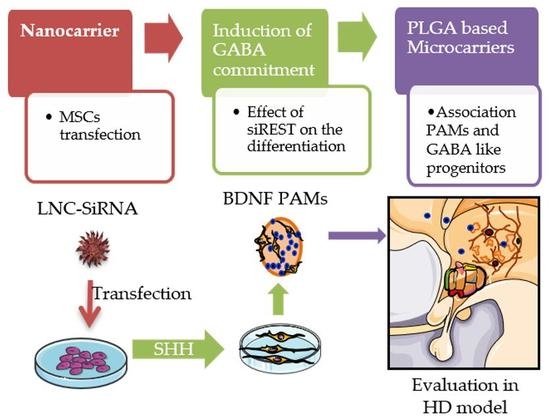
A Combinatorial Cell and Drug Delivery Strategy for Huntington’s Disease Using Pharmacologically Active Microcarriers and RNAi Neuronally-Committed Mesenchymal Stromal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. siRNA-LNCs
2.2. Fluorescent siRNA-LNCs-DiD
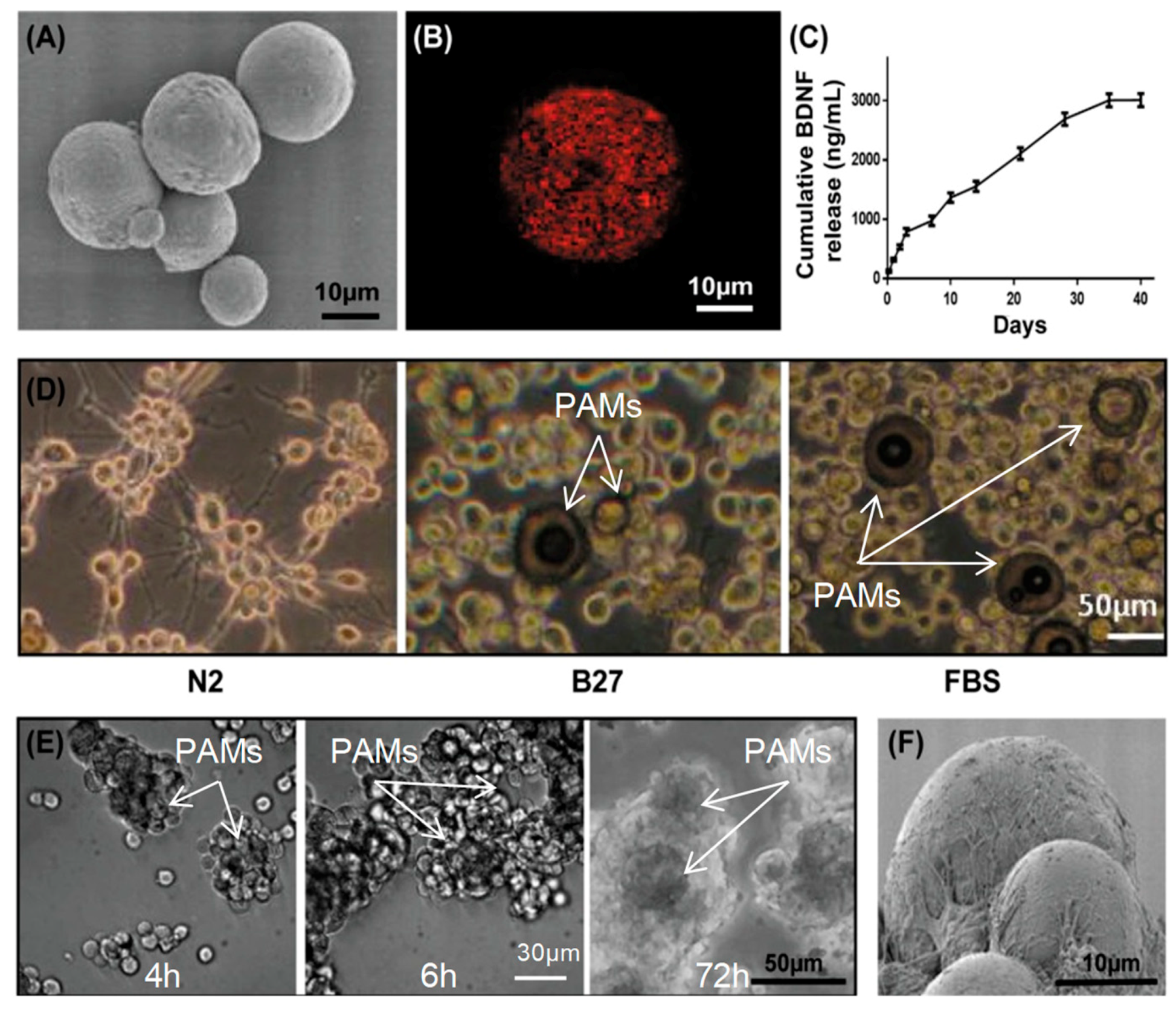
2.3. BDNF-Releasing, Laminin (LM)-Coated PAMs
2.4. LNC and PAM Characterization
2.5. MIAMI E/F Cells
2.6. MIAMI E/F Cell Transfection
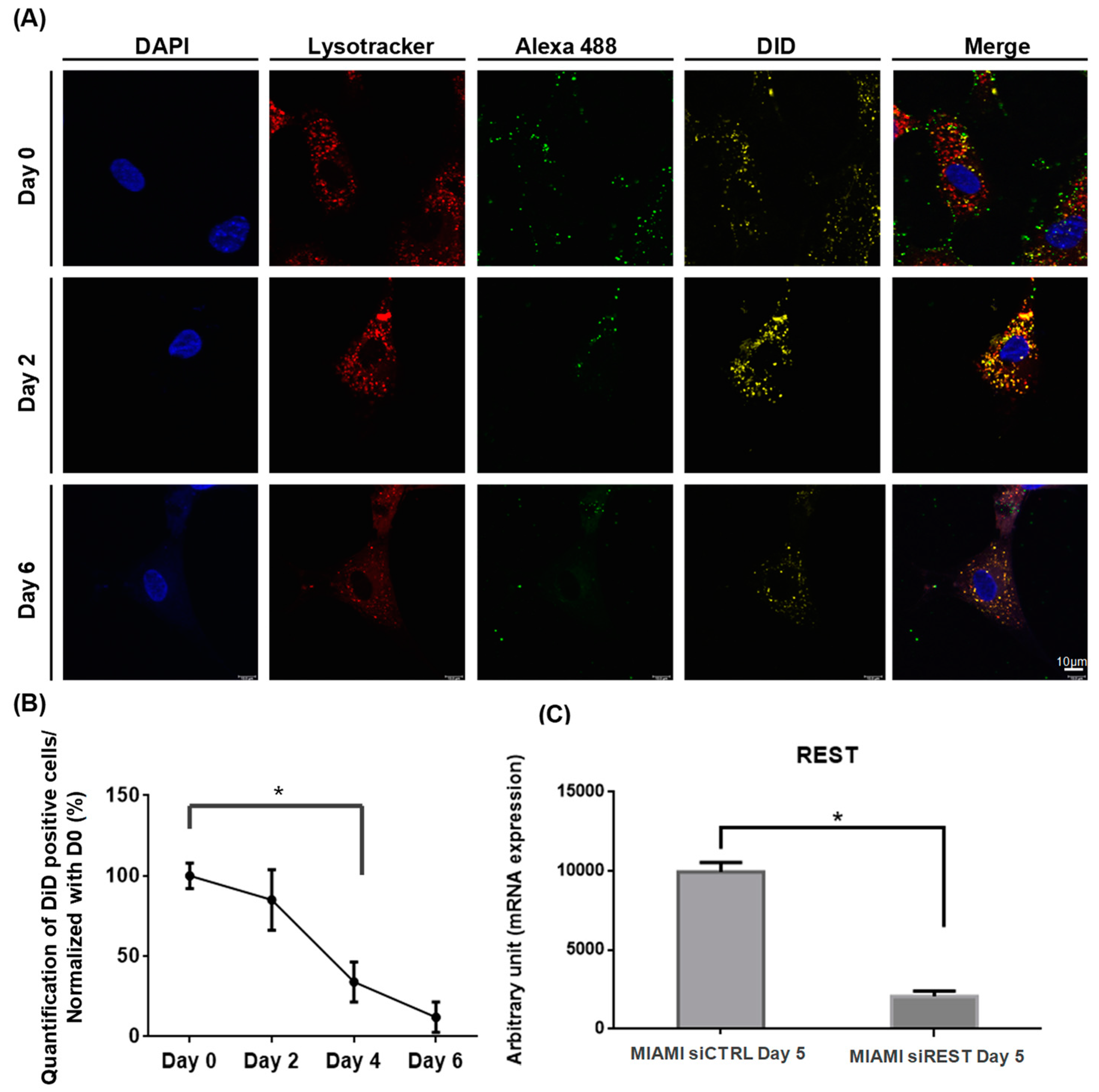
2.7. LNC Cell Time Retention
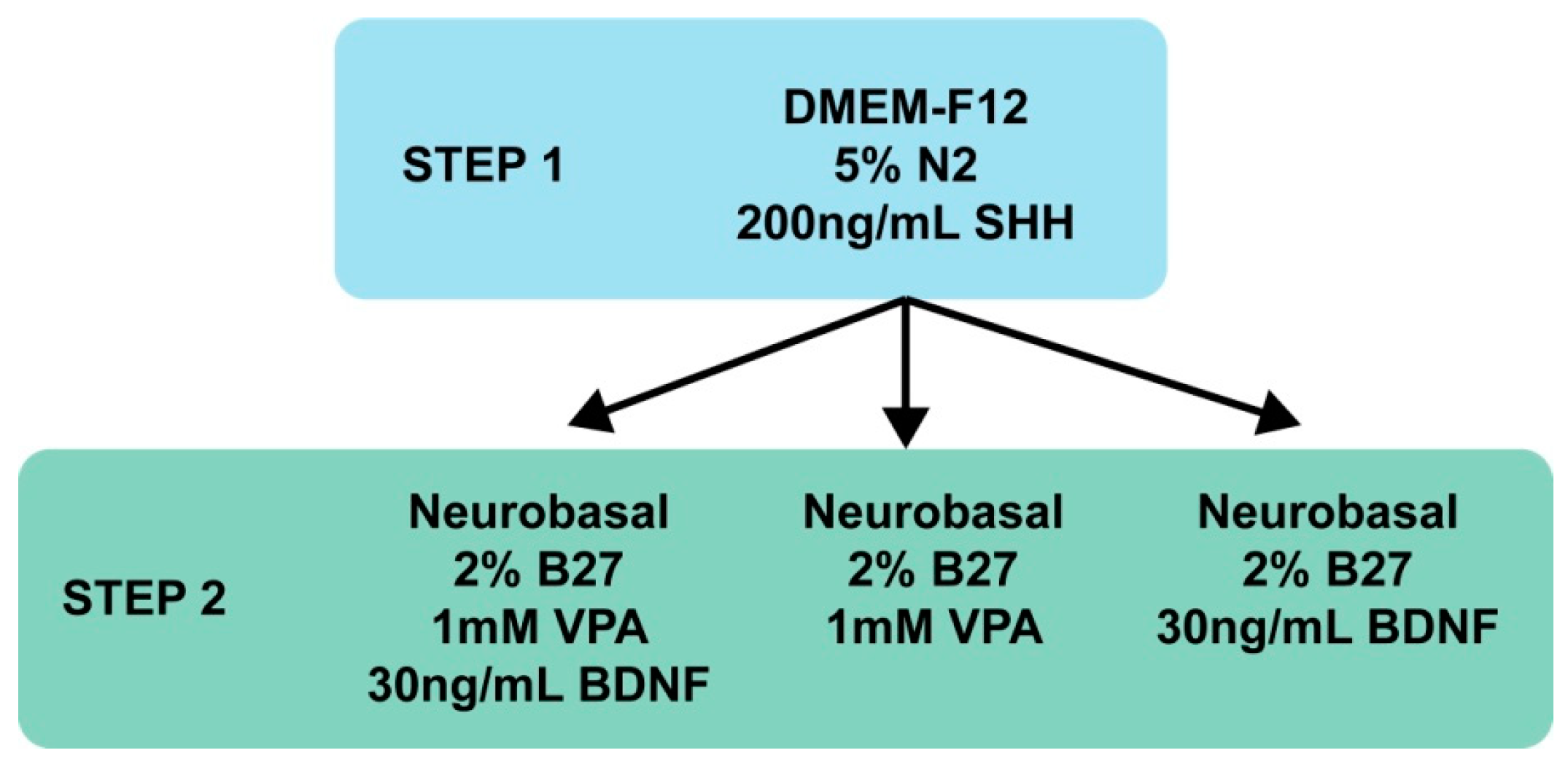
2.8. MIAMI E/F Cell Neuronal Differentiation
2.9. Formation of PAMs-Cell Constructs
2.10. Preparation of Brain Organotypic Slices
2.11. Injection of the Cells-PAMs Constructs into Organotypic Slides
2.12. Reverse Transcription and Real Time Quantitative PCR
2.13. Immunocytofluorescence
2.14. Immunofluorescence
2.15. MIAMI Cell Secretome Analysis
2.16. Data Analysis
3. Results
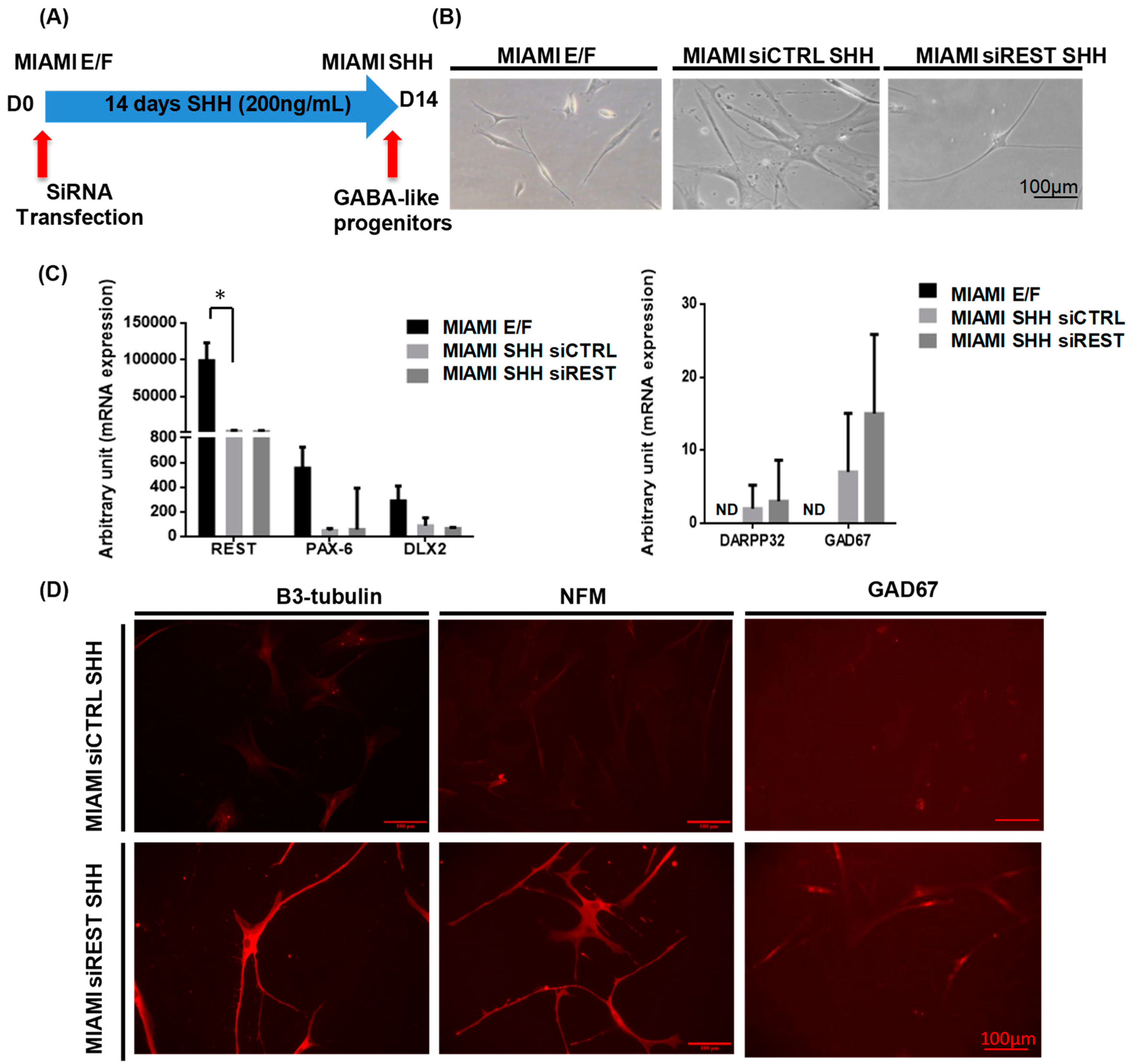
3.1. MIAMI E/F Cell Transfection
3.2. MIAMI E/F Cells Commitment into GABA-Like Progenitors
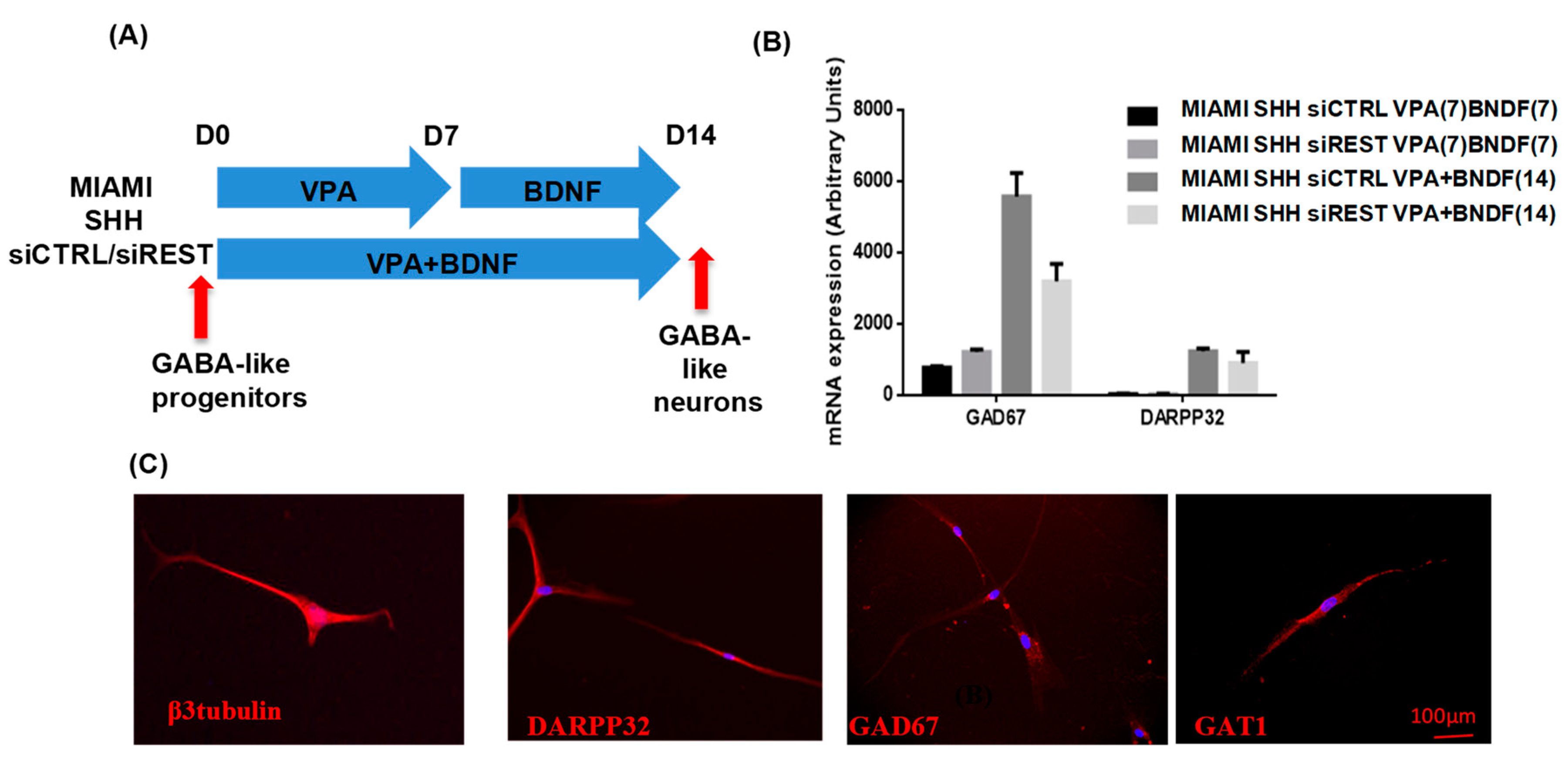
3.3. MIAMI E/F Cell Commitment into GABAergic-Like Neurons
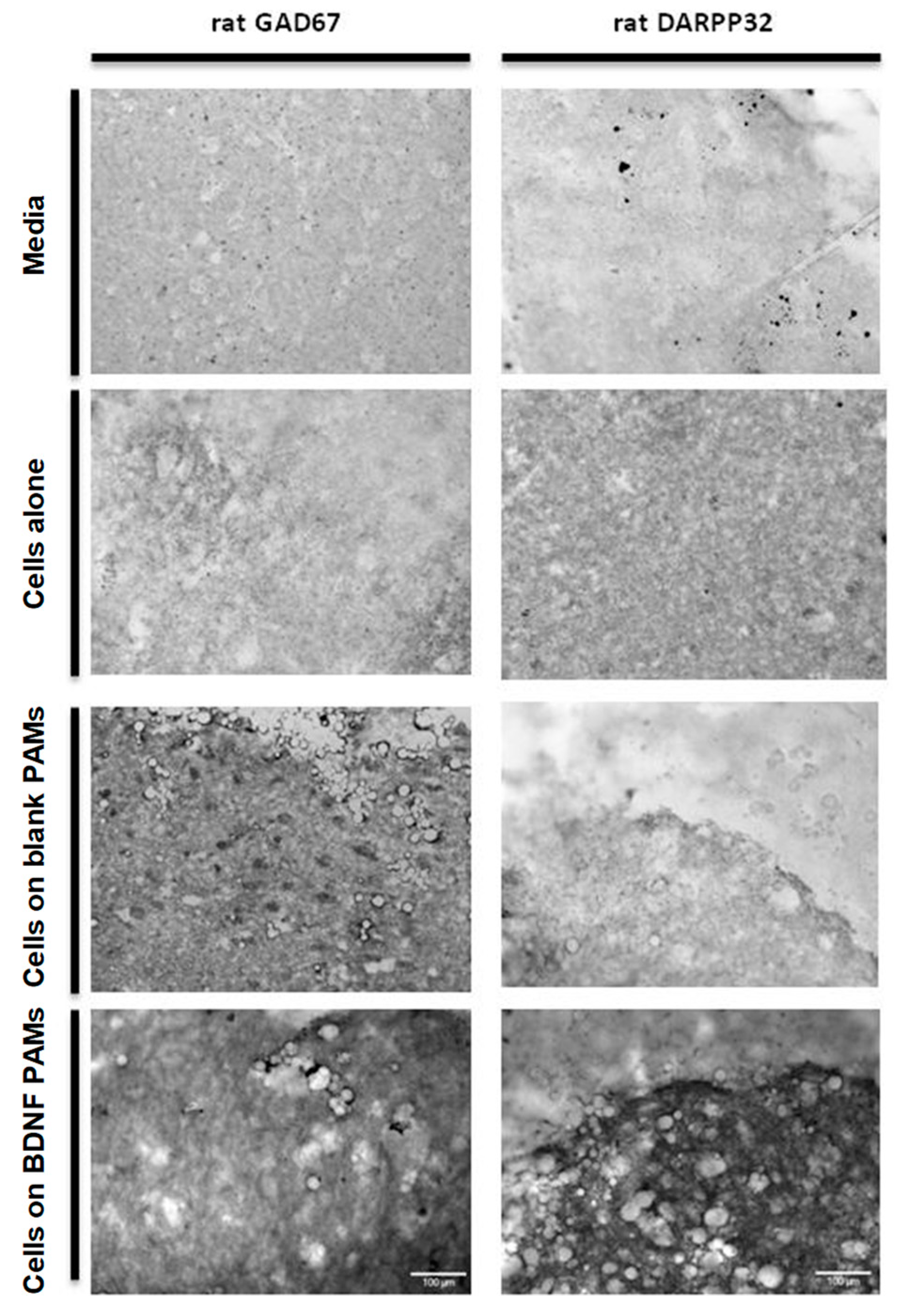
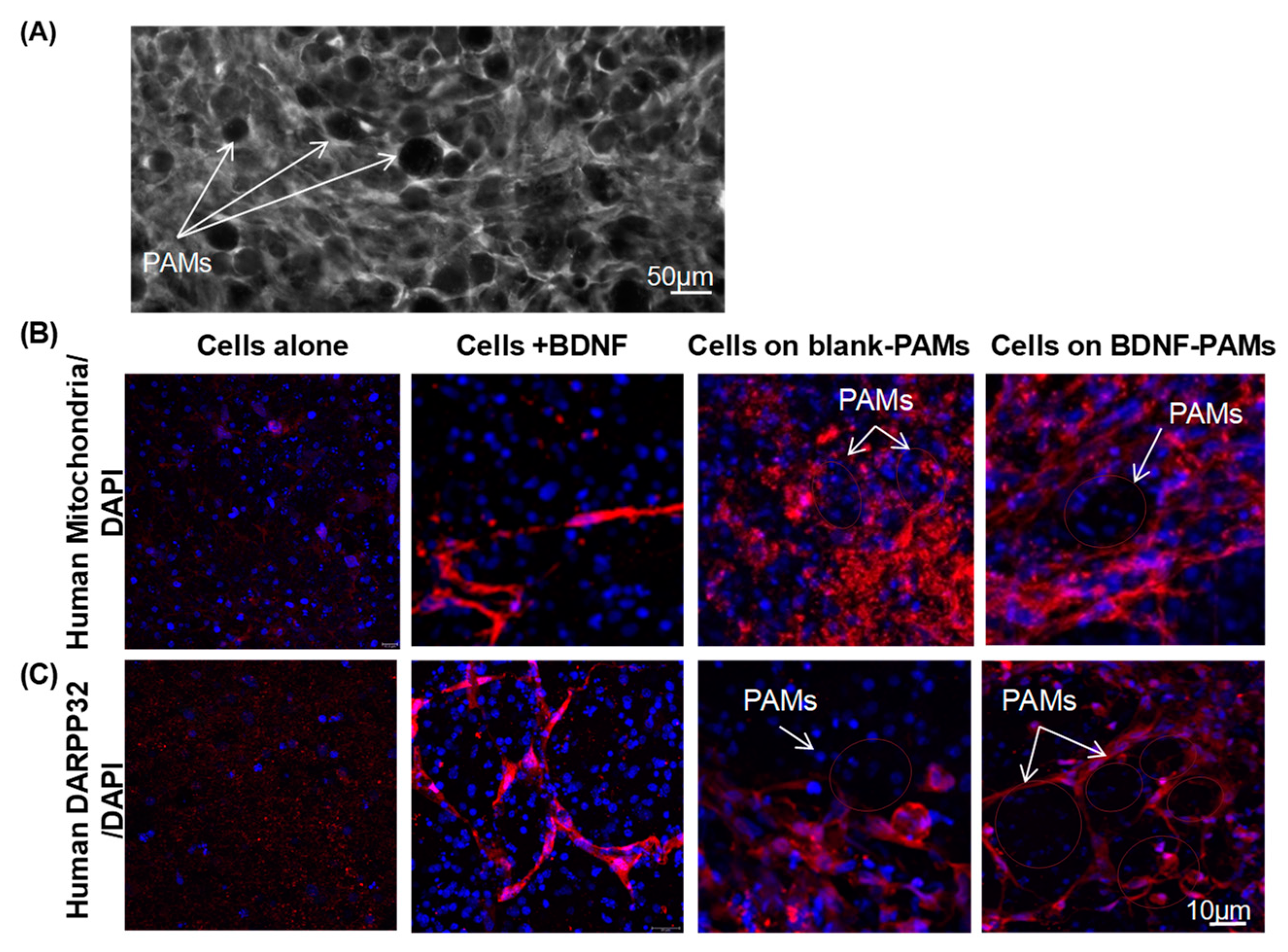
3.4. Adherence of MIAMI E/F SHH-siREST on PAMs
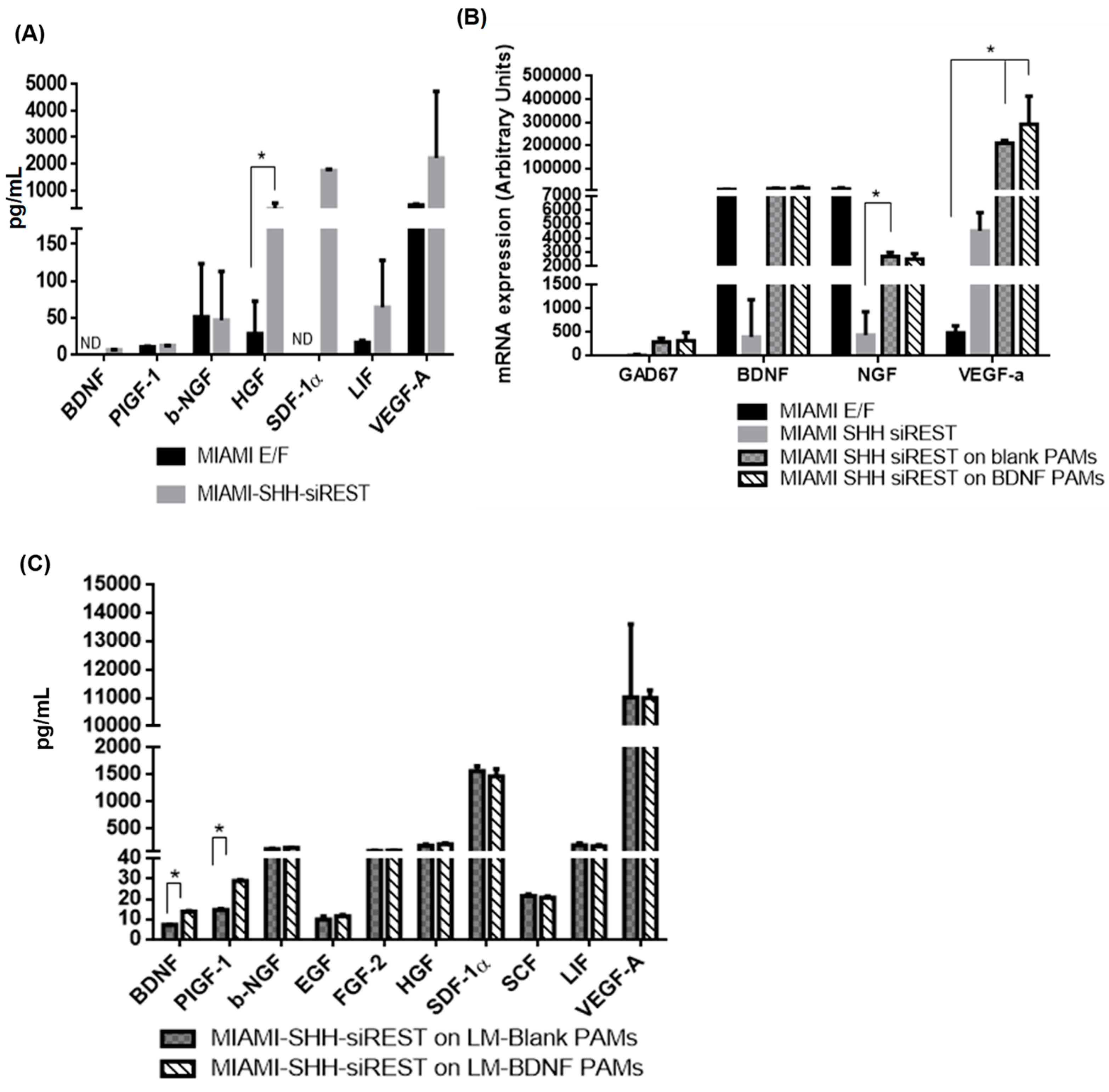
3.5. Characterization of MIAMI-SHH-siREST on PAMs
3.6. Behavior of PAMs and MIAMI-SHH-siREST in Huntington’s Disease Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Heading | BDNF | PIGF-1 | b-NGF | HGF | SDF-1 | LIF | VEGF-A |
|---|---|---|---|---|---|---|---|
| MIAMI E/F media (Control) | 4.55 ± 0.294 | 14.36 ± 0.20 | 0.00 | 124.93 ± 1.52 | 481.95 ± 3.27 | 49.35 ± 3.43 | 34.21 ± 0.676 |
| Supernatant MIAMI E/F | 1.85 ± 0.139 | 24.38 ± 1.25 | 51.07 ± 72.22 | 152.87 ± 42.87 | 385.73 ± 52.87 | 65.80 ± 2.54 | 482.53 ± 32.54 |
| Step 1 Media (Control) | 0.00 | 0.00 | 0.00 | 0.00 | 13.61 ±0.65 | 0.00 | 0.00 |
| supernatant MIAMI E/F SHH siREST | 5.95 ± 0.59 | 11.70 ± 0.26 | 46.68 ± 66.05 | 304.12 ± 216.21 | 1746.64 ± 63.87 | 63.78 ± 64.24 | 2205.48 ± 505.54 |
| Step 2 Media without BDNF (Control) | 0.00 | 0.00 | 0.00 | 0.00 | 14.76 ± 0.01 | 0.00 | 0.00 |
