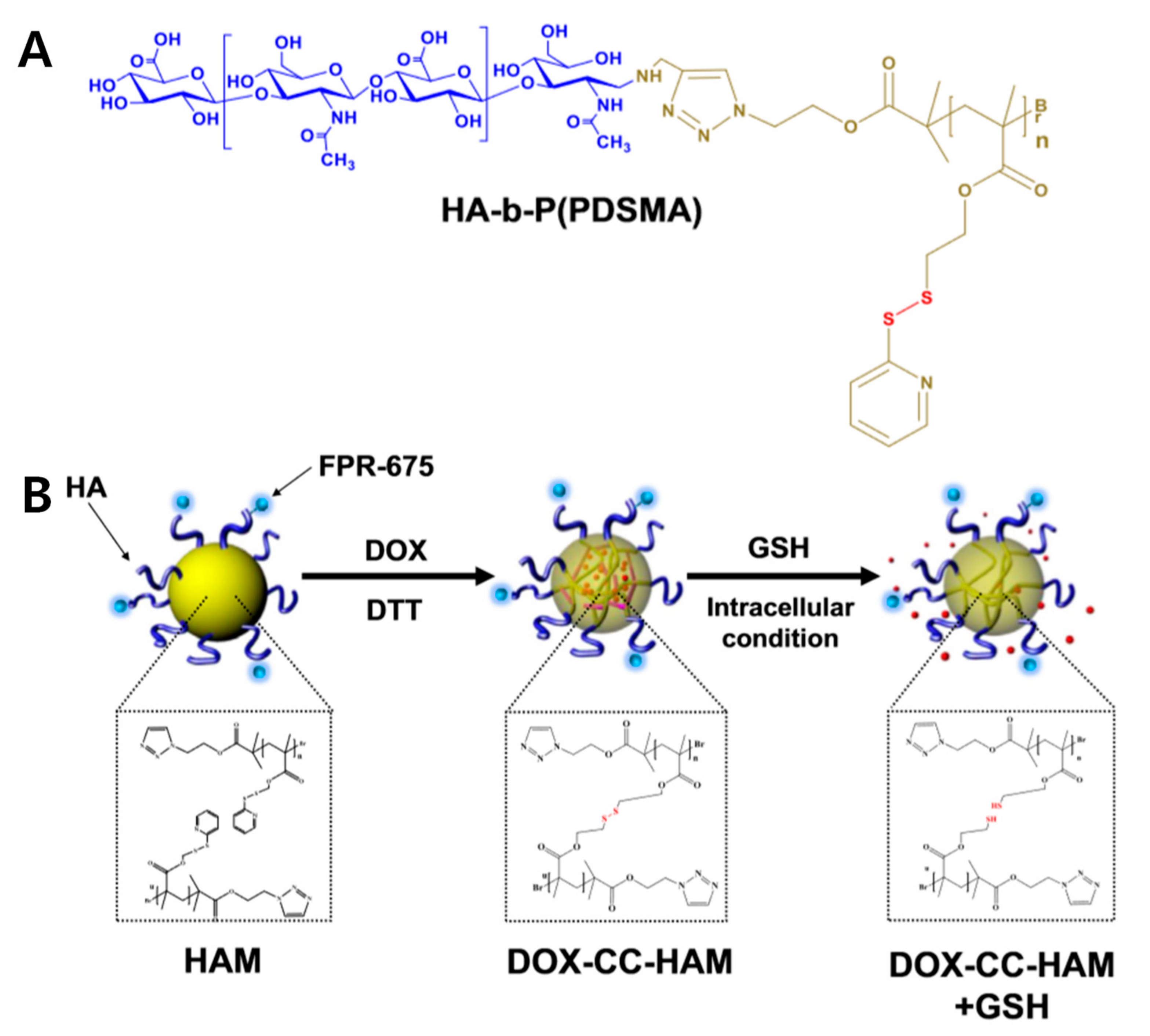
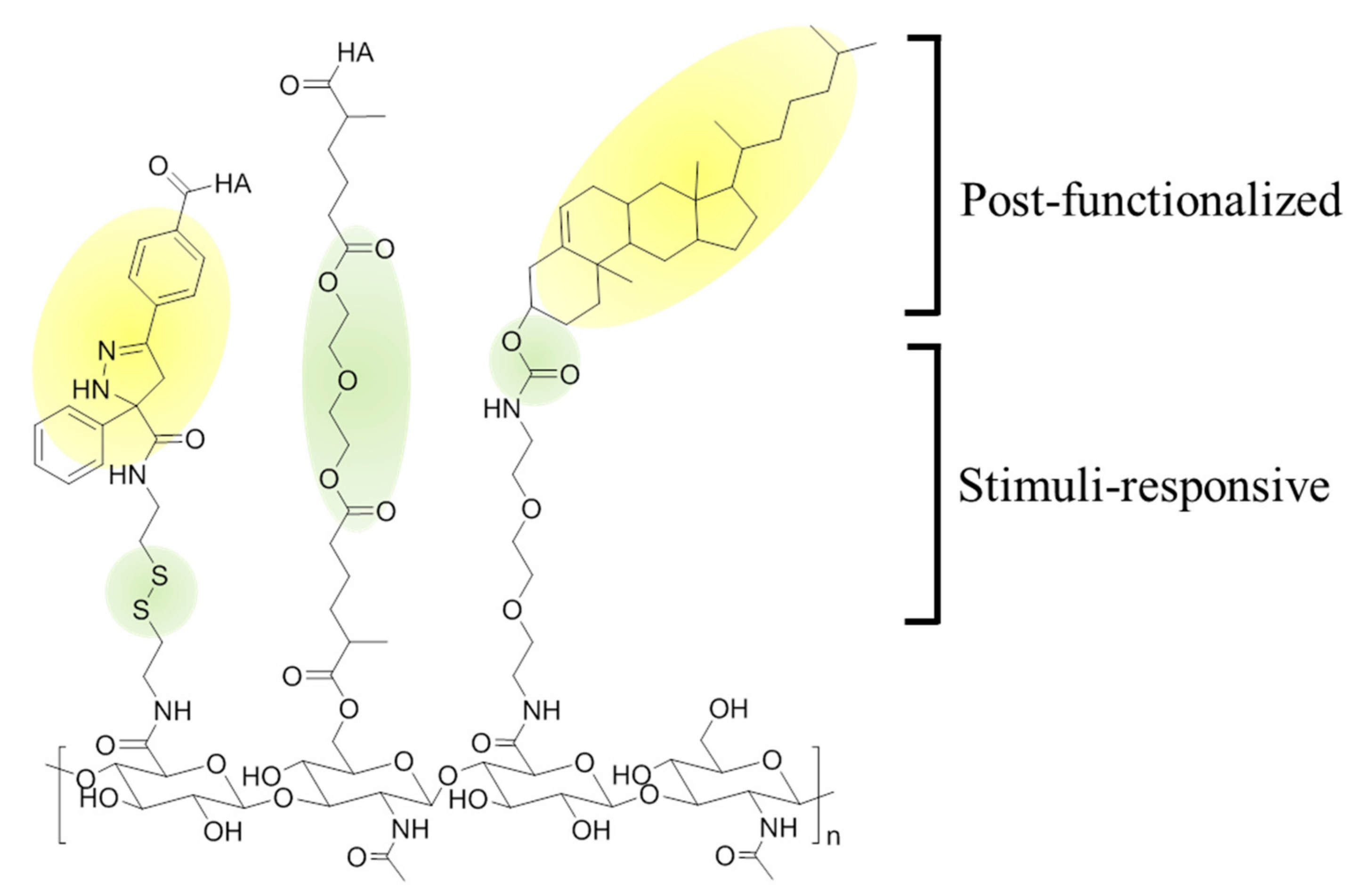
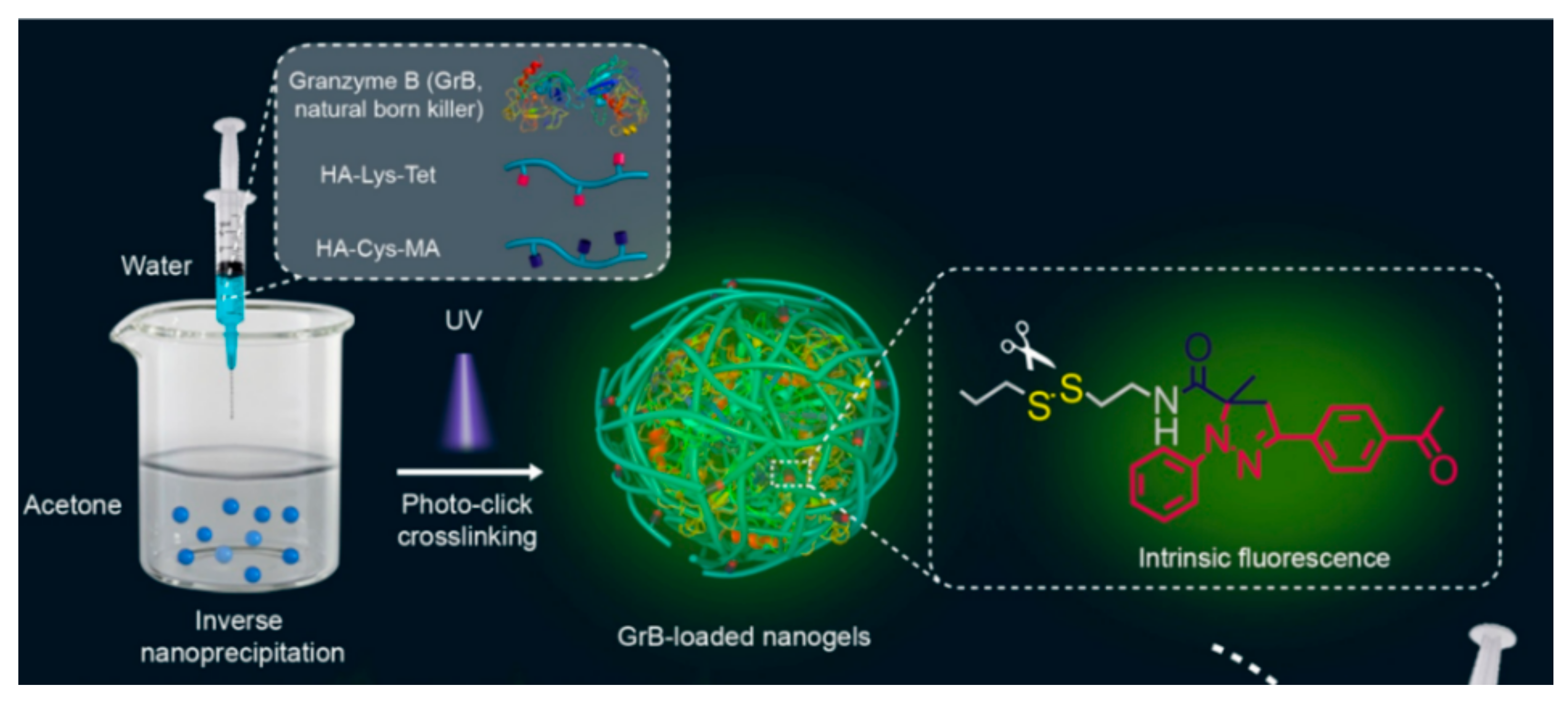
3.1. Methacrylate-Modified HA Nanogels
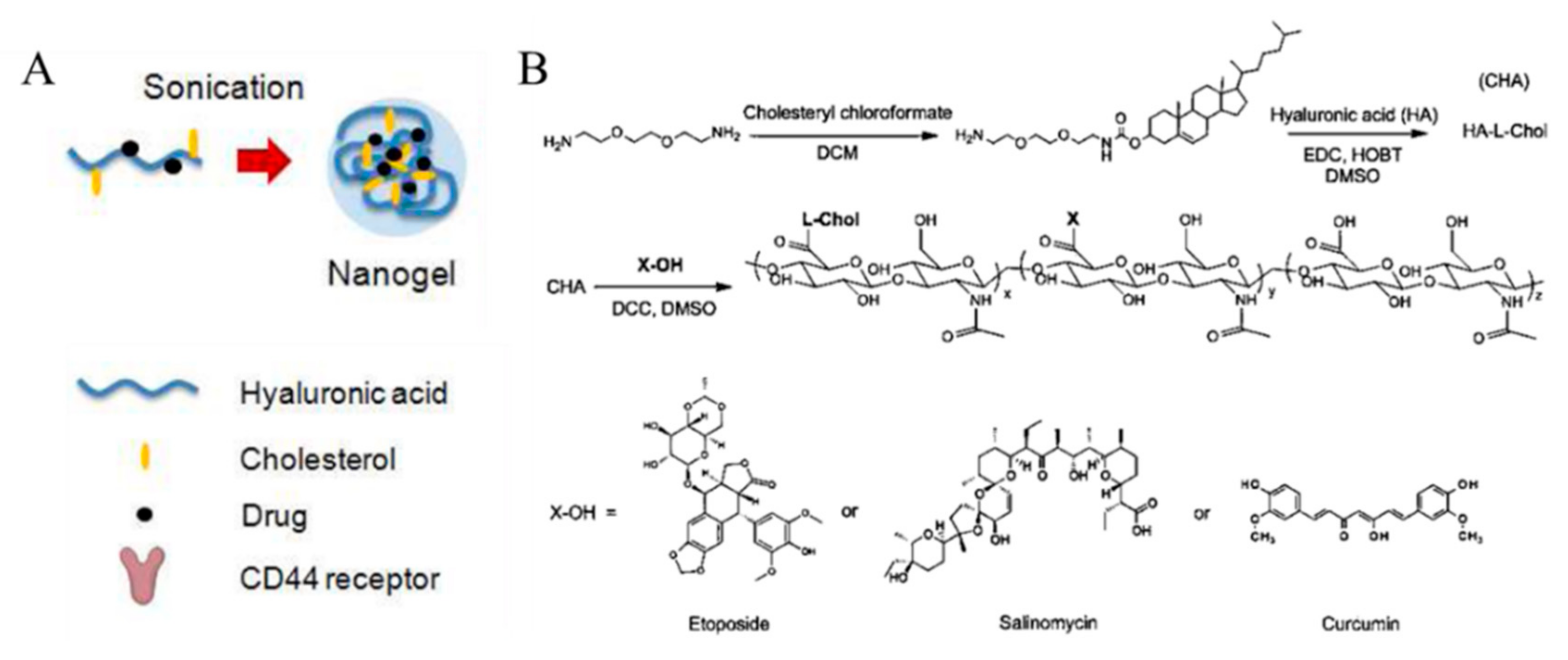
3.2. Cholesterol-HA Nanogels
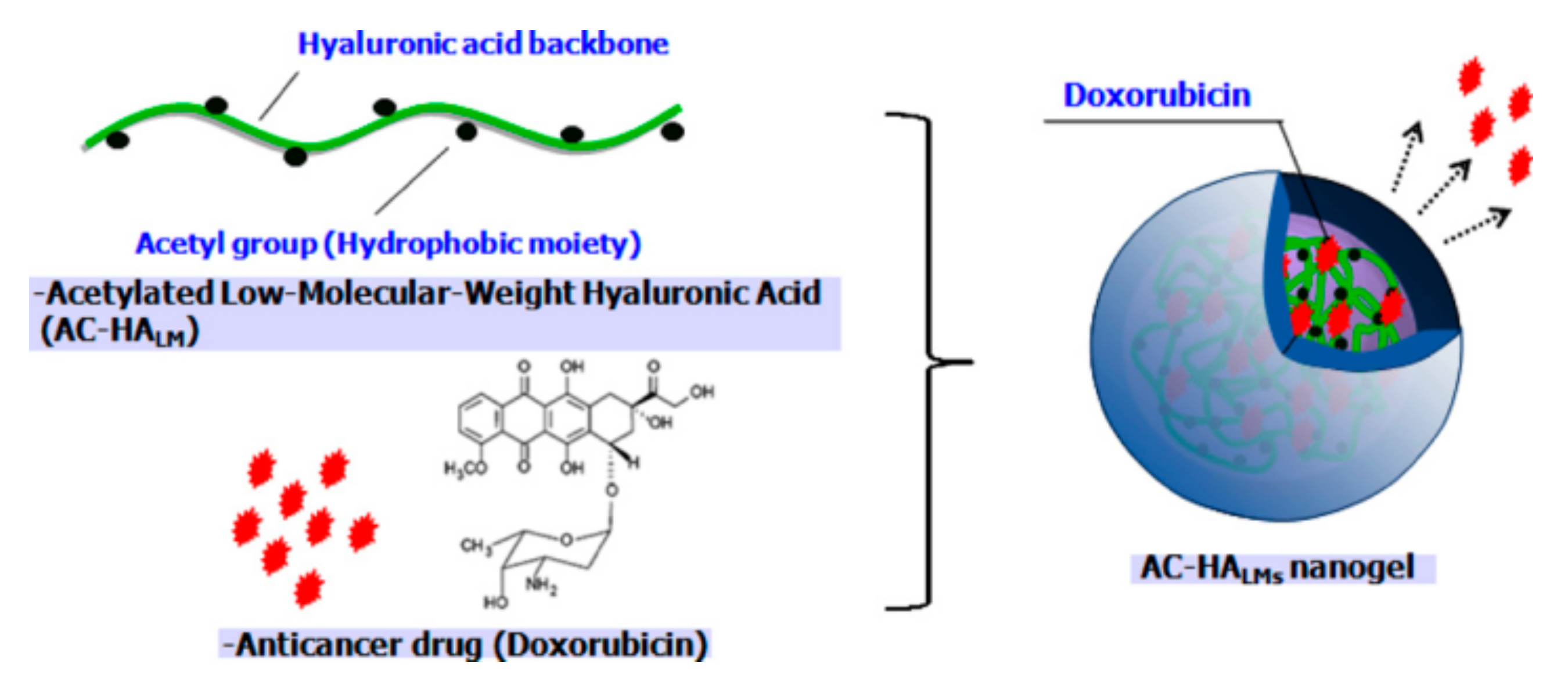
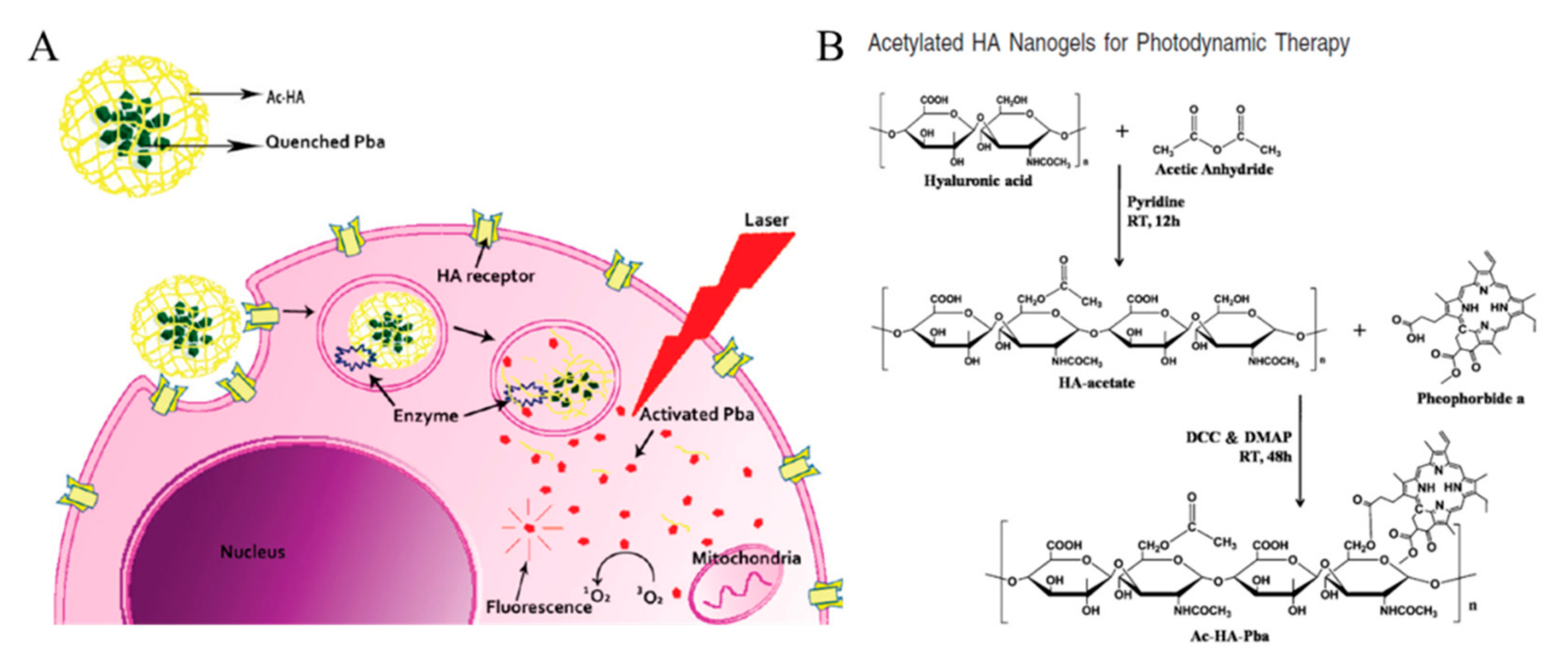
3.3. Acetylated HA Nanogels
3.4. Conclusion of HA Nanogels
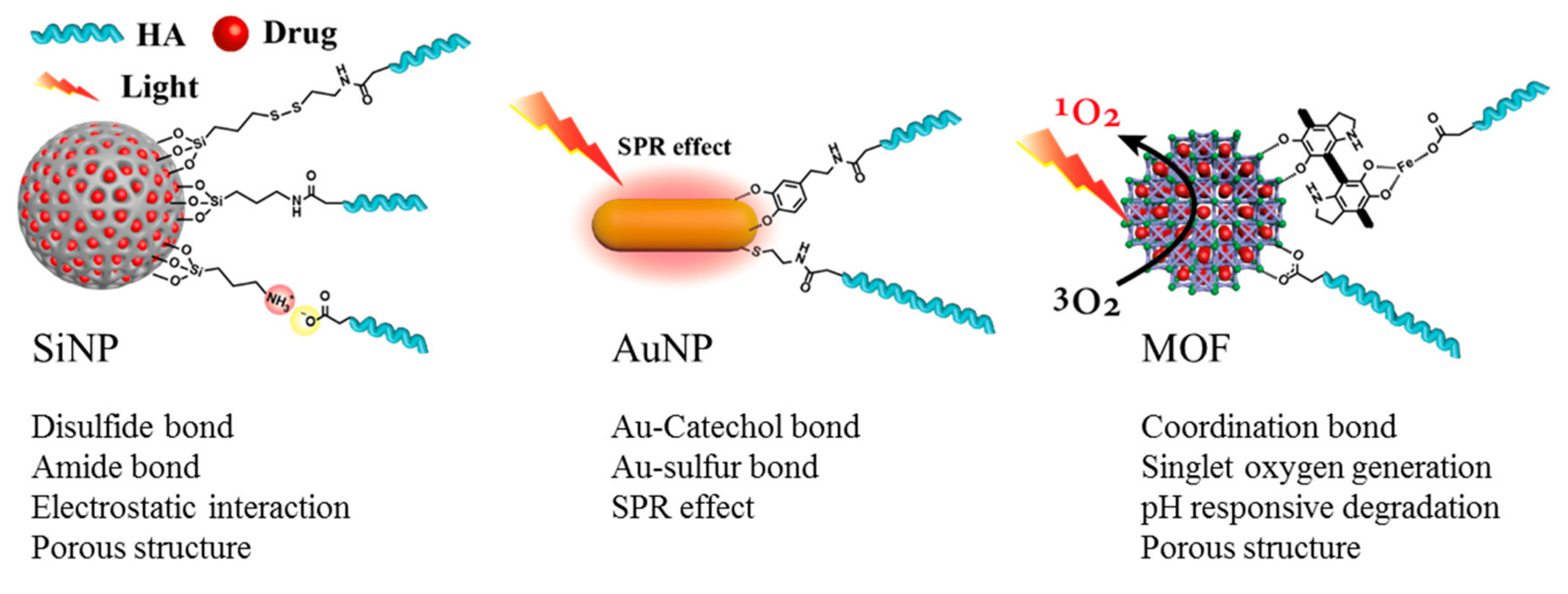
4. HA Inorganic Nanomedicines
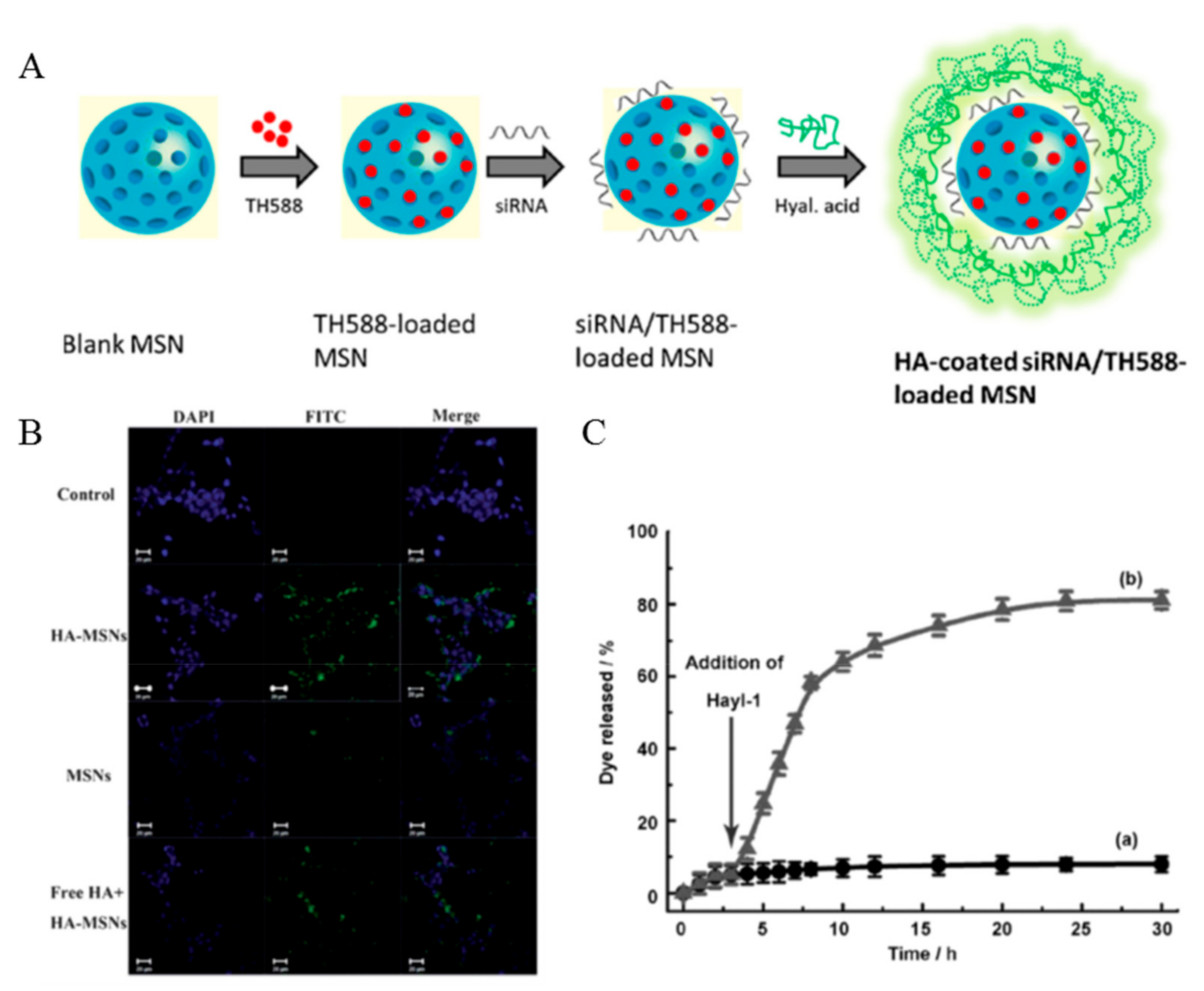
4.1. SiNPs
4.2. AuNPs
4.3. MOF Nanoparticle
4.4. Conclusion of HA Inorganic Nanomedicines
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Wickens, J.M.; Alsaab, H.O.; Kesharwani, P.; Bhise, K.; Amin, M.; Tekade, R.K.; Gupta, U.; Iyer, A.K. Recent advances in hyaluronic acid-decorated nanocarriers for targeted cancer therapy. Drug Discov. Today 2017, 22, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.; Laurent, T.; Laurent, U. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Park, K.; Kim, K.S.; Kim, J.; Yang, J.A.; Kong, J.H.; Lee, M.Y.; Hoffman, A.S.; Hahn, S.K. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J. Control. Release 2010, 141, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Chung, H.; Min, K.H.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Jeong, S.Y. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials 2010, 31, 106–114. [Google Scholar] [CrossRef]
- Huang, G.; Chen, J. Preparation and applications of hyaluronic acid and its derivatives. Int. J. Biol. Macromol. 2019, 125, 478–484. [Google Scholar] [CrossRef]
- Li, J.; Huo, M.; Wang, J.; Zhou, J.; Mohammad, J.M.; Zhang, Y.; Zhu, Q.; Waddad, A.Y.; Zhang, Q. Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials 2012, 33, 2310–2320. [Google Scholar] [CrossRef]
- Jin, Y.-J.; Ubonvan, T.; Kim, D.-D. Hyaluronic acid in drug delivery systems. J. Pharm. Investig. 2010, 40, 33–43. [Google Scholar] [CrossRef]
- Xiao, K.; Li, Y.; Luo, J.; Lee, J.S.; Xiao, W.; Gonik, A.M.; Agarwal, R.G.; Lam, K.S. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials 2011, 32, 3435–3446. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhu, F.; Wang, Q.; Liang, F.; Qu, X.; Gan, Z.; Yang, Z. Combinatorial targeting polymeric micelles for anti-tumor drug delivery. J. Mater. Chem. B 2015, 3, 4043–4051. [Google Scholar] [CrossRef]
- Ayre, A.; Kadam, V.; Dand, N.; Patel, P. Polymeric micelles as a drug carrier for tumor targeting. Chron. Young Sci. 2013, 4, 94. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, W.; Duan, X.; Zhu, L.; Fan, L.; Qiao, Y.; Wu, H. Preparation of two types of polymeric micelles based on poly(beta-L-malic acid) for antitumor drug delivery. PLoS ONE 2016, 11, e0162607. [Google Scholar]
- Chen, Z.G. Small-molecule delivery by nanoparticles for anticancer therapy. Trends Mol. Med. 2010, 16, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, S.; Kim, J.-H.; Park, K.; Kim, K.; Kwon, I.C. Polymeric nanomedicine for cancer therapy. Prog. Polym. Sci. 2008, 33, 113–137. [Google Scholar] [CrossRef]
- Varela-Moreira, A.; Shi, Y.; Fens, M.H.A.M.; Lammers, T.; Hennink, W.E.; Schiffelers, R.M. Clinical application of polymeric micelles for the treatment of cancer. Mater. Chem. Front. 2017, 1, 1485–1501. [Google Scholar] [CrossRef]
- Zhou, X.X.; Jin, L.; Qi, R.Q.; Ma, T. pH-responsive polymeric micelles self-assembled from amphiphilic copolymer modified with lipid used as doxorubicin delivery carriers. R. Soc. Open Sci. 2018, 5, 171654. [Google Scholar] [CrossRef]
