Investigation of Fugitive Aerosols Released into the Environment during High-Flow Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. HFNT Circuit and Interfaces
2.2. Inhaled Dose
2.3. Anatomical Models
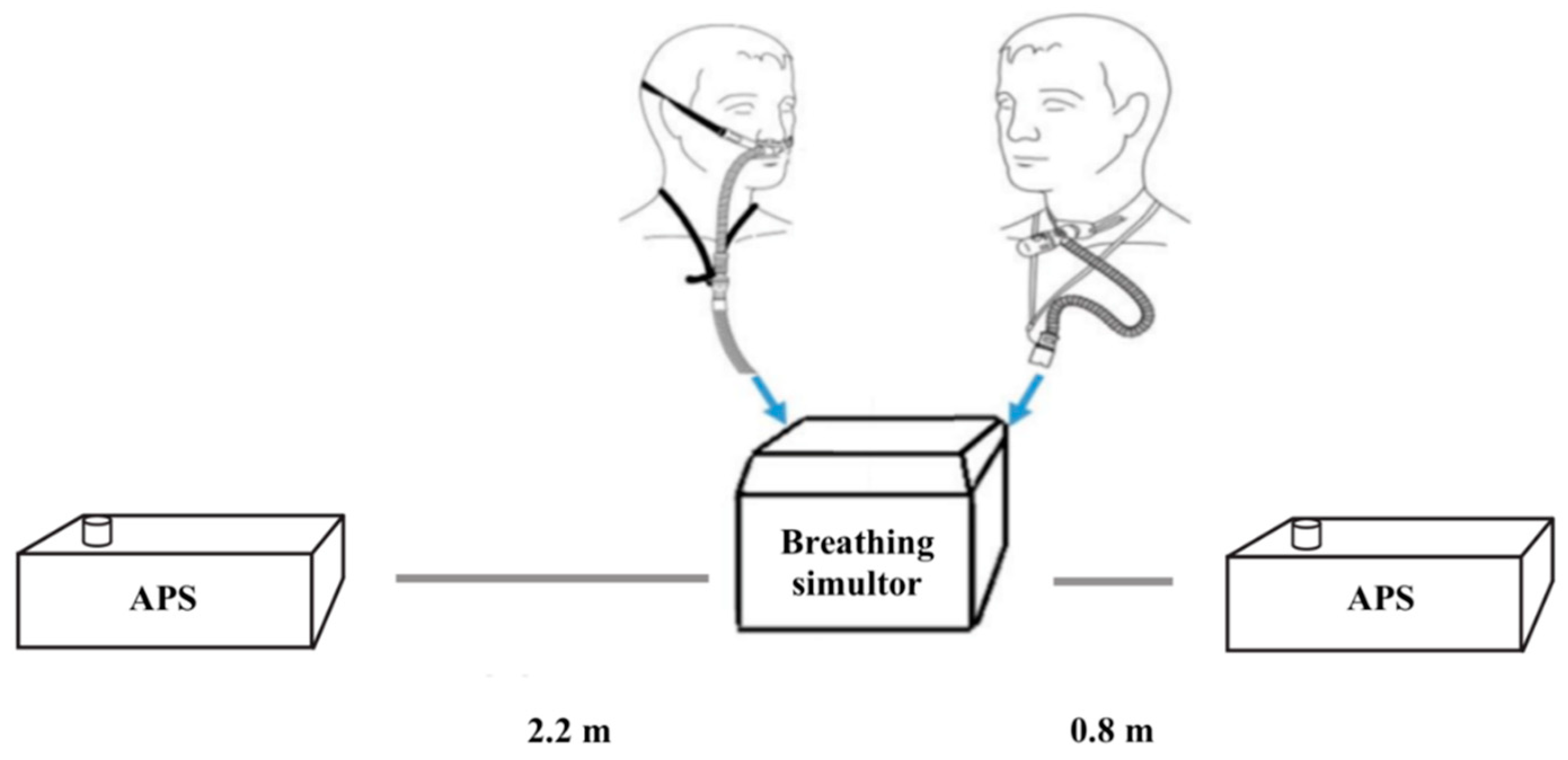
2.4. Characterising Fugitive Emissions
2.5. Temperature, Humidity and Airflow Characteristics
2.6. Data Analysis and Statistics
3. Results
3.1. Inhaled Dose
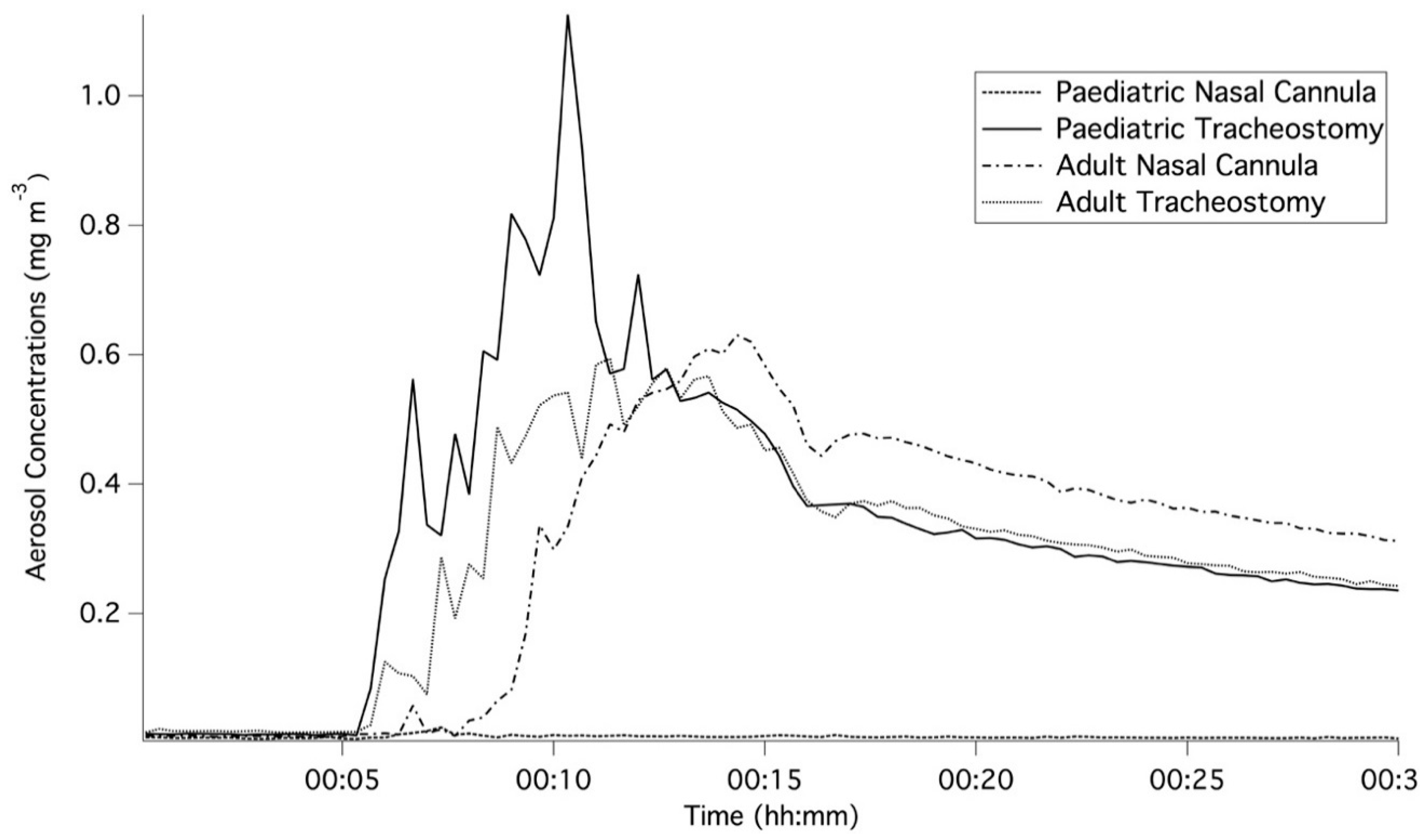
3.2. Peak Aerosol Concentrations
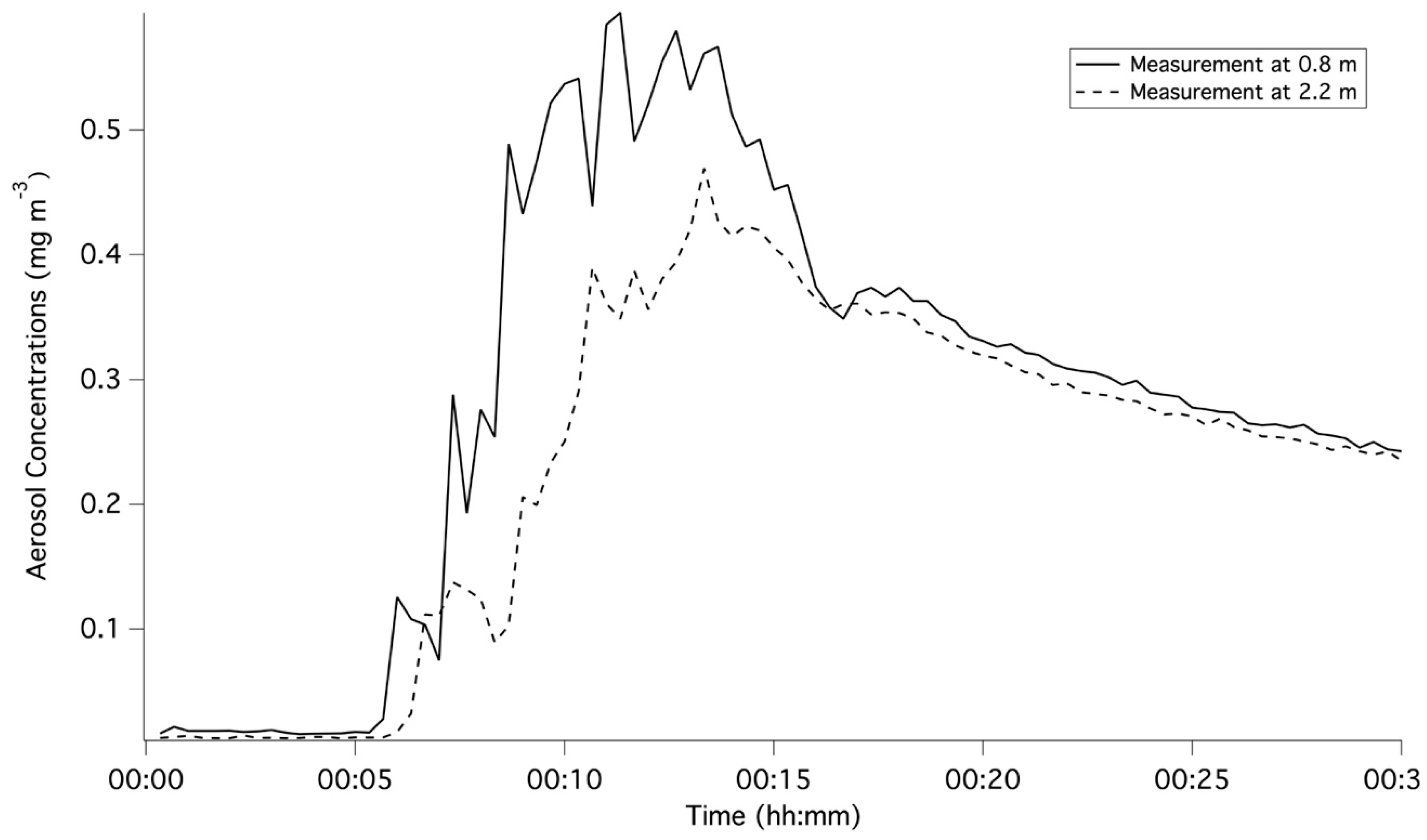
3.3. Time-Weighted Averaged Aerosol Concentrations
3.4. Aerosol Droplet Sizing
3.5. Potential Inhalation Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- ICRP. Human Respiratory Tract Model for Radiological Protection. J. Radiol. Prot. 1994, 24, 1–842. [Google Scholar]
- Nazaroff, W.W. Indoor particle dynamics. Indoor Air 2004, 14, 175–183. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Byrne, M.; Ashmore, M.; Terry, A.; Dimitroulopoulou, C. Development of a probabilistic multi-zone multi-source computational model and demonstration of its applications in predicting pm concentrations indoors. Sci. Total Environ. 2014, 490, 798–806. [Google Scholar] [CrossRef]
- He, C.; Morawska, L.; Gilbert, D. Particle deposition rates in residential houses. Atmos. Environ. 2005, 39, 3891–3899. [Google Scholar] [CrossRef]
- Dolovich, M.B.; Dhand, R. Aerosol drug delivery: Developments in device design and clinical use. Lancet (Lond. Engl.) 2011, 377, 1032–1045. [Google Scholar] [CrossRef]