| supernatant MIAMI E/F SHH siREST on LM-blanks PAMS | 7.21 ± 0.19 | 14.73 ± 0.46 | 128.04 ± 12.21 | 192.08 ± 25.37 | 1567.67 ± 92.07 | 195 ± 39.81 | 11,028.92 ± 2576.19 |
| supernatant MIAMI E/F SHH siREST on LM-BDNF PAMS | 13.77 ± 0.35 | 29.02 ± 0.41 | 148.30 ± 6.18 | 219.43 ± 15.76 | 1467 ± 138.48 | 180.11 ± 20.07 | 11,003.58 ± 1189.29 |
References
- MacDonald, M.E.; Ambrose, C.M.; Duyao, M.P.; Myers, R.H.; Lin, C.; Srinidhi, L.; Barnes, G.; Taylor, S.A.; James, M.; Groot, N.; et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 1993, 72, 971–983. [Google Scholar] [CrossRef]
- Cattaneo, E.; Rigamonti, D.; Goffredo, D.; Zuccato, C.; Squitieri, F.; Sipione, S. Loss of normal huntingtin function: new developments in Huntington’s disease research. Trends Neurosci. 2001, 24, 182–188. [Google Scholar] [CrossRef]
- Klempíř, J.; Klempířová, O.; Štochl, J.; Špačková, N.; Roth, J. The relationship between impairment of voluntary movements and cognitive impairment in Huntington’s disease. J. Neurol. 2009, 256, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Clabough, E.B. Huntington’s Disease: The Past, Present, and future search for Disease Modifiers. Yale J. Biol. Med. 2013, 86, 217. [Google Scholar] [PubMed]
- Fink, K.D.; Deng, P.; Torrest, A.; Stewart, H.; Pollock, K.; Gruenloh, W.; Annett, G.; Tempkin, T.; Wheelock, V.; Nolta, J.A. Developing stem cell therapies for juvenile and adult-onset Huntington’s disease. Regen. Med. 2015, 10, 623–646. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Kordower, J.H. Gene therapy for Huntington’s disease. Neurobiol. Dis. 2012, 48, 243–254. [Google Scholar] [CrossRef]
- Snyder, B.R.; Chiu, A.M.; Prockop, D.J.; Chan, A.W.S. Human multipotent stromal cells (MSCs) increase neurogenesis and decrease atrophy of the striatum in a transgenic mouse model for Huntington’s disease. PloS One 2010, 5, e9347. [Google Scholar] [CrossRef]
- Delcroix, G.J.-R.; Garbayo, E.; Sindji, L.; Thomas, O.; Vanpouille-Box, C.; Schiller, P.C.; Montero-Menei, C.N. The therapeutic potential of human multipotent mesenchymal stromal cells combined with pharmacologically active microcarriers transplanted in hemi-parkinsonian rats. Biomaterials 2011, 32, 1560–1573. [Google Scholar] [CrossRef]