- Shin, D.H.; Tam, Y.T.; Kwon, G.S. Polymeric micelle nanocarriers in cancer research. Front. Chem. Sci. Eng. 2016, 10, 348–359. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Li, S. Polymeric micelles: Nanocarriers for cancer-targeted drug delivery. AAPS Pharmscitech 2014, 15, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ahn, C.H.; Park, T.G. Poly[lactic-co-(glycolic acid)]-grafted hyaluronic acid copolymer micelle nanoparticles for target-specific delivery of doxorubicin. Macromol. Biosci. 2009, 9, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Vasi, A.M.; Popa, M.I.; Butnaru, M.; Dodi, G.; Verestiuc, L. Chemical functionalization of hyaluronic acid for drug delivery applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Wang, L.; Wang, X.; Tu, P. Comparison of hyaluronic acid-based micelles and polyethylene glycol-based micelles on reversal of multidrug resistance and enhanced anticancer efficacy in vitro and in vivo. Drug Deliv. 2018, 25, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-C.; Chen, J.-H.; Chen, Y.-H.; Teng, T.-m.; Su, C.-H.; Hsu, S.-h. Biodegradable micelles from a hyaluronan-poly(ε-caprolactone) graft copolymer as nanocarriers for fibroblast growth factor 1. J. Mater. Chem. B 2013, 1, 5977. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y.; Ko, H.; Jeon, J.; Saravanakumar, G.; Suh, Y.D.; Lee, D.S.; Park, J.H. Bioreducible core-crosslinked hyaluronic acid micelle for targeted cancer therapy. J. Control. Release 2015, 200, 158–166. [Google Scholar] [CrossRef]
- Han, H.S.; Thambi, T.; Choi, K.Y.; Son, S.; Ko, H.; Lee, M.C.; Jo, D.G.; Chae, Y.S.; Kang, Y.M.; Lee, J.Y.; et al. Bioreducible shell-cross-linked hyaluronic acid nanoparticles for tumor-targeted drug delivery. Biomacromolecules 2015, 16, 447–456. [Google Scholar] [CrossRef]
- Choi, K.Y.; Yoon, H.Y.; Kim, J.-H.; Bae, S.M.; Park, R.-W.; Kang, Y.M.; Kim, I.-S.; Kwon, I.C.; Choi, K.; Jeong, S.Y. Smart nanocarrier based on PEGylated hyaluronic acid for cancer therapy. ACS Nano 2011, 5, 8591–8599. [Google Scholar] [CrossRef]
- Zhong, L.; Xu, L.; Liu, Y.; Li, Q.; Zhao, D.; Li, Z.; Zhang, H.; Zhang, H.; Kan, Q.; Wang, Y.; et al. Transformative hyaluronic acid-based active targeting supramolecular nanoplatform improves long circulation and enhances cellular uptake in cancer therapy. Acta Pharm. Sin. B 2019, 9, 397–409. [Google Scholar] [CrossRef]
- Alves, C.G.; de Melo-Diogo, D.; Lima-Sousa, R.; Costa, E.C.; Correia, I.J. Hyaluronic acid functionalized nanoparticles loaded with IR780 and DOX for cancer chemo-photothermal therapy. Eur. J. Pharm. Biopharm. 2019, 137, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 2041731417726464. [Google Scholar] [CrossRef] [PubMed]
- Saadat, E.; Amini, M.; Khoshayand, M.R.; Dinarvand, R.; Dorkoosh, F.A. Synthesis and optimization of a novel polymeric micelle based on hyaluronic acid and phospholipids for delivery of paclitaxel, in vitro and in-vivo evaluation. Int. J. Pharm. 2014, 475, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Bongiovi, F.; Di Prima, G.; Palumbo, F.S.; Licciardi, M.; Pitarresi, G.; Giammona, G. Hyaluronic acid-based micelles as ocular platform to modulate the loading, release, and corneal permeation of corticosteroids. Macromol. Biosci. 2017, 17, 1700261. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Seymour, L.W.; Miyamoto, Y. Conjugates of anticancer agents and polymers: Advantages of macromolecular therapeutics in vivo. Bioconj. Chem. 1992, 3, 351–362. [Google Scholar] [CrossRef]
