High Potency of SN-38-Loaded Bovine Serum Albumin Nanoparticles Against Triple-Negative Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of sBSANP, BSANP, and Rho-sBSANP
2.3. Characterization of the sBSANPs
2.3.1. Size, Polydispersity Index, Zeta Potential
2.3.2. Particle Yield
2.3.3. Entrapment Efficiency Calculation
2.4. Fluorescence Quenching Study
2.5. FTIR
2.6. In Vitro Release Kinetics
2.7. Ex Vivo Hemolysis Assay
2.8. Cell Culture and Cytotoxicity Assay
2.8.1. Cell Culture
2.8.2. Cytotoxicity Assay
2.9. Cellular Uptake
2.9.1. Flow Cytometry
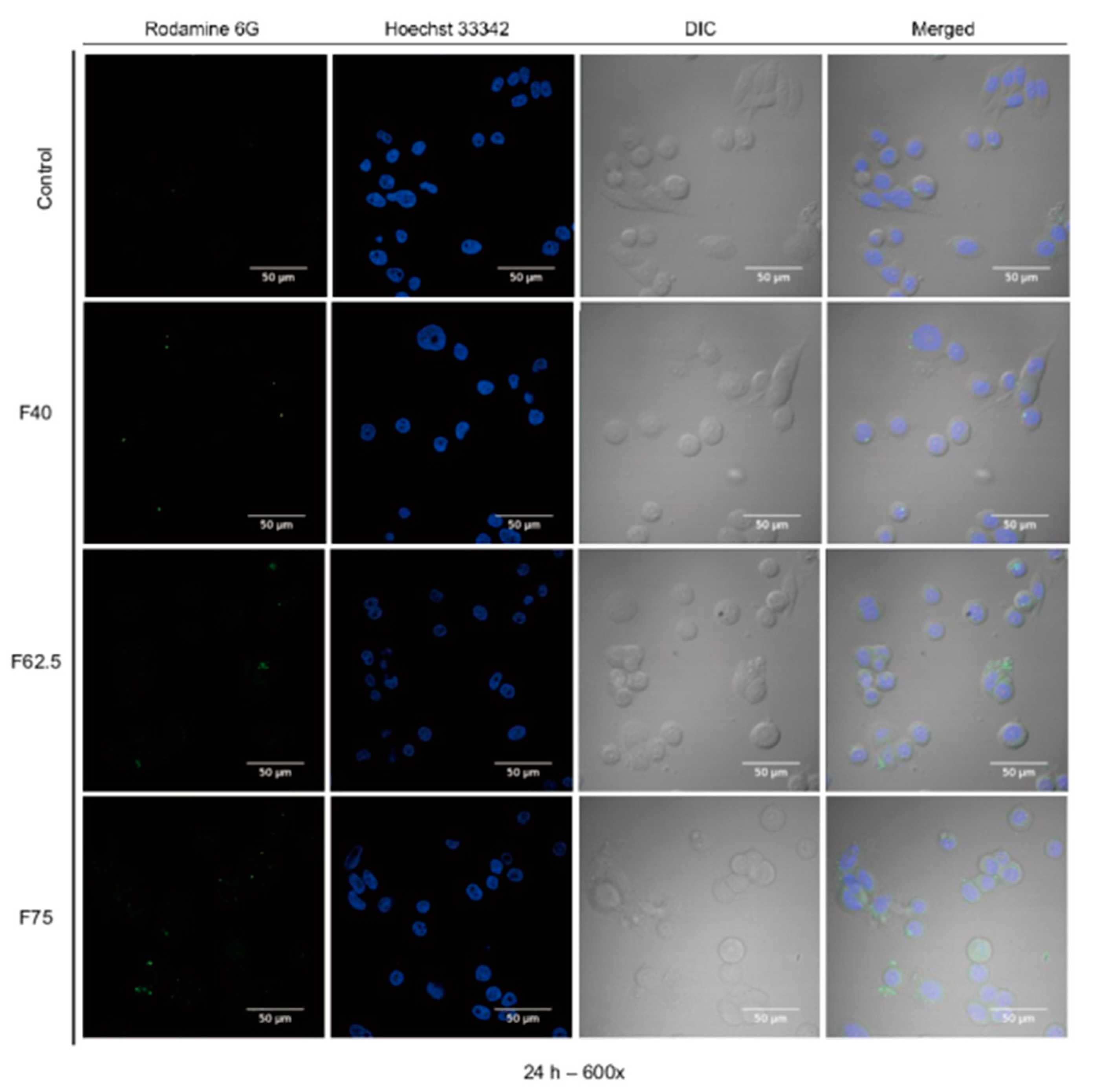
2.9.2. Confocal Laser Scanning Microscopy
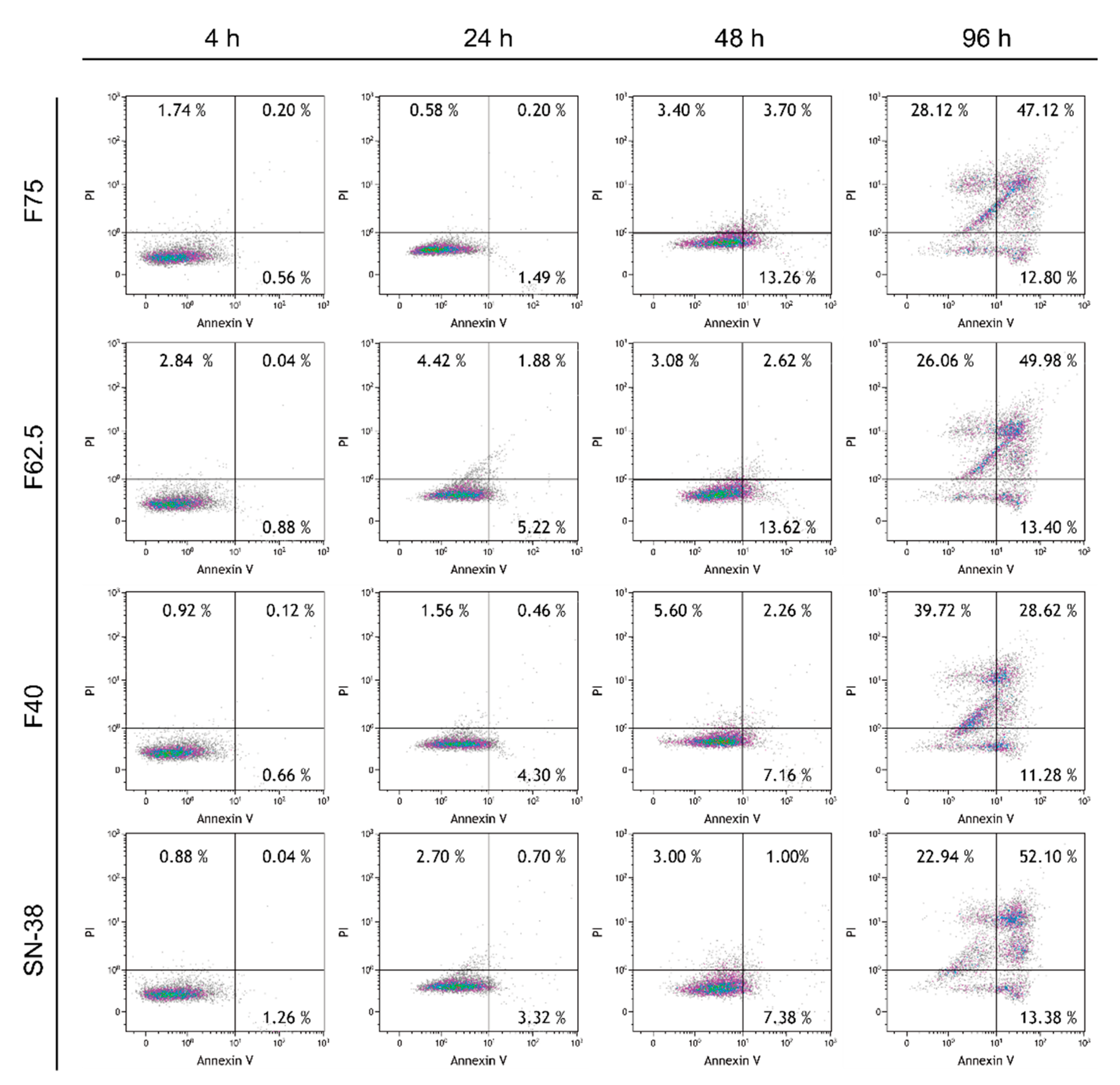
2.10. Annexin V–PI Apoptosis Assay
2.11. Pharmacokinetic Study
2.12. High Performance Liquid Chromatography (HPLC) Analysis
