Poloxamer Hydrogels for Biomedical Applications
Abstract
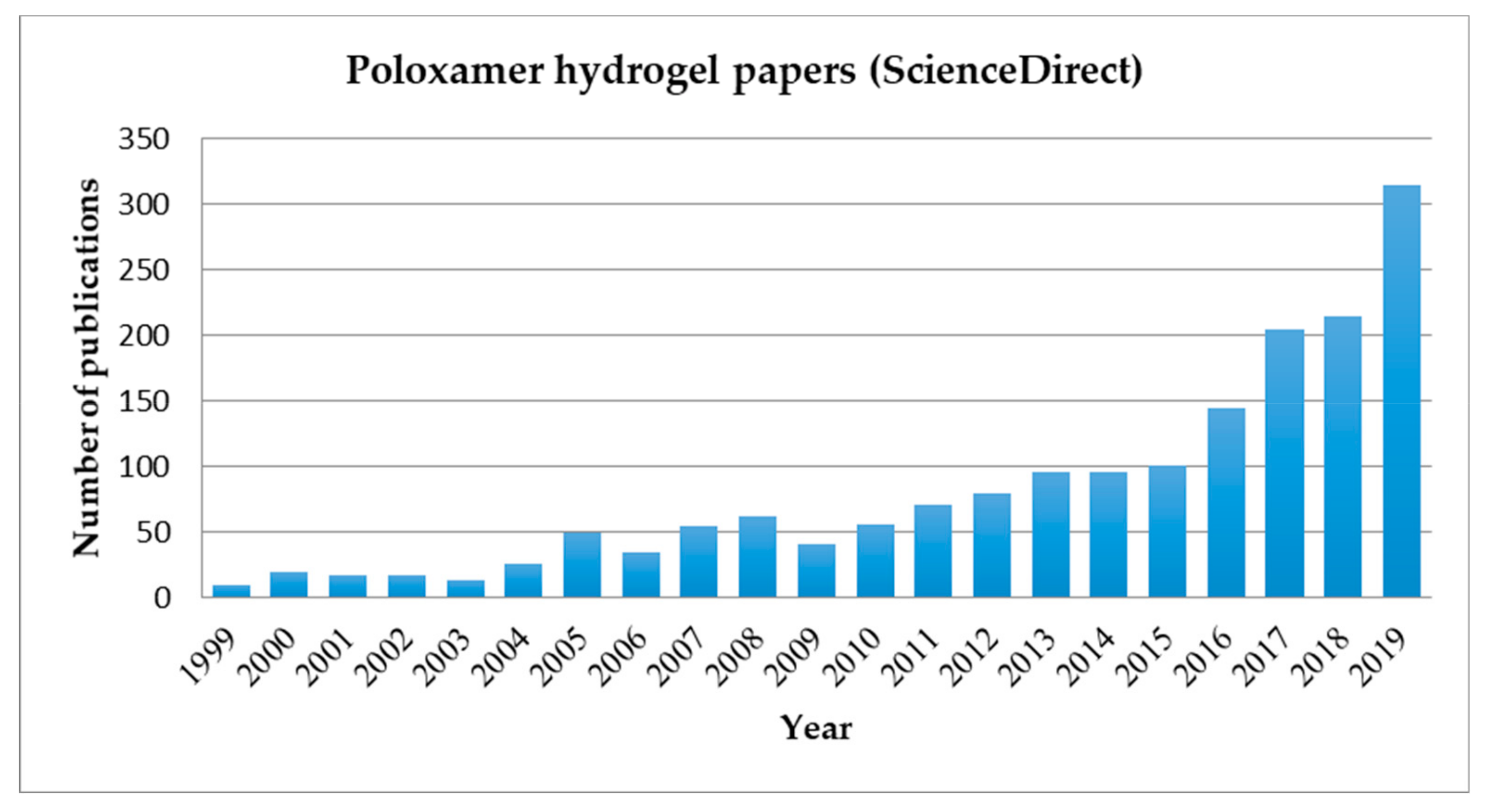
1. Introduction
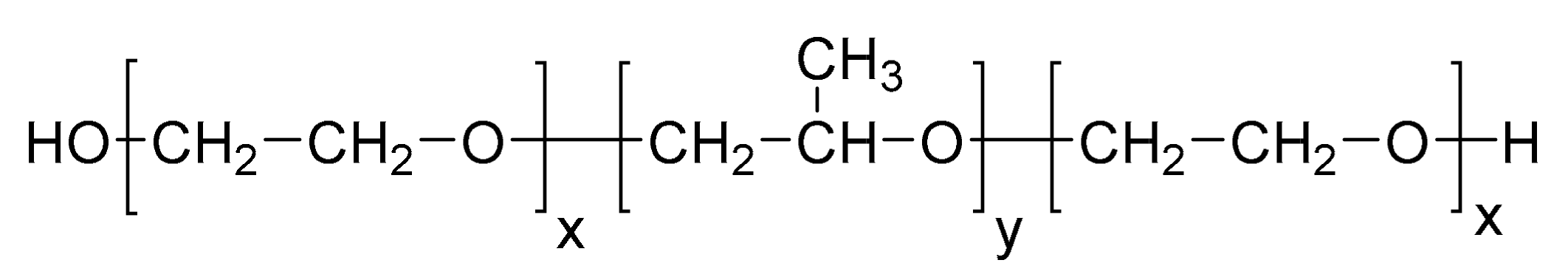
2. Poloxamers
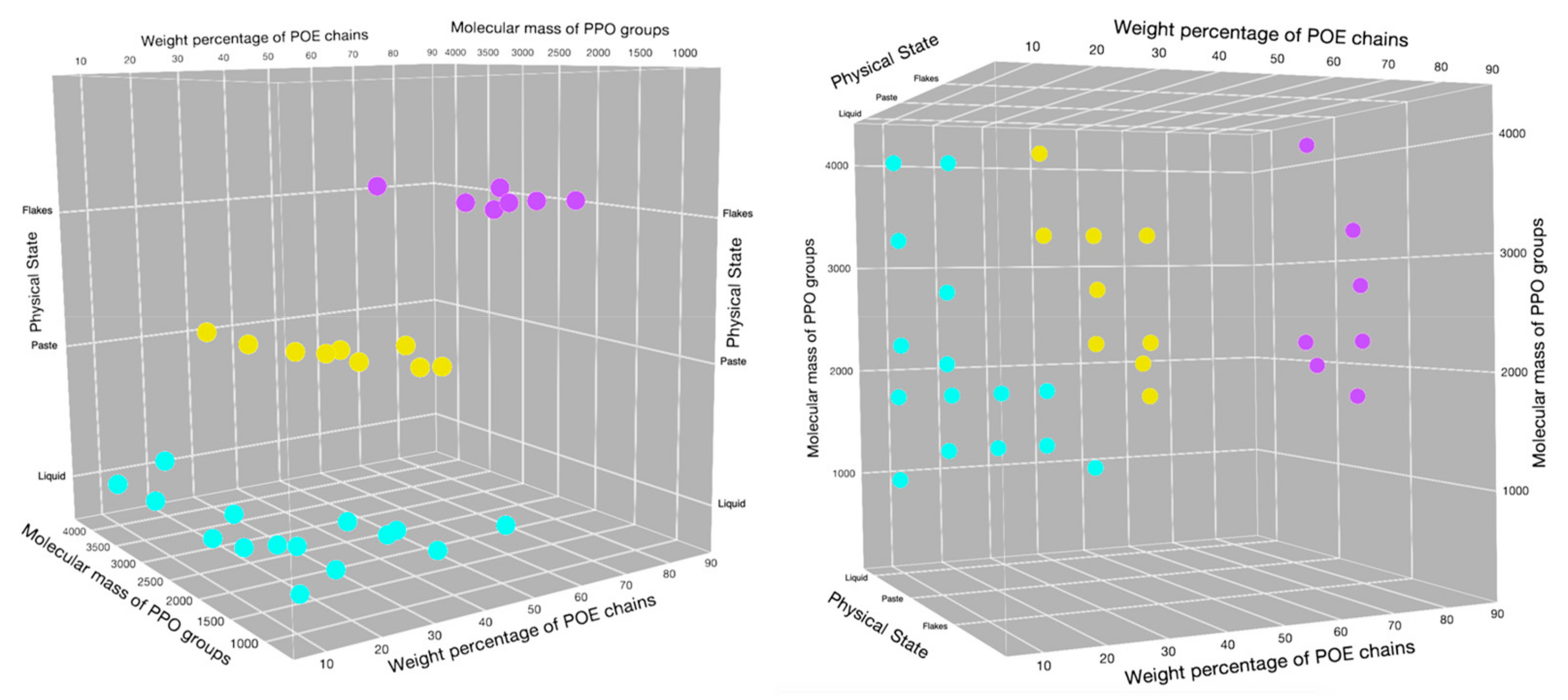
2.1. Poloxamers Properties
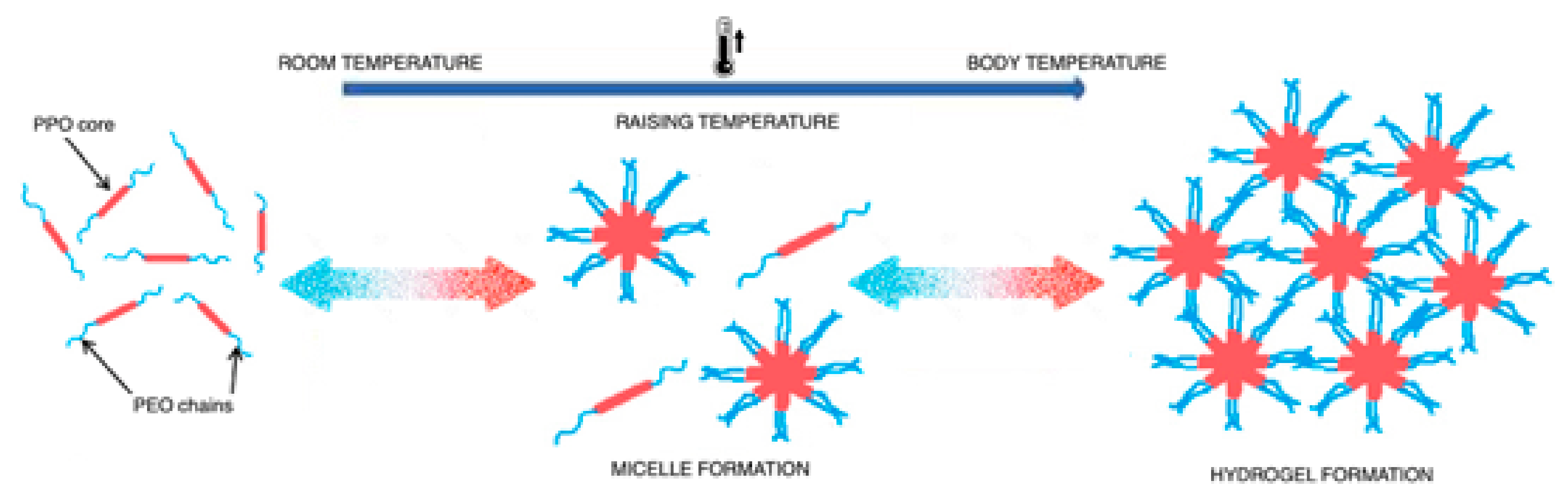
2.2. Poloxamers Behavior
3. Drug Delivery Systems (DDS)
3.1. Poloxamers for Oftalmic Administration
3.2. Poloxamers for Transdermal Administration
3.3. Poloxamers for Vaginal Administration
4. Tissue Regeneration Scaffolders
5. Poloxamer as Micellar Systems
6. Summary
Funding
Conflicts of Interest
References
- Wichterle, O.; Lim, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Gioffredi, E.; Boffito, M.; Calzone, S.; Giannitelli, S.M.; Rainer, A.; Trombetta, M.; Mozetic, P.; Chiono, V. Pluronic F127 hydrogel characterization and biofabrication in cellularized constructs for tissue engineering applications. Procedia Cirp 2016, 49, 125–132. [Google Scholar] [CrossRef]
- Almeida, M.; Magalhães, M.; Veiga, F.; Figueiras, A. Poloxamers, poloxamines and polymeric micelles: Definition, structure and therapeutic applications in cancer. J. Polym. Res. 2018, 25, 31. [Google Scholar] [CrossRef]
- Aguilar, M.R.; Elvira, C.; Gallardo, A.; Vázquez, B.; Román, J.S. Smart Polymers and Their Applications as Biomaterials. In Topics in Tissue Engineering; Ashammakhi, N., Reis, R.L., Chiellini, E., Eds.; Biomaterials and Tissue Engineering Group: Oulu, Finland, 2007; Volume 3. [Google Scholar]
- Johnston, T.P.; Palmer, W.K. Mechanism of poloxamer 407-induced hypertriglyceridemia in the rat. Biochem. Pharm. 1993, 46, 1037–1042. [Google Scholar] [CrossRef]
- Li, J.; Stachowski, M.; Zhang, Z. Application of responsive polymers in implantable medical devices and biosensors. In Switchable and Responsive Surfaces and Materials for Biomedical Applications; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2015. [Google Scholar]
- Nalbandian, R.M.; Henry, R.L.; Wilks, H.S. Artificial skin. II. Pluronic F-127 Silver nitrate or silver lactate gel in the treatment of thermal burns. J. Biomed. Mater. Res. 1972, 6, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Law, T.; Florence, A.T.; Whateley, T.L. Some chemically modified poloxamer hydrogels-controlled release of prostaglandin-e2 and testosterone. Int. J. Pharm. 1986, 33, 65–69. [Google Scholar] [CrossRef]
- Esposito, E.; Carotta, V.; Scabbia, A.; Trombelli, L.; D’Antona, P.; Menegatti, E.; Nastruzzi, C. Comparative analysis of tetracycline-containing dental gels: Poloxamer- and monoglyceride-based formulations. Int. J. Pharm. 1996, 142, 9–23. [Google Scholar] [CrossRef]
- Stratton, L.P.; Dong, A.; Manning, M.C.; Carpenter, J.F. Drug delivery matrix containing native protein precipitates suspended in a poloxamer gel. J. Pharm. Sci. 1997, 86, 1006–1010. [Google Scholar] [CrossRef]
- Xie, M.H.; Ge, M.; Peng, J.B.; Jiang, X.R.; Wang, D.S.; Ji, L.Q.; Ying, Y.; Wang, Z. In-vivo anti-tumor activity of a novel poloxamer-based thermosensitive in situ gel for sustained delivery of norcantharidin. Pharm. Dev. Technol. 2019, 24, 623–629. [Google Scholar] [CrossRef]
- He, C.; Ji, H.; Qian, Y.; Wang, Q.; Liu, X.; Zhao, W.; Zhao, C. Heparin-based and heparin-inspired hydrogels: Size-effect, gelation and biomedical applications. J. Mater. Chem. B 2019, 7, 1186–1208. [Google Scholar] [CrossRef]