- Yin, T.; Wang, J.; Yin, L.; Shen, L.; Zhou, J.; Huo, M. Redox-sensitive hyaluronic acid–paclitaxel conjugate micelles with high physical drug loading for efficient tumor therapy. Polym. Chem. 2015, 6, 8047–8059. [Google Scholar] [CrossRef]
- Zhong, Y.; Goltsche, K.; Cheng, L.; Xie, F.; Meng, F.; Deng, C.; Zhong, Z.; Haag, R. Hyaluronic acid-shelled acid-activatable paclitaxel prodrug micelles effectively target and treat CD44-overexpressing human breast tumor xenografts in vivo. Biomaterials 2016, 84, 250–261. [Google Scholar] [CrossRef]
- Ohya, Y.; Takeda, S.; Shibata, Y.; Ouchi, T.; Kano, A.; Iwata, T.; Mochizuki, S.; Taniwaki, Y.; Maruyama, A. Evaluation of polyanion-coated biodegradable polymeric micelles as drug delivery vehicles. J. Control. Release 2011, 155, 104–110. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Park, T.G. Hyaluronic acid− paclitaxel conjugate micelles: Synthesis, characterization, and antitumor activity. Bioconj. Chem. 2008, 19, 1319–1325. [Google Scholar] [CrossRef]
- Thomas, A.P.; Palanikumar, L.; Jeena, M.T.; Kim, K.; Ryu, J.H. Cancer-mitochondria-targeted photodynamic therapy with supramolecular assembly of HA and a water soluble NIR cyanine dye. Chem. Sci. 2017, 8, 8351–8356. [Google Scholar] [CrossRef]
- Choi, H.; Jeena, M.T.; Palanikumar, L.; Jeong, Y.; Park, S.; Lee, E.; Ryu, J.H. The HA-incorporated nanostructure of a peptide-drug amphiphile for targeted anticancer drug delivery. Chem. Commun. 2016, 52, 5637–5640. [Google Scholar] [CrossRef]
- Mao, H.L.; Qian, F.; Li, S.; Shen, J.W.; Ye, C.K.; Hua, L.; Zhang, L.Z.; Wu, D.M.; Lu, J.; Yu, R.T.; et al. Delivery of doxorubicin from hyaluronic acid-modified glutathione-responsive ferrocene micelles for combination cancer therapy. Mol. Pharm. 2019, 16, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, K.; Lan, L.; Ma, J.; Zeng, Y.; Xu, L.; Wu, D. Double-layered hyaluronic acid/stearic acid-modified polyethyleneimine nanoparticles encapsulating (-)-gossypol: A nanocarrier for chiral anticancer drugs. J. Mater. Chem. B 2014, 2, 5238–5248. [Google Scholar] [CrossRef]
- Li, S.; Cai, Y.; Cao, J.; Cai, M.; Chen, Y.; Luo, X. Phosphorylcholine micelles decorated by hyaluronic acid for enhancing antitumor efficiency. Polym. Chem. 2017, 8, 2472–2483. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New progress and prospects: The application of nanogel in drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Mok, H.; Lee, S.; Oh, Y.K.; Park, T.G. Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. J. Control. Release 2007, 119, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.; Goncalves, C.; Costa, C.; Sousa, J.; Silva-Gomes, R.; Castro, A.G.; Pedrosa, J.; Appelberg, R.; Gama, F.M. Delivery of LLKKK18 loaded into self-assembling hyaluronic acid nanogel for tuberculosis treatment. J. Control. Release 2016, 235, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Khatun, Z.; Nurunnabi, M.; Nafiujjaman, M.; Reeck, G.R.; Khan, H.A.; Cho, K.J.; Lee, Y.K. A hyaluronic acid nanogel for photo-chemo theranostics of lung cancer with simultaneous light-responsive controlled release of doxorubicin. Nanoscale 2015, 7, 10680–10689. [Google Scholar] [CrossRef]
- Chen, J.; Zou, Y.; Deng, C.; Meng, F.; Zhang, J.; Zhong, Z. Multifunctional click hyaluronic acid nanogels for targeted protein delivery and effective cancer treatment in vivo. Chem. Mater. 2016, 28, 8792–8799. [Google Scholar] [CrossRef]
