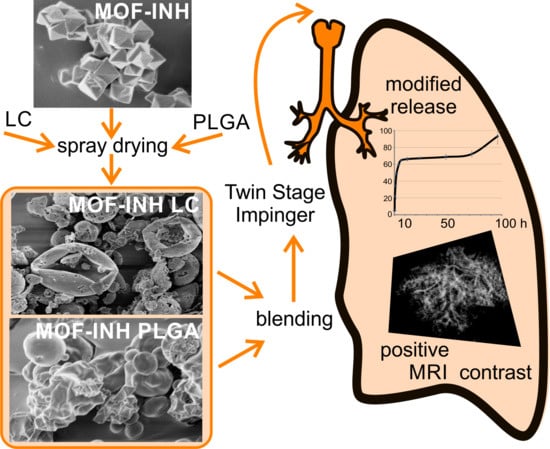
An Inhalable Theranostic System for Local Tuberculosis Treatment Containing an Isoniazid Loaded Metal Organic Framework Fe-MIL-101-NH2—From Raw MOF to Drug Delivery System
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. MOF Synthesis and Drug Incorporation
2.3. Preparation of Spray-Dried Particles
2.4. Design of Experiment (DOE)
2.5. Particle Characterization
2.5.1. In Vitro Aerodynamic Characterization
2.5.2. MOF and Drug Quantification
2.5.3. Sampling for Content Uniformity and Content Average
2.5.4. Particle Size and Morphology
2.6. In Vitro Drug Release Study
2.7. Statistical Analysis
2.8. Magnetic Resonance Imaging (MRI)
2.8.1. Preparation of Sponge Phantoms of Lung Tissue
2.8.2. Preparation of Lung Ex Vivo Samples
2.8.3. MR Imaging and Image Analysis
2.9. In Vitro Study—Cell Viability, Cytoxicity, and Cellular Uptake
3. Results
3.1. Experimental Design
3.2. Powder Characterization
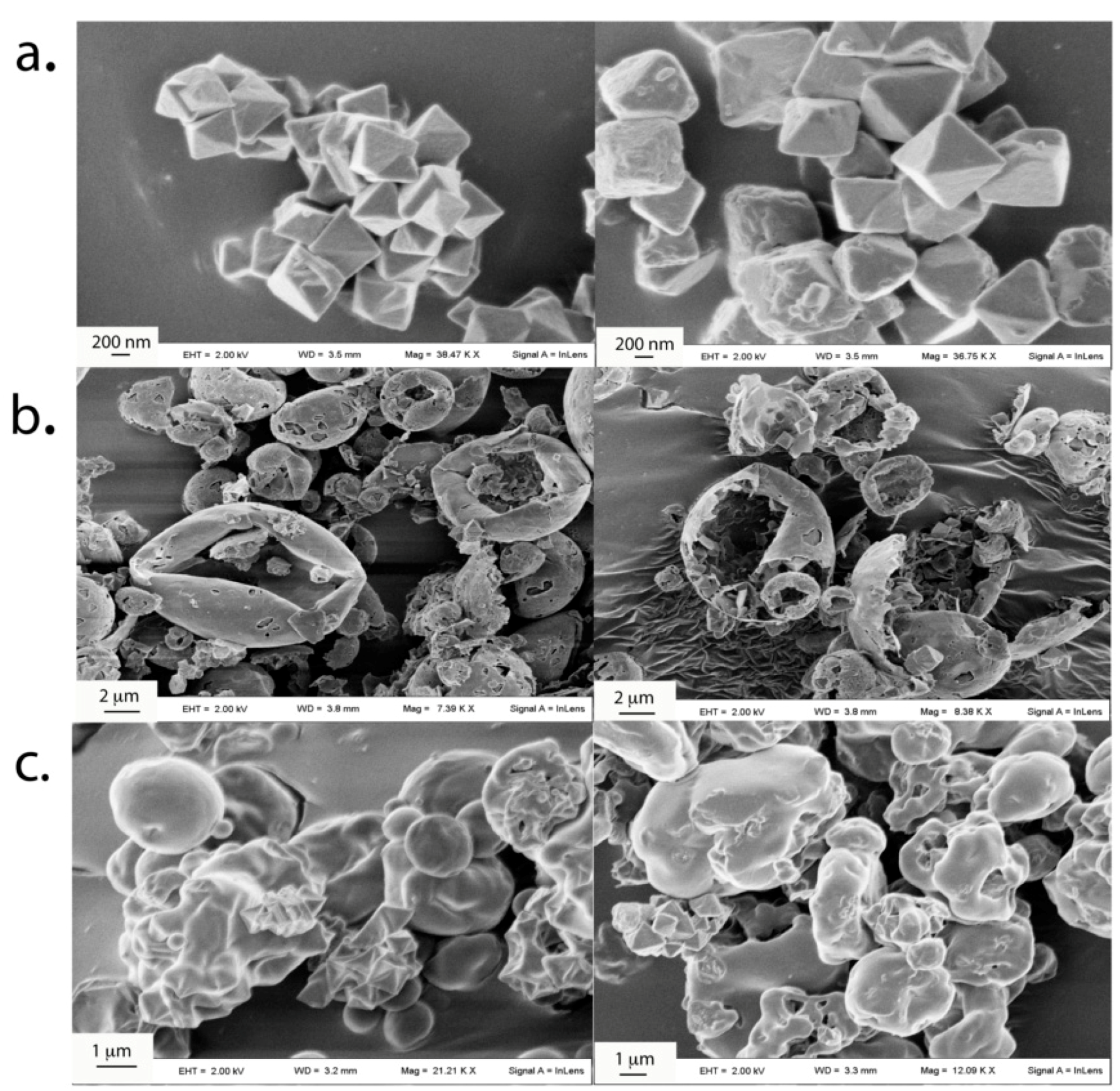
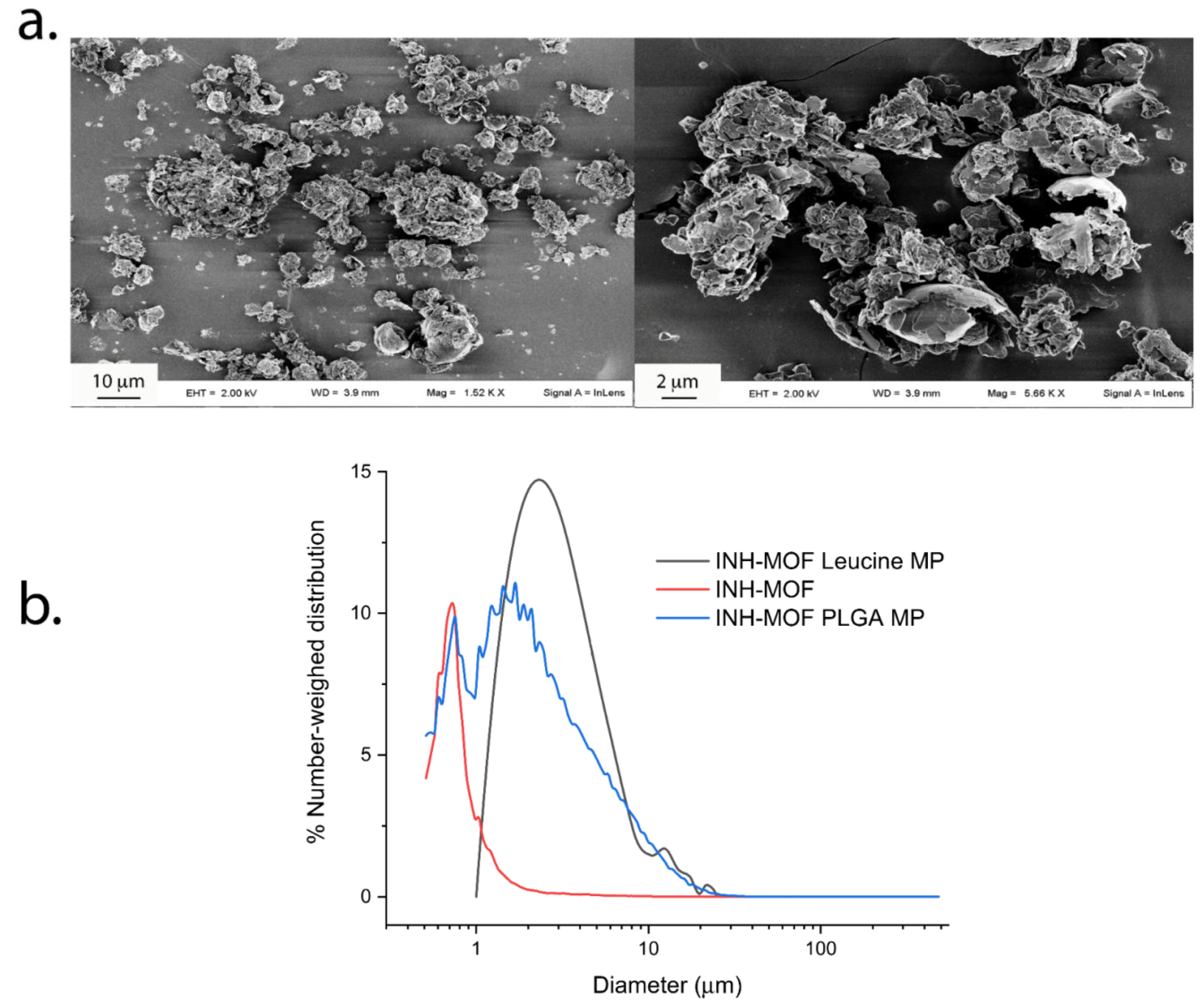
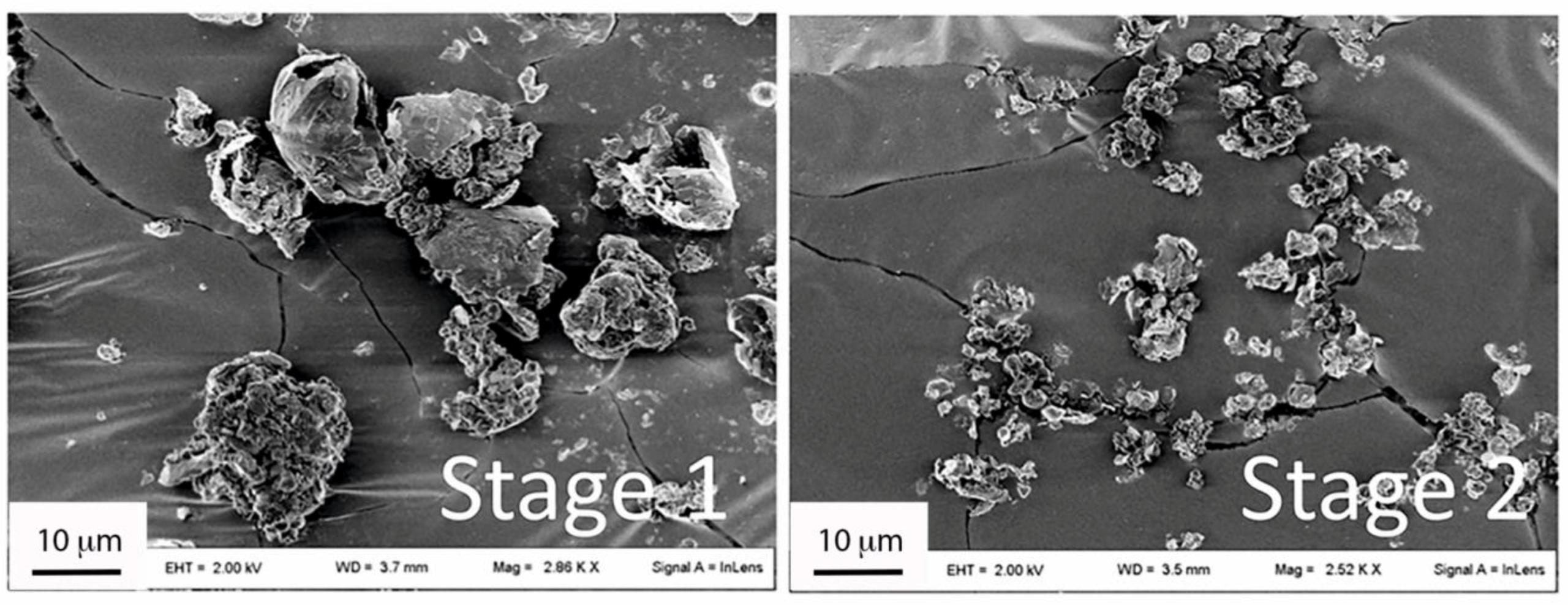
3.2.1. Morphology and Size
3.2.2. In Vitro Aerodynamic Characterization and Content Uniformity
3.3. In Vitro Drug Release Study
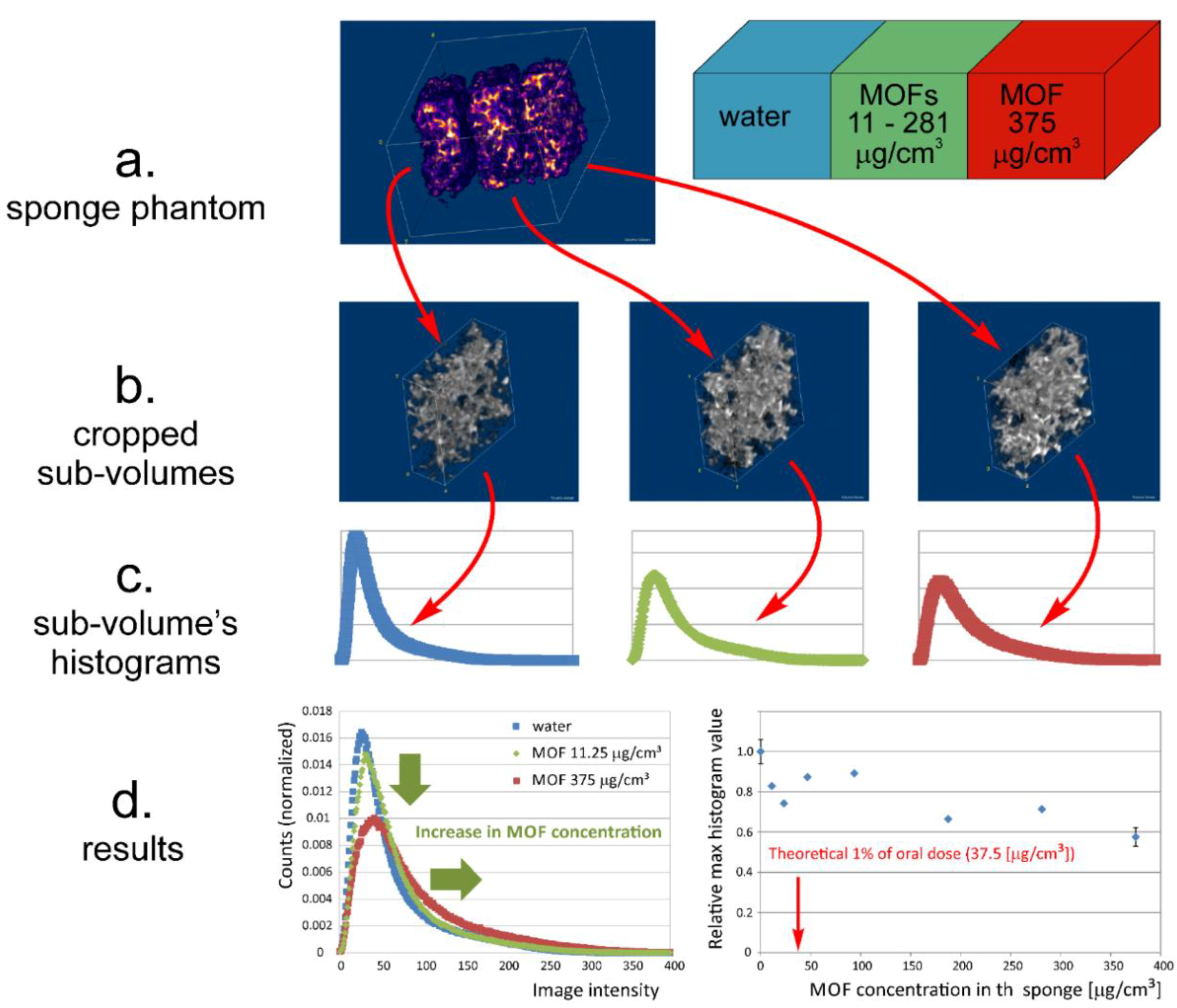
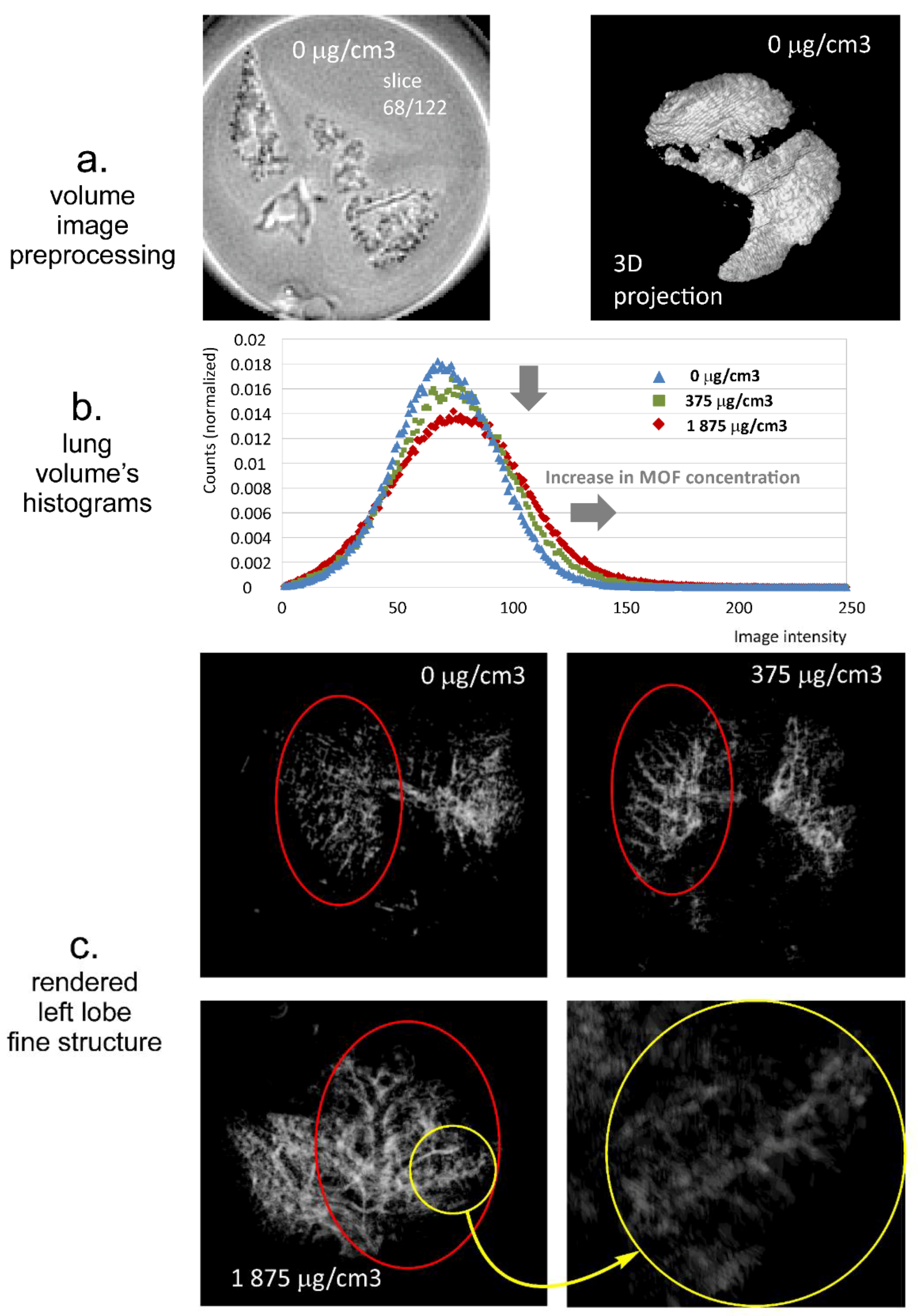
3.4. MRI Contrast Capabilities
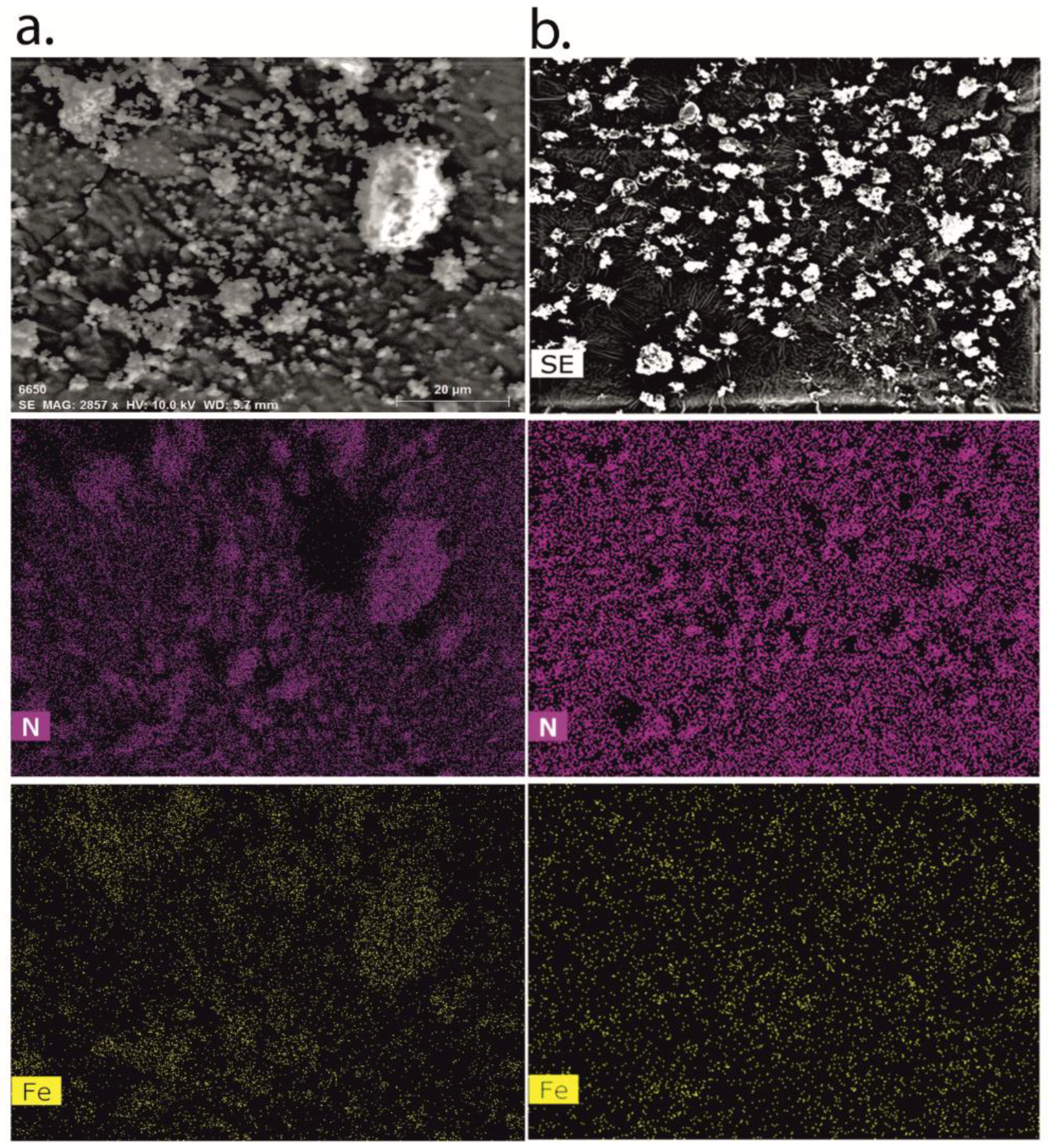
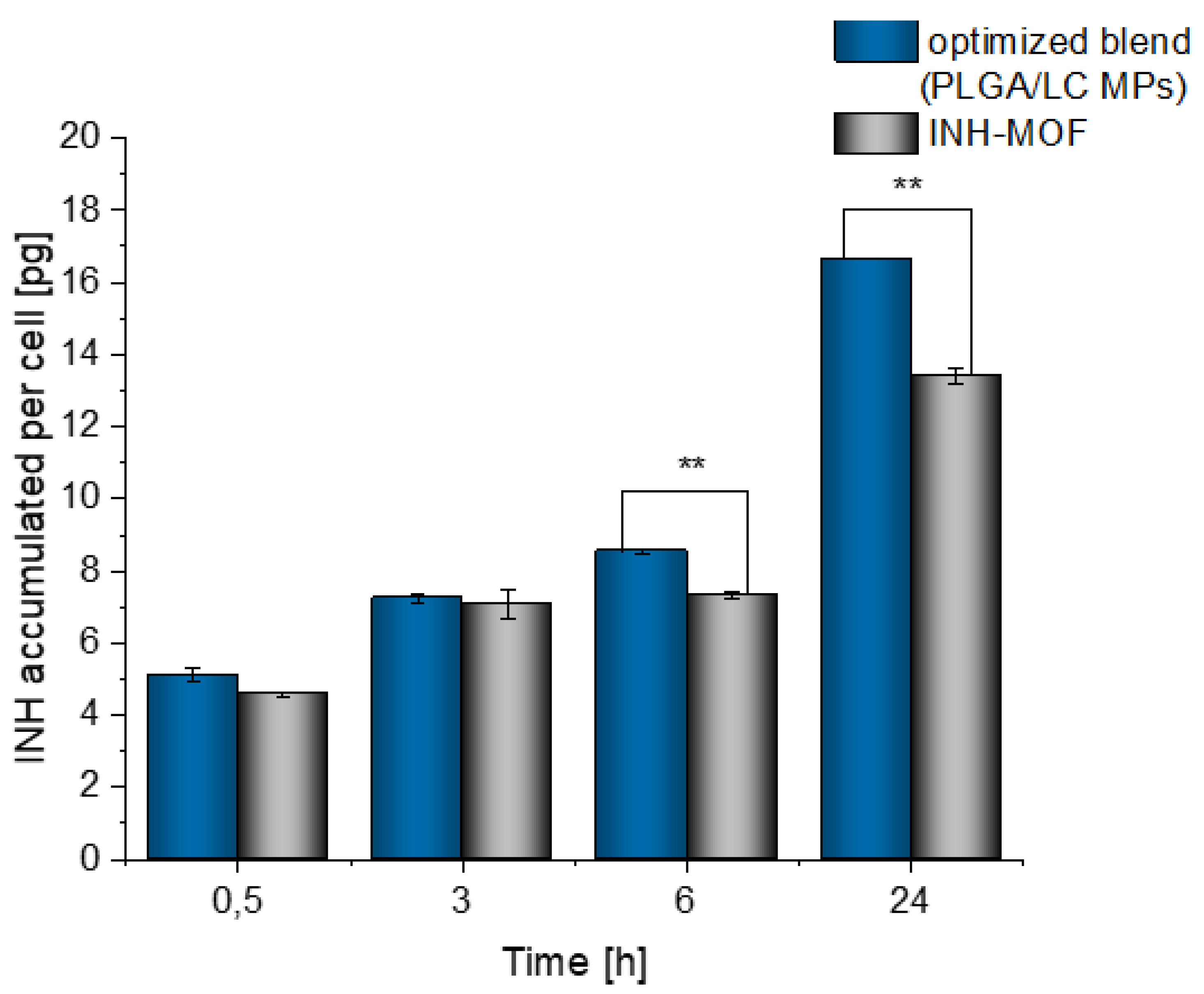
3.5. Time-Dependent Cellular Uptake
3.6. Cytotoxicity Study
4. Discussion
5. Conclusions
- Good aerodynamic properties combined with controlled release of INH;
- Improved absorption of the formulation by macrophages;
- Promising contrast properties enabling monitoring of the distribution of formulation within the inhomogeneous structure of the lungs.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| %ED | emitted dose; |
| %FPF | fine particle fraction; |
| 3D UTE | three-dimensional ultra short echo time; |
| ACN | acetonitrile; |
| INH | isoniazid; |
| INH-MOF | complex of isoniazid and MOF; |
| LC | leucine; |
| MOF | metal-organic framework; |
| MP | microparticles; |
| MRI | magnetic resonance imaging; |