- Lin, H.-L.; Wan, G.-H.; Chen, Y.-H.; Fink, J.B.; Liu, W.-Q.; Liu, K.-Y. Influence of nebulizer type with different pediatric aerosol masks on drug deposition in a model of a spontaneously breathing small child. Respir. Care 2012, 57, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, G.C. Advances in aerosols: Adult respiratory disease. J. Aerosol Med. 2006, 19, 36–46. [Google Scholar] [CrossRef]
- ElHansy, M.H.E.; Boules, M.E.; El Essawy, A.F.M.; Al-Kholy, M.B.; Abdelrahman, M.M.; Said, A.S.A.; Hussein, R.R.S.; Abdelrahim, M.E. Inhaled salbutamol dose delivered by jet nebulizer, vibrating mesh nebulizer and metered dose inhaler with spacer during invasive mechanical ventilation. Pulm. Pharmacol. Ther. 2017, 45, 159–163. [Google Scholar] [CrossRef]
- Hassan, A.; Salah Eldin, R.; Abdelrahman, M.M.; Abdelrahim, M.E. In-vitro/in-vivo comparison of inhaled salbutamol dose delivered by jet nebulizer, vibrating mesh nebulizer and metered dose inhaler with spacer during non-invasive ventilation. Exp. Lung Res. 2017, 43, 19–28. [Google Scholar] [CrossRef]
- Dysart, K.; Miller, T.L.; Wolfson, M.R.; Shaffer, T.H. Research in high flow therapy: Mechanisms of action. Respir. Med. 2009, 103, 1400–1405. [Google Scholar] [CrossRef]
- Bhashyam, A.R.; Wolf, M.T.; Marcinkowski, A.L.; Saville, A.; Thomas, K.; Carcillo, J.A.; Corcoran, T.E. Aerosol delivery through nasal cannulas: An in vitro study. J. Aerosol Med. Pulm. Drug Deliv. 2008, 21, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.; Joyce, M.; Sweeney, L.; MacLoughlin, R. In vitro determination of the main effects in the design of high-flow nasal therapy systems with respect to aerosol performance. Pulm. Ther. 2018, 4, 1–14. [Google Scholar] [CrossRef]