- André, E.M.; Passirani, C.; Seijo, B.; Sanchez, A.; Montero-Menei, C.N. Nano and microcarriers to improve stem cell behaviour for neuroregenerative medicine strategies: Application to Huntington’s disease. Biomaterials 2016, 83, 347–362. [Google Scholar] [CrossRef]
- Emerich, D.F.; Cain, C.K.; Greco, C.; Saydoff, J.A.; HU, Z.Y.; Liu, H.; Lindner, M.D. Cellular delivery of human CNTF prevents motor and cognitive dysfunction in a rodent model of Huntington’s disease. Cell Transplant. 1997, 249–266. [Google Scholar] [CrossRef]
- Jiang, Y.; Hailong, L.; Shanshan, H.; Tan, H.; Zhang, Y.; Li, H. Bone marrow mesenchymal stem cells can improve the motor function of a Huntington’s disease rat model. Neurol. Res. 2011, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huang, C.; Liu, F.; Lin, H.; Yang, X.; Zhang, Z. Comparison of the neuronal differentiation abilities of bone marrow-derived and adipose tissue-derived mesenchymal stem cells. Mol. Med. Rep. 2017, 16, 3877–3886. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ballas, N.; Mandel, G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr. Opin. Neurobiol. 2005, 15, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Ballas, N.; Grunseich, C.; Lu, D.D.; Speh, J.C.; Mandel, G. REST and Its Corepressors Mediate Plasticity of Neuronal Gene Chromatin throughout Neurogenesis. Cell 2005, 121, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Lv, Y.; Zhang, S.; Chen, L.; Bai, C.; Nan, X.; Yue, W.; Pei, X. NRSF silencing induces neuronal differentiation of human mesenchymal stem cells. Exp. Cell Res. 2008, 314, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.F.; Paquette, A.J.; Anderson, D.J. NRSF/REST is required in vivo for repression of multiple neuronal target genes during embryogenesis. Nat. Genet. 1998, 20, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Heurtault, B.; Saulnier, P.; Pech, B.; Proust, J.E.; Benoit, J.P. A novel phase inversion-based process for the preparation of lipid nanocarriers. Pharm. Res. 2002, 19, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Morille, M.; Montier, T.; Legras, P.; Carmoy, N.; Brodin, P.; Pitard, B.; Benoît, J.-P.; Passirani, C. Long-circulating DNA lipid nanocapsules as new vector for passive tumor targeting. Biomaterials 2010, 31, 321–329. [Google Scholar] [CrossRef]