| PLGA | poly (lactic-co-glycolic acid); |
| ROS | reactive oxygen species; |
| TB | tuberculosis; |
References
- Khadka, P.; Dummer, J.; Hill, P.C.; Das, S.C. Considerations in preparing for clinical studies of inhaled rifampicin to enhance tuberculosis treatment. Int. J. Pharm. 2018, 548, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Giovagnoli, S.; Schoubben, A.; Ricci, M. The long and winding road to inhaled TB therapy: Not only the bug’s fault. Drug Dev. Ind. Pharm. 2017, 43, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Brand, P.; Friemel, I.; Meyer, T.; Schulz, H.; Heyder, J.; Haussinger, K. Total deposition of therapeutic particles during spontaneous and controlled inhalations. J. Pharm. Sci. 2000, 89, 724–731. [Google Scholar] [CrossRef]
- Forbes, B.; Asgharian, B.; Dailey, L.A.; Ferguson, D.; Gerde, P.; Gumbleton, M.; Gustavsson, L.; Hardy, C.; Hassall, D.; Jones, R.; et al. Wolff, Challenges in inhaled product development and opportunities for open innovation. Adv. Drug Deliv. Rev. 2011, 63, 69–87. [Google Scholar] [CrossRef]
- Dubsky, S.; Fouras, A. Imaging regional lung function: A critical tool for developing inhaled antimicrobial therapies. Adv. Drug Deliv. Rev. 2015, 85, 100–109. [Google Scholar] [CrossRef]
- Yadav, A.B.; Singh, A.K.; Verma, R.K.; Mohan, M.; Agrawal, A.K.; Misra, A. The devil’s advocacy: When and why inhaled therapies for tuberculosis may not work. Tuberculosis 2011, 91, 65–66. [Google Scholar] [CrossRef]
- Robertson, H.T.; Buxton, R.B. Imaging for lung physiology: What do we wish we could measure? J. Appl. Physiol. 2012, 113, 317–327. [Google Scholar] [CrossRef]
- Yang, M.Y.; Chan, J.G.Y.; Chan, H.K. Pulmonary drug delivery by powder aerosols. J. Control. Release 2014, 193, 228–240. [Google Scholar] [CrossRef]
- Buzan, M.T.A.; Herta, H.; Coman, M.M.; Homorodean, D.; Sfrangeu, S.A.; Pop, C.M. MRI as indicator of pulmonary tuberculosis activity: From morphological to molecular level assessment—A case report. Rom. J. Morphol. Embryol. 2017, 58, 193–196. [Google Scholar]