- Perry, S.A.; Kesser, K.C.; Geller, D.E.; Selhorst, D.M.; Rendle, J.K.; Hertzog, J.H. Influences of cannula size and flow rate on aerosol drug delivery through the vapotherm humidified high-flow nasal cannula system. Pediatr. Crit. Care Med. 2013, 14, e250–e256. [Google Scholar] [CrossRef]
- Bennett, G.; Joyce, M.; Sweeney, L.; MacLoughlin, R. In vitro study of the effect of breathing pattern on aerosol delivery during high-flow nasal therapy. Pulm. Ther. 2019, 5, 43–54. [Google Scholar] [CrossRef]
- Ari, A.; Harwood, R.; Sheard, M.; Dailey, P.; Fink, J.B. In vitro comparison of heliox and oxygen in aerosol delivery using pediatric high flow nasal cannula. Pediatr. Pulm. 2011, 46, 795–801. [Google Scholar] [CrossRef]
- Wittgen, B.P.H.; Kunst, P.W.A.; van der Born, K.; van Wijk, A.W.; Perkins, W.; Pilkiewicz, F.G.; Perez-Soler, R.; Nicholson, S.; Peters, G.J.; Postmus, P.E. Phase i study of aerosolized slit cisplatin in the treatment of patients with carcinoma of the lung. Clin. Cancer Res. 2007, 13, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Shults, R.A.; Baron, S.; Decker, J.; Deitchman, S.D.; Connor, J.D. Health care worker exposure to aerosolized ribavirin: Biological and air monitoring. J. Occup. Environ. Med. 1996, 38, 257–263. [Google Scholar] [CrossRef]
- Croteau, G.A.; Martin, D.B.; Camp, J.; Yost, M.; Conrad, C.; Zeitlin, P.L.; Heald, A.E. Evaluation of exposure and health care worker response to nebulized administration of tgaavcf to patients with cystic fibrosis. Ann. Occup. Hyg. 2004, 48, 673–681. [Google Scholar]