- Tian, W.; Han, S.; Huang, X.; Han, M.; Cao, J.; Liang, Y.; Sun, Y. LDH hybrid thermosensitive hydrogel for intravaginal delivery of anti-HIV drugs. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Alexandridis, P.; Hatton, T.A. Poly(ethylene oxide)-poly(propylene oxide )-poly (ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Singh-Joy, S.D.; McLain, V.C. Safety Assessment of Poloxamers 101, 105, 108, 122, 123, 124, 181, 182, 183, 184, 185, 188, 212, 215, 217, 231, 234, 235, 237, 238, 282, 284, 288, 331, 333, 334, 335, 338, 401, 402, 403, and 407, Poloxamer 105 Benzoate, and Poloxamer 182 Dibenzoate as Used in Cosmetics. Int. J. Toxicol. 2008, 27, 93–128. [Google Scholar]
- Pitto-Barry, A.; Barry, N.P. Pluronic® block-copolymers in medicine: From chemical and biological versatility to rationalization and clinical advances. Polym. Chem. 2014, 5, 3281–3496. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 154–162. [Google Scholar] [CrossRef]
- Devi, D.R.; Sandhya, P.; Hari, B.V. Poloxamer: A novel functional molecule for drug delivery and gene therapy. J. Pharm. Sci. Res. 2013, 5, 159–165. [Google Scholar]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of Poly(Ethylene Oxide)-Poly(Propylene Oxide)-Poly(Ethylene Oxide) Triblock Copolymers in Aqueous-Solutions-Thermodynamics of Copolymer Association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Simões, S.M.; Figueiras, A.R.; Veiga, F.; Concheiro, A.; Alvarez-Lorenzo, C. Polymeric micelles for oral drug administration enabling loco regional and systemic treatments. Expert Opin. Drug Deliv. 2015, 12, 297–318. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Vadnere, M.; Amidon, G.L.; Lindenbaum, S.; Haslam, J.L. Thermodynamic studies on the gel-sol transition of some pluronic polyols. Int. J. Pharm. 1984, 22, 207. [Google Scholar] [CrossRef]
- Cho, C.W.; Cho, Y.S.; Lee, H.K.; Yeom, Y.I.; Park, S.N.; Yoon, D.Y. Improvement of receptor-mediated gene delivery to HepG2 cells using an amphiphilic gelling agent. Biotechnol. Appl. Biochem. 2000, 32, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Morishita, M.; Barichello, J.M.; Takayama, K.; Chiba, Y.; Tokwa, S.; Nagai, T. Pluronic F-127 gels incorporating highly purified unsaturated fatty acids for buccal delivery of insulin. Int. J. Pharm. 2001, 212, 289–293. [Google Scholar] [CrossRef]
- Johnston, T.P.; Punjabi, M.A.; Froelich, C.J. Sustained delivery of interleukin-2 from a Poloxamer 407 gel matrix following intraperitoneal injection in mice. Pharm. Res. 1992, 9, 425–434. [Google Scholar] [CrossRef]
- DiBiase, M.D.; Rhodes, C.T. Investigations of epidermal growth factor in semisolid formulations. Pharm. Acta Helv. 1991, 66, 165–169. [Google Scholar]
- Clokie, C.M.; Urist, M.R. Bone morphogenic protein excipients: Comparative observations on poloxamer. Plast. Reconstr. Surg. 2000, 105, 628–637. [Google Scholar] [CrossRef]
- Hom, D.B.; Medhi, K.; Assefa, G.; Juhn, S.K.; Johnston, T.P. Vascular effects of sustained-release fibroblast growth factors. Ann. Otol. Rhinol. Laryngol. 1996, 105, 109–116. [Google Scholar] [CrossRef]
- Al Khateb, K.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef]