- Rizzi, E.B.; Schinina, V.; Cristofaro, M.; Goletti, D.; Palmieri, F.; Bevilacqua, N.; Lauria, F.N.; Girardi, E.; Bibbolino, C. Detection of Pulmonary tuberculosis: Comparing MR imaging with HRCT. BMC Infect. Dis. 2011, 11. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, Z.; Shen, G.; Zhang, Y.; Li, L.; Wu, Z.; Luo, D.; Gu, Q.; Mao, H.; Wang, L. MRI evaluation of pulmonary lesions and lung tissue changes induced by tuberculosis. Int. J. Infect. Dis. 2019, 82, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Roach, D.J.; Cremillieux, Y.; Fleck, R.J.; Brody, A.S.; Serai, S.D.; Szczesniak, R.D.; Kerlakian, S.; Clancy, J.P.; Woods, J.C. Ultrashort Echo-Time Magnetic Resonance Imaging Is a Sensitive Method for the Evaluation of Early Cystic Fibrosis Lung Disease. Ann. Am. Thorac. Soc. 2016, 13, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Schloss, M.; Heckrodt, J.; Schneider, C.; Discher, T.; Krombach, G.A. Magnetic Resonance Imaging of the Lung as an Alternative for a Pregnant Woman with Pulmonary Tuberculosis. J. Radiol. Case Rep. 2015, 9, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.; Jeon, K.N.; Hwang, M.J.; Lee, J.S.; Ha, J.Y.; Ryu, K.H.; Kim, H.C. Comparison of lung imaging using three-dimensional ultrashort echo time and zero echo time sequences: Preliminary study. Eur. Radiol. 2019, 29, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- Oakes, J.M.; Scadeng, M.; Breen, E.C.; Prisk, G.K.; Darquenne, C. Regional Distribution of Aerosol Deposition in Rat Lungs Using Magnetic Resonance Imaging. Ann. Biomed. Eng. 2013, 41, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Oakes, J.M.; Breen, E.C.; Scadeng, M.; Tchantchou, G.S.; Darquenne, C. MRI-based measurements of aerosol deposition in the lung of healthy and elastase-treated rats. J. Appl. Physiol. 2014, 116, 1561–1568. [Google Scholar] [CrossRef]
- Wang, H.C.; Sebrie, C.; Ruaud, J.P.; Guillot, G.; Bouazizi-Verdier, K.; Willoquet, G.; Maitre, X.; Darrasse, L.; de Rochefort, L. Aerosol Deposition in the Lungs of Spontaneously Breathing Rats Using Gd-DOTA-Based Contrast Agents and Ultra-Short Echo Time MRI at 1.5 Tesla. Magn. Reson. Med. 2016, 75, 594–605. [Google Scholar] [CrossRef]