- O’Riordan, T.G.; Smaldone, G.C. Exposure of health care workers to aerosolized pentamidine. Chest 1992, 101, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Carnathan, B.; Martin, B.; Colice, G. Second hand (s)-albuterol: Rt exposure risk following racemic albuterol. Respir. Care 2001, 46, 1084. [Google Scholar]
- Simonds, A.K.; Hanak, A.; Chatwin, M.; Morrell, M.; Hall, A.; Parker, K.H.; Siggers, J.H.; Dickinson, R.J. Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: Implications for management of pandemic influenza and other airborne infections. Health Technol. Assess. (Winch. Engl.) 2010, 14, 131–172. [Google Scholar] [CrossRef]
- Somogyi, R.; Vesely, A.E.; Azami, T.; Preiss, D.; Fisher, J.; Correia, J.; Fowler, R.A. Dispersal of respiratory droplets with open vs closed oxygen delivery masks: Implications for the transmission of severe acute respiratory syndrome. Chest J. 2004, 125, 1155–1157. [Google Scholar] [CrossRef]
- Hui, D.S.; Chow, B.K.; Ng, S.S.; Chu, L.C.Y.; Hall, S.D.; Gin, T.; Sung, J.J.Y.; Chan, M.T.V. Exhaled air dispersion distances during noninvasive ventilation via different respironics face masks. Chest 2009, 136, 998–1005. [Google Scholar] [CrossRef]
- Hui, D.S.; Chow, B.K.; Lo, T.; Ng, S.S.; Ko, F.W.; Gin, T.; Chan, M.T.V. Exhaled air dispersion during noninvasive ventilation via helmets and a total facemask. Chest 2015, 147, 1336–1343. [Google Scholar] [CrossRef]
- Ari, A.; Fink, J.B.; Pilbeam, S.P. Secondhand aerosol exposure during mechanical ventilation with and without expiratory filters: An in-vitro study. Ind. J. Respir. Care 2016, 5, 677–682. [Google Scholar]