- Paillard, A.; Hindré, F.; Vignes-Colombeix, C.; Benoit, J.-P.; Garcion, E. The importance of endo-lysosomal escape with lipid nanocapsules for drug subcellular bioavailability. Biomaterials 2010, 31, 7542–7554. [Google Scholar] [CrossRef]
- D’Ippolito, G. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J. Cell Sci. 2004, 117, 2971–2981. [Google Scholar] [CrossRef]
- Roche, S.; D’Ippolito, G.; Gomez, L.A.; Bouckenooghe, T.; Lehmann, S.; Montero-Menei, C.N.; Schiller, P.C. Comparative analysis of protein expression of three stem cell populations: Models of cytokine delivery system in vivo. Int. J. Pharm. 2013, 440, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Daviaud, N.; Garbayo, E.; Sindji, L.; Martinez-Serrano, A.; Schiller, P.C.; Montero-Menei, C.N. Survival, Differentiation, and Neuroprotective Mechanisms of Human Stem Cells Complexed With Neurotrophin-3-Releasing Pharmacologically Active Microcarriers in an Ex Vivo Model of Parkinson’s Disease. Stem Cells Transl. Med. 2015, 4, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, G.J.-R.; Curtis, K.M.; Schiller, P.C.; Montero-Menei, C.N. EGF and bFGF pre-treatment enhances neural specification and the response to neuronal commitment of MIAMI cells. Differentiation 2010, 80, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Garbayo, E.; Raval, A.P.; Curtis, K.M.; Della-Morte, D.; Gomez, L.A.; D’Ippolito, G.; Reiner, T.; Perez-Stable, C.; Howard, G.A.; Perez-Pinzon, M.A.; et al. Neuroprotective properties of marrow-isolated adult multilineage-inducible cells in rat hippocampus following global cerebral ischemia are enhanced when complexed to biomimetic microcarriers: BMMs enhance MIAMI cell protection in cerebral ischemia. J. Neurochem. 2011, 119, 972–988. [Google Scholar] [CrossRef] [PubMed]
- Tatard, V.M.; D’Ippolito, G.; Diabira, S.; Valeyev, A.; Hackman, J.; McCarthy, M.; Bouckenooghe, T.; Menei, P.; Montero-Menei, C.N.; Schiller, P.C. Neurotrophin-directed differentiation of human adult marrow stromal cells to dopaminergic-like neurons. Bone 2007, 40, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Tatard, V.M.; Sindji, L.; Branton, J.(G.); Aubert-Pouëssel, A.; Colleau, J.; Benoit, J.-P.; Montero-Menei, C.N. Pharmacologically active microcarriers releasing glial cell line—derived neurotrophic factor: Survival and differentiation of embryonic dopaminergic neurons after grafting in hemiparkinsonian rats. Biomaterials 2007, 28, 1978–1988. [Google Scholar] [CrossRef]
