Be Aggressive! Amorphous Excipients Enabling Single-Step Freeze-Drying of Monoclonal Antibody Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Freeze-Drying
2.3. Differential Scanning Calorimetry
2.4. Freeze-Drying Microscopy
2.5. Visual Cake Appearance
2.6. Micro-Computed Tomography
2.7. Reconstitution Time
2.8. Specific Surface Area
2.9. Residual Moisture
2.10. Size-Exclusion Chromatography
3. Results
3.1. Thermal Properties of the Liquid Formulations
3.2. Impact of Freeze-Drying Parameters on Tp and Primary Drying Time
3.3. Cake Appearance and Structure
3.4. Other Product Quality Attributes
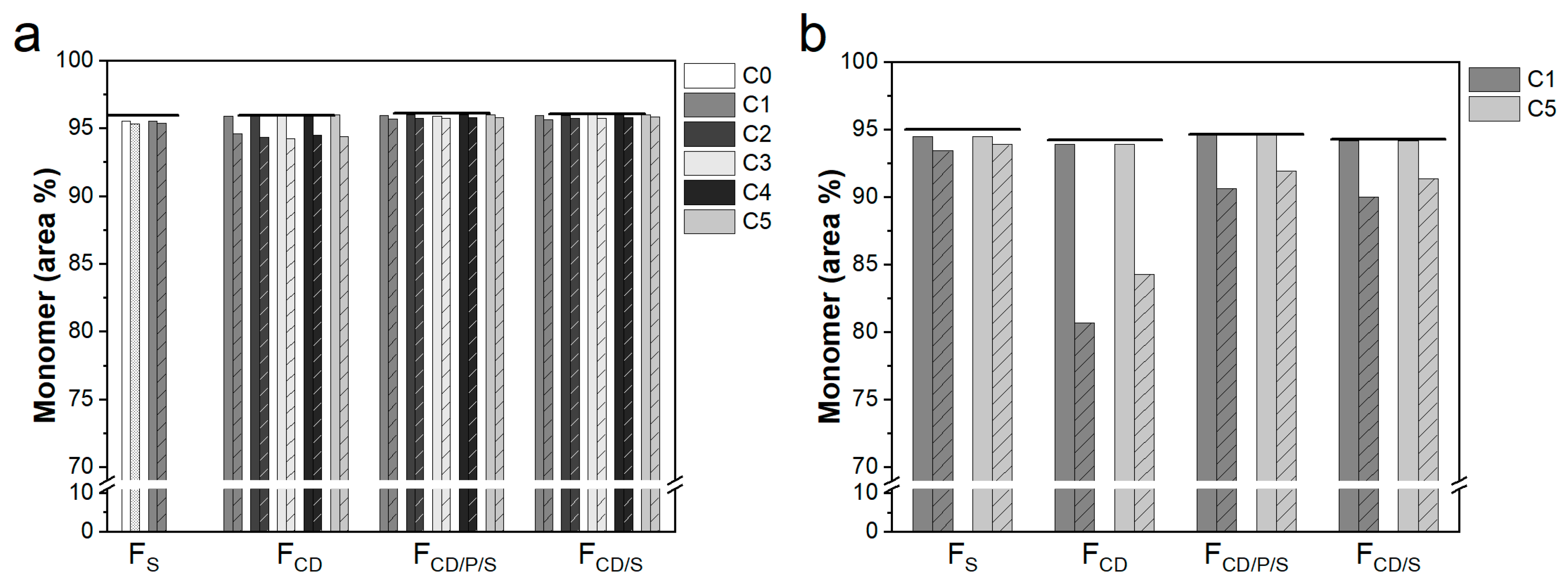
3.5. Protein Stability
4. Discussion
4.1. Correlation Between Tg’, Tc, Tp, and Cake Appearance
4.2. Impact of Process Parameters
4.3. Protein Stability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gervasi, V.; Agnol, R.D.; Cullen, S.; McCoy, T.; Vucen, S.; Crean, A. Parenteral protein formulations: An overview of approved products within the European Union. Eur. J. Pharm. Biopharm. 2018, 131, 8–24. [Google Scholar] [CrossRef]
- Constantino, H.R. Excipients for use in lyophilized pharmaceutical peptide, protein and other bioproducts. In Lyophilization of Biopharmaceuticals; Constantino, H.R., Pikal, M.J., Eds.; AAPS Press: Arlington, VA, USA, 2004; pp. 139–228. [Google Scholar]
- Tang, X.; Pikal, M.J. Design of freeze-drying processes for pharmaceuticals: Practical advice. Pharm. Res. 2004, 21, 191–200. [Google Scholar] [CrossRef]
