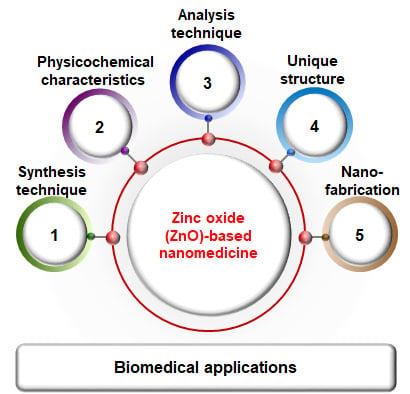
Synthesis, Characterization, and Three-Dimensional Structure Generation of Zinc Oxide-Based Nanomedicine for Biomedical Applications
Abstract
:1. Introduction
2. Synthesis Techniques for ZnO NPs
2.1. Conventional Methods
2.1.1. Physical Methods
2.1.2. Chemical Methods
2.1.3. Biological Methods
2.2. Non-Conventional Method: Microfluidic Reactor-Based Synthesis
3. Physicochemical Characterization and Tools
3.1. Appearance, Crystallinity, Particle Size, Morphology, and Porosity
3.2. Characterization Tools
3.2.1. X-ray Diffraction (XRD)
3.2.2. Scanning Electron Microscopy (SEM)
3.2.3. Transmission Electron Microscopy (TEM)
3.2.4. Brunauer–Emmett-Teller (BET) Analysis
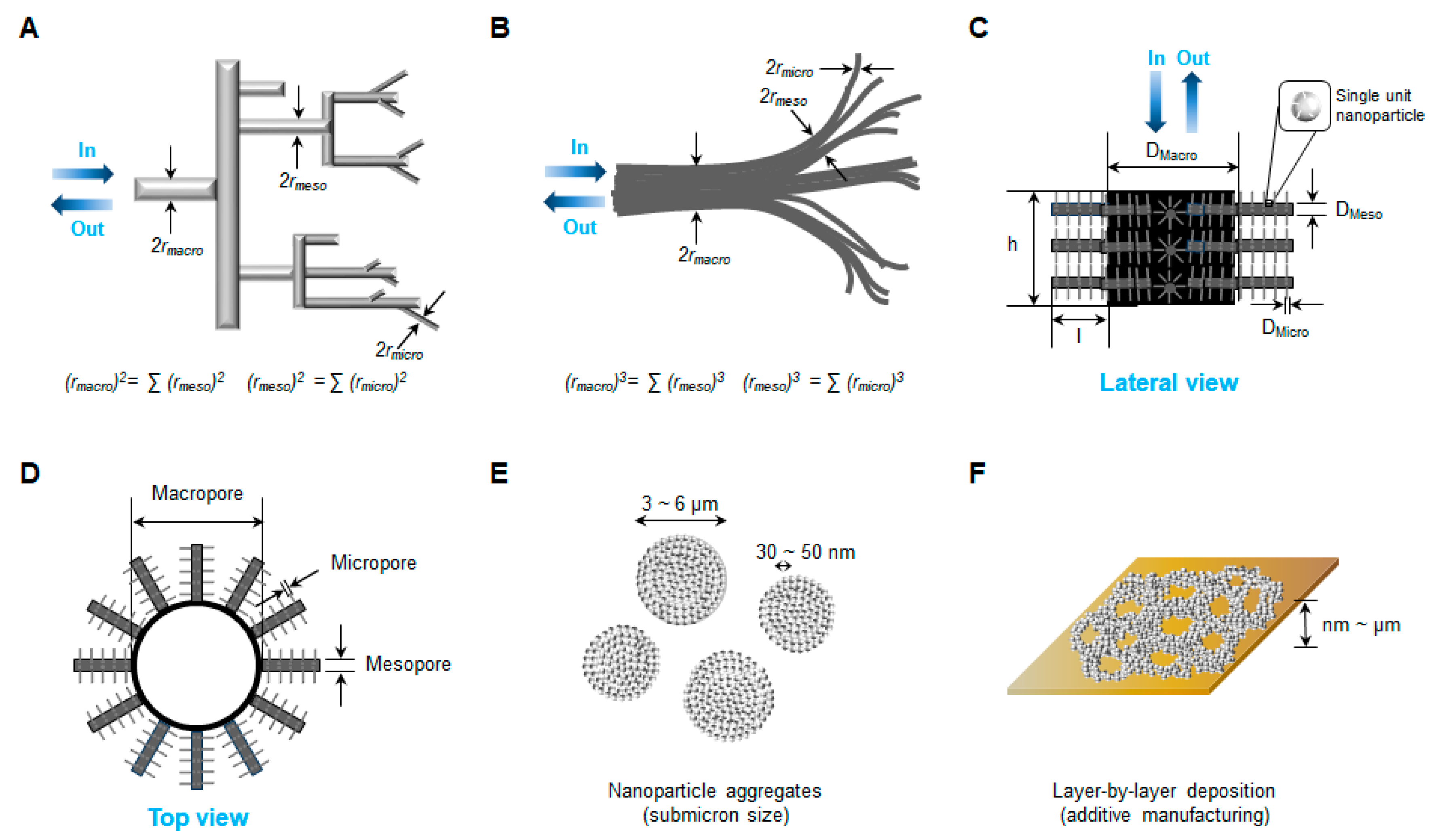
4. Three-Dimensional Structure Generation by Nanofabrication
4.1. Three-Dimensional Network Structure with Multilevel Porosity
4.2. Nanofabrication Techniques
4.2.1. Conventional Methods of Nanofabrication
4.2.2. Non-Conventional Methods of Nanofabrication
Biotemplating
Nanofabrication via Self-Assembly
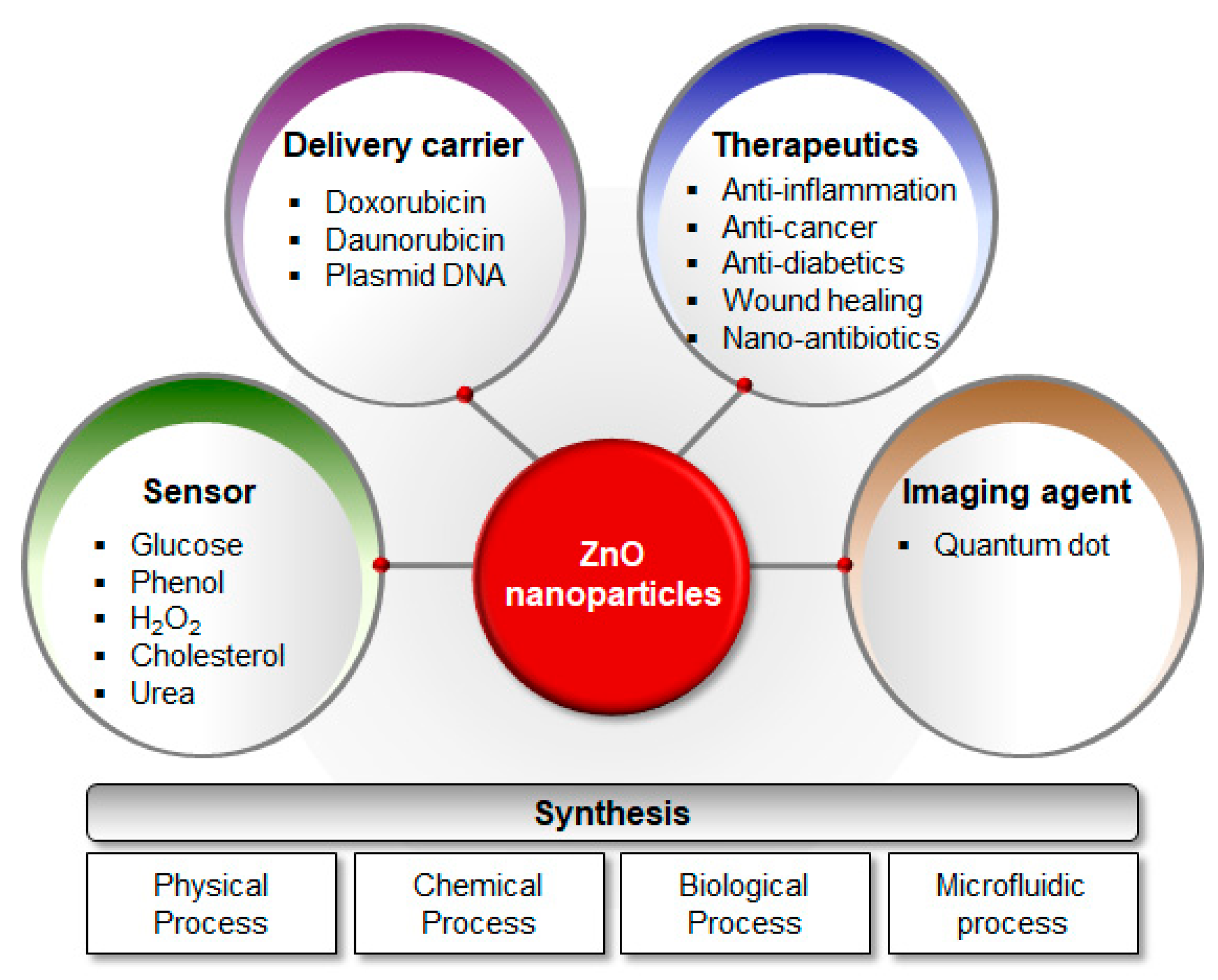
5. Biomedical Applications
5.1. Anticancer Activity
5.2. Antidiabetic Activity
5.3. Antimicrobial Activity
5.4. Anti-Inflammatory Activity
5.5. Wound Healing
5.6. Imaging Agents
5.7. Sensors
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bisht, G.; Rayamajhi, S. ZnO Nanoparticles: A Promising Anticancer Agent. Nanobiomedicine 2016, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Elshama, S.; Abdallah, M.; Abdel-Karim, R. Zinc Oxide Nanoparticles: Therapeutic Benefits and Toxicological Hazards. Open Nanomed. J. 2018, 5, 16–22. [Google Scholar] [CrossRef]
- Bharti, S.; Singh, S. Metal Based Drugs: Current Use and Future Potential. Der. Pharm. Lett. 2009, 1, 39–51. [Google Scholar]
- Theile, D. Under-Reported Aspects of Platinum Drug Pharmacology. Molecules 2017, 22, 382. [Google Scholar] [CrossRef]
- Das, S.; Mitra, S.; Khurana, S.M.P.; Debnath, N. Nanomaterials for biomedical applications. Front Life Sci. 2013, 7, 90–98. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Gun’ko, Y.; Vallet-Regí, M. ZnO Nanostructures for Drug Delivery and Theranostic Applications. Nanomaterials 2018, 8, 268. [Google Scholar] [CrossRef]
- Xiong, H.-M. ZnO Nanoparticles Applied to Bioimaging and Drug Delivery. Adv. Mater. 2013, 25, 5329–5335. [Google Scholar] [CrossRef]
- Davis, K.; Yarbrough, R.; Froeschle, M.; White, J.; Rathnayake, H. Band gap engineered zinc oxide nanostructures via a sol–gel synthesis of solvent driven shape-controlled crystal growth. RSC Adv. 2019, 9, 14638–14648. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Wiegand, C.; Hipler, U.-C.; Boldt, S.; Strehle, J.; Wollina, U. Skin-protective effects of a zinc oxide-functionalized textile and its relevance for atopic dermatitis. Clin. Cosmet Investig. Derm. 2013, 6, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Marks, T.J.; Facchetti, A. Metal oxides for optoelectronic applications. Nat. Mater. 2016, 15, 383. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tang, Y.; Liu, W. Efficient Dye-Sensitized Solar Cells Based on Nanoflower-like ZnO Photoelectrode. Molecules 2017, 22, 1284. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-E.; Jin, J.E.; Hwang, W.; Hong, S.W. Photocatalytic antibacterial application of zinc oxide nanoparticles and self-assembled networks under dual UV irradiation for enhanced disinfection. Int. J. Nanomed. 2019, 14, 1737–1751. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Canon, A.; Medina-Llamas, M.; Vezzoli, M.; Mattia, D. Multiscale design of ZnO nanostructured photocatalysts. Phys. Chem. Chem. Phys. 2018, 20, 6648–6656. [Google Scholar] [CrossRef] [Green Version]
- Münchow, E.; Albuquerque, M.T.; Zero, B.; Kamocki, K.; Piva, E.; Gregory, R.; Bottino, M. Development and characterization of novel ZnO-loaded electrospun membranes for periodontal regeneration. Dent. Mater. 2015, 31. [Google Scholar] [CrossRef]
- Gu, T.; Yao, C.; Zhang, K.; Li, C.; Ding, L.; Huang, Y.; Wu, M.; Wang, Y. Toxic effects of zinc oxide nanoparticles combined with vitamin C and casein phosphopeptides on gastric epithelium cells and the intestinal absorption of mice. Rsc. Adv. 2018, 8, 26078–26088. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Wang, J.; Lee, J.S.; Kim, D.; Zhu, L. Exploration of Zinc Oxide Nanoparticles as a Multitarget and Multifunctional Anticancer Nanomedicine. ACS Appl. Mater. Interfaces 2017, 9, 39971–39984. [Google Scholar] [CrossRef]
- Khan, S.T.; Musarrat, J.; Al-Khedhairy, A.A. Countering drug resistance, infectious diseases, and sepsis using metal and metal oxides nanoparticles: Current status. Colloids Surf. B Biointerfaces 2016, 146, 70–83. [Google Scholar] [CrossRef]
- Bouwmeester, H.; van der Zande, M.; Jepson, M.A. Effects of food-borne nanomaterials on gastrointestinal tissues and microbiota. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1481. [Google Scholar] [CrossRef]
- Balaure, P.C.; Holban, A.M.; Grumezescu, A.M.; Mogoşanu, G.D.; Bălşeanu, T.A.; Stan, M.S.; Dinischiotu, A.; Volceanov, A.; Mogoantă, L. In vitro and in vivo studies of novel fabricated bioactive dressings based on collagen and zinc oxide 3D scaffolds. Int. J. Pharm. 2019, 557, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Sabura Begum, P.M.; Mohammed Yusuff, K.K.; Joseph, R. Preparation and Use of Nano Zinc Oxide in Neoprene Rubber. Int. J. Polym. Mater. Polym. Biomater. 2008, 57, 1083–1094. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, X.; Xu, Z.; Yi, C. ZnO-based nanocarriers for drug delivery application: From passive to smart strategies. Int. J. Pharm. 2017, 534, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef]
- Zamani, M.; Rostami, M.; Aghajanzadeh, M.; Kheiri Manjili, H.; Rostamizadeh, K.; Danafar, H. Mesoporous titanium dioxide@ zinc oxide–graphene oxide nanocarriers for colon-specific drug delivery. J. Mater. Sci. 2018, 53, 1634–1645. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, C.; Sheng, P.; Chen, Y.; Yu, L.; Li, Q. Synthesis of ZnO hollow spheres through a bacterial template method and their gas sensing properties. Sens. Actuators B Chem. 2013, 181, 99–103. [Google Scholar] [CrossRef]