- Fathalla, Z.M.; Vangala, A.; Longman, M.; Khaled, K.A.; Hussein, A.K.; El-Garhy, O.H.; Alany, R.G. Poloxamer-based thermoresponsive ketorolac tromethamine in situ gel preparations: Design, characterisation, toxicity and transcorneal permeation studies. Eur. J. Pharm. Biopharm. 2017, 114, 119–134. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, X.; Tan, G.; Tian, L.; Liu, D.; Liu, Y.; Yang, X.; Pan, W. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr. Polym. 2017, 155, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Hu, W.; Tian, R.; Zhang, H.; Jia, Y.; Zhang, J.; Zhang, L. Optimization and evaluation of a thermoresponsive ophthalmic in situ gel containing curcumin-loaded albumin nanoparticles. Int. J. Nanomed. 2014, 9, 2517–2525. [Google Scholar]
- Almeida, H.; Lobão, P.; Frigerio, C.; Fonseca, J.; Silva, R.; Sousa Lobo, J.M.; Amaral, M.H. Preparation, characterization and biocompatibility studies of thermoresponsive eyedrops based on the combination of nanostructured lipid carriers (NLC) and the polymer Pluronic F-127 for controlled delivery of ibuprofen. Pharm. Dev. Technol. 2017, 22, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Cafaggi, S.; Russo, E.; Caviglioli, G.; Parodi, B.; Stefani, R.; Sillo, G.; Leardi, R.; Bignardi, G. Poloxamer 407 as a solubilising agent for tolfenamic acid and as a base for a gel formulation. Eur. J. Pharm. Sci. 2008, 35, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Ur-Rehman, T.; Tavelin, S.; Gröbner, G. Effect of DMSO on micellization, gelation and drugrelease profile of Poloxamer 407. Int. J. Pharm. 2010, 394, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Liaw, J.; Lin, Y.C. Evaluation of poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) (PEO–PPO–PEO) gels as a release vehicle for percutaneous fentanyl. J. Control. Release 2000, 68, 273–282. [Google Scholar] [CrossRef]
- Shin, S.C.; Cho, C.W.; Yang, K.H. Development of lidocaine gels for enhanced local anesthetic action. Int. J. Pharm. 2004, 287, 73–78. [Google Scholar] [CrossRef]
- Suedee, R.; Bodhibukkana, C.; Tangthong, N.; Amnuaikit, C.; Kaewnopparat, S.; Srichana, T. Development of a reservoir-type transdermal enantioselective-controlled delivery system for racemic propranolol using a molecularly imprinted polymer composite membrane. J. Control. Release 2008, 129, 170–178. [Google Scholar] [CrossRef]
- Stamatialis, D.F.; Rolevink, H.H.M.; Koops, G.H. Transdermal timolol delivery from a Pluronic gel. J. Control. Release 2006, 116, 53–65. [Google Scholar] [CrossRef]
- Nair, V.; Panchagnula, R. Poloxamer gel as vehicle for transdermal iontophoretic delivery of arginine vasopressin: Evaluation of in vivo performance in rats. Pharm. Res. 2003, 47, 555–562. [Google Scholar] [CrossRef]
- Pillai, O.; Panchagnula, R. Transdermal delivery of insulin from poloxamer gel: Ex vivo and in vivo skin permeation studies in rat using iontophoresis and chemical enhancers. J. Control. Release 2003, 89, 127–140. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Morrow, D.I.; McCarron, P.A.; Woolfson, A.D.; Morrissey, A.; Juzenas, P.; Juzeniene, A.; Iani, V.; McCarthy, H.O.; Moan, J. Microneedle-mediated intradermal delivery of 5-aminolevulinic acid: Potential for enhanced topical photodynamic therapy. J. Control. Release 2008, 129, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Flam, B.R.; Osborn, B.; Strom, J.A.; Bhansali, S. Skin penetration and fracture strength testing of silicon dioxide microneedles. Sens. Actuators A 2011, 170, 180–186. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Brazzle, J.D.; Frazier, A.B. Surface micromachined metallic microneedles. J. Microelectromech. Syst. 2003, 12, 281–288. [Google Scholar] [CrossRef]