- Passot, S.; Fonseca, F.; Barbouche, N.; Marin, M.; Alarcon-Lorca, M.; Rolland, D.; Rapaud, M. Effect of Product Temperature During Primary Drying on the Long-Term Stability of Lyophilized Proteins. Pharm. Dev. Technol. 2007, 12, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Lueckel, B.; Helk, B.; Bodmer, D.; Leuenberger, H. Effects of Formulation and Process Variables on the Aggregation of Freeze-Dried Interleukin-6 (IL-6) After Lyophilization and on Storage. Pharm. Dev. Technol. 1998, 3, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Q.; Hey, J.M.; Nail, S.L. Effect of collapse on the stability of freeze-dried recombinant factor VIII and alpha-amylase. J. Pharm. Sci. 2004, 93, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Schersch, K.; Betz, O.; Garidel, P.; Muehlau, S.; Bassarab, S.; Winter, G. Systematic Investigation of the Effect of Lyophilizate Collapse on Pharmaceutically Relevant Proteins, Part 2: Stability During Storage at Elevated Temperatures. J. Pharm. Sci. 2012, 101, 2288–2306. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.M.; Nail, S.L.; Pikal, M.J.; Geidobler, R.; Winter, G.; Hawe, A.; Davagnino, J.; Gupta, S.R. Lyophilized Drug Product Cake Appearance: What Is Acceptable? J. Pharm. Sci. 2017, 106, 1706–1721. [Google Scholar] [CrossRef]
- Horn, J.; Friess, W. Detection of Collapse and Crystallization of Saccharide, Protein, and Mannitol Formulations by Optical Fibers in Lyophilization. Front. Chem. 2018, 6, 4. [Google Scholar] [CrossRef]
- Gitter, J.H.; Geidobler, R.; Presser, I.; Winter, G. Significant Drying Time Reduction Using Microwave-Assisted Freeze-Drying for a Monoclonal Antibody. J. Pharm. Sci. 2018, 107, 2538–2543. [Google Scholar] [CrossRef]