- Chen, J.; Ouyang, J.; Chen, Q.; Deng, C.; Meng, F.; Zhang, J.; Cheng, R.; Lan, Q.; Zhong, Z. EGFR and CD44 dual-targeted multifunctional hyaluronic acid nanogels boost protein delivery to ovarian and breast cancers in vitro and in vivo. ACS Appl. Mater. Interfaces 2017, 9, 24140–24147. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Yao, X.; Zhang, Y.; Wu, W.; Jiang, X. Hyaluronic acid nanogels with enzyme-sensitive cross-linking group for drug delivery. J. Control. Release 2015, 205, 206–217. [Google Scholar] [CrossRef]
- Yang, C.; Li, C.; Zhang, P.; Wu, W.; Jiang, X. Redox responsive hyaluronic acid nanogels for treating RHAMM (CD168) over-expressive cancer, both primary and metastatic tumors. Theranostics 2017, 7, 1719–1734. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Fu, S.; Yao, W.; Wang, X.; Zha, Q.; Tang, R. Hyaluronic acid nanogels prepared via ortho ester linkages show pH-triggered behavior, enhanced penetration and antitumor efficacy in 3-D tumor spheroids. J. Coll. Interface Sci. 2017, 504, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Yeagle, P.L. Cholesterol and the cell membrane. Biochim. Biophys. Acta (BBA) Rev. Biomembr. 1985, 822, 267–287. [Google Scholar] [CrossRef]
- Wei, X.; Senanayake, T.H.; Warren, G.; Vinogradov, S.V. Hyaluronic acid-based nanogel-drug conjugates with enhanced anticancer activity designed for the targeting of CD44-positive and drug-resistant tumors. Bioconj. Chem. 2013, 24, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Senanayake, T.H.; Bohling, A.; Vinogradov, S.V. Targeted nanogel conjugate for improved stability and cellular permeability of curcumin: Synthesis, pharmacokinetics, and tumor growth inhibition. Mol. Pharm. 2014, 11, 3112–3122. [Google Scholar] [CrossRef] [PubMed]
- Nakai, T.; Hirakura, T.; Sakurai, Y.; Shimoboji, T.; Ishigai, M.; Akiyoshi, K. Injectable hydrogel for sustained protein release by salt-induced association of hyaluronic acid nanogel. Macromol. Biosci. 2012, 12, 475–483. [Google Scholar] [CrossRef]
- Park, W.; Kim, K.S.; Bae, B.C.; Kim, Y.H.; Na, K. Cancer cell specific targeting of nanogels from acetylated hyaluronic acid with low molecular weight. Eur. J. Pharm. Sci. 2010, 40, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Bae, B.-c.; Na, K. Acetylated hyaluronic acid/photosensitizer conjugate for the preparation of nanogels with controllable phototoxicity: Synthesis, characterization, autophotoquenching properties, and in vitro phototoxicity against HeLa cells. Bioconj. Chem. 2010, 21, 1312–1320. [Google Scholar] [CrossRef]
- Lee, C.S.; Na, K. Photochemically triggered cytosolic drug delivery using pH-responsive hyaluronic acid nanoparticles for light-induced cancer therapy. Biomacromolecules 2014, 15, 4228–4238. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Shi, J. Inorganic nanoparticle-based drug codelivery nanosystems to overcome the multidrug resistance of cancer cells. Mol. Pharm. 2014, 11, 2495–2510. [Google Scholar] [CrossRef]
- Huang, H.C.; Barua, S.; Sharma, G.; Dey, S.K.; Rege, K. Inorganic nanoparticles for cancer imaging and therapy. J. Control. Release 2011, 155, 344–357. [Google Scholar] [CrossRef]
- Sekhon, B.S.; Kamboj, S.R. Inorganic nanomedicine—Part 2. Nanomedicine 2010, 6, 612–618. [Google Scholar] [CrossRef]