- Tatard VM, V.-J.M.; Benoit JP, M.P. Montero-Menei CN In vivo evaluation of pharmacologically active microcarriers releasing nerve growth factor and conveying PC12 cells. Cell Transplant. 2004, 13, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Morille, M.; Van-Thanh, T.; Garric, X.; Cayon, J.; Coudane, J.; Noël, D.; Venier-Julienne, M.-C.; Montero-Menei, C.N. New PLGA-P188-PLGA matrix enhances TGF-β3 release from pharmacologically active microcarriers and promotes chondrogenesis of mesenchymal stem cells. J. Control. Release Off. J. Control. Release Soc. 2013, 170, 99–110. [Google Scholar] [CrossRef]
- Karam, J.-P.; Bonafè, F.; Sindji, L.; Muscari, C.; Montero-Menei, C.N. Adipose-derived stem cell adhesion on laminin-coated microcarriers improves commitment toward the cardiomyogenic lineage. J. Biomed. Mater. Res. A 2015, 103, 1828–1839. [Google Scholar] [CrossRef]
- Kandalam, S.; Sindji, L.; Delcroix, G.J.-R.; Violet, F.; Garric, X.; André, E.M.; Schiller, P.C.; Venier-Julienne, M.-C.; des Rieux, A.; Guicheux, J.; et al. Pharmacologically active microcarriers delivering BDNF within a hydrogel: Novel strategy for human bone marrow-derived stem cells neural/neuronal differentiation guidance and therapeutic secretome enhancement. Acta Biomater. 2017, 49, 167–180. [Google Scholar] [CrossRef]
- Simmons, D.A.; Rex, C.S.; Palmer, L.; Pandyarajan, V.; Fedulov, V.; Gall, C.M.; Lynch, G. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington’s disease knockin mice. Proc. Natl. Acad. Sci. USA 2009, 106, 4906–4911. [Google Scholar] [CrossRef] [PubMed]
- Baydyuk, M.; Xu, B. BDNF signaling and survival of striatal neurons. Front. Cell. Neurosci. 2014, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, C. Loss of Huntingtin-Mediated BDNF Gene Transcription in Huntington’s Disease. Science 2001, 293, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Canals, J.M.; Pineda, J.R.; Torres-Peraza, J.F.; Bosch, M.; Martín-Ibañez, R.; Muñoz, M.T.; Mengod, G.; Ernfors, P.; Alberch, J. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 7727–7739. [Google Scholar] [CrossRef] [PubMed]