- Colandene, J.D.; Maldonado, L.M.; Creagh, A.T.; Vrettos, J.S.; Goad, K.G.; Spitznagel, T.M. Lyophilization Cycle Development for a High-Concentration Monoclonal Antibody Formulation Lacking a Crystalline Bulking Agent. J. Pharm. Sci. 2007, 96, 1598–1608. [Google Scholar] [CrossRef]
- Bjelošević, M.; Seljak, K.B.; Trstenjak, U.; Logar, M.; Brus, B.; Grabnar, P.A. Aggressive conditions during primary drying as a contemporary approach to optimise freeze-drying cycles of biopharmaceuticals. Eur. J. Pharm. Sci. 2018, 122, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Depaz, R.A.; Pansare, S.; Patel, S.M. Freeze-Drying Above the Glass Transition Temperature in Amorphous Protein Formulations While Maintaining Product Quality and Improving Process Efficiency. J. Pharm. Sci. 2016, 105, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Horn, J.; Schanda, J.; Friess, W. Impact of fast and conservative freeze-drying on product quality of protein-mannitol-sucrose-glycerol lyophilizates. Eur. J. Pharm. Biopharm. 2018, 127, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Pansare, S.K.; Patel, S.M. Lyophilization Process Design and Development: A Single-Step Drying Approach. J. Pharm. Sci. 2019, 108, 1423–1433. [Google Scholar] [CrossRef]
- Izutsu, K.; Yoshioka, S.; Terao, T. Decreased Protein-Stabilizing Effects of Cryoprotectants Due to Crystallization. Pharm. Res. 1993, 10, 1232–1237. [Google Scholar] [CrossRef]
- Williams, N.A.; Dean, T. Vial breakage by frozen mannitol solutions: Correlation with thermal characteristics and effect of stereoisomerism, additives, and vial configuration. PDA J. Pharm. Sci. Technol. 1991, 45, 94–100. [Google Scholar]