- Yu, M.; Jambhrunkar, S.; Thorn, P.; Chen, J.; Gu, W.; Yu, C. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale 2013, 5, 178–183. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Lin, Y.; Yin, M.; Ren, J.; Qu, X. Bioresponsive hyaluronic acid-capped mesoporous silica nanoparticles for targeted drug delivery. Chemistry 2013, 19, 1778–1783. [Google Scholar] [CrossRef]
- Gary-Bobo, M.; Brevet, D.; Benkirane-Jessel, N.; Raehm, L.; Maillard, P.; Garcia, M.; Durand, J.O. Hyaluronic acid-functionalized mesoporous silica nanoparticles for efficient photodynamic therapy of cancer cells. Photodiag. Photodyn. Ther. 2012, 9, 256–260. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Tian, B.; Li, K.; Wang, L.; Liang, Y.; Han, J. Multifunctional mesoporous silica nanoparticles modified with tumor-shedable hyaluronic acid as carriers for doxorubicin. Coll. Surf. B Biointerfaces 2016, 144, 293–302. [Google Scholar] [CrossRef]
- Lee, D.; Beack, S.; Yoo, J.; Kim, S.-K.; Lee, C.; Kwon, W.; Hahn, S.K.; Kim, C. In vivo photoacoustic imaging of livers using biodegradable hyaluronic acid-conjugated silica nanoparticles. Adv. Funct. Mater. 2018, 28, 1800941. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Z.Q.; Wang, S.J.; Liu, P.; Qin, Y.H.; Ma, Y.; Li, X.C.; Huo, Z.J. Hyaluronic acid-tagged silica nanoparticles in colon cancer therapy: Therapeutic efficacy evaluation. Int. J. Nanomed. 2015, 10, 6445–6454. [Google Scholar]
- Amorim, S.; Martins, A.; Neves, N.M.; Reis, R.L.; Pires, R.A. Hyaluronic acid/poly-l-lysine bilayered silica nanoparticles enhance the osteogenic differentiation of human mesenchymal stem cells. J. Mater. Chem. B 2014, 2, 6939–6946. [Google Scholar] [CrossRef]
- Singh, S.P.; Sharma, M.; Gupta, P.K. Cytotoxicity of curcumin silica nanoparticle complexes conjugated with hyaluronic acid on colon cancer cells. Int. J. Biol. Macromol. 2015, 74, 162–170. [Google Scholar] [CrossRef]
- Shi, X.L.; Li, Y.; Zhao, L.M.; Su, L.W.; Ding, G. Delivery of MTH1 inhibitor (TH287) and MDR1 siRNA via hyaluronic acid-based mesoporous silica nanoparticles for oral cancers treatment. Coll. Surf. B Biointerfaces 2019, 173, 599–606. [Google Scholar] [CrossRef]
- Zhao, Q.; Geng, H.; Wang, Y.; Gao, Y.; Huang, J.; Wang, Y.; Zhang, J.; Wang, S. Hyaluronic acid oligosaccharide modified redox-responsive mesoporous silica nanoparticles for targeted drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 20290–20299. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, J.; Zhu, W.; Sun, C.; Di, D.; Zhang, Y.; Wang, P.; Wang, Z.; Wang, S. Dual-stimuli responsive hyaluronic acid-conjugated mesoporous silica for targeted delivery to CD44-overexpressing cancer cells. Acta Biomater. 2015, 23, 147–156. [Google Scholar] [CrossRef]
- Ma, M.; Chen, H.; Chen, Y.; Zhang, K.; Wang, X.; Cui, X.; Shi, J. Hyaluronic acid-conjugated mesoporous silica nanoparticles: Excellent colloidal dispersity in physiological fluids and targeting efficacy. J. Mater. Chem. 2012, 22, 5615. [Google Scholar] [CrossRef]
- Hocine, O.; Gary-Bobo, M.; Brevet, D.; Maynadier, M.; Fontanel, S.; Raehm, L.; Richeter, S.; Loock, B.; Couleaud, P.; Frochot, C.; et al. Silicalites and mesoporous silica nanoparticles for photodynamic therapy. Int. J. Pharm. 2010, 402, 221–230. [Google Scholar] [CrossRef]