2.12.1. In Vitro Quantitation
2.12.2. In Vivo Quantitation
2.13. Statistical Analysis
3. Results
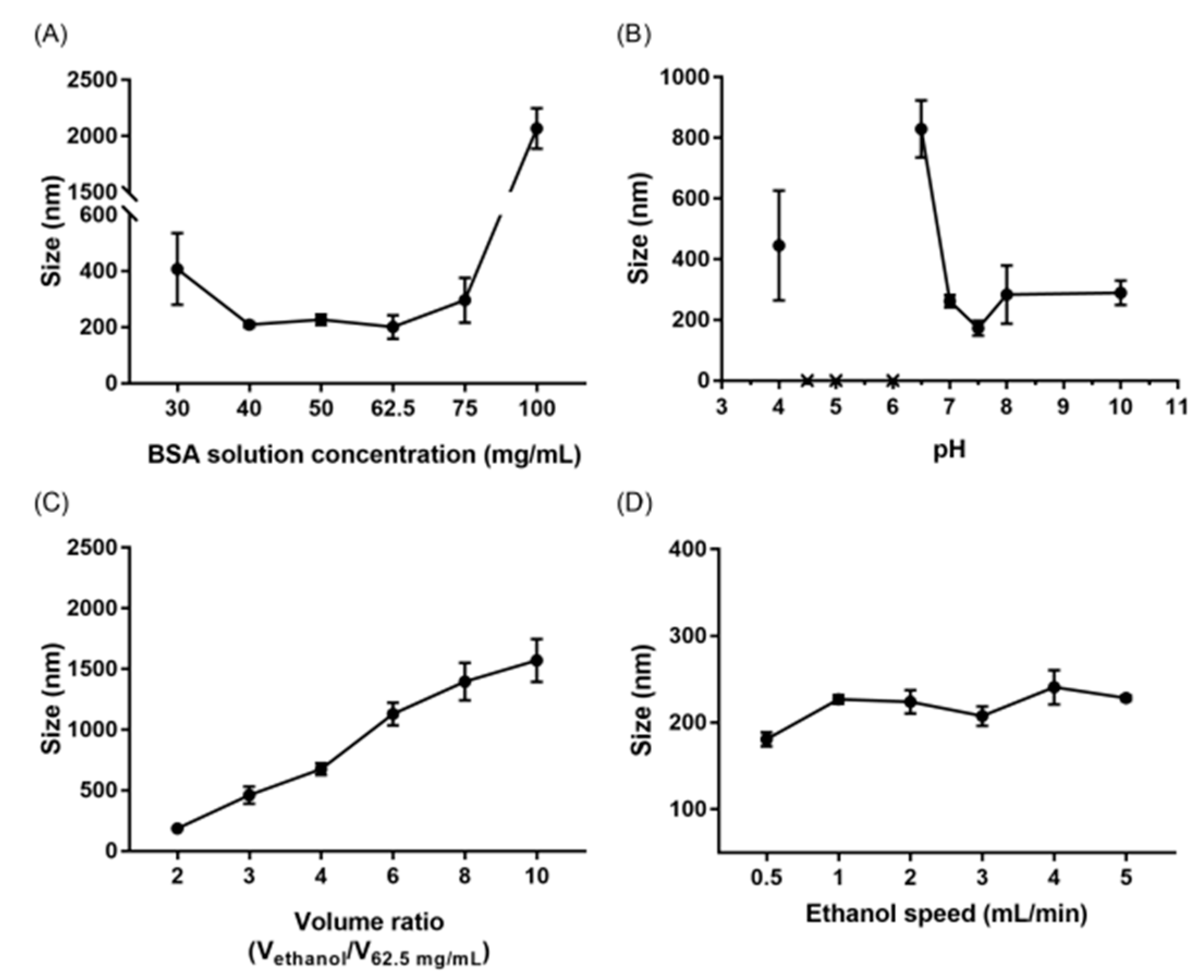
3.1. Attributes Affecting the Size of Albumin Nanoparticles
3.2. Characterization of sBSANPs
3.2.1. Particle Size, Zeta Potential, Entrapment Efficiency, and Particle Yield
3.2.2. Fluorescence Quenching Study
3.2.3. FTIR
3.3. In Vitro Release Kinetics
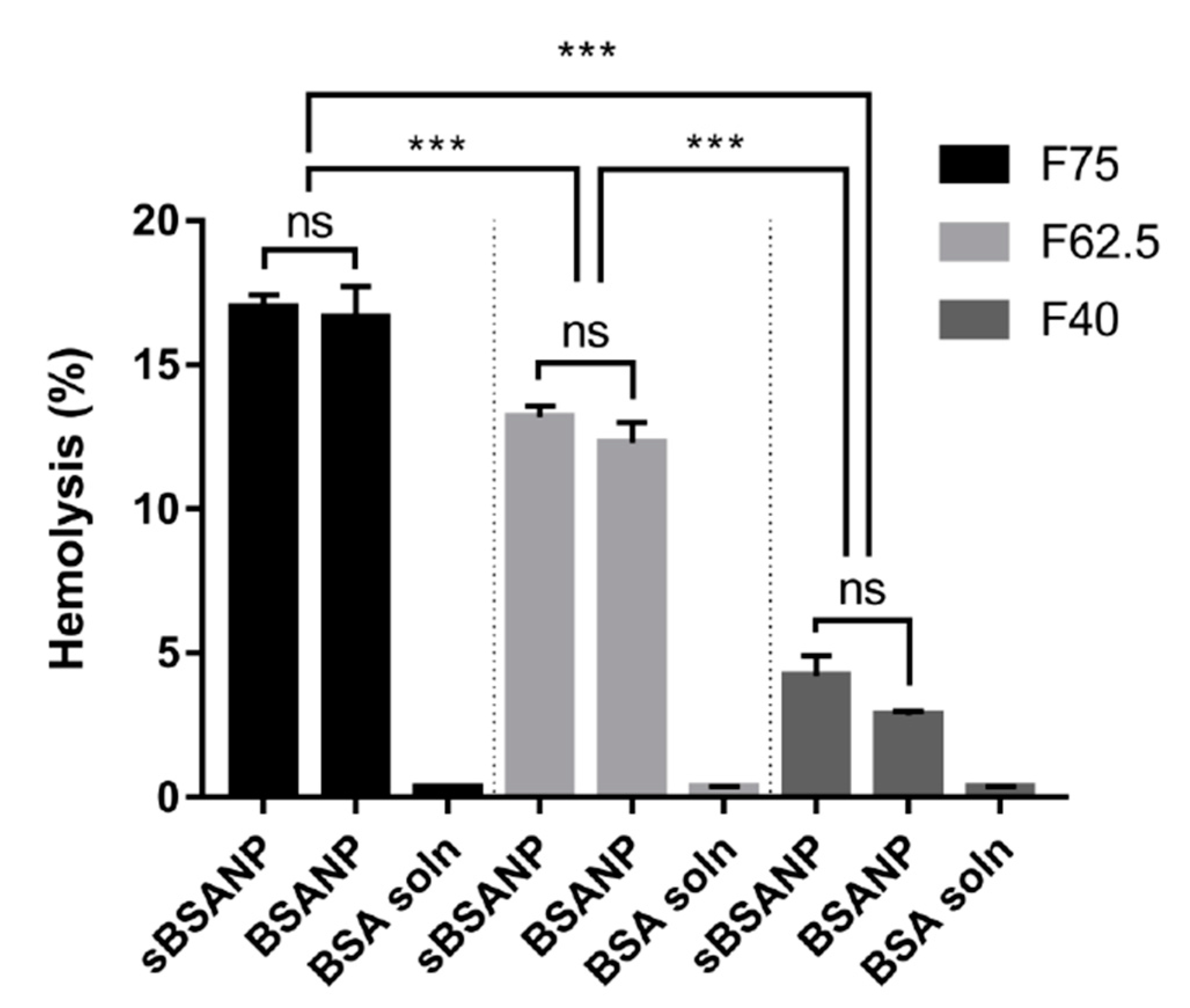
3.4. Ex Vivo Hemolysis Assay
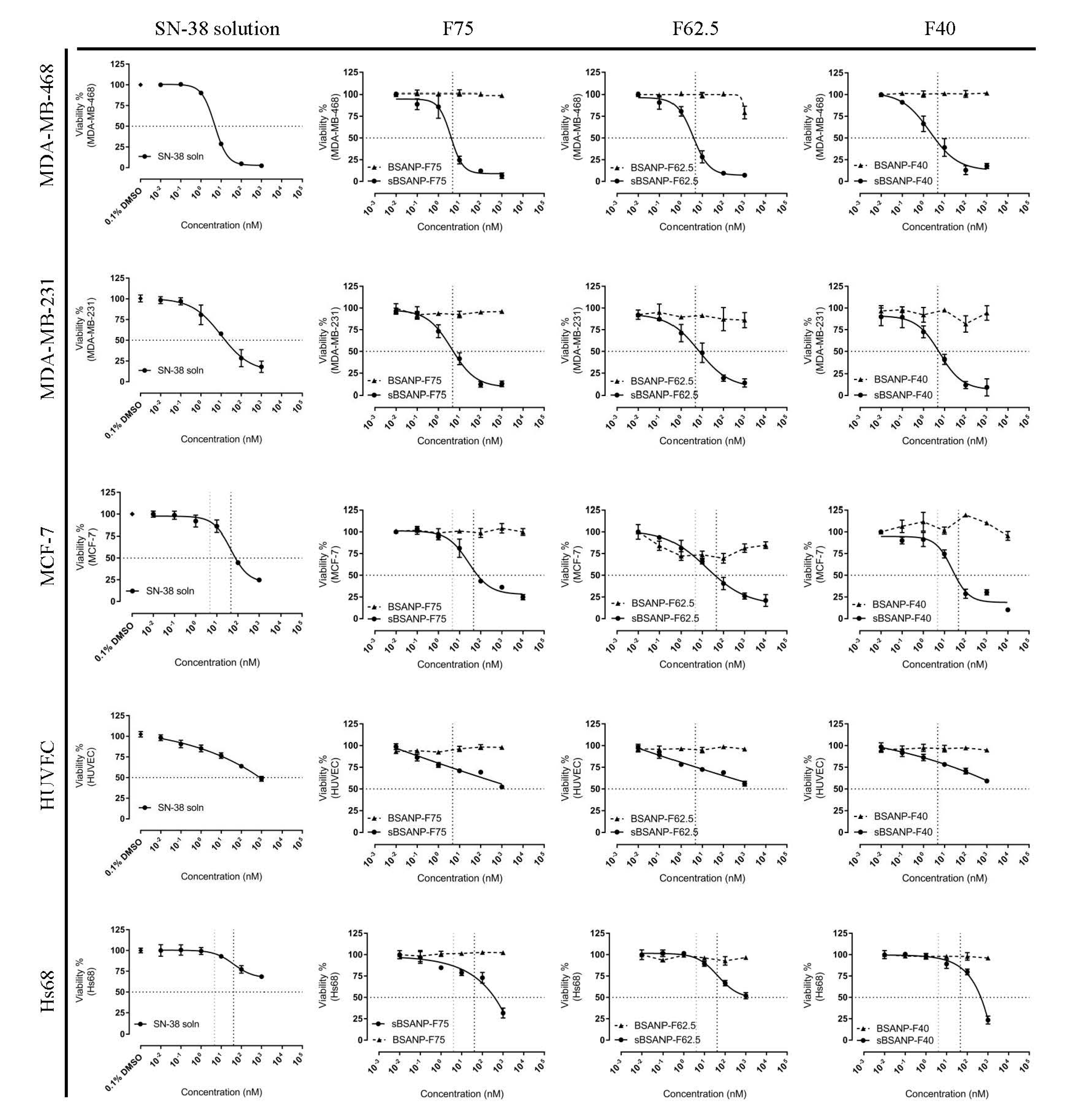
3.5. In Vitro Cytotoxicity and Safety Evaluation
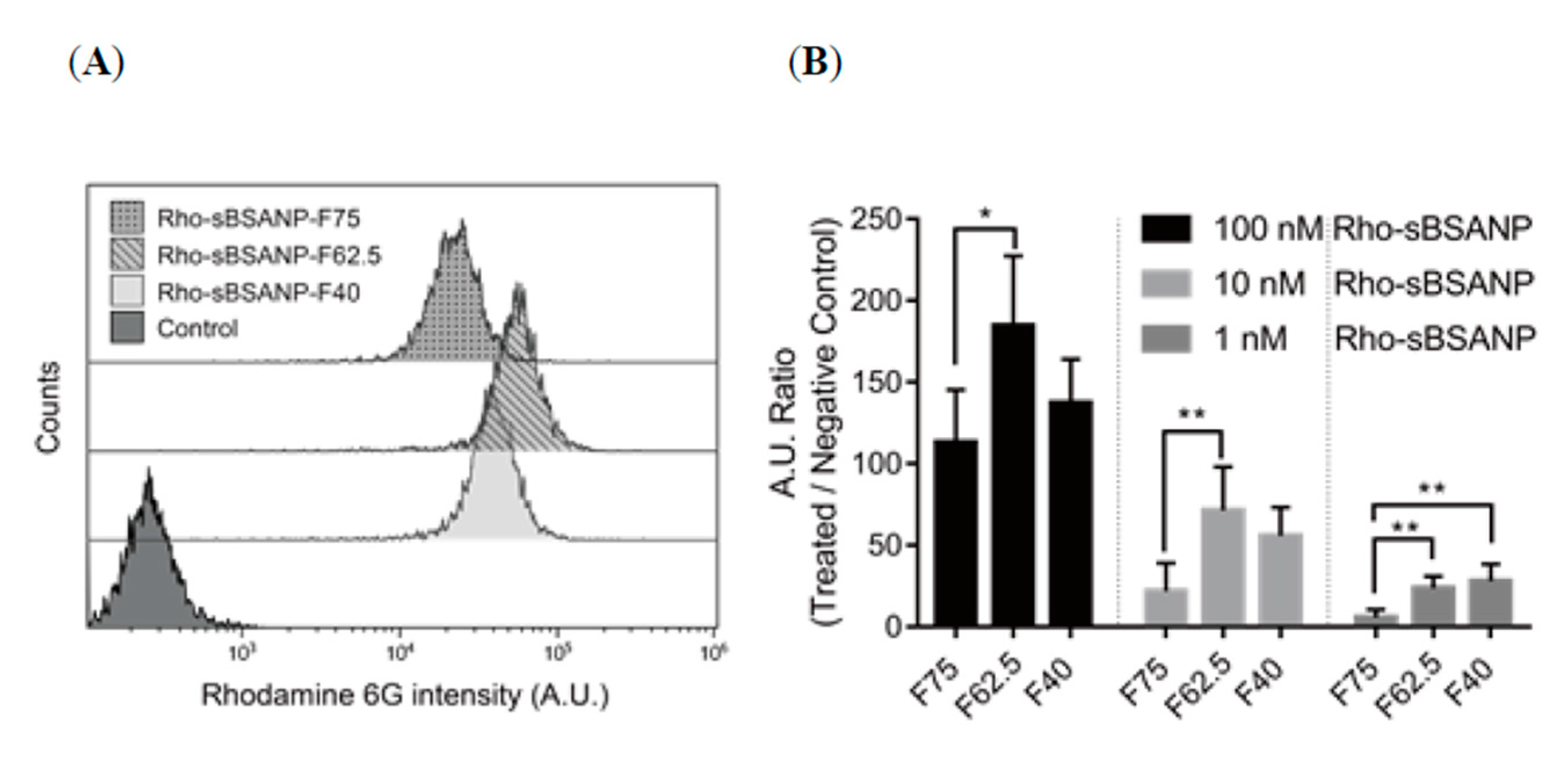
3.6. Cellular Uptake
3.7. Annexin V–PI Apoptosis Assay
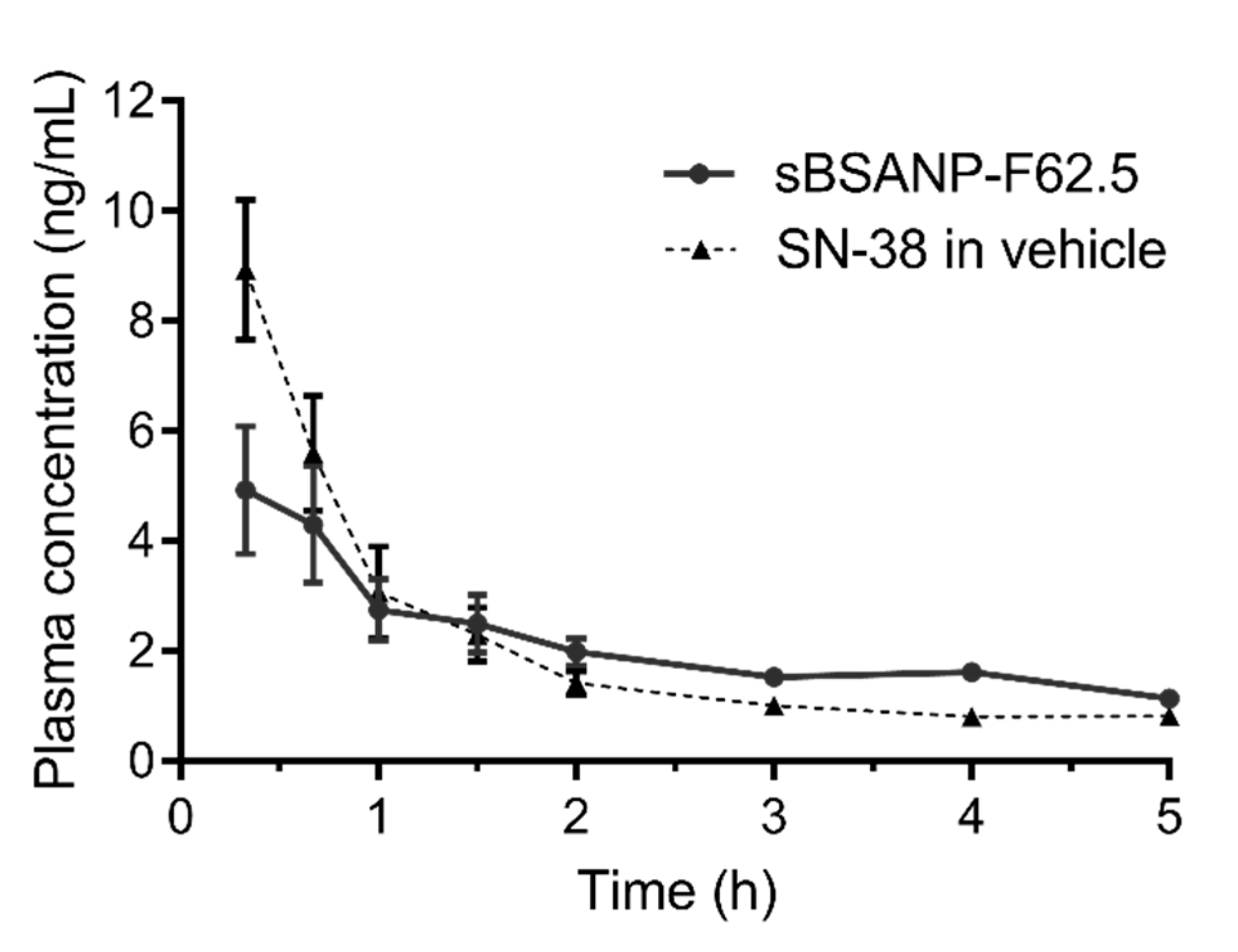
3.8. Pharmacokinetic Study
4. Discussion
4.1. Attributes Affecting the Size of Albumin Nanoparticles
4.2. Characterization of sBSANP