- Larsen, B.S.; Skytte, J.; Svagan, A.J.; Meng-Lund, H.; Grohganz, H.; Lobmann, K. Using dextran of different molecular weights to achieve faster freeze-drying and improved storage stability of lactate dehydrogenase. Pharm. Dev. Technol. 2019, 24, 323–328. [Google Scholar] [CrossRef]
- Haeuser, C.; Goldbach, P.; Huwyler, J.; Friess, W.; Allmendinger, A. Impact of dextran on thermal properties, product quality attributes, and monoclonal antibody stability in freeze-dried formulations. Eur. J. Pharm. Biopharm. 2019. under review. [Google Scholar]
- Wong, J.; Kipp, J.E.; Miller, R.L.; Nair, L.M.; Ray, G.J. Mechanism of 2-hydropropyl-β-cyclodextrin in the stabilization of frozen formulations. Eur. J. Pharm. Sci. 2014, 62, 281–292. [Google Scholar] [CrossRef]
- Meister, E.; Šaši, S.; Gieseler, H. Freeze-Dry Microscopy: Impact of Nucleation Temperature and Excipient Concentration on Collapse Temperature Data. AAPS PharmSciTech 2009, 10, 582–588. [Google Scholar] [CrossRef][Green Version]
- Serno, T.; Geidobler, R.; Winter, G. Protein stabilization by cyclodextrins in the liquid and dried state. Adv. Drug Deliv. Rev. 2011, 63, 1086–1106. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, N.; Bouchard, A.; Hofland, G.W.; Witkamp, G.-J.; Crommelin, D.J.; Jiskoot, W. Stabilization of IgG by supercritical fluid drying: Optimization of formulation and process parameters. Eur. J. Pharm. Biopharm. 2008, 68, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, H.; Merrikhihaghi, S.; Najafabadi, A.R.; Ramezani, V.; Sardari, S.; Vatanara, A. A comparative study to evaluate the effect of different carbohydrates on the stability of immunoglobulin G during lyophilization and following storage. Pharm. Sci. 2016, 22, 251. [Google Scholar] [CrossRef]