- Zhu, P.; Weng, Z.; Li, X.; Liu, X.; Wu, S.; Yeung, K.W.K.; Wang, X.; Cui, Z.; Yang, X.; Chu, P.K. Biomedical Applications of Functionalized ZnO Nanomaterials: From Biosensors to Bioimaging. Adv. Mater. Interfaces 2016, 3, 1500494. [Google Scholar] [CrossRef]
- Guo, D.; Wu, C.; Jiang, H.; Li, Q.; Wang, X.D.; Chen, B. Synergistic cytotoxic effect of different sized ZnO nanoparticles and daunorubicin against leukemia cancer cells under UV irradiation. J. Photochem. Photobiol. B 2008, 93, 119–126. [Google Scholar] [CrossRef]
- Liu, J.; Huang, H.; Zhao, H.; Yan, X.; Wu, S.; Li, Y.; Wu, M.; Chen, L.; Yang, X.; Su, B.-L. Enhanced Gas Sensitivity and Selectivity on Aperture-Controllable 3D Interconnected Macro–Mesoporous ZnO Nanostructures. ACS Appl. Mater. Interfaces 2016, 8, 8583–8590. [Google Scholar] [CrossRef]
- Nie, L.; Gao, L.; Feng, P.; Zhang, J.; Fu, X.; Liu, Y.; Yan, X.; Wang, T. Three-Dimensional Functionalized Tetrapod-like ZnO Nanostructures for Plasmid DNA Delivery. Small 2006, 2, 621–625. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A.M. Nanoparticles in biomedical applications. Adv. Phys. X 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Li, X.; Zhao, C.; Liu, X. A paper-based microfluidic biosensor integrating zinc oxide nanowires for electrochemical glucose detection. Microsyst. Nanoeng. 2015, 1, 15014. [Google Scholar] [CrossRef]
- Saravanan, A.; Huang, B.; Kathiravan, D.; Prasannan, A. Natural Biowaste-Cocoons Derived Granular Activated Carbon-Coated ZnO Nanorods: A Simple Route to Synthesis Core-Shell Structure and Their Highly Enhanced UV and Hydrogen Sensing Properties. ACS Appl. Mater. Interfaces 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; ur Rahman, A.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Wang, X.; Ahmad, M.; Sun, H. Three-Dimensional ZnO Hierarchical Nanostructures: Solution Phase Synthesis and Applications. Materials 2017, 10, 1304. [Google Scholar] [CrossRef]
- Pan, X.; Liu, X.; Bermak, A.; Fan, Z. Self-Gating Effect Induced Large Performance Improvement of ZnO Nanocomb Gas Sensors. ACS Nano 2013, 7, 9318–9324. [Google Scholar] [CrossRef]
- Xu, T.; Ji, P.; He, M.; Li, J. Growth and Structure of Pure ZnO Micro/Nanocombs. J. Nanomater. 2012, 2012, 5. [Google Scholar] [CrossRef]
- Tan, K.H.; Lim, F.S.; Toh, A.Z.Y.; Zheng, X.-X.; Dee, C.F.; Majlis, B.Y.; Chai, S.-P.; Chang, W.S. Tunable Spectrum Selectivity for Multiphoton Absorption with Enhanced Visible Light Trapping in ZnO Nanorods. Small 2018, 14, 1704053. [Google Scholar] [CrossRef]
- Xu, X.; Jia, Y.; Xiao, L.; Wu, Z. Strong vibration-catalysis of ZnO nanorods for dye wastewater decolorization via piezo-electro-chemical coupling. Chemosphere 2018, 193, 1143–1148. [Google Scholar] [CrossRef]
- Hu, L.; Yan, J.; Liao, M.; Xiang, H.; Gong, X.; Zhang, L.; Fang, X. An Optimized Ultraviolet-A Light Photodetector with Wide-Range Photoresponse Based on ZnS/ZnO Biaxial Nanobelt. Adv. Mater. 2012, 24, 2305–2309. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Li, X. Water Molecule-Induced Stiffening in ZnO Nanobelts. Nano Lett. 2011, 11, 2845–2848. [Google Scholar] [CrossRef]
- Li, Q.; Wei, L.; Xie, Y.; Zhang, K.; Liu, L.; Zhu, D.; Jiao, J.; Chen, Y.; Yan, S.; Liu, G.; et al. ZnO nanoneedle/H2O solid-liquid heterojunction-based self-powered ultraviolet detector. Nanoscale Res. Lett. 2013, 8, 415. [Google Scholar] [CrossRef]
- Yao, Y.-F.; Tu, C.-G.; Chang, T.-W.; Chen, H.-T.; Weng, C.-M.; Su, C.-Y.; Hsieh, C.; Liao, C.-H.; Kiang, Y.-W.; Yang, C.C. Growth of Highly Conductive Ga-Doped ZnO Nanoneedles. ACS Appl. Mater. Interfaces 2015, 7, 10525–10533. [Google Scholar] [CrossRef]
- Errico, V.; Arrabito, G.; Fornetti, E.; Fuoco, C.; Testa, S.; Saggio, G.; Rufini, S.; Cannata, S.; Desideri, A.; Falconi, C.; et al. High-Density ZnO Nanowires as a Reversible Myogenic–Differentiation Switch. ACS Appl. Mater. Interfaces 2018, 10, 14097–14107. [Google Scholar] [CrossRef]
- Mead, J.L.; Xie, H.; Wang, S.; Huang, H. Enhanced adhesion of ZnO nanowires during in situ scanning electron microscope peeling. Nanoscale 2018, 10, 3410–3420. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Tang, F.; Ma, J.; Zhou, X. A Facile Approach for the Preparation of Nano-size Zinc Oxide in Water/Glycerol with Extremely Concentrated Zinc Sources. Nanoscale Res. Lett. 2018, 13. [Google Scholar] [CrossRef]
- Yu, W.-C.; Sabastian, N.; Chang, W.-C.; Tsia, C.-Y.; Lin, C.-M. Electrochemical Deposition of ZnO Porous Nanoplate Network for Dye-Sensitized Solar Cells. J. Nanosci. Nanotechnol. 2018, 18, 56–61. [Google Scholar] [CrossRef]
- Gopala Krishna, P.; Ananthaswamy, P.; Yadavalli, T.; Nagabhushana, B.; Ananda, S.; Yogisha, S. ZnO nanopellets have selective anticancer activity. Mater. Sci. Eng. C 2016, 62. [Google Scholar] [CrossRef]
- Akhtar, N.; Metkar, S.; Girigoswami, A.; Girigoswami, K. ZnO nanoflower based sensitive nano-biosensor for amyloid detection. Mater. Sci. Eng. C 2017, 78. [Google Scholar] [CrossRef]
- Li, C.; Li, G.; Shen, C.; Hui, C.; Tian, J.; Du, S.; Zhang, Z.; Gao, H.-J. Atomic-scale tuning of self-assembled ZnO microscopic patterns: From dendritic fractals to compact island. Nanoscale 2010, 2, 2557–2560. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, H.; Wang, Y.; Miao, Y.; Gu, L.; Jiao, Z. Self-assembly and template-free synthesis of ZnO hierarchical nanostructures and their photocatalytic properties. J. Colloid Interface Sci. 2015, 448C, 367–373. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, V.; Shanmugam, R. A review on green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour. Effic. Technol. 2017, 3. [Google Scholar] [CrossRef]