- Saeed, H.; Mohsen, M.; Fink, J.B.; Dailey, P.; Salah Eldin, A.; Abdelrahman, M.M.; Elberry, A.A.; Rabea, H.; Hussein, R.R.S.; Abdelrahim, M.E.A. Fill volume, humidification and heat effects on aerosol delivery and fugitive emissions during noninvasive ventilation. J. Drug Deliv. Sci. Technol. 2017, 39, 372–378. [Google Scholar] [CrossRef]
- Elmashae, Y.; Yermakov, M.; Frank, E.; Benjamin, M.; Maier, A.; Newman, N.; Reponen, T.; Grinshpun, S.A. Exposure of home-attending healthcare workers to aerosolized medications (simulation study). J. Aerosol Sci. 2019, 133, 49–55. [Google Scholar] [CrossRef]
- McGrath, J.A.; O’Sullivan, A.; Bennett, G.; O’Toole, C.; Joyce, M.; Byrne, M.A.; MacLoughlin, R. Investigation of the quantity of exhaled aerosols released into the environment during nebulisation. Pharmaceutics 2019, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, L.; Joyce, M.; Bennett, G.; MacLoughlin, R. Assessment of the effect of cannula choice and gas flow rate on aerosol delivery during high flow nasal therapy. In Proceedings of the Drug to the Delivery Lungs 2018, Edinburgh, UK, 7 August 2018. [Google Scholar]
- RDD Onine. Rdd Online Respiratory Drug Delivery. Pediatric Upper Airway Models. 2019. Available online: https://www.rddonline.com/resources/tools/pediatric_upper_airway_models.php (accessed on 7 May 2019).
- Sherman, M. In Simplified modeling for infiltration and radon entry. In Proceedings of the Thermal Performance of the Exterior Envelopes of Buildings Conference V, Clearwater Beach, FL, USA, 7–10 December 1992. [Google Scholar]
- U.S. EPA. Exposure Factors Handbook: 2011 Edition; US Environmental Protection Agency: Washington, DC, USA, 2011.