- Ressing, M.E.; Jiskoot, W.; Talsma, H.; Van Ingen, C.W.; Beuvery, E.C.; Crommelin, D.J. The influence of sucrose, dextran, and hydroxypropyl-β-cyclodextrin as lyoprotectants for a freeze-dried mouse IgG2a monoclonal antibody (MN12). Pharm. Res. 1992, 9, 266–270. [Google Scholar] [CrossRef]
- Haeuser, C.; Goldbach, P.; Huwyler, J.; Friess, W.; Allmendinger, A. Excipients for room temperature stable freeze-dried monoclonal antibody formulations. J. Pharm. Sci. 2019. [Google Scholar] [CrossRef]
- Nunes, C.; Mahendrasingam, A.; Suryanarayanan, R. Quantification of Crystallinity in Substantially Amorphous Materials by Synchrotron X-ray Powder Diffractometry. Pharm. Res. 2005, 22, 1942–1953. [Google Scholar] [CrossRef]
- Padilla, A.M.; Ivanisevic, I.; Yang, Y.; Engers, D.; Bogner, R.H.; Pikal, M.J. The Study of Phase Separation in Amorphous Freeze-Dried Systems. Part I: Raman Mapping and Computational Analysis of XRPD Data in Model Polymer Systems. J. Pharm. Sci. 2011, 100, 206–222. [Google Scholar] [CrossRef]
- Bandi, N.; Wei, W.; Roberts, C.B.; Kotra, L.P.; Kompella, U.B. Preparation of budesonide- and indomethacin-hydroxypropyl-β-cyclodextrin (HPBCD) complexes using a single-step, organic-solvent-free supercritical fluid process. Eur. J. Pharm. Sci. 2004, 23, 159–168. [Google Scholar] [CrossRef]
- Geidobler, R. Cyclodextrins as Excipients in Drying of Proteins and Controlled Ice Nucleation in Freeze-Drying. Ph.D. Thesis, Ludwig-Maximilians-University, Munich, Germany, 2014. [Google Scholar]
- Haeuser, C.; Goldbach, P.; Huwyler, J.; Friess, W.; Allmendinger, A. Imaging Techniques to Characterize Cake Appearance of Freeze-Dried Products. J. Pharm. Sci. 2018, 107, 2810–2822. [Google Scholar] [CrossRef]