- Brayner, R.; Dahoumane, S.A.; Yéprémian, C.; Djediat, C.; Meyer, M.; Couté, A.; Fiévet, F. ZnO Nanoparticles: Synthesis, Characterization, and Ecotoxicological Studies. Langmuir 2010, 26, 6522–6528. [Google Scholar] [CrossRef]
- Malfatti, L.; Pinna, A.; Enzo, S.; Falcaro, P.; Marmiroli, B.; Innocenzi, P. Tuning the phase transition of ZnO thin films through lithography: An integrated bottom-up and top-down processing. J. Synchrotron Radiat. 2015, 22, 165–171. [Google Scholar] [CrossRef]
- Krupiński, P.; Kornowicz, A.; Sokołowski, K.; Cieślak, A.M.; Lewiński, J. Applying Mechanochemistry for Bottom-Up Synthesis and Host–Guest Surface Modification of Semiconducting Nanocrystals: A Case of Water-Soluble β-Cyclodextrin-Coated Zinc Oxide. Chem. Eur. J. 2016, 22, 7817–7823. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, N.B.; Singh, A.; Singh, H.; Singh, S. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 2016, 38, 545–560. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Park, J.-H.; Hahn, Y.-B. A comprehensive biosensor integrated with a ZnO nanorod FET array for selective detection of glucose, cholesterol and urea. Chem. Comm. 2015, 51, 11968–11971. [Google Scholar] [CrossRef]
- Khan, W.; Ajmal, M.; Khan, F.; Huda, N.; Kim, S.-D. Induced Photonic Response of ZnO Nanorods Grown on Oxygen Plasma-Treated Seed Crystallites. Nanomaterials 2018, 8, 371. [Google Scholar] [CrossRef]
- Sharifalhoseini, Z.; Entezari, M.; Shahidi, M. Synergistic effect of low and high intensity ultrasonic irradiation on the direct growth of ZnO nanostructures on the galvanized steel surface: Investigation of the corrosion behavior. Ultrason. Sonochem. 2018, 44. [Google Scholar] [CrossRef]
- Zeng, H.; Cai, W.; Li, Y.; Hu, J.; Liu, P. Composition/Structural Evolution and Optical Properties of ZnO/Zn Nanoparticles by Laser Ablation in Liquid Media. J. Phys. Chem. B 2005, 109, 18260–18266. [Google Scholar] [CrossRef]
- Chang Dr, I. Plasma synthesis of metal nanopowders. Adv. Powder Metall. Prop. Process. Appl. 2013, 69–85. [Google Scholar] [CrossRef]
- Peng, H.; Fangli, Y.; Liuyang, B.; Jinlin, L.; Yunfa, C. Plasma Synthesis of Large Quantities of Zinc Oxide Nanorods. J. Phys. Chem. C 2007, 111, 194–200. [Google Scholar] [CrossRef]
- Fouad, O.; Ismail, A.; Zaki, Z.; Mohamed, R.M. Zinc Oxide Thin Films Prepared by Thermal Evaporation Deposition and Its Photocatalytic Activity. Appl. Catal. B 2006, 62, 144–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Liu, X.; Yan, Y.; Chen, C.; Zhu, J. Synthesis of Nano/Micro Zinc Oxide Rods and Arrays by Thermal Evaporation Approach on Cylindrical Shape Substrate. J. Phys. Chem. B 2005, 109, 13091–13093. [Google Scholar] [CrossRef]
- Lyu, S.C.; Zhang, Y.; Lee, C.J.; Ruh, H.; Lee, H.J. Low-Temperature Growth of ZnO Nanowire Array by a Simple Physical Vapor-Deposition Method. Chem. Mater. 2003, 15, 3294–3299. [Google Scholar] [CrossRef]
- Yadav, R.S.; Mishra, P.; Pandey, A.C. Tuning the band gap of ZnO nanoparticles by ultrasonic irradiation. Inorg. Mater. 2010, 46, 163–167. [Google Scholar] [CrossRef]
- Thareja, R.K.; Shukla, S. Synthesis and characterization of zinc oxide nanoparticles by laser ablation of zinc in liquid. Appl. Surf. Sci. 2007, 253, 8889–8895. [Google Scholar] [CrossRef]
- Naveed Ul Haq, A.; Nadhman, A.; Ullah, I.; Mustafa, G.; Yasinzai, M.; Khan, I. Synthesis Approaches of Zinc Oxide Nanoparticles: The Dilemma of Ecotoxicity. J. Nanomater. 2017, 2017, 14. [Google Scholar] [CrossRef]
- Fricke, M.; Voigt, A.; Veit, P.; Sundmacher, K. Miniemulsion-Based Process for Controlling the Size and Shape of Zinc Oxide Nanoparticles. Ind. Eng. Chem. Res. 2015, 54, 10293–10300. [Google Scholar] [CrossRef]
- Valdez, C.N.; Schimpf, A.M.; Gamelin, D.R.; Mayer, J.M. Low Capping Group Surface Density on Zinc Oxide Nanocrystals. ACS Nano 2014, 8, 9463–9470. [Google Scholar] [CrossRef]
- Oliveira, A.P.A.; Hochepied, J.-F.; Grillon, F.; Berger, M.-H. Controlled Precipitation of Zinc Oxide Particles at Room Temperature. Chem. Mater. 2003, 15, 3202–3207. [Google Scholar] [CrossRef]
- Demir, M.M.; Muñoz-Espí, R.; Lieberwirth, I.; Wegner, G. Precipitation of monodisperse ZnO nanocrystals via acid-catalyzed esterification of zinc acetate. J. Mater. Chem. 2006, 16, 2940–2947. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Song, Y. Microfluidic Synthesis of Nanohybrids. Small 2017, 13, 1604084. [Google Scholar] [CrossRef]
- Aneesh, P.M.; Vanoja, M.A.; Jayaraj, M. Synthesis of ZnO nanoparticles by hydrothermal method. Nanophotonic Mater. IV 2007. [Google Scholar] [CrossRef]
- Santos, L.; Nunes, D.; Calmeiro, T.; Branquinho, R.; Salgueiro, D.; Barquinha, P.; Pereira, L.; Martins, R.; Fortunato, E. Solvothermal Synthesis of Gallium–Indium-Zinc-Oxide Nanoparticles for Electrolyte-Gated Transistors. ACS Appl. Mater. Interfaces 2015, 7, 638–646. [Google Scholar] [CrossRef]
- Noothongkaew, S.; Pukird, S.; Sukkabot, W.; Seok An, K. Zinc Oxide Nano Walls Synthesized by Chemical Vapor Deposition. Key Eng. Mater. 2014, 608, 127–131. [Google Scholar] [CrossRef]
- Agarwal, H.; Menon, S.; Kumar, V.; Shanmugam, R. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. Biol. Interact. 2018, 286. [Google Scholar] [CrossRef]
- Ishwarya, R.; Vaseeharan, B.; Kalyani, S.; Banumathi, B.; Govindarajan, M.; Alharbi, N.; Km, S.N.; Al-anbr, M.; Khaled, J.; Benelli, G. Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and its evaluation of photocatalytic, antibiofilm and larvicidal activity: Impact on mosquito morphology and biofilm architecture. J. Photochem. Photobiol. B 2017, 178. [Google Scholar] [CrossRef]
- Raja, A.; Ashokkumar, S.; Pavithra Marthandam, R.; Jayachandiran, J.; Khatiwada, C.P.; Kaviyarasu, K.; Ganapathi Raman, R.; Swaminathan, M. Eco-friendly preparation of zinc oxide nanoparticles using Tabernaemontana divaricata and its photocatalytic and antimicrobial activity. J. Photochem. Photobiol. B 2018, 181, 53–58. [Google Scholar] [CrossRef]
- Taran, M.; Rad, M.; Alavi, M. Biosynthesis of TiO2 and ZnO nanoparticles by Halomonas elongata IBRC-M 10214 in different conditions of medium. BioImpacts 2018, 8, 81–89. [Google Scholar] [CrossRef]