- Gill, H.S.; Prausnitz, M.R. Coating formulations for microneedles. Pharm. Res. 2007, 24, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.R.; Rao, M.P.; Turner, K.L.; Meinhart, C.D.; MacDonald, N.C. Bulk micromachined titanium microneedles. J. Microelectromech. Syst. 2007, 16, 289–295. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Morrow, D.I.; Singh, T.R.; Migalska, K.; McCarron, P.A. Processing difficulties and instability of carbohydrate microneedle arrays. Drug Deliv. Ind. Pharm. 2009, 35, 1242–1254. [Google Scholar] [CrossRef]
- Lee, K.; Lee, C.Y.; Jung, H. Dissolving microneedles for transdermal drug administration prepared by stepwise controlled drawing of maltose. Biomaterials 2011, 32, 3134–3140. [Google Scholar] [CrossRef]
- Li, G.; Badkar, A.; Nema, S.; Kolli, C.S.; Banga, A.K. In vitro transdermal delivery of therapeutic antibodies using maltose microneedles. Int. J. Pharm. 2009, 368, 109–115. [Google Scholar] [CrossRef]
- Wang, P.M.; Cornwell, M.; Hill, J.; Prausnitz, M.R. Precise microinjection into skin using hollow microneedles. J. Investig. Derm. 2006, 126, 1080–1087. [Google Scholar] [CrossRef]
- Bystrova, S.; Luttge, R. Micromolding for ceramic microneedle arrays. Microelectron. Eng. 2011, 88, 1681–1684. [Google Scholar] [CrossRef]
- Ito, Y.; Murano, H.; Hamasaki, N.; Fukushima, K.; Takada, K. Incidence of low bioavailability of leuprolide acetate after percutaneous administration to rats by dissolving microneedles. Int. J. Pharm. 2011, 407, 126–131. [Google Scholar] [CrossRef]
- Noh, Y.W.; Kim, T.H.; Baek, J.S.; Park, H.H.; Lee, S.S.; Han, M.; Shin, S.C.; Cho, C.W. In vitro characterization of the invasiveness of polymer microneedle against skin. Int. J. Pharm. 2010, 397, 201–205. [Google Scholar] [CrossRef]
- Sachdeva, V.; Banga, A.K. Microneedles and their applications. Recent Pat. Drug Deliv. Formul. 2011, 5, 95–132. [Google Scholar] [CrossRef]
- Thakur, R.R.S.; Fallows, S.J.; McMillan, H.L.; Donnelly, R.F.; Jones, D.S. Microneedle-mediated intrascleral delivery of in situ forming thermoresponsive implants for sustained ocular drug delivery. J. Pharm Pharm. Pharmacol. 2014, 66, 584–595. [Google Scholar] [CrossRef]
- Gilger, B.C.; Abarca, E.M.; Salmon, J.H.; Patel, S. Treatment of acute posterior uveitis in a porcine model by injection of triamcinolone acetonide into the suprachoroidal space using microneedles. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2483–2492. [Google Scholar] [CrossRef]
- Patel, S.R.; Lin, A.S.; Edelhauser, H.F.; Prausnitz, M.R. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm. Res. 2011, 28, 166–176. [Google Scholar] [CrossRef]