- Meister, E.; Gieseler, H. Freeze-Dry Microscopy of Protein/Sugar Mixtures: Drying Behavior, Interpretation of Collapse Temperatures and a Comparison to Corresponding Glass Transition Data. J. Pharm. Sci. 2009, 98, 3072–3087. [Google Scholar] [CrossRef]
- Pikal, M.J.; Shah, S. The collapse temperature in freeze drying: Dependence on measurement methodology and rate of water removal from the glassy phase. Int. J. Pharm. 1990, 62, 165–186. [Google Scholar] [CrossRef]
- Greco, K.; Mujat, M.; Galbally-Kinney, K.L.; Hammer, D.X.; Ferguson, R.D.; Iftimia, N.; Mulhall, P.; Sharma, P.; Kessler, W.J.; Pikal, M.J.; et al. Accurate Prediction of Collapse Temperature using Optical Coherence Tomography-Based Freeze-Drying Microscopy. J. Pharm. Sci. 2013, 102, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.; Seyferth, S.; Lee, G. Measurement of shrinkage and cracking in lyophilized amorphous cakes. Part I: Final-product assessment. J. Pharm. Sci. 2015, 104, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.; Seyferth, S.; Lee, G. Measurement of shrinkage and cracking in lyophilized amorphous cakes. Part II: Kinetics. Pharm. Res. 2015, 32, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.; Patapoff, T.W. Split-cakes, still delicious. PDA J. Pharm. Sci. Technol. 2019, 73, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Esfandiary, R.; Gattu, S.K.; Stewart, J.M.; Patel, S.M. Effect of Freezing on Lyophilization Process Performance and Drug Product Cake Appearance. J. Pharm. Sci. 2016, 105, 1427–1433. [Google Scholar] [CrossRef]