- Wang, H.C.; Sebrie, C.; Jude, S.; Maurin, A.; Retif, S.; Le Mee, M.; Julea, F.; Dubuisson, R.M.; Willoquet, G.; Bouazizi, K.; et al. Quantitative Gd-DOTA-based aerosol deposition mapping in the lungs of asthmatic rats using 3D UTE-MRI. NMR Biomed. 2018, 31. [Google Scholar] [CrossRef]
- Sethi, T.; Agrawal, A. Structure and function of the tuberculous lung: Considerations for inhaled therapies. Tuberculosis 2011, 91, 67–70. [Google Scholar] [CrossRef]
- Ahmed, N.; Fessi, H.; Elaissari, A. Theranostic applications of nanoparticles in cancer. Drug Discov. Today 2012, 17, 928–934. [Google Scholar] [CrossRef]
- Picard, F.J.; Bergeron, M.G. Rapid molecular theranostics in infectious diseases. Drug Discov. Today 2002, 7, 1092–1101. [Google Scholar] [CrossRef]
- Terreno, E.; Uggeri, F.; Aime, S. Image guided therapy: The advent of theranostic agents. J. Control. Release 2012, 161, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Funkhouser, J. Reinventing pharma: The theranostic revolution. Curr. Drug Discov. 2002, 2, 17–19. [Google Scholar]
- Sumer, B.; Gao, J. Theranostic nanomedicine for cancer. Nanomedicine 2008, 3, 137–140. [Google Scholar] [CrossRef]
- Lee, D.Y.; Li, K.C.P. Molecular Theranostics: A Primer for the Imaging Professional. Am. J. Roentgenol. 2011, 197, 318–324. [Google Scholar] [CrossRef]
- Furukawa, S.; Reboul, J.; Diring, S.; Sumida, K.; Kitagawa, S. Structuring of metal-organic frameworks at the mesoscopic/macroscopic scale. Chem. Soc. Rev. 2014, 43, 5700–5734. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Wyszogrodzka, G.; Dorozynski, P.; Gil, B.; Roth, W.J.; Strzempek, M.; Marszalek, B.; Weglarz, W.P.; Menaszek, E.; Strzempek, W.; Kulinowski, P. Iron-Based Metal-Organic Frameworks as a Theranostic Carrier for Local Tuberculosis Therapy. Pharm. Res. 2018, 35, 144. [Google Scholar] [CrossRef]
- Wu, M.X.; Yang, Y.W. Metal-Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29, 20. [Google Scholar] [CrossRef]