- Rajabairavi, N.; Raju, C.S.; Karthikeyan, C.; Varutharaju, K.; Nethaji, S.; Hameed, A.S.H.; Shajahan, A. Biosynthesis of Novel Zinc Oxide Nanoparticles (ZnO NPs) Using Endophytic Bacteria Sphingobacterium thalpophilum. Recent Trends in Mater. Sci. Appl. 2017, 245–254. [Google Scholar] [CrossRef]
- Rauf, M.A.; Owais, M.; Rajpoot, R.; Ahmad, F.; Khan, N.; Zubair, S. Biomimetically synthesized ZnO nanoparticles attain potent antibacterial activity against less susceptible S. aureus skin infection in experimental animals. RSC Adv. 2017, 7, 36361–36373. [Google Scholar] [CrossRef] [Green Version]
- Kalpana, V.N.; Kataru, B.A.S.; Sravani, N.; Vigneshwari, T.; Panneerselvam, A.; Devi Rajeswari, V. Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. OpenNano 2018, 3, 48–55. [Google Scholar] [CrossRef]
- Shamsuzzaman; Mashrai, A.; Khanam, H.; Aljawfi, R.N. Biological synthesis of ZnO nanoparticles using C. albicans and studying their catalytic performance in the synthesis of steroidal pyrazolines. Arab. J. Chem. 2017, 10, S1530–S1536. [Google Scholar] [CrossRef] [Green Version]
- Moghaddam, A.B.; Moniri, M.; Azizi, S.; Rahim, R.A.; Ariff, A.B.; Saad, W.Z.; Namvar, F.; Navaderi, M. Biosynthesis of ZnO Nanoparticles by a New Pichia kudriavzevii Yeast Strain and Evaluation of Their Antimicrobial and Antioxidant Activities. Molecules 2017, 22, 872. [Google Scholar] [CrossRef]
- Chauhan, R.; Reddy, A.; Abraham, J. Biosynthesis of silver and zinc oxide nanoparticles using Pichia fermentans JA2 and their antimicrobial property. Appl. Nanosci. 2014. [Google Scholar] [CrossRef]
- Rao, M.D.; Gautam, P. Synthesis and characterization of ZnO nanoflowers using Chlamydomonas reinhardtii: A green approach. Env. Prog. Sustain. Energy 2016, 35, 1020–1026. [Google Scholar] [CrossRef]
- Azizi, S.; Ahmad, M.B.; Namvar, F.; Mohamad, R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater. Lett. 2014, 116, 275–277. [Google Scholar] [CrossRef]
- Żelechowska, K.; Karczewska-Golec, J.; Karczewski, J.; Łoś, M.; Kłonkowski, A.M.; Węgrzyn, G.; Golec, P. Phage-Directed Synthesis of Photoluminescent Zinc Oxide Nanoparticles under Benign Conditions. Bioconjug. Chem. 2016, 27, 1999–2006. [Google Scholar] [CrossRef]
- Li, N.; Gao, Y.; Hou, L.; Gao, F. DNA-Based Toolkit for Directed Synthesis of Zinc Oxide Nanoparticle Chains and Understanding the Quantum Size Effects in ZnO Nanocrystals. J. Phys. Chem. C 2011, 115, 25266–25272. [Google Scholar] [CrossRef]
- Gharagozlou, M.; Baradaran, Z.; Bayati, R. A green chemical method for synthesis of ZnO nanoparticles from solid-state decomposition of Schiff-bases derived from amino acid alanine complexes. Ceram. Int. 2015, 41, 8382–8387. [Google Scholar] [CrossRef]
- Divya, M.; Vaseeharan, B.; Abinaya, M.; Sekar, V.; Govindarajan, M.; Alharbi, N.; Km, S.; Khaled, J.; Benelli, G. Biopolymer gelatin-coated zinc oxide nanoparticles showed high antibacterial, antibiofilm and anti-angiogenic activity. J. Photochem. Photobiol. B 2017, 178. [Google Scholar] [CrossRef] [PubMed]
- Ambika, S.; Sundrarajan, M. Green biosynthesis of ZnO nanoparticles using Vitex negundo L. extract: Spectroscopic investigation of interaction between ZnO nanoparticles and human serum albumin. J. Photochem. Photobiol. B 2015, 149. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.; Kamilya, T.; Saha, S. Optical and Structural Properties of Protein Capped ZnO Nanoparticles and Its Antimicrobial Activity. J. Adv. Biol. Biotechnol. 2016, 10, 1–9. [Google Scholar] [CrossRef]
- Gawade, V.V.; Gavade, N.L.; Shinde, H.M.; Babar, S.B.; Kadam, A.N.; Garadkar, K.M. Green synthesis of ZnO nanoparticles by using Calotropis procera leaves for the photodegradation of methyl orange. J. Mater. Sci. 2017, 28, 14033–14039. [Google Scholar] [CrossRef]
- Ogunyemi, S.O.; Abdallah, Y.; Zhang, M.; Fouad, H.; Hong, X.; Ibrahim, E.; Masum, M.M.I.; Hossain, A.; Mo, J.; Li, B. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif. Cells Nanomed. Biotechnol. 2019, 47, 341–352. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Mulvey, P.; Kumar, A.; Prasad, K.; Bazaka, K.; Warner, J.; Jacob, M.V. Eco-friendly nanocomposites derived from geranium oil and zinc oxide in one step approach. Sci. Rep. 2019, 9, 5973. [Google Scholar] [CrossRef]
- Xing, Y.; Dittrich, P.S. One-Dimensional Nanostructures: Microfluidic-Based Synthesis, Alignment and Integration towards Functional Sensing Devices. Sensors 2018, 18, 134. [Google Scholar] [CrossRef]
- deMello, A.J. Control and detection of chemical reactions in microfluidic systems. Nature 2006, 442, 394–402. [Google Scholar] [CrossRef]
- Janasek, D.; Franzke, J.; Manz, A. Scaling and the design of miniaturized chemical-analysis systems. Nature 2006, 442, 374–380. [Google Scholar] [CrossRef]
- Li, L.-L.; Li, X.; Wang, H. Microfluidic Synthesis of Nanomaterials for Biomedical Applications. Small Methods 2017, 1, 1700140. [Google Scholar] [CrossRef]
- Elvira, K.S.; i Solvas, X.C.; Wootton, R.C.R.; deMello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, I.; Habba, Y.G.; Capochichi-Gnambodoe, M.; Marty, F.; Vial, J.; Leprince-Wang, Y.; Bourouina, T. Zinc oxide nano-enabled microfluidic reactor for water purification and its applicability to volatile organic compounds. Microsyst. Nanoeng. 2018, 4, 17093. [Google Scholar] [CrossRef]
- Makgwane, P.; Sinha Ray, S. Synthesis of Nanomaterials by Continuous-Flow Microfluidics: A Review. J. Nanosci. Nanotechnol. 2014, 14, 1338–1363. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Chow, B.Y.; Prakash, M.; Boyden, E.S.; Jacobson, J.M. Face-selective electrostatic control of hydrothermal zinc oxide nanowire synthesis. Nat. Mater. 2011, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Kraus, I.; Li, S.; Knauer, A.; Schmutz, M.; Faerber, J.; Serra, C.A.; Köhler, M. Continuous-Microflow Synthesis and Morphological Characterization of Multiscale Composite Materials Based on Polymer Microparticles and Inorganic Nanoparticles. J. Flow Chem. 2014, 4, 72–78. [Google Scholar] [CrossRef]
