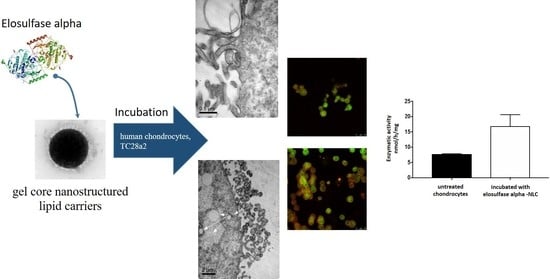
Enzyme-Loaded Gel Core Nanostructured Lipid Carriers to Improve Treatment of Lysosomal Storage Diseases: Formulation and In Vitro Cellular Studies of Elosulfase Alfa-Loaded Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Elosulfase Alfa-Loaded NLC
2.2.2. Determination of Particle Size and ζ Potential
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. X-ray Diffraction Analysis (XRD)
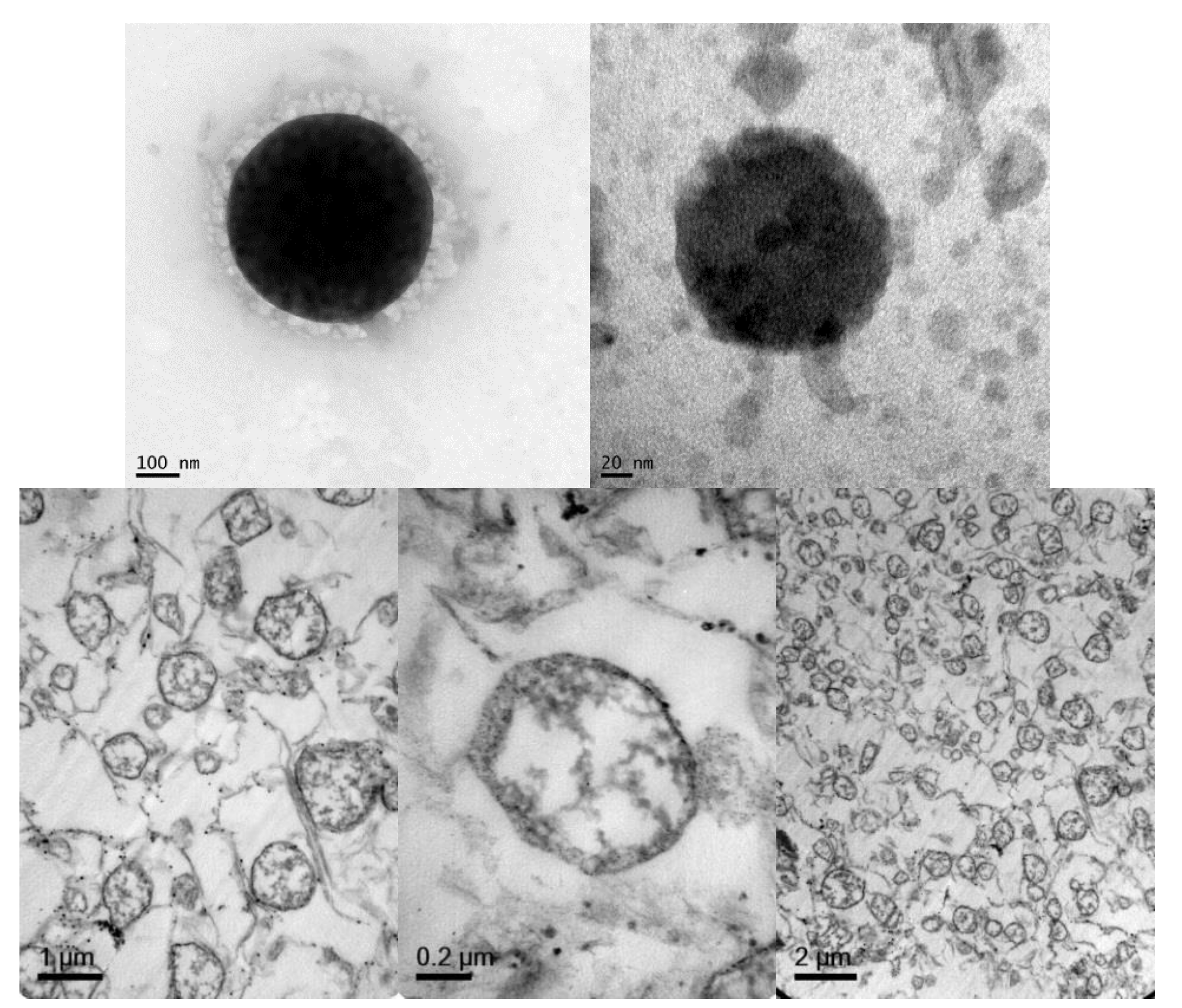
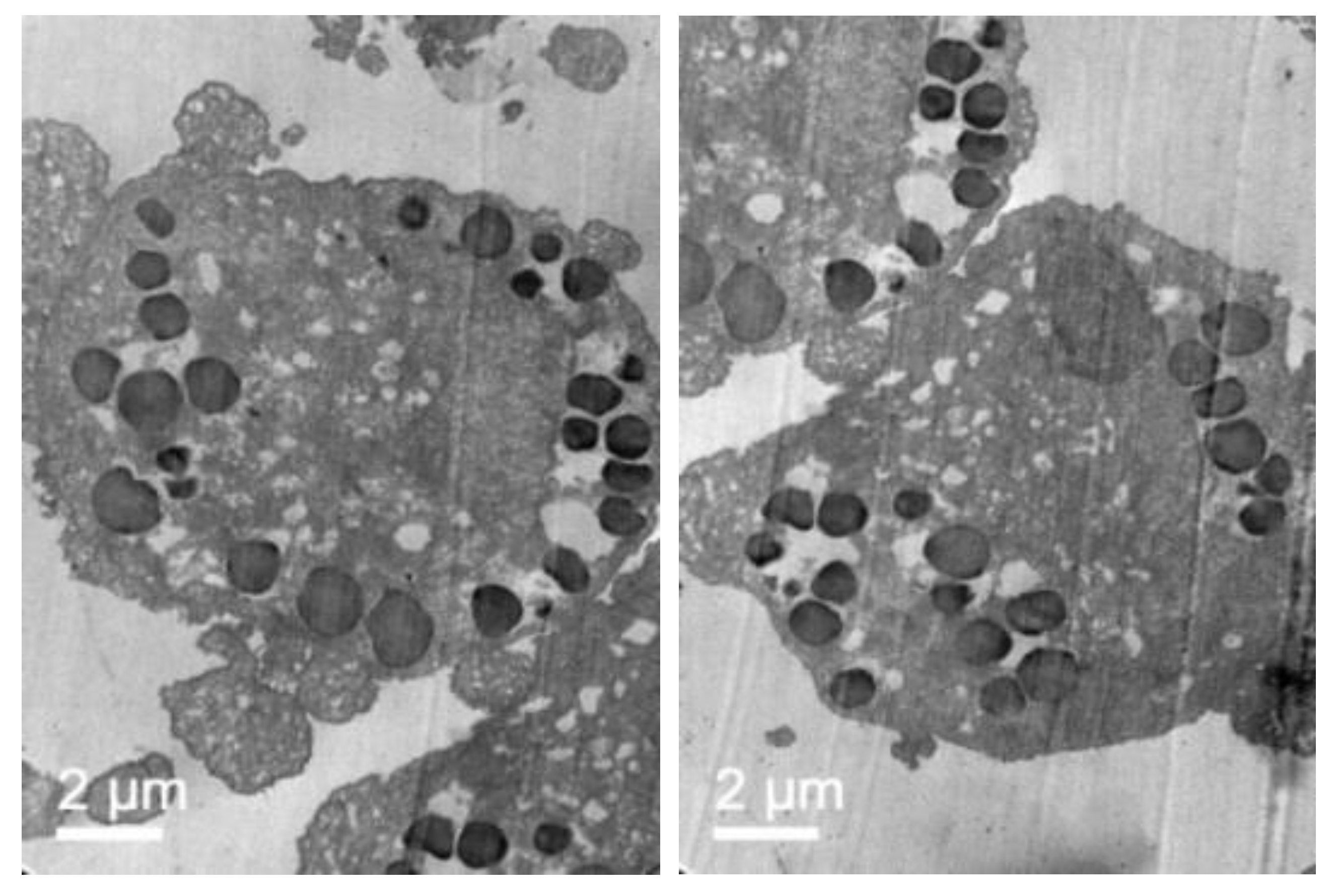
2.2.5. Transmission Electron Microscopy (TEM)
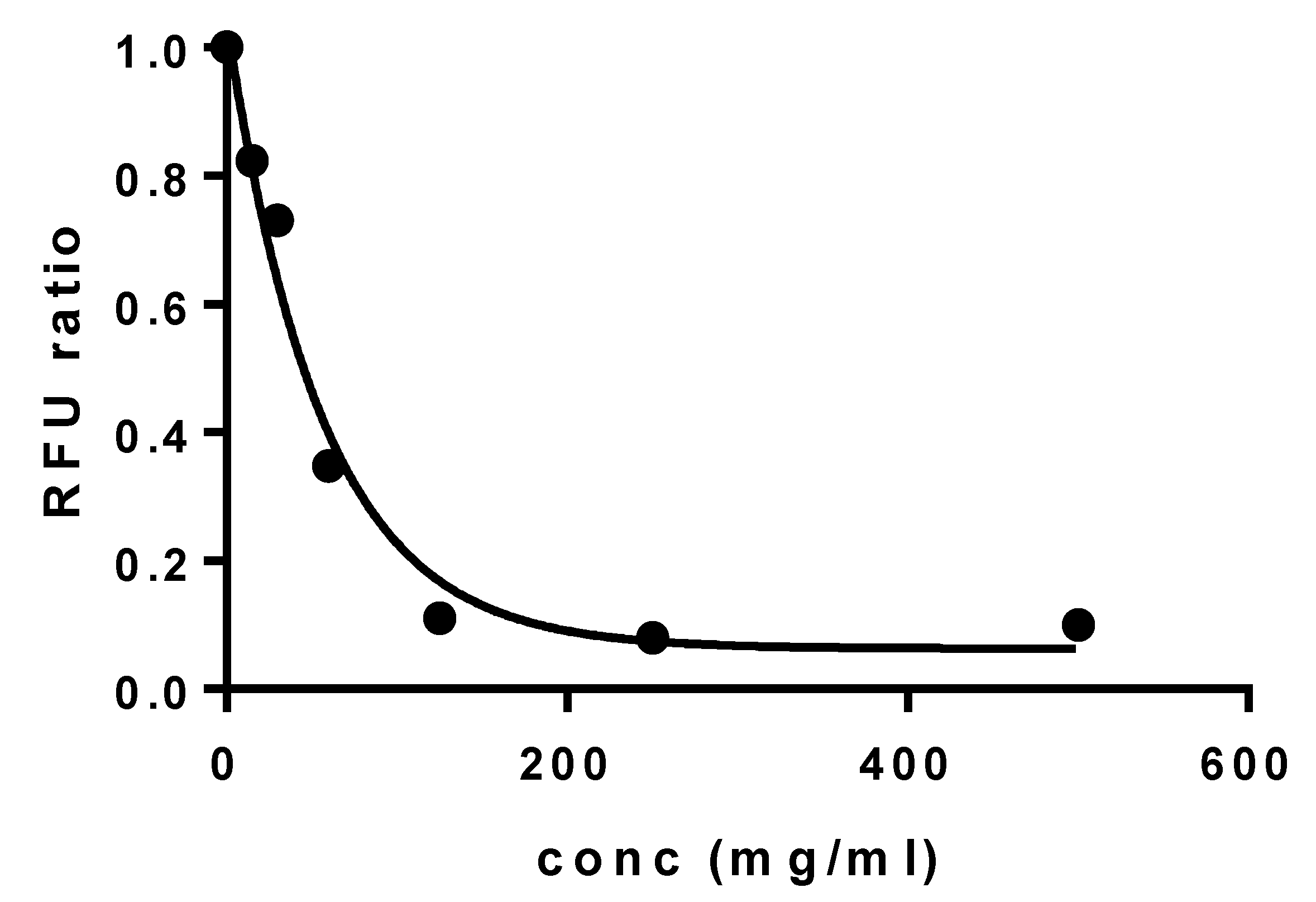