- Wyszogrodzka, G.; Marszalek, B.; Gil, B.; Dorozynski, P. Metal-organic frameworks: Mechanisms of antibacterial action and potential applications. Drug Discov. Today 2016, 21, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.Q.; Zhang, Y.N.; Liu, L.F.; Wan, W.; Guo, P.; Nystrom, A.M.; Zou, X.D. One-pot Synthesis of Metal Organic Frameworks with Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Hickey, A.J.; Durham, P.G.; Dharmadhikari, A.; Nardell, E.A. Inhaled drug treatment for tuberculosis: Past progress and future prospects. J. Control. Release 2016, 240, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Hickey, A.J.; Rossi, C.; Borchard, G.; Terada, H.; Makino, K.; Fourie, P.B.; Colombo, P. Inhaled drug therapy for treatment of tuberculosis. Tuberculosis 2011, 91, 71–81. [Google Scholar] [CrossRef]
- Bauer, S.; Serre, C.; Devic, T.; Horcajada, P.; Marrot, J.; Ferey, G.; Stock, N. High-throughput assisted rationalization of the formation of metal organic frameworks in the iron(III) aminoterephthalate solvothermal system. Inorg. Chem. 2008, 47, 7568–7576. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous optimization of several response variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Kleinman, L.I.; Radford, E.P., Jr. Ventilation standards for small mammals. J. Appl. Physiol. 1964, 19, 360–362. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.; Cardona, A.; Sebastian, H. Seung, Trainable Weka Segmentation: A machine learning tool for microscopy pixel classification. Bioinformatics 2017, 33, 15. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical Particle Engineering via Spray Drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharm. 2003, 56, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Rees, P.J.; Clark, T.J.; Morén, F. The importance of particle size in response to inhaled bronchodilators. Eur. J. Respir. Dis. Suppl. 1982, 119, 73–78. [Google Scholar] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.D.; Fattal, E.; Tsapis, N. Pulmonary drug delivery systems for tuberculosis treatment. Int. J. Pharm. 