- Zukas, B.G.; Gupta, N.R. Interphase Synthesis of Zinc Oxide Nanoparticles in a Droplet Flow Reactor. Ind. Eng. Chem. Res. 2017, 56, 7184–7191. [Google Scholar] [CrossRef]
- OECD. Physical-Chemical Decision Framework to Inform Decisions for Risk Assessment of Manufactrued Nanomaterials; Organization for Economic Co-operation and Development: Paris, France, 2019; Volume 90. [Google Scholar]
- Rasmussen, K.; Rauscher, H.; Mech, A.; Riego Sintes, J.; Gilliland, D.; González, M.; Kearns, P.; Moss, K.; Visser, M.; Groenewold, M.; et al. Physico-chemical properties of manufactured nanomaterials-Characterisation and relevant methods. An outlook based on the OECD Testing Programme. Regul. Toxicol. Pharmacol. 2018, 92, 8–28. [Google Scholar] [CrossRef]
- Noh, H.W.; Jeong, S.M.; Cho, J.; Hong, J.-I. Ultrahigh photosensitivity of the polar surfaces of single crystalline ZnO nanoplates. Nanoscale 2018, 10, 6801–6805. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, G.; Wang, C.; Li, Y.; Dunphy, D.; Hasan, T.; Brinker, C.J.; Su, B.-L. Bio-inspired Murray materials for mass transfer and activity. Nat. Commun. 2017, 8, 14921. [Google Scholar] [CrossRef] [Green Version]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. App. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and Enhanced Photocatalytic Activity of Zinc Oxide Nanoparticles Toward Organosulfur Pollutants. Sci. Rep. 2019, 9, 6866. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, P.; Hong, R. Preparation and application of aluminum-doped zinc oxide powders via precipitation and plasma processing method. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Abdullayeva, N.; Altaf, C.T.; Mintas, M.; Ozer, A.; Sankir, M.; Kurt, H.; Sankir, N.D. Investigation of Strain Effects on Photoelectrochemical Performance of Flexible ZnO Electrodes. Sci. Rep. 2019, 9, 11006. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-E.; Hwang, W.; Lee, H.J.; Jin, H.-E. Dual UV irradiation-based metal oxide nanoparticles for enhanced antimicrobial activity in Escherichia coli and M13 bacteriophage. Int. J. Nanomed. 2017, 12, 8057–8070. [Google Scholar] [CrossRef]
- Zhao, Z.; Lei, W.; Zhang, X.; Wang, B.; Jiang, H. ZnO-Based Amperometric Enzyme Biosensors. Sensors 2010, 10, 1216–1231. [Google Scholar] [CrossRef]
- Li, X.; Cheng, S.; Deng, S.; Wei, X.; Zhu, J.; Chen, Q. Direct Observation of the Layer-by-Layer Growth of ZnO Nanopillar by In Situ High Resolution Transmission Electron Microscopy. Sci. Rep. 2017, 7, 40911. [Google Scholar] [CrossRef]
- Ludi, B.; Niederberger, M. Zinc oxide nanoparticles: Chemical mechanisms and classical and non-classical crystallization. Dalton Trans. 2013, 42, 12554–12568. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Laurenti, M.; Cauda, V. Porous Zinc Oxide Thin Films: Synthesis Approaches and Applications. Coatings 2018, 8, 67. [Google Scholar] [CrossRef]
- Zafar, M.N.; Dar, Q.; Nawaz, F.; Zafar, M.N.; Iqbal, M.; Nazar, M.F. Effective adsorptive removal of azo dyes over spherical ZnO nanoparticles. J. Mater. Res. Technol. 2019, 8, 713–725. [Google Scholar] [CrossRef]
- Lu, X.; Kanamori, K.; Nakanishi, K. Preparation of zinc oxide with a three-dimensionally interconnected macroporous structure via a sol–gel method accompanied by phase separation. New J. Chem. 2019, 43, 11720–11726. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; van der Wal, A.; Presser, V.; Biesheuvel, P.M. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef] [Green Version]
- Zdravkov, B.D.; Čermák, J.J.; Šefara, M.; Janků, J. Pore classification in the characterization of porous materials: A perspective. CEJC 2007, 5, 385–395. [Google Scholar] [CrossRef]
- Sun, M.-H.; Huang, S.-Z.; Chen, L.-H.; Li, Y.; Yang, X.-Y.; Yuan, Z.-Y.; Su, B.-L. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef]
- Akita, D.; Kunita, I.; Fricker, M.D.; Kuroda, S.; Sato, K.; Nakagaki, T. Experimental models for Murray’s law. J. Phys. D Appl. Phys. 2016, 50, 024001. [Google Scholar] [CrossRef]
- Parlett, C.M.A.; Wilson, K.; Lee, A.F. Hierarchical porous materials: Catalytic applications. Chem. Soc. Rev. 2013, 42, 3876–3893. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Liu, J.; Wang, F.; Kong, J.; Qiu, S.; He, C.; Luan, L. Synthesis of Nestlike ZnO Hierarchically Porous Structures and Analysis of Their Gas Sensing Properties. ACS Appl. Mater. Interfaces 2012, 4, 817–825. [Google Scholar] [CrossRef]
- Lei, C.; Pi, M.; Jiang, C.; Cheng, B.; Yu, J. Synthesis of hierarchical porous zinc oxide (ZnO) microspheres with highly efficient adsorption of Congo red. J. Colloid Interface. Sci. 2017, 490, 242–251. [Google Scholar] [CrossRef]
- Leone, F.; Cataldo, R.; Mohamed, S.S.Y.; Manna, L.; Banchero, M.; Ronchetti, S.; Mandras, N.; Tullio, V.; Cavalli, R.; Onida, B. Nanostructured ZnO as Multifunctional Carrier for a Green Antibacterial Drug Delivery System-A Feasibility Study. Nanomaterials 2019, 9, 407. [Google Scholar] [CrossRef]
- Pérez, R.; Sanchez-Salcedo, S.; Lozano, D.; Heras, C.; Esbrit, P.; Vallet-Regí, M.; Salinas, A.J. Osteogenic Effect of ZnO-Mesoporous Glasses Loaded with Osteostatin. Nanomaterials 2018, 8, 592. [Google Scholar] [CrossRef] [PubMed]
- Aizenberg, J.; Fratzl, P. Biological and Biomimetic Materials. Adv. Mater. 2009, 21, 387–388. [Google Scholar] [CrossRef]
- Fratzl, P. Biomimetic materials research: What can we really learn from nature’s structural materials? J. R. Soc. Interface 2007, 4, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef]
- Lakes, R. Materials with structural hierarchy. Nature 1993, 361, 511–515. [Google Scholar] [CrossRef]
- Messersmith, P.B. Multitasking in Tissues and Materials. Science 2008, 319, 1767–1768. [Google Scholar] [CrossRef]