- Kells, A.P.; Henry, R.A.; Connor, B. AAV–BDNF mediated attenuation of quinolinic acid-induced neuropathology and motor function impairment. Gene Ther. 2008, 15, 966–977. [Google Scholar] [CrossRef]
- Terzić, J.; Saraga-Babić, M. Expression pattern of PAX3 and PAX6 genes during human embryogenesis. Int. J. Dev. Biol. 1999, 43, 501–508. [Google Scholar] [PubMed]
- Garel, S.; Yun, K.; Grosschedl, R.; Rubenstein, J.L.R. The early topography of thalamocortical projections is shifted in Ebf1 and Dlx1/2 mutant mice. Development 2002, 129, 5621–5634. [Google Scholar] [CrossRef]
- Andre, E.M.; Pensado, A.; Resnier, P.; Braz, L.; da Costa, A.R.; Passirani, C.; Sanchez, A.; Montero-Menei, C.N. Characterization and comparison of two novel nanosystems associated with siRNA for cellular therapy. Int. J. Pharm. 2015, 497, 255–267. [Google Scholar] [CrossRef]
- André, E.M.; Daviaud, N.; Sindji, L.; Cayon, J.; Perrot, R.; Montero-Menei, C.N. A novel ex vivo Huntington’s disease model for studying GABAergic neurons and cell grafts by laser microdissection. PLoS ONE 2018, 13, e0193409. [Google Scholar] [CrossRef]
- Resnier, P.; LeQuinio, P.; Lautram, N.; André, E.; Gaillard, C.; Bastiat, G.; Benoit, J.-P.; Passirani, C. Efficient in vitro gene therapy with PEG siRNA lipid nanocapsules for passive targeting strategy in melanoma. Biotechnol. J. 2014, 9, 1389–1401. [Google Scholar] [CrossRef]
- Aubry, L.; Bugi, A.; Lefort, N.; Rousseau, F.; Peschanski, M.; Perrier, A.L. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc. Natl. Acad. Sci. USA 2008, 105, 16707–16712. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Hu, B.; Liu, Y.; Vermilyea, S.C.; Liu, H.; Gao, L.; Sun, Y.; Zhang, X.; Zhang, S.-C. Human Embryonic Stem Cell-Derived GABA Neurons Correct Locomotion Deficits in Quinolinic Acid-Lesioned Mice. Cell Stem Cell 2012, 10, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Palm, K.; Belluardo, N.; Metsis, M.; Timmusk, T. Neuronal expression of zinc finger transcription factor REST/NRSF/XBR gene. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 1280–1296. [Google Scholar] [CrossRef]