- Roxhed, N.; Griss, P.; Stemme, G. Membrane-sealed hollow microneedles and related administration schemes for transdermal drug delivery. Biomed. Microdevices 2008, 10, 271–279. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Chichkov, B.; Mente, P.; Monteiro-Riviere, N.A.; Doraiswamy, A.; Narayan, R.J. Two photon polymerization of polymer–ceramic hybrid materials for transdermal drug delivery. Int. J. Appl. Ceram Technol. 2007, 4, 22–29. [Google Scholar] [CrossRef]
- Sivaraman, A.; Banga, A.K. Novel in situ forming hydrogel microneedles for transdermal drug delivery. Drug Deliv. Transl. Res. 2017, 7, 16–26. [Google Scholar] [CrossRef]
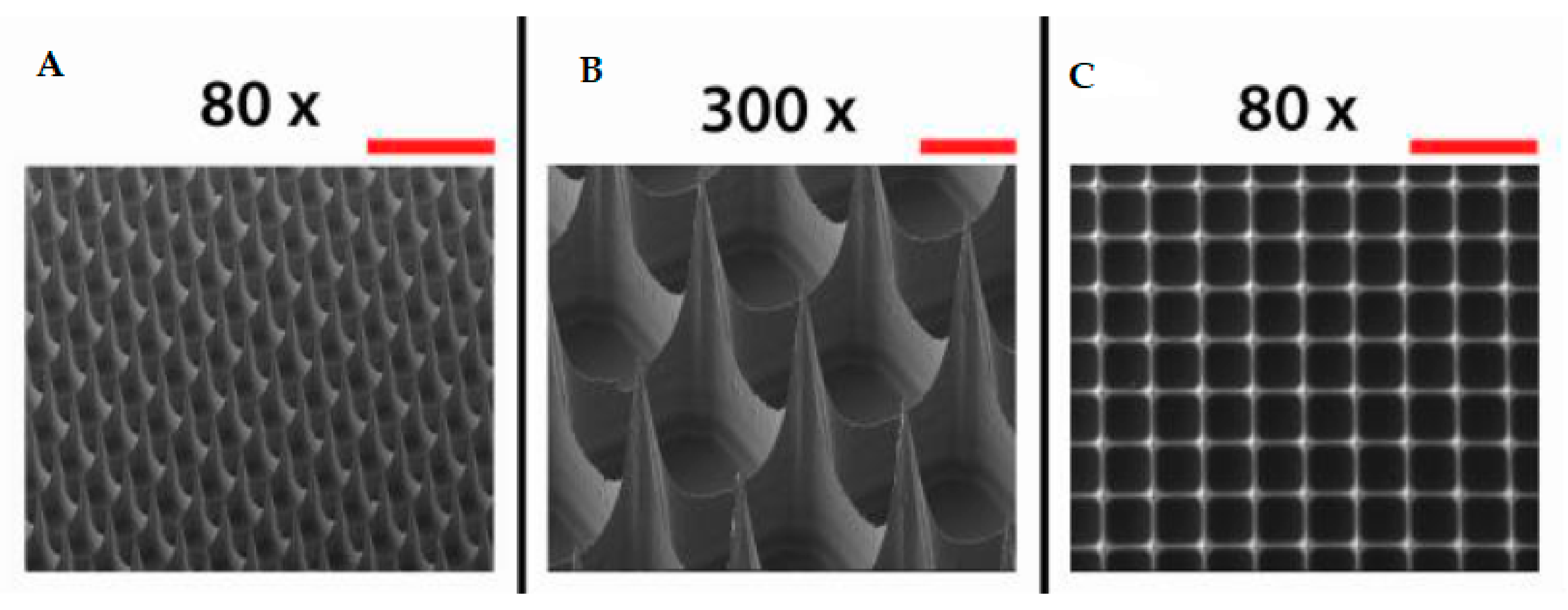
- Khan, S.; Minhas, M.U.; Tekko, I.A.; Donnelly, R.F.; Thakur, R.R.S. Evaluation of microneedles-assisted in situ depot forming poloxamer gels for sustained transdermal drug delivery. Drug Deliv. Transl. Res. 2019, 9, 764–782. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; van Oorschot, B.H.; Nejadnik, M.R.; Bocchino, A.; Rosato, M.; Kersten, G.; O’Mahony, C.; Bouwstra, J.; van der Maaden, K. Universal applicator for digitally-controlled pressing force and impact velocity insertion of microneedles into skin. Pharmaceutics 2018, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, C.K.; Nayak, P.K.; Sarangi, D.K.; Sahoo, T.K. Intra vaginal drug delivery system: An overview. Am. J. Adv. Drug Deliv. 2013, 1, 43–55. [Google Scholar]
- Ndesendo, V.M.; Pillay, V.; Choonara, Y.E.; Buchmann, E.; Bayever, D.N.; Meyer, L.C. A review of current intravaginal drug delivery approaches employed for the prophylaxis of HIV/AIDS and prevention of sexually transmitted infections. AAPS Pharmscitech 2008, 9, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Ahsan, F. The vagina as a route for systemic drug delivery. J. Control. Release 2005, 103, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Taurin, S.; Almomen, A.A.; Pollak, T.; Kim, S.J.; Maxwell, J.; Peterson, C.M.; Owen, S.C.; Janát-Amsbury, M.M. Thermosensitive hydrogels a versatile concept adapted to vaginal drug delivery. J. Drug Target. 2018, 26, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef]
- Ci, T.; Yuan, L.; Bao, X.; Hou, Y.; Wu, H.; Sun, H.; Cao, D.; Ke, X. Development and anti-Candida evaluation of the vaginal delivery system of amphotericin B nanosuspension-loaded thermogel. J. Drug Target. 2018, 26, 829–839. [Google Scholar] [CrossRef]
- Ibrahim, E.S.; Ismail, S.; Fetih, G.; Shaaban, O.; Hassanein, K.; Abdellah, N. Development and characterization of thermosensitive pluronic-based metronidazole in situ gelling formulations for vaginal application. Acta Pharma. 2012, 62, 59–70. [Google Scholar] [CrossRef]
- Xuan, J.-J.; Yan, Y.D.; Oh, D.H.; Choi, Y.K.; Yong, C.S.; Choi, H.G. Development of thermosensitive injectable hydrogel with sustained release of doxorubicin: Rheological characterization and in vivo evaluation in rats. Drug Deliv. 2011, 18, 305–311. [Google Scholar] [CrossRef]
- Hani, U.; Shivakamuar, H.G. Development of miconazole nitrate thermosensitive bioadhesive vaginal gel for vaginal candidiasis. Am. J. Adv. Drug Deliv. 2013, 3, 358–368. [Google Scholar]