- Ohori, R.; Akita, T.; Yamashita, C. Effect of temperature ramp rate during the primary drying process on the properties of amorphous-based lyophilized cake, Part 2: Successful lyophilization by adopting a fast ramp rate during primary drying in protein formulations. Eur. J. Pharm. Biopharm. 2018, 130, 83–95. [Google Scholar] [CrossRef]
- Ohori, R.; Yamashita, C. Effects of temperature ramp rate during the primary drying process on the properties of amorphous-based lyophilized cake, Part 1: Cake characterization, collapse temperature and drying behavior. J. Drug Deliv. Sci. Technol. 2017, 39, 131–139. [Google Scholar] [CrossRef]
- Pikal, M.J.; Roy, M.L.; Shah, S. Mass and Heat Transfer in Vial Freeze-Drying of Pharmaceuticals: Role of the Vial. J. Pharm. Sci. 1984, 73, 1224–1237. [Google Scholar] [CrossRef]
- Lewis, L.M.; Johnson, R.E.; Oldroyd, M.E.; Ahmed, S.S.; Joseph, L.; Saracovan, I.; Sinha, S. Characterizing the Freeze–Drying Behavior of Model Protein Formulations. AAPS PharmSciTech 2010, 11, 1580–1590. [Google Scholar] [CrossRef]
- Cleland, J.L.; Lam, X.; Kendrick, B.; Yang, J.; Yang, T.; Overcashier, D.; Brooks, D.; Hsu, C.; Carpenter, J.F. A specific molar ratio of stabilizer to protein is required for storage stability of a lyophilized monoclonal antibody. J. Pharm. Sci. 2001, 90, 310–321. [Google Scholar] [CrossRef]
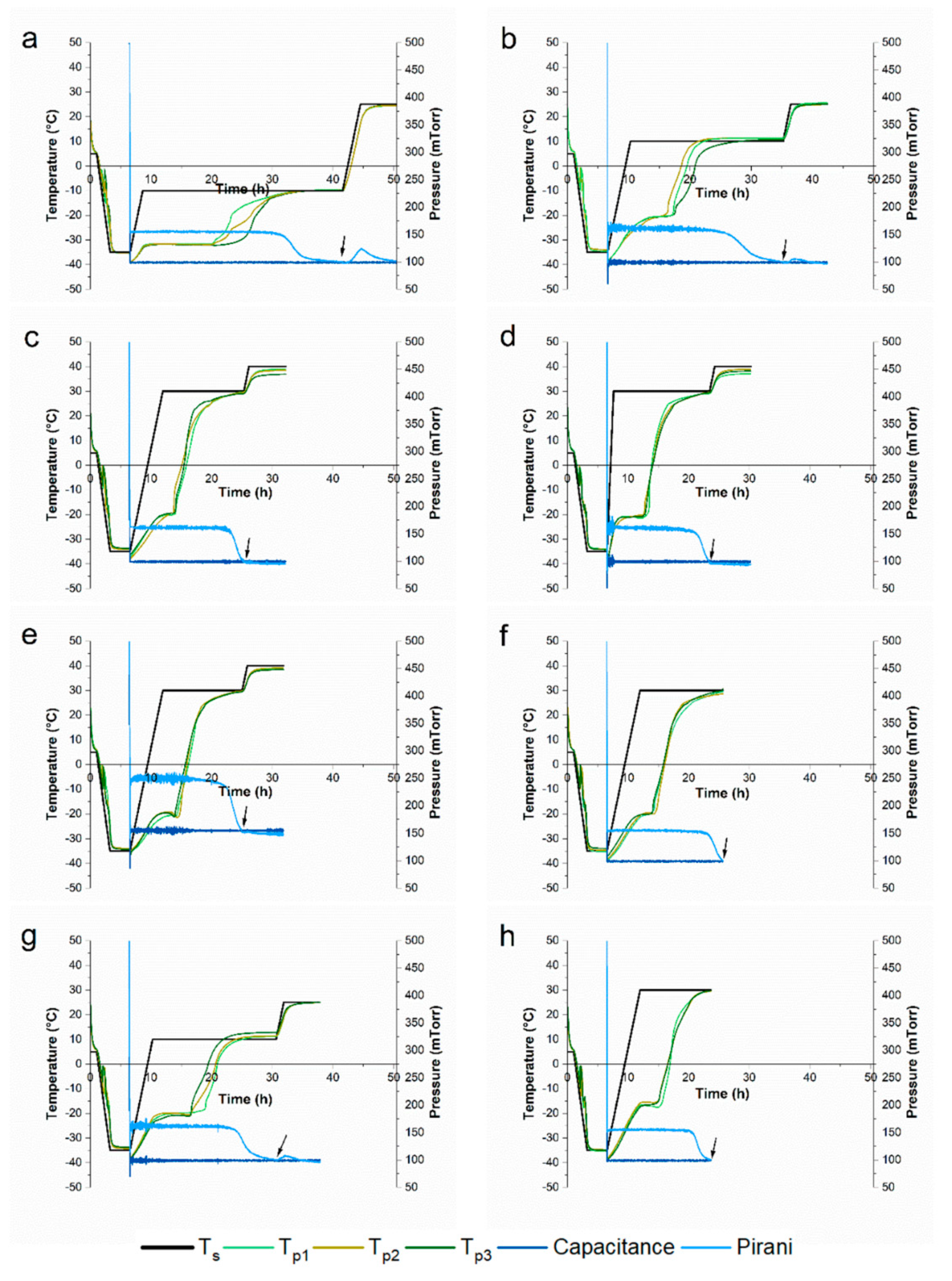
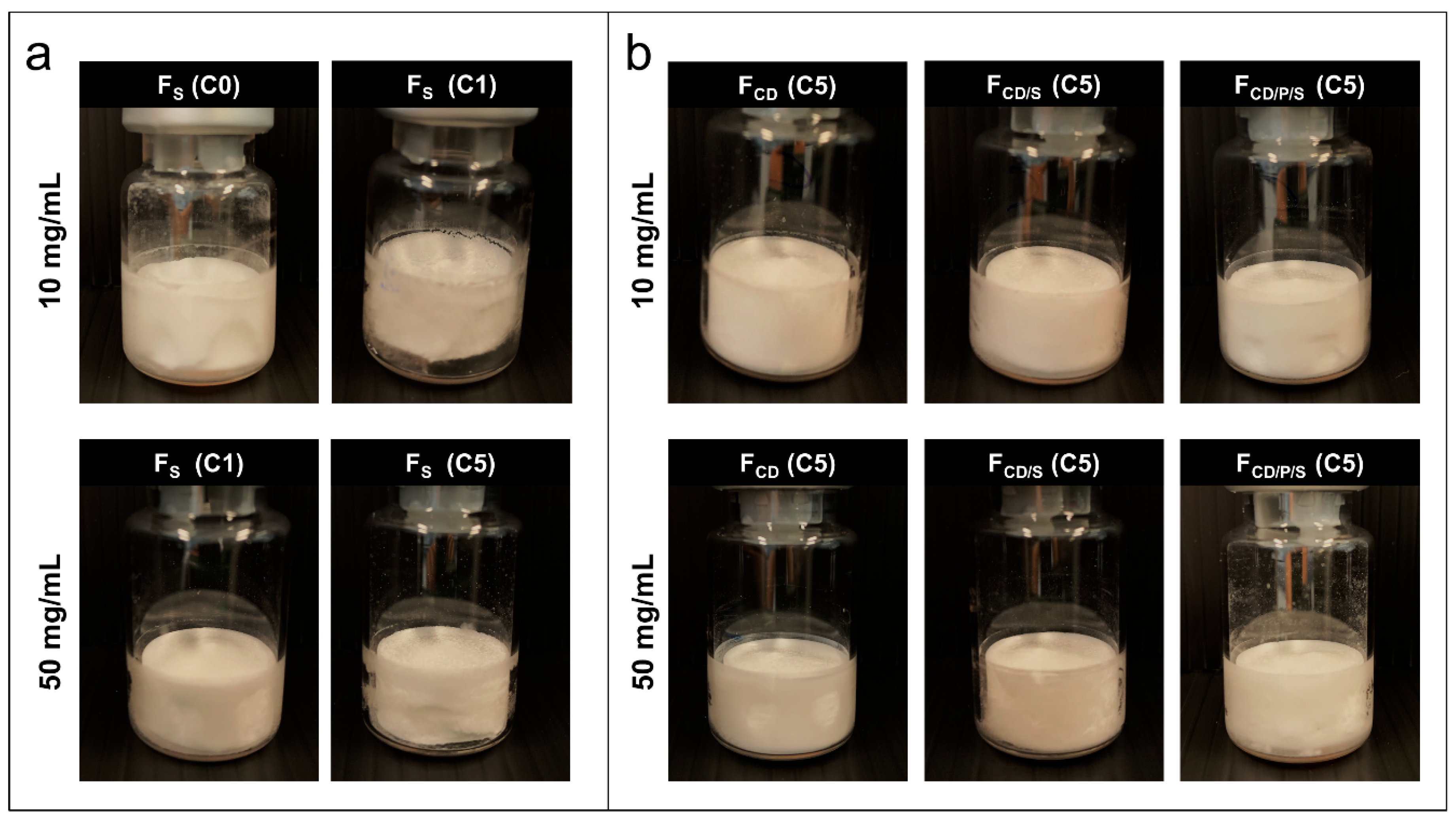
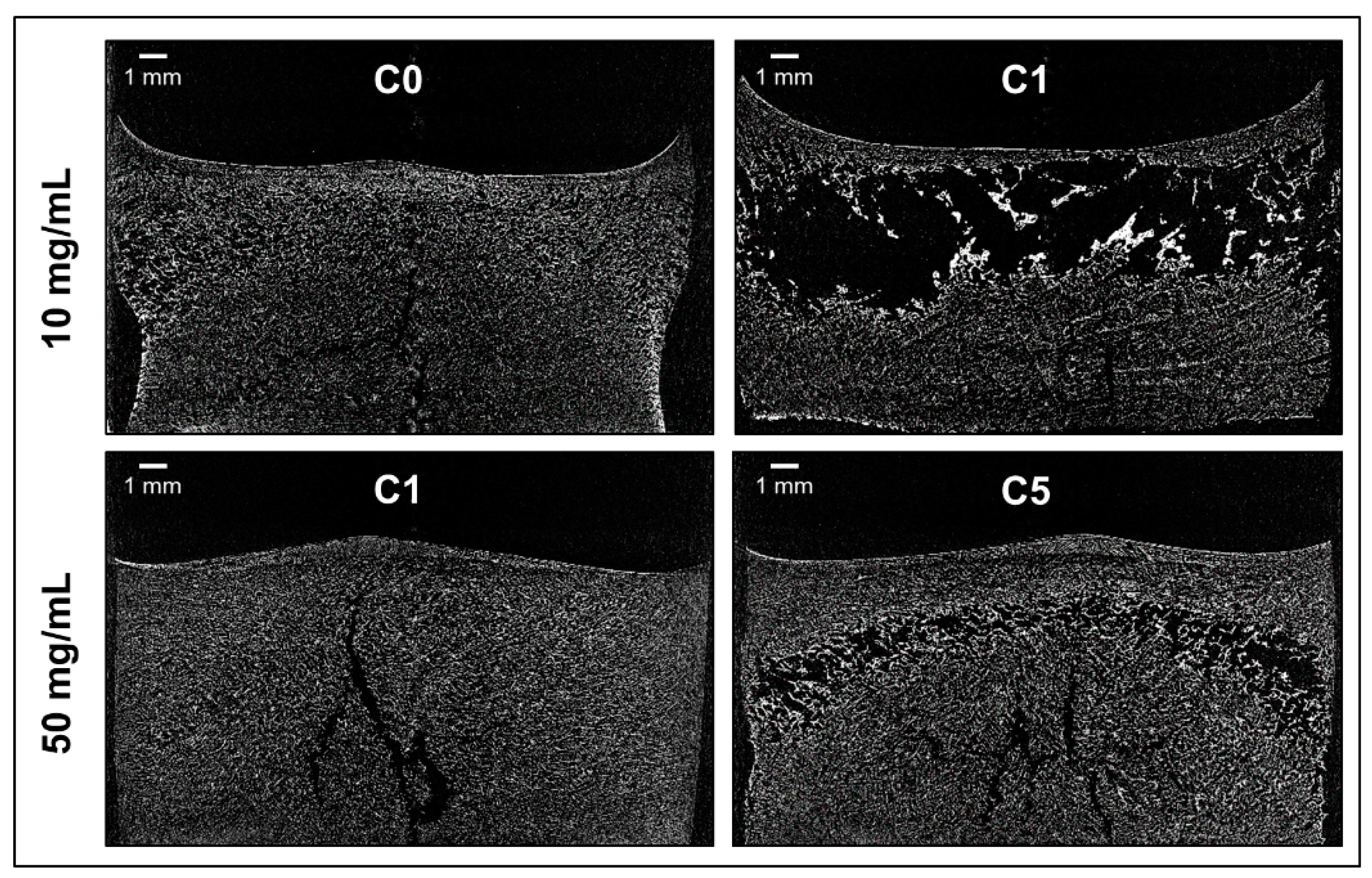

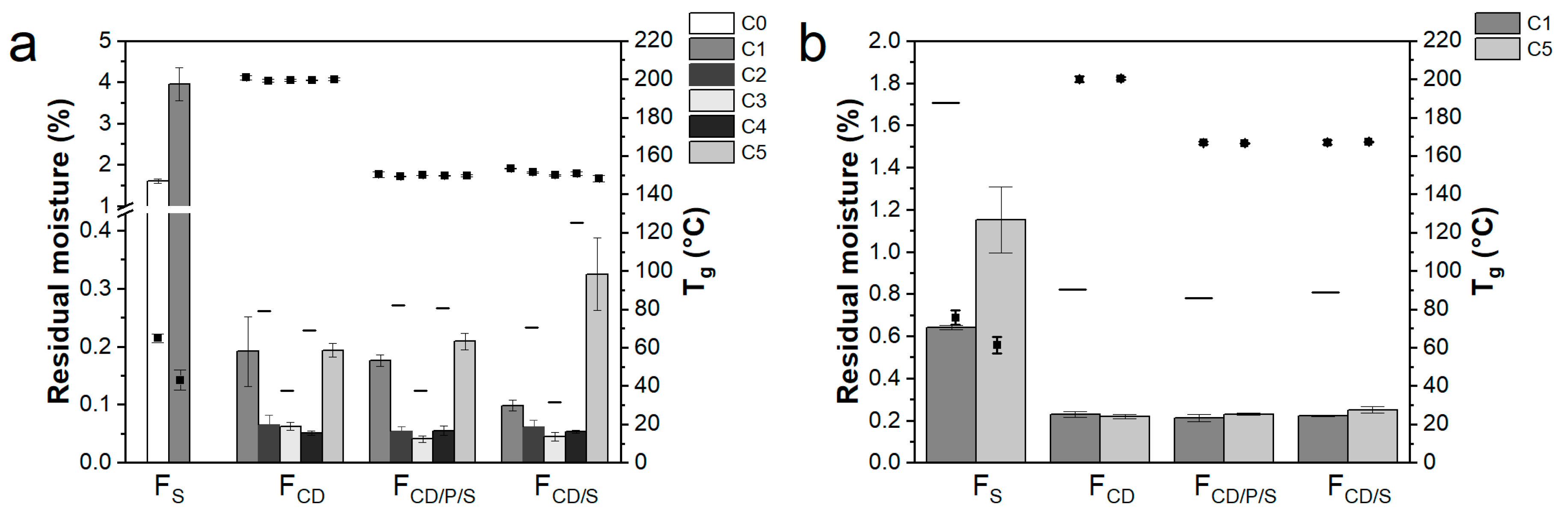
| Cycle | Freezing | Primary Drying | Secondary Drying | |||||
|---|---|---|---|---|---|---|---|---|
| Ramp (°C/min) | Ts (°C) | pc (mTorr) | Ramp (°C/min) | Ts (°C) | Hold Time (min) | Pressure (mTorr) | ||
| 0 | Ramp 0.3 °C/min Temp. −35 °C Hold 180 min | 0.2 | −10 | 100 | 0.2 | +25 | 360 | 100 |