- Munch, E.; Launey, M.E.; Alsem, D.H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Tough, Bio-Inspired Hybrid Materials. Science 2008, 322, 1516–1520. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C.; Arribart, H.; Madeleine Giraud Guille, M. Biomimetism and bioinspiration as tools for the design of innovative materials and systems. Nat. Mater. 2005, 4, 277–288. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Chen, L.-H.; Li, Y.; Rooke, J.C.; Sanchez, C.; Su, B.-L. Hierarchically porous materials: Synthesis strategies and structure design. Chem. Soc. Rev. 2017, 46, 481–558. [Google Scholar] [CrossRef]
- Żelechowska, K. Methods of ZnO nanoparticles synthesis. Biotechnologia 2015, 95, 150–159. [Google Scholar] [CrossRef]
- Prakash, T.; Rajan, J.; Sathya Raj, D.; Kumar, S.; Donato, N.; Spadaro, D.; Neri, G. Sensing properties of ZnO nanoparticles synthesized by using albumen as a biotemplate for acetic acid monitoring in aqueous mixture. Sens. Actuators B Chem. 2013, 176, 560–568. [Google Scholar] [CrossRef]
- Fang, K.-M.; Wang, Z.-Z.; Zhang, M.; Wang, A.-J.; Meng, Z.-Y.; Feng, J.-J. Gelatin-assisted Hydrothermal Synthesis of Single Crystalline ZnO Nanostars and Their Photocatalytic Properties. J. Colloid Interface Sci. 2013, 402. [Google Scholar] [CrossRef] [PubMed]
- Oudhia, A.; Sharma, S.; Kulkarni, P.; Lalwani, R. Blue emitting ZnO nanostructures grown through cellulose bio-templates. Luminescence 2015, 31. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Su, H.; Xu, J.; Song, W.; Gu, Y.; Chen, Y.; Moon, W.-J.; Zhang, D. Silk-mediated synthesis and modification of photoluminescent ZnO nanoparticles. J. Nanopart. Res. 2012, 14, 726. [Google Scholar] [CrossRef]
- Camaratta, R.; Orozco-Messana, J.; Bergmann, C. Synthesis of ZnO through biomimetization of eggshell membranes using different precursors and its characterization. Ceram. Int. 2015, 41. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Hussein, M.; Taufiq-Yap, Y.H. Hydrothermal synthesis of zinc oxide nanoparticles using rice as soft biotemplate. Chem. Cent. J. 2013, 7, 136. [Google Scholar] [CrossRef]
- Upneja, A.; Dou, G.; Gopu, C.; Johnson, C.A.; Newman, A.; Suleimenov, A.; Goldfarb, J.L. Sustainable waste mitigation: Biotemplated nanostructured ZnO for photocatalytic water treatment via extraction of biofuels from hydrothermal carbonization of banana stalk. RSC Adv. 2016, 6, 92813–92823. [Google Scholar] [CrossRef]
- Selvakumar, R.; Seethalakshmi, N.; Thavamani, P.; Naidu, R.; Megharaj, M. Recent advances in the synthesis of inorganic nano/microstructures using microbial biotemplates and their applications. RSC Adv. 2014, 4, 52156–52169. [Google Scholar] [CrossRef] [Green Version]
- Stitz, N.; Eiben, S.; Atanasova, P.; Domingo, N.; Leineweber, A.; Burghard, Z.; Bill, J. Piezoelectric Templates – New Views on Biomineralization and Biomimetics. Sci. Rep. 2016, 6, 26518. [Google Scholar] [CrossRef]
- Levchenko, I.; Bazaka, K.; Keidar, M.; Xu, S.; Fang, J. Hierarchical Multicomponent Inorganic Metamaterials: Intrinsically Driven Self-Assembly at the Nanoscale. Adv. Mater. 2018, 30, 1702226. [Google Scholar] [CrossRef]
- Sofos, M.; Goldberger, J.; Stone, D.A.; Allen, J.E.; Ma, Q.; Herman, D.J.; Tsai, W.-W.; Lauhon, L.J.; Stupp, S.I. A synergistic assembly of nanoscale lamellar photoconductor hybrids. Nat. Mater. 2008, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.-X.; Liu, B. In situ etching-induced self-assembly of metal cluster decorated one-dimensional semiconductors for solar-powered water splitting: Unraveling cooperative synergy by photoelectrochemical investigations. Nanoscale 2017, 9, 17118–17132. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhao, R.; Chen, S.; Wang, H.; Li, J.; Zhu, Z. Self-Assembly of Gridlike Zinc Oxide Lamellae for Chemical-Sensing Applications. ACS Appl. Mater. Interfaces 2015, 7, 5870–5878. [Google Scholar] [CrossRef] [PubMed]
- Zena, D.M.; Chiu, J.-M.; Tai, Y. Self-assembled monolayer assisted fabrication of zinc oxide nanorods. Cryst. Eng. Comm. 2013, 15, 4189–4195. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, L.; Guo, L. Precursor-Directed Self-Assembly of Porous ZnO Nanosheets as High-Performance Surface-Enhanced Raman Scattering Substrate. Small 2014, 10, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Müller, W.; Wang, X.; Lilja, L.; Shen, Z. Porous titania surfaces on titanium with hierarchical macro- and mesoporosities for enhancing cell adhesion, proliferation and mineralization. Mater. Sci. Eng. C 2014, 47. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 18. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO Photocatalyst for the Efficient and Rapid Photocatalytic Degradation of Azo Dyes. Nanoscale Res. Lett. 2017, 12. [Google Scholar] [CrossRef]
- Falgenhauer, J.; Fiehler, F.; Richter, C.; Rudolph, M.; Schlettwein, D. Consequences of changes in the ZnO trap distribution on the performance of dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2017, 19, 16159–16168. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Harnisch, W.; Froelich, K.; Ginzkey, C.; Koehler, C.; Hagen, R.; Kleinsasser, N. Antitumor activity of photo-stimulated zinc oxide nanoparticles combined with paclitaxel or cisplatin in HNSCC cell lines. J. Photochem. Photobiol. B 2012, 114, 87–93. [Google Scholar] [CrossRef]
- Peng, H.; Cui, B.; Li, G.; Wang, Y.; Li, N.; Chang, Z.; Wang, Y. A multifunctional β-CD-modified Fe3O4@ZnO:Er3+,Yb3+ nanocarrier for antitumor drug delivery and microwave-triggered drug release. Mater. Sci. Eng. C 2015, 46. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Kumar, K.; Choudhary, C.; Mishra, P.K.; Vaidya, B. Development and characterization of metal oxide nanoparticles for the delivery of anticancer drug. Artif. Cells Nanomed. Biotechnol. 2016, 44, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, N.; Ahmad, R.; Ko, H.A.; Khang, G.; Hahn, Y.-B. Enhanced anticancer potency using an acid-responsive ZnO-incorporated liposomal drug-delivery system. Nanoscale 2015, 7, 4088–4096. [Google Scholar] [CrossRef] [PubMed]