- Nairi, V.; Magnolia, S.; Piludu, M.; Nieddu, M.; Caria, C.A.; Sogos, V.; Vallet-Regì, M.; Monduzzi, M. Mesoporous silica nanoparticles functionalized with hyaluronic acid. Effect of the biopolymer chain length on cell internalization. Coll. Surf. B Biointerfaces 2018, 168, 50–59. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Yang, J.-A.; Jung, H.S.; Beack, S.; Choi, J.E.; Hur, W.; Koo, H.; Kim, K.; Yoon, S.K.; Hahn, S.K. Hyaluronic acid–gold nanoparticle/interferon α complex for targeted treatment of hepatitis C virus infection. ACS Nano 2012, 6, 9522–9531. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Kim, I.K.; Park, T.G. Synthesis, characterization, and in vivo diagnostic applications of hyaluronic acid immobilized gold nanoprobes. Biomaterials 2008, 29, 4709–4718. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Liu, Z.; Shi, P.; Dong, K.; Ju, E.; Ren, J.; Qu, X. A multi-stimuli responsive gold nanocage-hyaluronic platform for targeted photothermal and chemotherapy. Biomaterials 2014, 35, 9678–9688. [Google Scholar] [CrossRef]
- Xu, W.; Qian, J.; Hou, G.; Suo, A.; Wang, Y.; Wang, J.; Sun, T.; Yang, M.; Wan, X.; Yao, Y. Hyaluronic acid-functionalized gold nanorods with pH/NIR dual-responsive drug release for synergetic targeted photothermal chemotherapy of breast cancer. ACS Appl. Mater. Interfaces 2017, 9, 36533–36547. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef]
- Bergen, J.M.; Von Recum, H.A.; Goodman, T.T.; Massey, A.P.; Pun, S.H. Gold nanoparticles as a versatile platform for optimizing physicochemical parameters for targeted drug delivery. Macromol. Biosci. 2006, 6, 506–516. [Google Scholar] [CrossRef]
- Manju, S.; Sreenivasan, K. Gold nanoparticles generated and stabilized by water soluble curcumin-polymer conjugate: Blood compatibility evaluation and targeted drug delivery onto cancer cells. J. Coll. Interface Sci. 2012, 368, 144–151. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, H.; Li, X.; Lv, Y.; Ma, T.; Guo, S.; Huang, Z.; Wang, X.; Xu, P. Dual targeting hyaluronic acid—RGD mesoporous silica coated gold nanorods for chemo-photothermal cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 261–270. [Google Scholar] [CrossRef]
- Xu, W.; Qian, J.; Hou, G.; Wang, Y.; Wang, J.; Sun, T.; Ji, L.; Suo, A.; Yao, Y. A dual-targeted hyaluronic acid-gold nanorod platform with triple-stimuli responsiveness for photodynamic/photothermal therapy of breast cancer. Acta Biomater. 2019, 83, 400–413. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Li, L.; Shi, W.; Li, X.; Ma, H. Gold nanoparticles functionalized with cresyl violet and porphyrin via hyaluronic acid for targeted cell imaging and phototherapy. Chem. Commun. 2014, 50, 15696–15698. [Google Scholar] [CrossRef]
- Yang, S.; Palanikumar, L.; Jeong, S.; Kim, K.; Lee, J.; Jeoung, E.; Kim, C.; Ryu, J.H.; Park, M.H. Synergistic effect of photothermal therapy and chemotherapy using camptothecin-conjugated gold nanorods. Part. Part. Syst. Char. 2018, 35, 1700307. [Google Scholar] [CrossRef]
- Kim, K.; Jo, M.-C.; Jeong, S.; Palanikumar, L.; Rotello, V.M.; Ryu, J.-H.; Park, M.-H. Externally controlled drug release using a gold nanorod contained composite membrane. Nanoscale 2016, 8, 11949–11955. [Google Scholar] [CrossRef]
- Guerrero, A.R.; Hassan, N.; Escobar, C.A.; Albericio, F.; Kogan, M.J.; Araya, E. Gold nanoparticles for photothermally controlled drug release. Nanomedicine 2014, 9, 2023–2039. [Google Scholar] [CrossRef]
- Della Rocca, J.; Liu, D.; Lin, W. Nanoscale metal–organic frameworks for biomedical imaging and drug delivery. Acc. Chem. Res. 2011, 44, 957–968. [Google Scholar] [CrossRef]