4.3. In Vitro Release Kinetics
4.4. Ex Vivo Hemolysis Assay
4.5. In Vitro Cytotoxicity and Safety Evaluation
4.6. Pharmacokinetic Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lin, N.U.; Vanderplas, A.; Hughes, M.E.; Theriault, R.L.; Edge, S.B.; Wong, Y.N.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; Weeks, J.C.; et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012, 118, 5463–5472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Adamson, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Wright, G.S.; et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Zsila, F. Subdomain IB is the third major drug binding region of human serum albumin: Toward the three-sites model. Mol. Pharm. 2013, 10, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, F.; Kroetz, D.L.; Schuetz, E.; Dolan, M.E.; Ramírez, J.; Relling, M. Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J. Clin. Oncol. 2009, 27, 2604–2614. [Google Scholar] [CrossRef]
- Femke, M.; Goey, A.K.; van Schaik, R.H.; Mathijssen, R.H.; Bins, S. Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics. Clin. Pharmacokinet. 2018, 57, 1229–1254. [Google Scholar] [Green Version]
- Thomas, L.L.; David, A.W. Foye’s Principles of Medicinal Chemistry, 7th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2008; p. 1188. [Google Scholar]
- Yao, Y.; Su, X.; Xie, Y.; Wang, Y.X.; Kang, T.; Gou, L.; Yi, C.; Yang, G.L. Synthesis, characterization, and antitumor evaluation of the albumin-SN38 conjugate. Anticancer. Drugs 2013, 24, 270–277. [Google Scholar] [CrossRef]