- Mruthyunjaya, S.; Manchanda, R.; Godbole, R.; Pujari, R.; Shiras, A.; Shastry, P. Laminin-1 induces neurite outgrowth in human mesenchymal stem cells in serum/differentiation factors-free conditions through activation of FAK-MEK/ERK signaling pathways. Biochem. Biophys. Res. Commun. 2010, 391, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Brännvall, K.; Bergman, K.; Wallenquist, U.; Svahn, S.; Bowden, T.; Hilborn, J.; Forsberg-Nilsson, K. Enhanced neuronal differentiation in a three-dimensional collagen-hyaluronan matrix. J. Neurosci. Res. 2007, 85, 2138–2146. [Google Scholar] [CrossRef]
- Bhattacharyya, B.J.; Banisadr, G.; Jung, H.; Ren, D.; Cronshaw, D.G.; Zou, Y.; Miller, R.J. The chemokine stromal cell-derived factor-1 regulates GABAergic inputs to neural progenitors in the postnatal dentate gyrus. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 6720–6730. [Google Scholar] [CrossRef]
- Yanagawa, Y.; Iwabuchi, K.; Onoé, K. Enhancement of stromal cell-derived factor-1alpha-induced chemotaxis for CD4/8 double-positive thymocytes by fibronectin and laminin in mice. Immunology 2001, 104, 43–49. [Google Scholar] [CrossRef]
- Jin, K.; Zhu, Y.; Sun, Y.; Mao, X.O.; Xie, L.; Greenberg, D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11946–11950. [Google Scholar] [CrossRef]
- Luttun, A.; Tjwa, M.; Carmeliet, P. Placental growth factor (PlGF) and its receptor Flt-1 (VEGFR-1): novel therapeutic targets for angiogenic disorders. Ann. N. Y. Acad. Sci. 2002, 979, 80–93. [Google Scholar] [CrossRef]
- Diao, Y.-P.; Cui, F.-K.; Yan, S.; Chen, Z.-G.; Lian, L.-S.; Guo, L.-L.; Li, Y.-J. Nerve Growth Factor Promotes Angiogenesis and Skeletal Muscle Fiber Remodeling in a Murine Model of Hindlimb Ischemia. Chin. Med. J. (Engl.) 2016, 129, 313–319. [Google Scholar] [CrossRef]
| Gene | Full name | NM number | Sequences |
|---|---|---|---|
| ACTB | Actin | NM_001101.3 | F: CCAGATCATGTTTGAGACCT |
| R: GGCATACCCCTCGTAGAT | |||
| BDNF | Brain-derived neurotrophic factor | NM_001143816 | F: CAAACATCCGAGGACAAGG |
| R: TACTGAGCATCACCCTGG | |||
| DARPP32 | Dopamine- and cAMP-regulated phosphoprotein | NM_181505 | F: GAGAGCCTCAGGAGAGGG |
| R: CTCATTCAAATTGCTGATAGACTGC | |||
| Dlx2 | Distal-less homeobox 2 | NM_004405 | F: GACCTTGAGCCTGAAATTCG |
| R: ACCTGAGTCTGGGTGAGG | |||