- Simon-Yarza, T.; Mielcarek, A.; Couvreur, P.; Serre, C. Nanoparticles of metal-organic frameworks: On the road to in vivo efficacy in biomedicine. Adv. Mater. 2018, 30, e1707365. [Google Scholar] [CrossRef]
- Shu, F.; Lv, D.; Song, X.-L.; Huang, B.; Wang, C.; Yu, Y.; Zhao, S.-C. Fabrication of a hyaluronic acid conjugated metal organic framework for targeted drug delivery and magnetic resonance imaging. RSC Adv. 2018, 8, 6581–6589. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, Y.; Phua, S.Z.F.; Lim, W.Q.; Zhao, Y. ZnO–DOX@ ZIF-8 core–shell nanoparticles for pH-responsive drug delivery. ACS Biomater. Sci. Eng. 2017, 3, 2223–2229. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nyström, A.M.; Zou, X. One-pot synthesis of metal–organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.; Liu, R.; Zhang, X.; Li, Z.; Luan, Y. A versatile prodrug strategy to in situ encapsulate drugs in MOF nanocarriers: A case of cytarabine-IR820 prodrug encapsulated ZIF-8 toward chemo-photothermal therapy. Adv. Funct. Mater. 2018, 28, 1802830. [Google Scholar] [CrossRef]
- Cai, W.; Gao, H.; Chu, C.; Wang, X.; Wang, J.; Zhang, P.; Lin, G.; Li, W.; Liu, G.; Chen, X. Engineering phototheranostic nanoscale metal-organic frameworks for multimodal imaging-guided cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 2040–2051. [Google Scholar] [CrossRef]
- Chen, Z.-X.; Liu, M.-D.; Zhang, M.-K.; Wang, S.-B.; Xu, L.; Li, C.-X.; Gao, F.; Xie, B.-R.; Zhong, Z.-L.; Zhang, X.-Z. Interfering with lactate-fueled respiration for enhanced photodynamic tumor therapy by a porphyrinic MOF nanoplatform. Adv. Funct. Mater. 2018, 28, 1803498. [Google Scholar] [CrossRef]
- Almalik, A.; Benabdelkamel, H.; Masood, A.; Alanazi, I.O.; Alradwan, I.; Majrashi, M.; Alfadda, A.A.; Alghamdi, W.M.; Alrabiah, H.; Tirelli, N.; et al. Hyaluronic acid coated chitosan nanoparticles reduced the immunogenicity of the formed protein corona. Sci. Rep. 2017, 7, 10542. [Google Scholar] [CrossRef]
- Varnamkhasti, B.S.; Hosseinzadeh, H.; Azhdarzadeh, M.; Vafaei, S.Y.; Esfandyari-Manesh, M.; Mirzaie, Z.H.; Amini, M.; Ostad, S.N.; Atyabi, F.; Dinarvand, R. Protein corona hampers targeting potential of MUC1 aptamer functionalized SN-38 core-shell nanoparticles. Int. J. Pharm. 2015, 494, 430–444. [Google Scholar] [CrossRef] [PubMed]
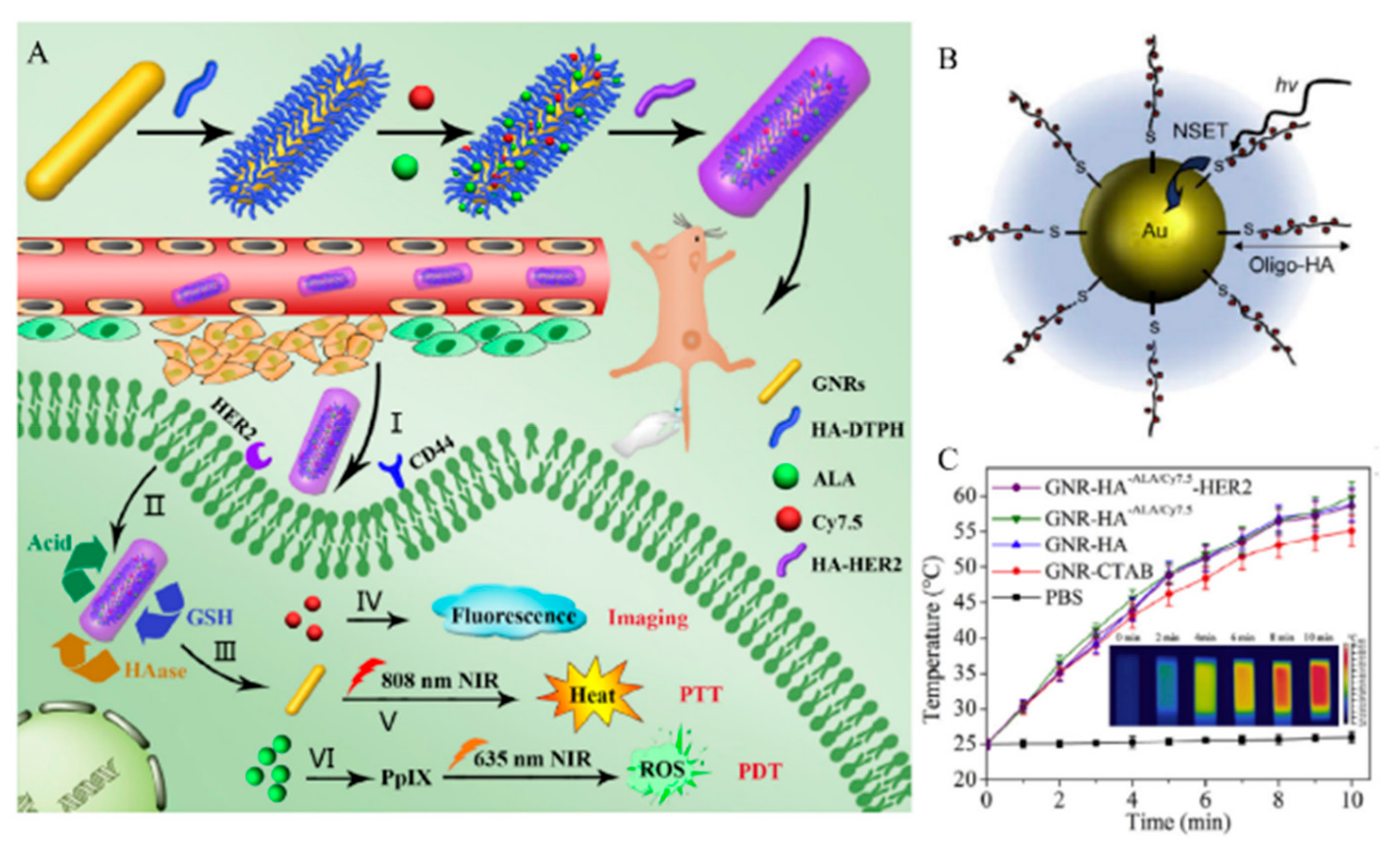
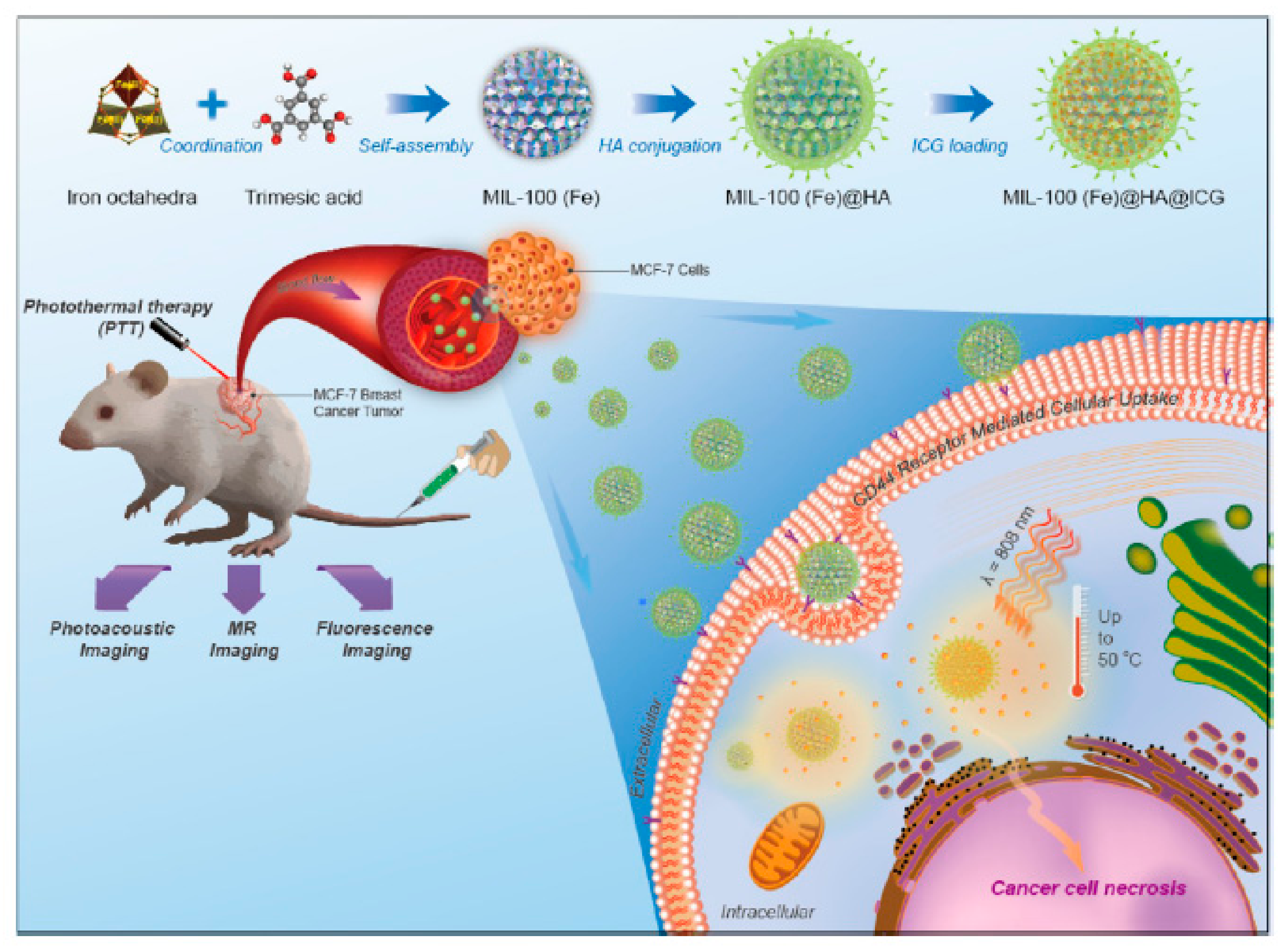
| Component | Cargo | Drug loading Content (%) | Responsive Stimuli | Therapy Method | Size (nm) | Surface Charge (mV) | HA MW (kDa) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2-(Pyridine-2-yldisulfanyl)-ethyl methacrylate | Dox | 8 | GSH | Chemo | 148–215 | 7.4 | [27] | |
| Poly(ethylene glycol) | CPTDox | 32–34 | HAdase | Chemo | 320 | 234 | [29] | |
| β-Dendritic oligoglycerol | PTX | 20.6 | pH | Chemo | 120 | −21 | 9.5 | [37] |
| Deoxycholic acid (DOCA) | PTX | 34.1 | GSH | Chemo | 130–300 | −31–−37 | 11 | [9] |
| Hexadecylamine | Dexamethasone, triamcinolone, triamcinolone acetonide | 2–6 | HAdase | Chemo | 190–−350 | −15–−46 | <10 | [34] |
| Ferrocenium tetradecyl | Dox | 5.97 | GSH | Chemo | 117 | −25.7 | 100 | [42] |
| Indocyanine dye | IR-Pyr | HAdase, ROS | PDT | 30 | −40 | [40] | ||
| Peptide-drug | CPT | HAdase | Chemo | 44 | −9.4 | [41] | ||
| Polyethylene-imine (PEI) -stearic acid | (−)-Gossypol | HAdase | Chemo | 110.9 | −29.6 | >100 | [43] |
| Component | Cargo | Drug Loading Content (%) | Responsive Stimuli | Therapy Method | Size (nm) | Surface Charge (mV) | HA MW (kDa) | Ref. |