- El-Gharbawy, R.; Emara, A.; Abu-Risha, S. Zinc oxide nanoparticles and a standard antidiabetic drug restore the function and structure of beta cells in Type-2 diabetes. Biomed. Pharm. 2016, 84, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Umrani, R.; Paknikar, K. Zinc oxide nanoparticles show antidiabetic activity in streptozotocin-induced Type 1 and 2 diabetic rats. Nanomedicine 2014, 9, 89–104. [Google Scholar] [CrossRef]
- Chausmer, A.B. Zinc, Insulin and Diabetes. J. Am. Coll Nutr. 1998, 17, 109–115. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Shanmugam, V.K. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: A review. Biomed. Pharm. 2019, 109, 2561–2572. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Cha, S.J.; Yang, I.J.; Sreekanth, T.V.M.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B 2015, 146, 10–17. [Google Scholar] [CrossRef]
- Yao, S.; Feng, X.; Lu, J.; Zheng, Y.; Wang, X.; Volinsky, A.A.; Wang, L.-N. Antibacterial activity and inflammation inhibition of ZnO nanoparticles embedded TiO2 nanotubes. Nanotechnology 2018, 29, 244003. [Google Scholar] [CrossRef]
- Dong, H.; Li, Q.; Tan, C.; Bai, N.; Cai, P. Bi-directional controlled release of ibuprofen and Mg2+ from magnesium alloys coated by multifunctional composite. Mater. Sci. Eng. C 2016, 68. [Google Scholar] [CrossRef]
- Lin, P.-H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2018, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef] [PubMed]
- Ågren, M.S.; Chvapil, M.; Franzén, L. Enhancement of re-epithelialization with topical zinc oxide in porcine partial-thickness wounds. J. Surg. Res. 1991, 50, 101–105. [Google Scholar] [CrossRef]
- Rajendran, N.K.; Sundar, S.; Houreld, N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44. [Google Scholar] [CrossRef]
- Gao, Y.; Han, Y.; Cui, M.; Tey, H.L.; Wang, L.; Xu, C. ZnO nanoparticles as an antimicrobial tissue adhesive for skin wound closure. J. Mater. Chem. B 2017, 5, 4535–4541. [Google Scholar] [CrossRef]
- Mohandas, A.; Kumar, P.T.S.; Raja, B.; Lakshmanan, V.-K.; Jayakumar, R. Exploration of alginate hydrogel/nano zinc oxide composite bandages for infected wounds. Int. J. Nanomed. 2015, 10 (Suppl. 1), 53–66. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.E.; Ahn, H.S.; Kim, J.H.; Arai, Y.; Lee, S.H.; Yoon, T.J.; Hwang, S.J.; Sung, J.H. Boiling Method-Based Zinc Oxide Nanorods for Enhancement of Adipose-Derived Stem Cell Proliferation. Tissue Eng. Part C Methods 2016, 22, 847–855. [Google Scholar] [CrossRef]
- Lansdown, A. Metallothioneins: Potential therapeutic aids for wound healing in the skin. Wound Repair Regen 2002, 10, 130–132. [Google Scholar] [CrossRef]
- Kietzmann, M.; Braun, M. Effects of the zinc oxide and cod liver oil containing ointment Zincojecol in an animal model of wound healing. Dtw Dtsch Tierarztl Wochenschr 2006, 113, 331–334. [Google Scholar]
- Bajwa, N.; Mehra, N.; Jain, K.; Jain, N. Pharmaceutical and biomedical applications of quantum dots. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1–11. [Google Scholar] [CrossRef]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomed. 2017, 12, 5421–5431. [Google Scholar] [CrossRef]
- Liu, T.-M.; Conde, J.; Lipiński, T.; Bednarkiewicz, A.; Huang, C.-C. Revisiting the classification of NIR-absorbing/emitting nanomaterials for in vivo bioapplications. NPG Asia Mater. 2016, 8, e295. [Google Scholar] [CrossRef]
- Jia, Z.; Misra, R.D.K. Tunable ZnO quantum dots for bioimaging: Synthesis and photoluminescence. Mater. Technol. 2013, 28, 221–227. [Google Scholar] [CrossRef]
- Chaudhary, S.; Umar, A.; Bhasin, K.K.; Baskoutas, S. Chemical Sensing Applications of ZnO Nanomaterials. Materials 2018, 11, 287. [Google Scholar] [CrossRef]
- Nasiri, N.; Clarke, C. Nanostructured Gas Sensors for Medical and Health Applications: Low to High Dimensional Materials. Biosensors 2019, 9, 43. [Google Scholar] [CrossRef]
- Shankar, P.; Rayappan, J.B.B. Monomer: A Designer of ZnO Nanostructures (Nanobush & Nanowire) and their Room Temperature Ethanol Vapor Sensing Signatures. ACS Appl. Mater. Interfaces 2017, 9. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Wang, Z.; Bian, Y.; Wang, Y.; Han, N.; Chen, Y. Transilient Response to Acetone Gas Using the Interlocking p+n Field-Effect Transistor Circuit. Sensors 2018, 18, 1914. [Google Scholar] [CrossRef]
- Mohsin, M.A.; Liu, B.D.; Zhang, X.L.; Yang, W.J.; Liu, L.S.; Jiang, X. Cellular-membrane inspired surface modification of well aligned ZnO nanorods for chemosensing of epinephrine. RSC Adv. 2017, 7, 3012–3020. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, N.R.; Muthukumar, S.; Prasad, S. Ultrasensitive and low-volume point-of-care diagnostics on flexible strips-a study with cardiac troponin biomarkers. Sci. Rep. 2016, 6, 33423. [Google Scholar] [CrossRef]
- Perumal, V.; Hashim, U.; Gopinath, S.C.B.; Haarindraprasad, R.; Liu, W.-W.; Poopalan, P.; Balakrishnan, S.R.; Thivina, V.; Ruslinda, A.R. Thickness dependent nanostructural, morphological, optical and impedometric analyses of zinc oxide-gold hybrids: Nanoparticle to thin film. PLoS ONE 2015, 10, e0144964. [Google Scholar] [CrossRef]
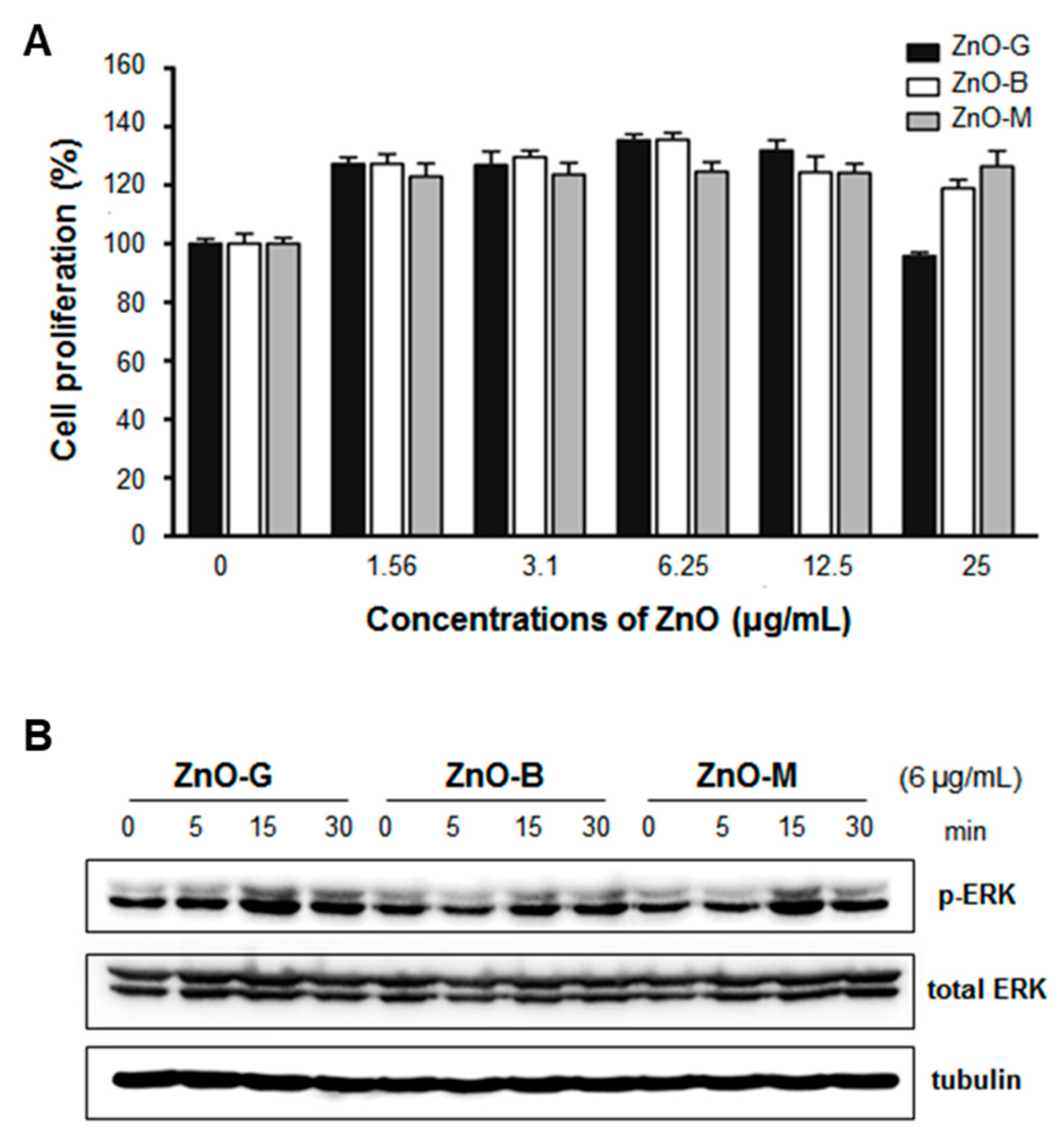
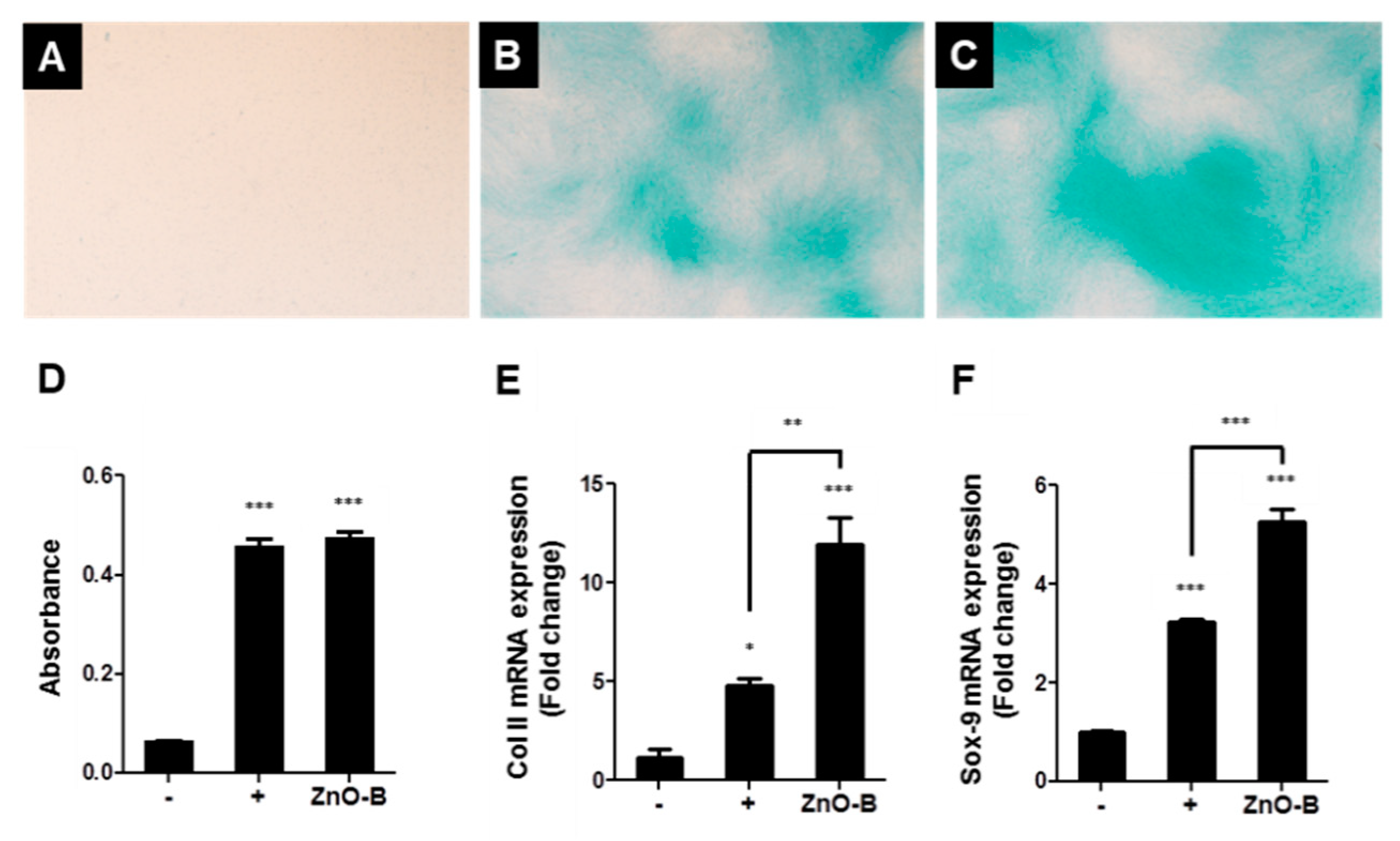
| Category | Applications | References |
|---|---|---|
| Pharmaceuticals |
| [20] |
| Cosmetics—hair and skin care products |
| [20,21] |
| Medical devices |
| [22] |
| Synthesis Technique | Advantages | Disadvantages | References | |
|---|---|---|---|---|
| Physical methods |
|
|
| [9,54,55,56,57,58,59,60,61,62,63,64,65,66,67] |
| Chemical methods |
|
|
| [9,35,55,68,69,70,71,72,73,74,75,76] |
| Biological methods (green synthesis) |
|
|
| [9,52,56,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97] |
| Microfluidic reactor-based methods |
|
|
| [98,99,100,101,102,103,104,105,106,107] |
| Physicochemical Characteristics | Analysis Techniques |
|---|---|
| Agglomeration/aggregation | SEM (++), TEM (++), SPM (++), MALS (+), SAXS (+/−), SMPS (++) |