- Bala, V.; Rao, S.; Li, P.; Wang, S.; Prestidge, C.A. Lipophilic prodrugs of SN38: Synthesis and in vitro characterization toward oral chemotherapy. Mol. Pharm. 2016, 13, 287–294. [Google Scholar] [CrossRef]
- Palakurthi, S. Challenges in SN38 drug delivery: Current success and future directions. Expert Opin. Drug Deliv. 2015, 12, 1911–1921. [Google Scholar] [CrossRef]
- Watcharin, W.; Schmithals, C.; Pleli, T.; Köberle, V.; Korkusuz, H.; Huebner, H.; Zeuzem, S.; Korf, S.W.; Vogl, T.J.; Rittmeyer, C.; et al. Biodegradable human serum albumin nanoparticles as contrast agents for the detection of hepatocellular carcinoma by magnetic resonance imaging. Eur. J. Pharm. Biopharm. 2014, 87, 132–141. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Plasmonics in biology and plasmon-controlled fluorescence. Plasmonics. 2006, 1, 5–33. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.P.; Wu, P.C.; Tsai, Y.H.; Huang, Y.B. Physicochemical and safety evaluation of 5-aminolevulinic acid in novel liposomes as carrier for skin delivery. J. Liposome Res. 2008, 18, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, B.; Knupp, L.; Northcott, C.A. Femoral Arterial and venous Ccatheterization for blood sampling, drug administration and conscious blood pressure and heart rate measurements. J. Vis. Exp. 2012, 59, e3496. [Google Scholar]
- Dosing Techniques and Limits. Animal Care and Use Program. Available online: https://acuc.berkeley.edu/guidelines/dosing.pdf (accessed on 22 December 2018).
- Recommended dose volumes for common laboratory animals. Available online: https://iqconsortium.org/images/LG-3Rs/IQ-CRO_Recommended_Dose_Volumes_for_Common_Laboratory_Animals_June_2016_(2).pdf (accessed on 28 December 2018).