| 1 | 0.2 | +10 | 100 | 0.2 | +25 | 360 | 100 | |
| 2 | 0.2 | +30 | 100 | 0.2 | +40 | 360 | 100 | |
| 3 | 1.0 | +30 | 100 | 0.2 | +40 | 360 | 100 | |
| 4 | 0.2 | +30 | 155 | 0.2 | +40 | 360 | 155 | |
| 5 | 0.2 | +30 | 100 | - | ||||
| Formulation | mAb Concentration(mg/mL) | Tg’ (°C) | Tc (°C) |
|---|---|---|---|
| FS | 10 | −29.5 | −31.0 |
| 50 | −25.4 | −20.4 | |
| FCD | 10 | −10.9 | −10.1 |
| 50 | −8.3 | −6.3 | |
| FCD/P/S | 10 | −19.2 | −18.5 |
| 50 | −16.5 | −14.6 | |
| FCD/S | 10 | −18.6 | −16.0 |
| 50 | −15.2 | −10.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haeuser, C.; Goldbach, P.; Huwyler, J.; Friess, W.; Allmendinger, A. Be Aggressive! Amorphous Excipients Enabling Single-Step Freeze-Drying of Monoclonal Antibody Formulations. Pharmaceutics 2019, 11, 616. https://doi.org/10.3390/pharmaceutics11110616
Haeuser C, Goldbach P, Huwyler J, Friess W, Allmendinger A. Be Aggressive! Amorphous Excipients Enabling Single-Step Freeze-Drying of Monoclonal Antibody Formulations. Pharmaceutics. 2019; 11(11):616. https://doi.org/10.3390/pharmaceutics11110616
Chicago/Turabian StyleHaeuser, Christina, Pierre Goldbach, Joerg Huwyler, Wolfgang Friess, and Andrea Allmendinger. 2019. "Be Aggressive! Amorphous Excipients Enabling Single-Step Freeze-Drying of Monoclonal Antibody Formulations" Pharmaceutics 11, no. 11: 616. https://doi.org/10.3390/pharmaceutics11110616
APA StyleHaeuser, C., Goldbach, P., Huwyler, J., Friess, W., & Allmendinger, A. (2019). Be Aggressive! Amorphous Excipients Enabling Single-Step Freeze-Drying of Monoclonal Antibody Formulations. Pharmaceutics, 11(11), 616. https://doi.org/10.3390/pharmaceutics11110616