| Composition | Neutron/electron scattering (+), XRD (+), ICP-MS/OES (++), SP ICP-MS (++), EDS (+), NMR (++), XRF (++), SIMS (+), EELS (+), TOF-MS/ATOF-MS (++), FTIR/RS (++), UV–Vis (+), AES (+/−) |
| Crystalline phase | SEM (+), TEM (+), Neutron/electron scattering (++), XRD (++), FTIR/RS (+; RS), TGA/DSC (+) |
| Dustiness | SD/VS (+) |
| Solubility | DLS/PCS/QELS (++), MALS (++) |
| Dispersibility | DLS/PCS/QELS (++), MALS (++) |
| Stability | DLS/PCS/QELS (++), MALS (++), ELS (++), TGA/DSC (++) |
| Particle size/size distribution | SEM (++), TEM (++), SPM (++), DLS/PCS/QELS (++), MALS (++), SAXS (+), XRD (+; volume weighted primary crystals), SP ICP-MS (++), TOF-MS/ATOF-MS (+; coupled with FFF), FTIR/RS (+; RS), UV–Vis (+; for plasmonic materials), CHDF (++), FFF/A4F/FlFFF (++), BET (+/−), CLS (++), SMPS (++) |
| Shape | SEM (++), TEM (++), SPM (++) |
| Specific surface area | TEM (+; electron tomography), SAXS (+/−), BET (++) |
| Surface chemistry | ICP-MS/OES (+/−), EDS (+), NMR (+), XPS (++), SIMS (++), EELS (++), TOF-MS/ATOF-MS (++), FTIR/RS (+), AES (++), TGA/DSC (++) |
| Surface charge/zeta potential | SPM (+/−), DLS/PCS/QELS (+), ELS (++) |
| Porosity | BET (++), Mercury intrusion (++) |
| Biomedical Application | Morphology/Structure | Test System | References |
|---|---|---|---|
| Anticancer activity | [1,2,5] | ||
| Paclitaxel or cisplatin-ZnO | Photo-stimulated paclitaxel or cisplatin-ZnO NPs under UV-A irradiation | HNSCC cells | [161] |
| VP-16-Fe3O4@ZnO:Er3+,Yb3+@β-CD | VP-16 released from Fe3O4@ZnO:Er3+,Yb3+@β-CD NPs after microwave-triggering | MCF-7 cells | [162] |
| Doxorubicin-ZnO | Starch-stabilized ZnO NPs | MCF-7 cells | [163] |
| Daunorubicin-ZnO | Multilamellar liposomes with hexagonal ZnO NP cores | A549 (non-small cell lung carcinoma) cells | [164] |
| Aminopolysiloxane-capped ZnO NPs | K562 (sensitive leukemia) and K562/A02 (resistant leukemia) cells | [28] | |
| Antidiabetic activity | [165,166,167] | ||
| Vildagliptin + ZnO | Hexagonal ZnO NPs (mixed shape,~20 nm) | Rats, type 2 diabetes | [165] |
| ZnO | Hexagonal ZnO NPs (spherical shape, 10–15 nm) | Rats, type 1 and 2 diabetes | [166] |
| Antimicrobial activity | [6,34] | ||
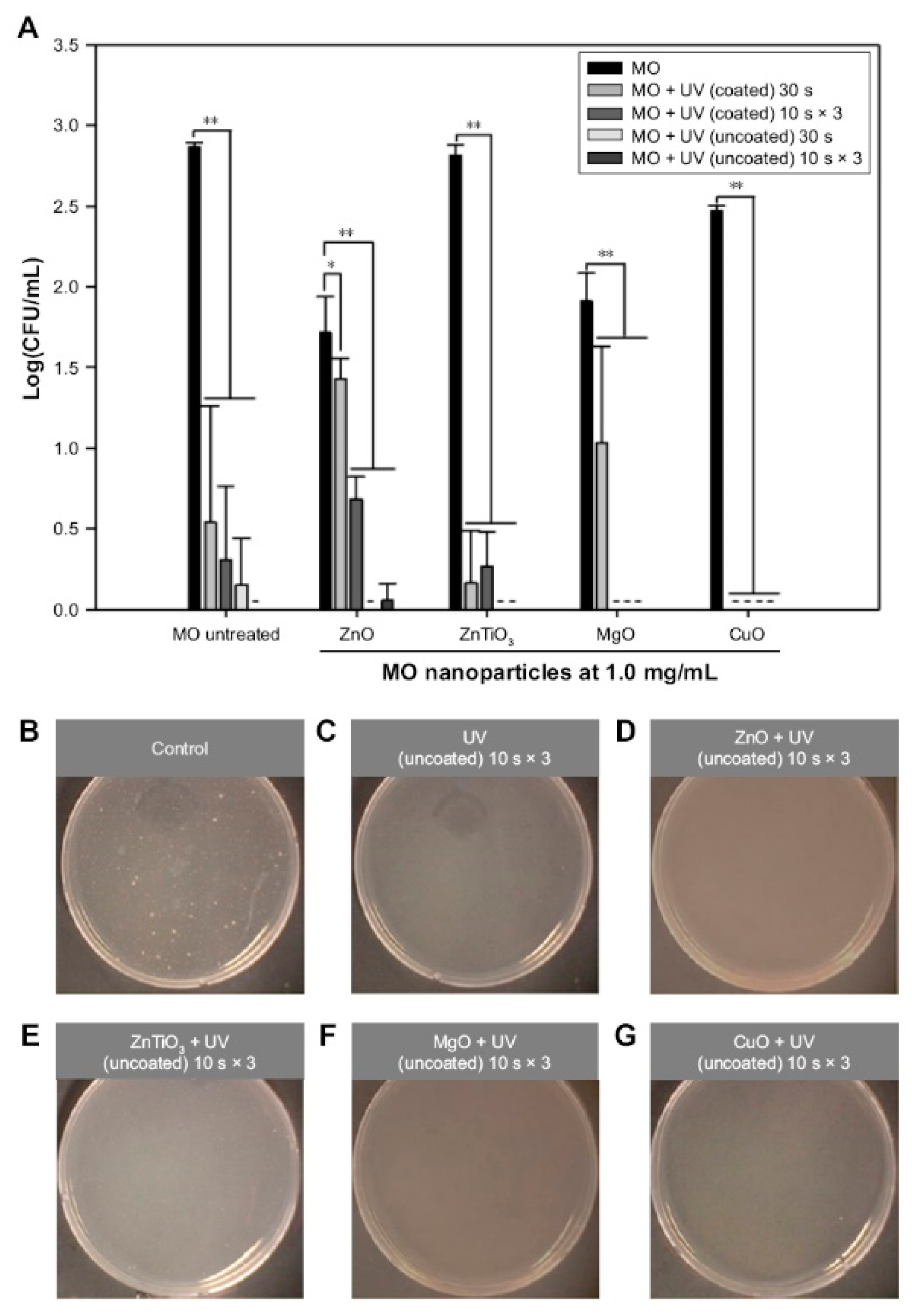
| ZnO | Self-assembled ZnO NP network structure on Si wafer under dual UV irradiation (ZnO 0.05 mg/mL, UV 10 sec, 5 or 120 min incubation) | E. coli | [13] |
| ZnO | Hexagonal ZnO NPs with/without dual UV irradiation (~100 nm, ZnO 1.0 mg/mL, UV 30 sec, 30 min incubation) | Escherichia coli, M13 bacteriophages | [116] |
| Gentamicin + ZnO | Mesoporous ZnO structures on Si substrates (guest-host structures) | In vitro release for 7 days | [121] |
| Anti-inflammatory activity | [168,169] | ||
| ZnO (74% Lyocell fiber, 19% Smart Cell sensitive fiber, and 7% spandex) | ZnO-functionalized textile (Benevit Zink+) | Staphylococcus aureus, Klebsiella pneumoniae (for atopic dermatitis patients) | [10] |
| ZnO–TiO2 | ZnO NP-embedded TiO2 nanotubes | Macrophage-like RAW 264.7 (murine leukemic monocyte) cells, S. aureus | [170] |
| Magnesium/epoxy resin-ZnO/poly-capro- lactone-ibuprofen | Multifunctional microstructure (coating) | In vitro release | [171] |
| Wound healing | [172,173,174,175] | ||
| ZnO | ZnO NPs (antimicrobial tissue adhesive, 71.1 nm) | Skin wound closure (E. coli and adhesion test) | [176] |
| Alginate/ZnO | Alginate/nano-ZnO composite bandages | Infected wounds (S. aureus and E. coli) | [177] |
| ZnO | ZnO NPs (boiling method-based synthesis) | Wound dressing (adipocyte-derived stem cell proliferation) | [178] |
| ZnO | Topical ZnO formulations (Increased local Zn and basal cell metallothionein in wound margins for accelerated wound healing) | Wound dressing (surgical wound model in Sprague-Dawley rat) | [179] |
| Cod liver oil/ZnO | Zincojecol (ointment containing cod liver oil and ZnO) | Wound dressing (tail skin, retarded wound model by dexamethasone) | [180] |
| Imaging agents | [181,182] | ||
| Folic acid-ZnO QD | Folic acid-modified ZnO nanocrystals (NIR excitation) | KB (oral carcinoma) cells | [183] |
| ZnO QD | ZnO QDs (3–4 nm) immobilized on silica nanospheres (~150–200 nm) (photoluminescence) | Photoluminescence intensity | [184] |
| Sensors | [185,186] | ||
| ZnO | Three-dimensional interconnected ZnO nanostructures (macro-mesoporosity) | Acetone/methanol detection | [29] |
| ZnO | ZnO nano-brush and pearl chain-like nanowire | Selective/sensitive ethanol sensing | [187] |
| Mn-ZnO | Interlocking p + n field-effect transistor circuit of Mn-doped ZnO NPs | Acetone sensing (> 2 ppm) | [188] |
| ZnO | Aligned ZnO nanorods | Epinephrine sensing | [189] |
| ZnO | ZnO electrodes on flexible porous polyimide substrates | Cardiac troponin sensing | [190] |
| ZnO | ZnO nanorod field-effect transistors (FETs) | Glucose, cholesterol, and urea sensing | [57] |
| Au–ZnO | Gold (Au)–ZnO hybrid NP films | Optical and impedimetric analyses | [191] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.-E.; Jin, H.-E. Synthesis, Characterization, and Three-Dimensional Structure Generation of Zinc Oxide-Based Nanomedicine for Biomedical Applications. Pharmaceutics 2019, 11, 575. https://doi.org/10.3390/pharmaceutics11110575
Jin S-E, Jin H-E. Synthesis, Characterization, and Three-Dimensional Structure Generation of Zinc Oxide-Based Nanomedicine for Biomedical Applications. Pharmaceutics. 2019; 11(11):575. https://doi.org/10.3390/pharmaceutics11110575
Chicago/Turabian StyleJin, Su-Eon, and Hyo-Eon Jin. 2019. "Synthesis, Characterization, and Three-Dimensional Structure Generation of Zinc Oxide-Based Nanomedicine for Biomedical Applications" Pharmaceutics 11, no. 11: 575. https://doi.org/10.3390/pharmaceutics11110575
APA StyleJin, S.-E., & Jin, H.-E. (2019). Synthesis, Characterization, and Three-Dimensional Structure Generation of Zinc Oxide-Based Nanomedicine for Biomedical Applications. Pharmaceutics, 11(11), 575. https://doi.org/10.3390/pharmaceutics11110575