| GAD67 | Glutamic Acid Decarboxylase 67 | NM_000817 | F: GGTGGCTCCAAAAATCAAAGC |
| R: CAATGTCAGACTGGGTAGCG | |||
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase | NM_001289745.1 | F: CAAAAGGGTCATCATCTCTGC |
| R: AGTTGTCATGGATGACCTTGG | |||
| GDNF | Glial cell line-derived neurotrophic factor | NM_011675.2 | Qiagen, ref #QT00001589 |
| Pax6 | Paired box 6 | NM_000280 | F: TTTCAGCACCAGTGTCTACC |
| R: TAGGTATCATAACTCCGCCC | |||
| NGF | Nerve growth factor | NM_002506 | Qiagen, ref #QT00043330 |
| REST | RE1-silencing transcription factor | NM_001193508.1 | F: ACTCATACAGGAGAACGCC |
| R: GTGAACCTGTCTTGCATGG | |||
| VEGFA | Vascular endothelial growth factor A | NM_001204384 | F: CAGCGCAGCTACTGCCATCCA |
| R: CAGTGGGCACACACTCCAGGC | |||
| ACTB | Actin | NM_001101.3 | F: CCAGATCATGTTTGAGACCT |
| R: GGCATACCCCTCGTAGAT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
André, E.M.; Delcroix, G.J.; Kandalam, S.; Sindji, L.; Montero-Menei, C.N. A Combinatorial Cell and Drug Delivery Strategy for Huntington’s Disease Using Pharmacologically Active Microcarriers and RNAi Neuronally-Committed Mesenchymal Stromal Cells. Pharmaceutics 2019, 11, 526. https://doi.org/10.3390/pharmaceutics11100526
André EM, Delcroix GJ, Kandalam S, Sindji L, Montero-Menei CN. A Combinatorial Cell and Drug Delivery Strategy for Huntington’s Disease Using Pharmacologically Active Microcarriers and RNAi Neuronally-Committed Mesenchymal Stromal Cells. Pharmaceutics. 2019; 11(10):526. https://doi.org/10.3390/pharmaceutics11100526
Chicago/Turabian StyleAndré, Emilie M., Gaëtan J. Delcroix, Saikrishna Kandalam, Laurence Sindji, and Claudia N. Montero-Menei. 2019. "A Combinatorial Cell and Drug Delivery Strategy for Huntington’s Disease Using Pharmacologically Active Microcarriers and RNAi Neuronally-Committed Mesenchymal Stromal Cells" Pharmaceutics 11, no. 10: 526. https://doi.org/10.3390/pharmaceutics11100526
APA StyleAndré, E. M., Delcroix, G. J., Kandalam, S., Sindji, L., & Montero-Menei, C. N. (2019). A Combinatorial Cell and Drug Delivery Strategy for Huntington’s Disease Using Pharmacologically Active Microcarriers and RNAi Neuronally-Committed Mesenchymal Stromal Cells. Pharmaceutics, 11(10), 526. https://doi.org/10.3390/pharmaceutics11100526