- Mei, L.; Chen, J.; Yu, S.; Huang, Y.; Xie, Y.; Wang, H.; Pan, X.; Wu, C. Expansible thermal gelling foam aerosol for vaginal drug delivery. Drug Deliv. 2017, 24, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Holland, I.; Logan, J.; Shi, J.; McCormick, C.; Liu, D.; Shu, W. 3D biofabrication for tubular tissue engineering. Bio-Des. Manuf. 2018, 1, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Doğan, A.; Yalvaç, M.E.; Şahin, F.; Kabanov, A.V.; Palotás, A.; Rizvanov, A.A. Differentiation of human stem cells is promoted by amphiphilic pluronic block copolymers. Int. J. Nanomed. 2012, 7, 4849–4860. [Google Scholar]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from laboratory to clinic, part II (BMP delivery). J. Tissue Eng. Regen. Med. 2008, 2, 81–96. [Google Scholar] [CrossRef]
- Gittens, S.A.; Uludag, H. Growth factor delivery for bone tissue engineering. J. Drug Target. 2001, 9, 407–429. [Google Scholar] [CrossRef]
- Han, Q.Q.; Du, Y.; Yang, P.S. The role of small molecules in bone regeneration. Future Med. Chem. 2013, 5, 1671–1684. [Google Scholar] [CrossRef]
- Laurencin, C.T.; Ashe, K.M.; Henry, N.; Kan, H.M.; Lo, K.W.-H. Delivery of small molecules for bone regenerative engineering: Preclinical studies and potential clinical applications. Drug Discov. Today 2014, 19, 794–800. [Google Scholar] [CrossRef]
- Zhang, Y.; Bradley, A.D.; Wang, D.; Reinhardt, R.A. Statins, bone metabolism and treatment of bone catabolic diseases. Pharm. Res. 2014, 88, 53–61. [Google Scholar] [CrossRef]
- Rezazadeh, M.; Parandeh, M.; Akbari, V.; Ebrahimi, Z.; Taheri, A. Incorporation of rosuvastatin-loaded chitosan/ chondroitin sulfate nanoparticles into a thermosensitive hydrogel for bone tissue engineering: Preparation, characterization, and cellular behavior. Pharm. Dev. Technol. 2019, 24, 357–367. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; Greco, F.; Busilacchi, A.; Sollazzo, V.; Gigante, A. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: A review. Carbohydr. Polym. 2012, 89, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Temofeew, A.; Hixon, K.R.; McBride-Gagyi, S.H.; Scott, A. Sell The fabrication of cryogel scaffolds incorporated with poloxamer 407 for potential use in the regeneration of the nucleus pulposus. J. Mater. Sci. Mater. Med. 2017, 28, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Pattappa, G.; Li, Z.; Peroglio, M.; Wismer, N.; Alini, M.; Grad, S. Diversity of intervertebral disc cells: Phenoztype and function. J. Anat. 2012, 221, 480–496. [Google Scholar] [CrossRef] [PubMed]
- Suntornnond, R.; An, J.; Chua, C.K. Bioprinting of Thermoresponsive Hydrogels for Next Generation Tissue Engineering: A Review. Macromol. Mater. Eng. 2017, 302, 1600266. [Google Scholar] [CrossRef]
- Chung, J.H.; Naficy, S.; Yue, Z.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-ink properties and printability for extrusion printing living cells. Biomater. Sci. 2013, 1, 763. [Google Scholar] [CrossRef]
- Nicmodeus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev. 2008, 14, 149–165. [Google Scholar]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater. 2013, 25, 3124–3130. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef]
- Aliabadi, H.M.; Lavasanifar, A. Polymeric micelles for drug delivery. Expert Opin. Drug Deliv. 2006, 3, 139–162. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Lemieux, P.; Vinogradov, S.; Alakhov, V. Pluronic block copolymers: Novel functional molecules for gene therapy. Adv. Drug Deliv. Rev. 2002, 54, 223–233. [Google Scholar] [CrossRef]