- Berlinski, A. Pediatric aerosol therapy. Respir. Care 2017, 62, 662–677. [Google Scholar] [CrossRef] [PubMed]
- Berlinski, A. Nebulized albuterol delivery in a model of spontaneously breathing children with tracheostomy. Respir. Care 2013, 58, 2076–2086. [Google Scholar] [CrossRef]
- Hess, D.R. Aerosol delivery devices in the treatment of asthma. Respir. Care 2008, 53, 699–725. [Google Scholar]
- Dailey, P.A.; Harwood, R.; Walsh, K.; Fink, J.B.; Thayer, T.; Gagnon, G.; Ari, A. Aerosol delivery through adult high flow nasal cannula with heliox and oxygen. Respir. Care 2017, 69, 1186–1192. [Google Scholar] [CrossRef]
- Ari, A.; Atalay, O.T.; Harwood, R.; Sheard, M.M.; Aljamhan, E.A.; Fink, J.B. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir. Care 2010, 55, 845–851. [Google Scholar] [PubMed]
- Berlinski, A.; Willis, J.R. Effect of tidal volume and nebulizer type and position on albuterol delivery in a pediatric model of mechanical ventilation. Respir. Care 2015, 60, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Dolovich, M.A. Influence of inspiratory flow rate, particle size, and airway caliber on aerosolized drug delivery to the lung. Respir. Care 2000, 45, 597–608. [Google Scholar] [PubMed]
- Golshahi, L.; Tian, G.; Azimi, M.; Son, Y.-J.; Walenga, R.; Longest, P.W.; Hindle, M. The use of condensational growth methods for efficient drug delivery to the lungs during noninvasive ventilation high flow therapy. Pharm. Res. 2013, 30, 2917–2930. [Google Scholar] [CrossRef] [PubMed]
- Long, C.M.; Suh, H.H.; Catalano, P.J.; Koutrakis, P. Using time-and size-resolved particulate data to quantify indoor penetration and deposition behavior. Environ. Sci. Technol. 2001, 35, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.A.; Byrne, M.A.; Ashmore, M.R.; Terry, A.C.; Dimitroulopoulou, C. A simulation study of the changes in pm2.5 concentrations due to interzonal airflow variations caused by internal door opening patterns. Atmos. Environ. 2014, 87, 183–188. [Google Scholar] [CrossRef]
- Ciuzas, D.; Prasauskas, T.; Krugly, E.; Sidaraviciute, R.; Jurelionis, A.; Seduikyte, L.; Kauneliene, V.; Wierzbicka, A.; Martuzevicius, D. Characterization of indoor aerosol temporal variations for the real-time management of indoor air quality. Atmos. Environ. 2015, 118, 107–117. [Google Scholar] [CrossRef]
- McGrath, J.A.; Bennett, G.; O’Toole, C.; Byrne, M.A.; Joyce, M.; MacLoughlin, R. Investigation of the quantity of exhaled aerosols released into the environ-ment during jet nebulization. In Proceedings of the Indoor Air 2018—15th International Conference on Indoor Air Quality and Climate, Philadelphia, PA, USA, 22–27 July 2018. [Google Scholar]
- Acevedo-Bolton, V.; Ott, W.R.; Cheng, K.C.; Jiang, R.T.; Klepeis, N.E.; Hildemann, L.M. Controlled experiments measuring personal exposure to pm2.5 in close proximity to cigarette smoking. Indoor Air 2014, 24, 199–212. [Google Scholar] [CrossRef]
- McGrath, J.A.; Sheahan, J.N.; Dimitroulopoulou, C.; Ashmore, M.R.; Terry, A.C.; Byrne, M.A. Pm exposure variations due to different time activity profile simulations within a single dwelling. Build. Environ. 2017, 116, 55–63. [Google Scholar] [CrossRef]