2015, 478, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Hasegawa, T.; Nakajima, T.; Inagawa, H.; Kohchi, C.; Soma, G.I.; Makino, K.; Terada, H. Delivery of rifampicin-PLGA microspheres into alveolar macrophages is promising for treatment of tuberculosis. J. Control. Release 2010, 142, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Nicolete, R.; dos Santos, D.F.; Faccioli, L.H. The uptake of PLGA micro or nanoparticles by macrophages provokes distinct in vitro inflammatory response. Int. Immunopharmacol. 2011, 11, 1557–1563. [Google Scholar] [CrossRef]
- Takeuchi, I.; Taniguchi, Y.; Tamura, Y.; Ochiai, K.; Makino, K. Effects of L-leucine on PLGA microparticles for pulmonary administration prepared using spray drying: Fine particle fraction and phagocytotic ratio of alveolar macrophages. Colloids Surf. A-Phys. Eng. Asp. 2018, 537, 411–417. [Google Scholar] [CrossRef]
- Bryk, R.; Lima, C.D.; Erdjument-Bromage, H.; Tempst, P.; Nathan, C. Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein. Science 2002, 295, 1073–1077. [Google Scholar] [CrossRef]
- Jaeger, T.; Budde, H.; Flohe, L.; Menge, U.; Singh, M.; Trujillo, M.; Radi, R. Multiple thioredoxin-mediated routes to detoxify hydroperoxides in Mycobacterium tuberculosis. Arch. Biochem. Biophys. 2004, 423, 182–191. [Google Scholar] [CrossRef]
- Newton, G.L.; Arnold, K.; Price, M.S.; Sherrill, C.; Delcardayre, S.B.; Aharonowitz, Y.; Cohen, G.; Davies, J.; Fahey, R.C.; Davis, C. Distribution of thiols in microorganisms: Mycothiol is a major thiol in most actinomycetes. J. Bacteriol. 1996, 178, 1990–1995. [Google Scholar] [CrossRef]
- Vilcheze, C.; Hartman, T.; Weinrick, B.; Jacobs, W.R. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat. Commun. 2013, 4, 1881. [Google Scholar] [CrossRef]
- Voskuil, M.I.; Bartek, I.L.; Visconti, K.; Schoolnik, G.K. The response of Mycobacterium tuberculosis to reactive oxygen and nitrogen species. Front. Microbiol. 2011, 2, 105. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, N.P.; Durham, P.; Hickey, A.J. The role of particle physico-chemical properties in pulmonary drug delivery for tuberculosis therapy. J. Microencapsul. 2014, 31, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.C.; Pulliam, B.L.; Edwards, D.A. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007, 25, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.L.; Zhang, X.M. A lung phantom model to study pulmonary edema using lung ultrasound surface wave elastography. Ultrasound Med. Biol. 2018, 44, 2400–2405. [Google Scholar] [CrossRef] [PubMed]