|---|---|---|---|---|---|---|---|---|
| HA-Cys-methacrylate, HA-Lys-tetrazole | Cytochrome C, granzyme B | 89.2 | GSH | Protein (Induce apoptosis) | 150 | −17.6 | 35 | [49] |
| Methacrylated HA, DEGDA | Dox | 16 | Enzyme (lipase and HAdase) | Chemo | 50 | −45.0 | 7 | [51] |
| Membrano-tropic cholesteryl-HA, cholesterol-modified HA | Etoposide, salinomycin, and curcumin | 20 | Hydrolysis (ester linkage) | Chemo | 20–40 | −31.6–−41.4 | 62 | [55] |
| Methacrylated HA | Dox | 24 | GSH (Disulfide bond) | Chemo | 79 | −40 | 0.5 | [58] |
| Cholesteryl-HA | Curcumin | 20 | pH, proteolytic enzyme | Chemo | 29.2 | −38.4 | 0.58 | [59] |
| Acetic anhydride | Dox | 93.1 | Chemo | 275 | −31 | 7 | [52] | |
| Acetic anhydride | Pheophorbide | 0.31 per 1 unit of HA | Enzyme | Photo-dynamic | 125–150 | −21–−34 | 62 | [56] |
| Bond | Pore | Cargo | Drug Loading Content (%) | Responsive Stimuli | Therapy Method | Size (nm) | Surface Charge (mV) | HA MW (kDa) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Amide | O | Dox | 1.2 | X | Chemo | 70–100 | −18.8 | 200 | [64] |
| Electrostatic interaction | O | TH287, MDR1 siRNA | 8.91 | X | Chemo | 184 | −18.4 | [72] | |
| Amide | O | 6-Mercapto-purine | GSH | Chemo | 80 | −25.5–−27.9 | 10 | [73] | |
| Amide | O | CPT | 0.96 | X | Chemo | 100 | −14.9 | 18 | [75] |
| Amide | O | Dox | 0.01 | HAdase | Chemo | 232 | −30 | 100 | [65] |
| Amide | X | 5-Fluoro-uracil | 15 | X | Chemo | 138 | 35 | [69] |
| Bond | Pore | Cargo | Drug Loading Content (%) | Responsive Stimuli | Therapy Method | Size (nm) | Surface Charge (mV) | HA MW (kDa) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Au-sulfur. | X | Hilyte-647 | X | HAdase, ROS | Diagnosis | 16 | 1790 | [79] | |
| Au-catechol | O | Dox | X | HAdase, NIR | Chemo, PTT | 50 | −25.7 | 100 | [80] |
| Au-catechol | X | Dox | X | pH, NIR | Chemo, PTT | 71 | −11.4 | 8 | [81] |
| Au-sulfur | X | ALA, Cy7.5 | X | HAdase, GSH, pH | PTT, PDT | 72 | −13.8 | 8 | [87] |
| Bond | Pore | Cargo | Drug Loading Content (%) | Responsive Stimuli | Therapy Method | Size (nm) | Surface Charge (mV) | HA MW (kDa) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Coordination | O | Dox | pH | Chemo, MRI | 150 | −30.2 | X | [94] | |
| O | Indocyanine green | 42 | NIR | PTT, MRI, PA imaging | 106 | −25.4 | X | [98] | |
| O | α-Cyano-4-hydroxy-cinnamate | HAdase, Light | PDT | 152 | −13.9 | X | [99] | ||
| Coordination | O | Cytarabine, indocyanine green | 39.8 | pH | Chemo, PTT | 135 | 10 | [97] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Choi, H.; Choi, E.S.; Park, M.-H.; Ryu, J.-H. Hyaluronic Acid-Coated Nanomedicine for Targeted Cancer Therapy. Pharmaceutics 2019, 11, 301. https://doi.org/10.3390/pharmaceutics11070301
Kim K, Choi H, Choi ES, Park M-H, Ryu J-H. Hyaluronic Acid-Coated Nanomedicine for Targeted Cancer Therapy. Pharmaceutics. 2019; 11(7):301. https://doi.org/10.3390/pharmaceutics11070301
Chicago/Turabian StyleKim, Kibeom, Huyeon Choi, Eun Seong Choi, Myoung-Hwan Park, and Ja-Hyoung Ryu. 2019. "Hyaluronic Acid-Coated Nanomedicine for Targeted Cancer Therapy" Pharmaceutics 11, no. 7: 301. https://doi.org/10.3390/pharmaceutics11070301
APA StyleKim, K., Choi, H., Choi, E. S., Park, M.-H., & Ryu, J.-H. (2019). Hyaluronic Acid-Coated Nanomedicine for Targeted Cancer Therapy. Pharmaceutics, 11(7), 301. https://doi.org/10.3390/pharmaceutics11070301