- Wu, C.H.; Coumar, M.S.; Chu, C.Y.; Lin, W.H.; Chen, Y.R.; Chen, C.T.; Shiao, H.Y.; Rafi, S.; Wang, S.Y.; Hsu, H.; et al. Design and synthesis of tetrahydropyridothieno [2,3-d] pyrimidine scaffold based epidermal growth factor receptor (EGFR) kinase inhibitors: The role of side chain chirality and Michael acceptor group for maximal potency. J. Med. Chem. 2010, 53, 7316–7326. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Piao, H.; Gao, Y.; Xu, C.; Tian, Y.; Wang, L.; Liu, J.; Tang, B.; Zou, M.J.; Cheng, G.; et al. Comparison of two self-assembled macromolecular prodrug micelles with different conjugate positions of SN38 for enhancing antitumor activity. Int. J. Nanomedicine 2015, 10, 2295. [Google Scholar]
- Zhang, J.A.; Xuan, T.; Parmar, T.; Ma, L.; Ugwu, S.; Ali, S.; Ahmad, I. Development and characterization of a novel liposome-based formulation of SN-38. Int. J. Pharm. 2004, 270, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Kouchakzadeh, H.; Shojaosadati, S.A.; Shokri, F. Efficient loading and entrapment of tamoxifen in human serum albumin based nanoparticulate delivery system by a modified desolvation technique. Chem. Eng. Res. Des. 2014, 92, 1681–1692. [Google Scholar] [CrossRef]
- Bovine albumin product information. Available online: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Product_Information_Sheet/a4919pis.pdf (accessed on 25 December 2018).
- Li, Y.; Zhao, X.; Zu, Y.; Han, X.; Ge, Y.; Wang, W.; Yu, X. A novel active targeting preparation, vinorelbine tartrate (VLBT) encapsulated by folate-conjugated bovine serum albumin (BSA) nanoparticles: Preparation, characterization and in vitro release study. Materials 2012, 5, 2403–2422. [Google Scholar] [CrossRef]
- Ruttala, H.B.; Ko, Y.T. Liposome encapsulated albumin-paclitaxel nanoparticle for enhanced antitumor efficacy. Pharm. Res. 2015, 32, 1002–1016. [Google Scholar] [CrossRef]
- Galisteo-González, F.; Molina-Bolívar, J.A. Systematic study on the preparation of BSA nanoparticles. Colloid. Surf. B Biointerfaces. 2014, 123, 286–292. [Google Scholar] [CrossRef]
- Yang, L.; Cui, F.; Cun, D.; Tao, A.; Shi, K.; Lin, W. Preparation, characterization and biodistribution of the lactone form of 10-hydroxycamptothecin (HCPT)-loaded bovine serum albumin (BSA) nanoparticles. Int. J. Pharm. 2007, 340, 163–172. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Supko, J.G. Current perspectives on the clinical experience, pharmacology, and continued development of the camptothecins. Clin. Cancer Res. 2002, 8, 641–661. [Google Scholar]
- Jahanban-Esfahlan, A.; Dastmalchi, S.; Davaran, S. A simple improved desolvation method for the rapid preparation of albumin nanoparticles. Int. J. Biol. Macromol. 2016, 91, 703–709. [Google Scholar] [CrossRef]
- Li, F.Q.; Su, H.; Wang, J.; Liu, J.Y.; Zhu, Q.G.; Fei, Y.B.; Pan, Y.H.; Hu, J.H. Preparation and characterization of sodium ferulate entrapped bovine serum albumin nanoparticles for liver targeting. Int. J. Pharm. 2008, 349, 274–282. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, V.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Taneja, N.; Singh, K.K. Rational design of polysorbate 80 stabilized human serum albumin nanoparticles tailored for high drug loading and entrapment of irinotecan. Int. J. Pharm. 2018, 536, 82–94. [Google Scholar] [CrossRef]
- Paul, B.K.; Ray, D.; Guchhait, N. Unraveling the binding interaction and kinetics of a prospective anti-HIV drug with a model transport protein: Results and challenges. Phys. Chem. Chem. Phys. 2013, 15, 1275–1287. [Google Scholar] [CrossRef]
- Zhuang, X.; Ha, T.; Kim, H.D.; Centner, T.; Labeit, S.; Chu, S. Fluorescence quenching: A tool for single-molecule protein-folding study. Proc. Natl. Acad. Sci. USA 2000, 97, 14241–14244. [Google Scholar] [CrossRef] [Green Version]
- Lakshmi, P.; Mondal, M.; Ramadas, K.; Natarajan, S. Molecular interaction of 2,4-diacetylphloroglucinol (DAPG) with human serum albumin (HSA): The spectroscopic, calorimetric and computational investigation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 183, 90–102. [Google Scholar]
- Martin Hof, R.M. Quenching of fluorescence. 2016. Available online: https://www.chem.uzh.ch/dam/jcr:571775eb-8b0e-4930-8aa4-ad0da5c11aec/FluorescenceQuenching_HS16.pdf (accessed on 11 December 2018).