- Molinari, F.; Madhuranthakam, A.J.; Lenkinski, R.; Bankier, A.A. Ultrashort echo time MRI of pulmonary water content: Assessment in a sponge phantom at 1.5 and 3.0 Tesla. Diagn. Interv. Radiol. 2014, 20, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, Y.; Biggs, D.L.; Manning, M.C.; Randolph, T.W.; Christians, U.; Hybertson, B.M.; Ng, K. Microparticle-based lung delivery of INH decreases INH metabolism and targets alveolar macrophages. J. Control. Release 2005, 107, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.K.; Kaur, J.; Kumar, K.; Yadav, A.B.; Misra, A. Intracellular time course, pharmacokinetics, and biodistribution of isoniazid and rifabutin following pulmonary delivery of inhalable microparticles to mice. Antimicrob. Agents Chemother. 2008, 52, 3195–3201. [Google Scholar] [CrossRef]
- Sen, H.; Jayanthi, S.; Sinha, R.; Muttil, P. Microsphere of Two Anti-Tubercular Drugs and a Biodegradable Polymer Administred by Inhalation Alone or Combined with Oral Route and Process Thereoff. Patent Application WO 2005/039529 A1, 6 May 2005. [Google Scholar]
- Monteiro, A.; Smith, R.L. Bronchial tree Architecture in Mammals of Diverse Body Mass. Int. J. Morphol. 2014, 32, 312–318. [Google Scholar] [CrossRef]
- Verma, R.K.; Mukker, J.K.; Singh, R.S.P.; Kumar, K.; Verma, P.R.P.; Misra, A. Partial Biodistribution and Pharmacokinetics of Isoniazid and Rifabutin Following Pulmonary Delivery of Inhalable Microparticles to Rhesus Macaques. Mol. Pharm. 2012, 9, 1011–1016. [Google Scholar] [CrossRef]
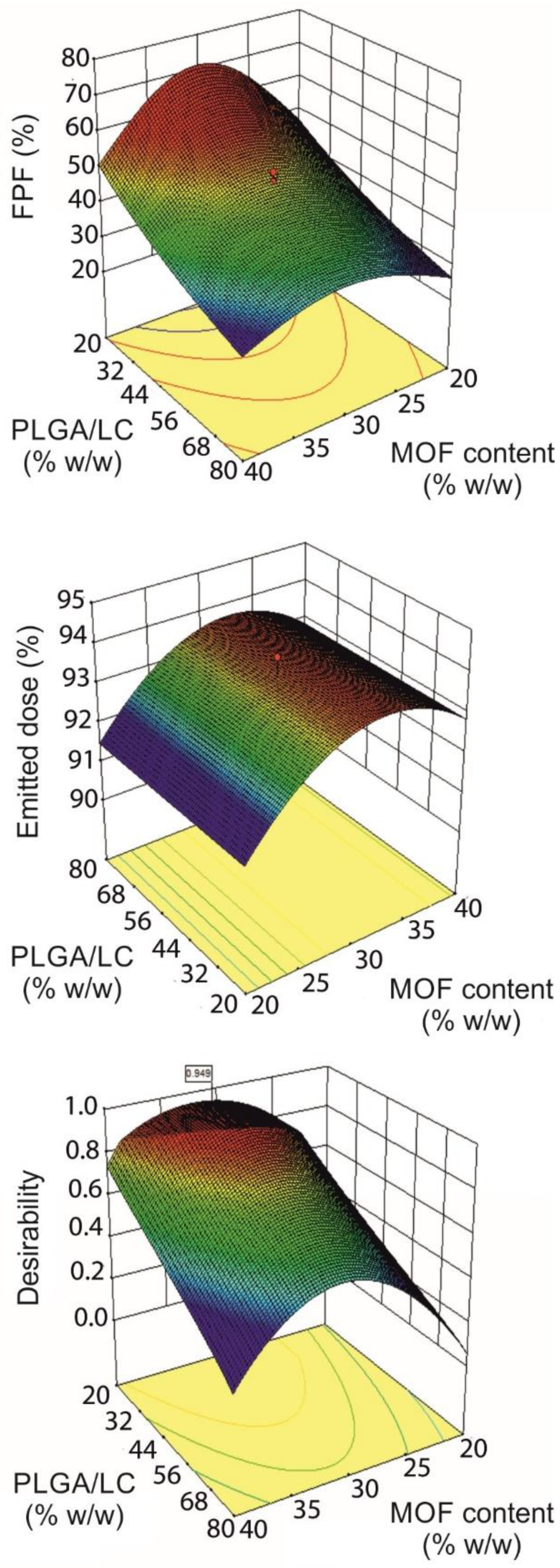



| Run | Factors | Responses | Content Homogeneity p-Value (n = 9) | ||||
|---|---|---|---|---|---|---|---|
| A | B | C | |||||
| INH-MOF Content [% w/w] | Blending Ratio [% w/w] | Blending Time [min] | %FPF | %ED | |||
| PLGA | LC | ||||||
| 1 | 20 | 20 | 80 | 5 | 48.06 | 91.29 | 0.078 |
| 2 | 20 | 80 | 20 | 5 | 25.99 | 90.91 | 0.840 |