- Anderson, R.A. Micelle formation by oxyethylene-oxypropylene polymers. Pharm. Acta Helv. 1972, 47, 304–308. [Google Scholar] [PubMed]
- Rey-Rico, A.; Cucchiarini, M. Controlled release strategies for raav-mediated gene delivery. Acta Biomater. 2016, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Niidome, T.; Huang, L. Gene therapy progress and prospects: Nonviral vectors. Gene Ther. 2002, 9, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Cucchiarini, M. Recent tissue engineering-based advances for effective rAAV-mediated gene transfer in the musculoskeletal system. Bioengineered 2016, 7, 175–188. [Google Scholar] [CrossRef][Green Version]
- De Laporte, L.; Cruz Rea, J.; Shea, L.D. Design of modular non-viral gene therapy vectors. Biomaterials 2006, 27, 947–954. [Google Scholar] [CrossRef]
- Wang, W.; Li, W.; Ma, N.; Steinhoff, G. Non-viral gene delivery methods. Curr. Pharm. Biotechnol. 2013, 14, 46–60. [Google Scholar]
- Cucchiarini, M.; Madry, H. Gene therapy for cartilage defects. J. Gene Med. 2005, 7, 1495–1509. [Google Scholar] [CrossRef]
- Madry, H.; Cucchiarini, M. Advances and challenges in gene-based approaches for osteoarthritis. J. Gene Med. 2013, 15, 343–355. [Google Scholar] [CrossRef]
- Johnstone, B.; Alini, M.; Cucchiarini, M.; Dodge, G.R.; Eglin, D.; Guilak, F.; Madry, H.; Mata, A.; Mauck, R.L.; Semino, C.E.; et al. Tissue engineering for articular cartilage repair—The state of the art. Eur. Cell Mater. 2013, 25, 248–267. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Venkatesan, J.K.; Frisch, J.; Rial-Hermida, I.; Schmitt, G.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. PEO–PPO–PEO micelles as effective rAAV-mediated gene delivery systems to target human mesenchymal stem cells without altering their differentiation potency. Acta Biomater. 2015, 27, 42–52. [Google Scholar] [CrossRef]
- Höfig, I.; Atkinson, M.J.; Mall, S.; Krackhardt, A.M.; Thirion, C.; Anastasov, N. Poloxamer synperonic F108 improves cellular transduction with lentiviral vectors. J. Gene Med. 2012, 14, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Millington, M.; Aendt, A.; Boyd, M.; Applegate, T.; Shen, S. Towards a clinically relevant lentiviral transduction protocol for primary human CD34 haematopoietic stem/progenitor cells. PLoS ONE 2009, 4, 6461. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, A.; Anderson, E.; Sullivan, K.; Reynolds, A.; Boese, Q.; Leake, D.; Karpilow, J.; Khvorova, A. A protocol for designing siRNAs with high functionality and specificity. Nat. Protoc. 2007, 9, 2068–2078. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Rey-Rico, A.; Sosnik, A.; Taboada, P.; Concheiro, A. Poloxamine-based nanomaterials for drug delivery. Front. Biosci. 2012, 337, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Chitgupi, U.; Nyayapathi, N.; Kim, J.; Wang, D.; Sun, B.; Li, C.; Carter, K.; Huang, W.C.; Kim, C.; Xia, J.; et al. Surfactant-Stripped Micelles for NIR-II Photoacoustic Imaging through 12 cm of Breast Tissue and Whole Human Breasts. Adv. Mater. 2019, 31, 1–10. [Google Scholar] [CrossRef]