- Della Porta, V.; Bramanti, E.; Campanella, B.; Tiné, M.R.; Duce, C. Conformational analysis of bovine serum albumin adsorbed on halloysite nanotubes and kaolinite: A Fourier transform infrared spectroscopy study. R.S.C. Adv. 2016, 6, 72386–72398. [Google Scholar] [CrossRef]
- Gokhale, A. Achieving zero-order release kinetics using multi-step diffusion-based drug delivery. Pharm. Technol. 2014, 26, 38–42. [Google Scholar]
- Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER). Guidance for industry nonclinical studies for the safety evaluation of pharmaceutical excipients. In Pharmacology/Toxicology; US Department of Health Human Services, Food Drug Administration: Rockville, MD, USA, 2005. [Google Scholar]
- Lin, Y.S.; Haynes, C.L. Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J. Am. Chem. Soc. 2010, 132, 4834–4842. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Clogston, J.D.; Neun, B.W.; Hall, J.B.; Patri, A.K.; McNeil, S.E. Method for analysis of nanoparticle hemolytic properties in vitro. Nano lett. 2008, 8, 2180–2187. [Google Scholar] [CrossRef]
- Barshtein, G.; Arbell, D.; Yedgar, S. Hemolytic effect of polymeric nanoparticles: Role of albumin. IEEE Trans. Nanobioscience 2011, 10, 259–261. [Google Scholar] [CrossRef]
- Lebert, J.M.; Lester, R.; Powell, E.; Seal, M.; McCarthy, J. Advances in the systemic treatment of triple-negative breast cancer. Curr. Oncol. 2018, 25, S142–S150. [Google Scholar] [CrossRef]
- Liu, F.W.; Liu, F.C.; Wang, Y.R.; Tsai, H.I.; Yu, H.P. Aloin protects skin fibroblasts from heat stress-induced oxidative stress damage by regulating the oxidative defense system. PLoS ONE 2015, 10, e0143528. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Cardillo, T.M.; Govindan, S.V.; Rossi, E.A.; Sharkey, R.M. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 2015, 6, 22496–22512. [Google Scholar] [CrossRef]
- Sepehri, N.; Rouhani, H.; Tavassolian, F.; Montazeri, H.; Khoshayand, M.R.; Ghahremani, M.H.; Ostad, S.N.; Atyabi, F.; Dinarvand, R.; et al. SN38 polymeric nanoparticles: in vitro cytotoxicity and in vivo antitumor efficacy in xenograft balb/c model with breast cancer versus irinotecan. Int. J. Pharm. 2014, 471, 485–497. [Google Scholar] [CrossRef]
- Kasper, D.; Zaleznik, D. Gas gangrene, antibiotic-associated colitis, and other clostridial infections. Harrison’s Princ. Intern. Med. 1998, 906–909. [Google Scholar]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef]



| Formulation | Particle Size (nm) | PDI | Zeta Potential (mV) | Entrapment Efficiency (%) | Particle Yield (%) |
|---|---|---|---|---|---|
| sBSANP-F75 | 264.07 ± 13.08 | 0.23 ± 0.01 | −37.16 ± 1.86 | 71.57 ± 0.84 | 86.75 ± 1.53 |
| sBSANP-F62.5 | 223.77 ± 2.36 | 0.21 ± 0.04 | −38.30 ± 2.33 | 63.28 ± 0.62 | 81.71 ± 5.49 |
| sBSANP-F40 | 134.37 ± 4.48 | 0.22 ± 0.02 | −40.34 ± 0.30 | 59.14 ± 3.14 | 65.91± 0.78 |
| BSANP-F75 | 288.23 ± 9.50 | 0.23 ± 0.06 | −41.31 ± 4.76 | - | 91.63 ± 5.51 |
| BSANP-F62.5 | 202.03 ± 17.30 | 0.14 ± 0.02 | −53.81 ± 3.39 | - | 85.65 ± 1.43 |
| BSANP-F40. | 124.47 ± 1.21 | 0.15 ± 0.04 | −53.16 ± 5.33 | - | 43.02 ± 2.33 |
| Cell Lines | IC50 (nM) | |||
|---|---|---|---|---|
| SN-38 Solution | sBSANP F75 | sBSANP F62.5 | sBSANP F40 | |
| MDA-MB-468 | 4.75 ± 0.17 | 3.86 ± 0.85 | 3.65 ± 0.60 | 2.01 ± 0.67 |
| MDA-MB-231 | 9.93 ± 3.16 | 4.16 ± 0.75 | 6.82 ± 1.97 | 5.72 ± 1.44 |
| MCF-7 | 46.22 ± 9.80 | 26.92 ± 6.30 | 15.94 ± 5.96 | 23.24 ± 6.30 |
| Pharmacokinetics Parameters | sBSANP-F62.5 | SN-38 Control | Significance |
|---|---|---|---|
| Vd (mL) | 1643.78 ± 245.46 | 650.75 ± 64.92 | *** |
| CL (mL/h) | 444.14 ± 66.61 | 483.66 ± 80.49 | ns |
| AUC (h × ng/mL) | 23.22 ± 3.54 | 21.19 ± 3.11 | ns |
| t½ (h) | 7.04 ± 1.38 | 7.35 ± 2.22 | ns |
| Cmax (ng/mL) | 6.29 ± 1.06 | 15.54 ± 1.70 | *** |
| AUMC (h × h × ng/mL) | 213.54 ± 67.83 | 154.92 ± 67.47 | ns |
| MRT (h) | 8.91 ± 1.46 | 7.04 ± 2.57 | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-C.; Chuang, C.-H.; Cheng, M.-H.; Lin, Y.-C.; Fang, Y.-P. High Potency of SN-38-Loaded Bovine Serum Albumin Nanoparticles Against Triple-Negative Breast Cancer. Pharmaceutics 2019, 11, 569. https://doi.org/10.3390/pharmaceutics11110569
Lin H-C, Chuang C-H, Cheng M-H, Lin Y-C, Fang Y-P. High Potency of SN-38-Loaded Bovine Serum Albumin Nanoparticles Against Triple-Negative Breast Cancer. Pharmaceutics. 2019; 11(11):569. https://doi.org/10.3390/pharmaceutics11110569
Chicago/Turabian StyleLin, Hsin-Che, Chih-Hung Chuang, Meng-Hsuan Cheng, Yu-Chih Lin, and Yi-Ping Fang. 2019. "High Potency of SN-38-Loaded Bovine Serum Albumin Nanoparticles Against Triple-Negative Breast Cancer" Pharmaceutics 11, no. 11: 569. https://doi.org/10.3390/pharmaceutics11110569