- Golshahi, L.; Walenga, R.L.; Longest, P.W.; Hindle, M. Development of a transient flow aerosol mixer-heater system for lung delivery of nasally administered aerosols using a nasal cannula. Aerosol Sci. Technol. 2014, 48, 1009–1021. [Google Scholar] [CrossRef]
| Breathing Pattern/Gas-Flow Rate | % Inhaled Dose | |
|---|---|---|
| Nasal Cannula | Tracheostomy | |
| Paediatric/2 L/min | 3.23 ± 1.58 | N/A |
| Paediatric/10 L/min | 0.42 ± 0.06 | 16.77 ± 0.26 |
| Paediatric/20 L/min | 1.17 ± 0.28 | 10.65 ± 2.54 |
| Adult/10 L/min | 5.35 ± 2.81 | 6.39 ± 1.58 |
| Adult/40 L/min | 2.56 ± 1.38 | 1.49 ± 0.30 |
| Adult/60 L/min | 1.01 ± 0.26 | 1.36 ± 0.14 |
| Breathing Pattern/Gas-Flow Rate | Peak Aerosol Concentration (mg m−3) | |
|---|---|---|
| Nasal Cannula | Tracheostomy | |
| Paediatric/2 L/min | 0.081 ± 0.016 | N/A |
| Paediatric/10 L/min | 0.025 ± 0.008 | 1.353 ± 0.212 |
| Paediatric/20 L/min | 0.017 ± 0.006 | 1.241 ± 0.160 |
| Adult/10 L/min | 0.636 ± 0.067 | 0.730 ± 0.073 |
| Adult/40 L/min | 0.196 ± 0.049 | 0.278 ± 0.013 |
| Adult/60 L/min | 0.090 ± 0.004 | 0.188 ± 0.039 |
| Breathing Pattern/Gas-Flow Rate | Average Mass Concentration (mg·m−3) | |||
|---|---|---|---|---|
| Measurements at the Distance of 0.8 m | Measurements at the Distance of 2.2 m | |||
| Nasal Cannula | Tracheostomy | Nasal Cannula | Tracheostomy | |
| Paediatric/2 L/min | 0.037 ± 0.005 | N/A | 0.027 ± 0.008 | N/A |
| Paediatric/10 L/min | 0.009 ± 0.001 | 0.334 ± 0.028 | 0.008 ± 0.002 | 0.211 ± 0.008 |
| Paediatric/20 L/min | 0.005 ± 0.001 | 0.370 ± 0.046 | 0.005 ± 0.002 | 0.210 ± 0.040 |
| Adult/10 L/min | 0.302 ± 0.027 | 0.290 ± 0.027 | 0.277 ± 0.050 | 0.236 ± 0.124 |
| Adult/40 L/min | 0.093 ± 0.006 | 0.138 ± 0.005 | 0.109 ± 0.026 | 0.176 ± 0.039 |
| Adult/60 L/min | 0.050 ± 0.003 | 0.101 ± 0.011 | 0.061 ± 0.042 | 0.127 ± 0.030 |
| Breathing Pattern/Gas-Flow Rate | Average MMAD (µm) | |
|---|---|---|
| Nasal Cannula | Tracheostomy | |
| Paediatric/2 L/min | 1.331 ± 0.043 | N/A |
| Paediatric/10 L/min | 2.070 ± 0.531 | 1.784 ± 0.033 |
| Paediatric/20 L/min | 3.200 ± 0.532 | 1.577 ± 0.134 |
| Adult/10 L/min | 1.722 ± 0.216 | 1.793 ± 0.111 |
| Adult/40 L/min | 1.259 ± 0.197 | 1.248 ± 0.131 |
| Adult/60 L/min | 1.350 ± 0.034 | 1.300 ± 0.023 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGrath, J.A.; O’Toole, C.; Bennett, G.; Joyce, M.; Byrne, M.A.; MacLoughlin, R. Investigation of Fugitive Aerosols Released into the Environment during High-Flow Therapy. Pharmaceutics 2019, 11, 254. https://doi.org/10.3390/pharmaceutics11060254
McGrath JA, O’Toole C, Bennett G, Joyce M, Byrne MA, MacLoughlin R. Investigation of Fugitive Aerosols Released into the Environment during High-Flow Therapy. Pharmaceutics. 2019; 11(6):254. https://doi.org/10.3390/pharmaceutics11060254
Chicago/Turabian StyleMcGrath, James A., Ciarraí O’Toole, Gavin Bennett, Mary Joyce, Miriam A. Byrne, and Ronan MacLoughlin. 2019. "Investigation of Fugitive Aerosols Released into the Environment during High-Flow Therapy" Pharmaceutics 11, no. 6: 254. https://doi.org/10.3390/pharmaceutics11060254
APA StyleMcGrath, J. A., O’Toole, C., Bennett, G., Joyce, M., Byrne, M. A., & MacLoughlin, R. (2019). Investigation of Fugitive Aerosols Released into the Environment during High-Flow Therapy. Pharmaceutics, 11(6), 254. https://doi.org/10.3390/pharmaceutics11060254