- Sousa, C.; Gouveia, L.F.; Kreutzer, B.; Silva-Lima, B.; Maphasa, R.E.; Dube, A.; Videira, M. Polymeric Micellar Formulation Enhances Antimicrobial and Anticancer Properties of Salinomycin. Pharm. Res. 2019, 36, 83. [Google Scholar] [CrossRef]
- Naujokat, C.; Steinhart, R. Salinomycin as a drug for targeting human cancer stem cells. J. Biomed. Biotechnol. 2012, 20, 44–46. [Google Scholar] [CrossRef]
- Steel, G.G.; Peckham, M.J. Exploitable Mechanisms in Combined Radiotherapy-Chemotherapy: The Concept of Additivity. Int. J. Radiat. Oncol. Biol. Phys. 1979, 5, 85–91. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Salama, J.K.; Vokes, E.E. The Concurrent Chemoradiation Paradigm—General Principles. Nat. Rew. Clin. Oncol. 2007, 4, 86–100. [Google Scholar] [CrossRef]
- DuRoss, A.N.; Neufeld, M.J.; Landry, M.R.; Rosch, J.G.; Eaton, C.T.; Sahay, G.; Thomas, C.R., Jr.; Sun, C. Micellar Formulation of Talazoparib and Buparlisib for Enhanced DNA Damage in Breast Cancer Chemoradiotherapy. ACS Appl. Mater. Interfaces 2019, 11, 12342–12356. [Google Scholar] [CrossRef] [PubMed]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule Formation by Interfacial Polymer Deposition Following Solvent Displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
| Poloxamer | Pluronic | PEO% | Average Molecular Weight | Melting Point (°C) | Viscosity (Pa·s) | Surface Tension (dyn cm−1) | HLB |
|---|---|---|---|---|---|---|---|
| P105 | L35 | 50 | 1900 | 7 | 0.375 | 49 | 18–23 |
| P108 | F38 | 80 | 4700 | 48 | 0.260 | 52 | >24 |
| P122 | L42 | 20 | 1630 | −26 | 0.280 | 46 | 7–12 |
| P123 | L43 | 30 | 1850 | −1 | 0.310 | 47 | 7–12 |
| P124 | L44 | 40 | 2200 | 16 | 0.440 | 45 | 12–18 |
| P182 | L62 | 20 | 2500 | −4 | 0.450 | 43 | 1–7 |
| P183 | L63 | 30 | 2650 | 10 | 0.490 | 43 | 7–12 |
| P184 | L64 | 40 | 2900 | 16 | 0.850 | 43 | 12–18 |
| P185 | P65 | 50 | 3400 | 27 | 0.180 | 46 | 12–18 |
| P188 | F68 | 80 | 8400 | 52 | 1.000 | 50 | >24 |
| P212 | L72 | 20 | 2750 | −7 | 0.510 | 39 | 1–7 |
| P215 | P75 | 50 | 4150 | 27 | 0.250 | 43 | 12–18 |
| P217 | F77 | 70 | 6600 | 48 | 0.480 | 47 | >24 |
| P234 | P84 | 40 | 4200 | 34 | 0.280 | 42 | 12–18 |
| P235 | P85 | 50 | 4600 | 34 | 0.310 | 42 | 12–18 |
| P237 | F87 | 70 | 7700 | 49 | 0.700 | 44 | >24 |
| P238 | F88 | 80 | 11,400 | 54 | 2.300 | 48 | >24 |
| P288 | F98 | 80 | 13,000 | 58 | 2.700 | 43 | >24 |
| P333 | P103 | 30 | 4950 | 30 | 0.285 | 34 | 7–12 |
| P334 | P104 | 40 | 5900 | 32 | 0.390 | 33 | 12–18 |
| P335 | P105 | 50 | 6500 | 35 | 0.750 | 39 | 12–18 |
| P338 | F108 | 80 | 14,600 | 57 | 2.800 | 41 | >24 |
| P402 | L122 | 20 | 5000 | 20 | 1.750 | 33 | 1–7 |
| P403 | P123 | 30 | 5750 | 31 | 0.350 | 34 | 7–12 |
| P407 | F127 | 70 | 12600 | 56 | 3.100 | 41 | 18–23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. https://doi.org/10.3390/pharmaceutics11120671
Russo E, Villa C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics. 2019; 11(12):671. https://doi.org/10.3390/pharmaceutics11120671
Chicago/Turabian StyleRusso, Eleonora, and Carla Villa. 2019. "Poloxamer Hydrogels for Biomedical Applications" Pharmaceutics 11, no. 12: 671. https://doi.org/10.3390/pharmaceutics11120671
APA StyleRusso, E., & Villa, C. (2019). Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics, 11(12), 671. https://doi.org/10.3390/pharmaceutics11120671