| 3 | 20 | 20 | 80 | 15 | 46.50 | 91.90 | 0.711 |
| 4 | 20 | 80 | 20 | 15 | 26.86 | 91.85 | 0.368 |
| 5 | 40 | 20 | 80 | 5 | 47.17 | 93.19 | 0.103 |
| 6 | 40 | 80 | 20 | 5 | 28.18 | 92.00 | 0.483 |
| 7 | 40 | 20 | 80 | 15 | 54.78 | 93.30 | 0.069 |
| 8 | 40 | 80 | 20 | 15 | 28.06 | 92.97 | 0.380 |
| 9 | 30 | 50 | 50 | 10 | 52.29 | 94.01 | 0.740 |
| 10 | 30 | 50 | 50 | 10 | 45.75 | 93.35 | 0.192 |
| 11 | 30 | 50 | 50 | 10 | 49.43 | 93.50 | 0.128 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyszogrodzka-Gaweł, G.; Dorożyński, P.; Giovagnoli, S.; Strzempek, W.; Pesta, E.; Węglarz, W.P.; Gil, B.; Menaszek, E.; Kulinowski, P. An Inhalable Theranostic System for Local Tuberculosis Treatment Containing an Isoniazid Loaded Metal Organic Framework Fe-MIL-101-NH2—From Raw MOF to Drug Delivery System. Pharmaceutics 2019, 11, 687. https://doi.org/10.3390/pharmaceutics11120687
Wyszogrodzka-Gaweł G, Dorożyński P, Giovagnoli S, Strzempek W, Pesta E, Węglarz WP, Gil B, Menaszek E, Kulinowski P. An Inhalable Theranostic System for Local Tuberculosis Treatment Containing an Isoniazid Loaded Metal Organic Framework Fe-MIL-101-NH2—From Raw MOF to Drug Delivery System. Pharmaceutics. 2019; 11(12):687. https://doi.org/10.3390/pharmaceutics11120687
Chicago/Turabian StyleWyszogrodzka-Gaweł, Gabriela, Przemysław Dorożyński, Stefano Giovagnoli, Weronika Strzempek, Edyta Pesta, Władysław P. Węglarz, Barbara Gil, Elżbieta Menaszek, and Piotr Kulinowski. 2019. "An Inhalable Theranostic System for Local Tuberculosis Treatment Containing an Isoniazid Loaded Metal Organic Framework Fe-MIL-101-NH2—From Raw MOF to Drug Delivery System" Pharmaceutics 11, no. 12: 687. https://doi.org/10.3390/pharmaceutics11120687
APA StyleWyszogrodzka-Gaweł, G., Dorożyński, P., Giovagnoli, S., Strzempek, W., Pesta, E., Węglarz, W. P., Gil, B., Menaszek, E., & Kulinowski, P. (2019). An Inhalable Theranostic System for Local Tuberculosis Treatment Containing an Isoniazid Loaded Metal Organic Framework Fe-MIL-101-NH2—From Raw MOF to Drug Delivery System. Pharmaceutics, 11(12), 687. https://doi.org/10.3390/pharmaceutics11120687