2.2.6. Determination of Elosulfase Alfa Activity
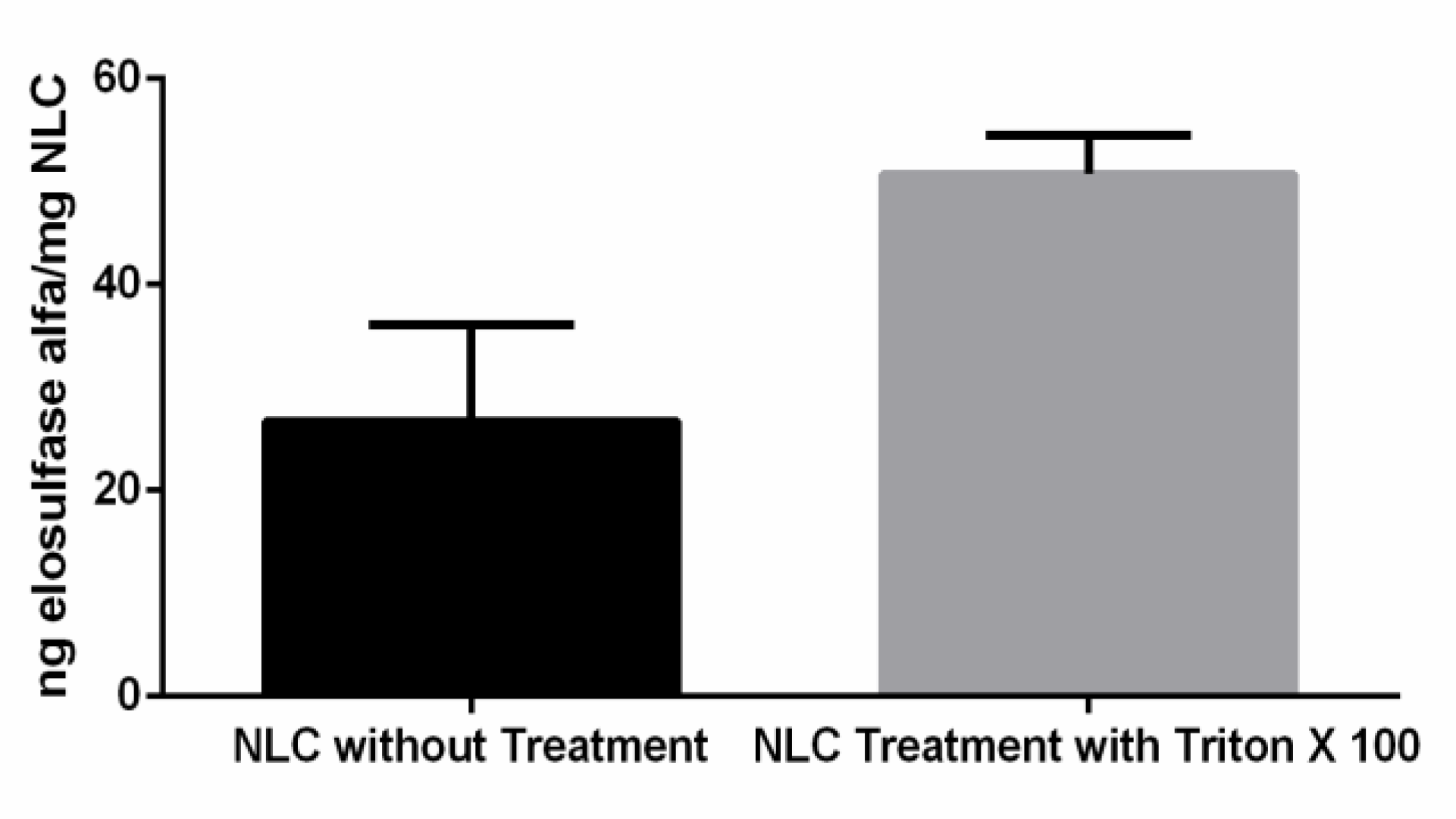
2.2.7. Determination of Elosulfase Alfa Loaded in NLC
2.2.8. Stability Study of NLCs in Human Plasma
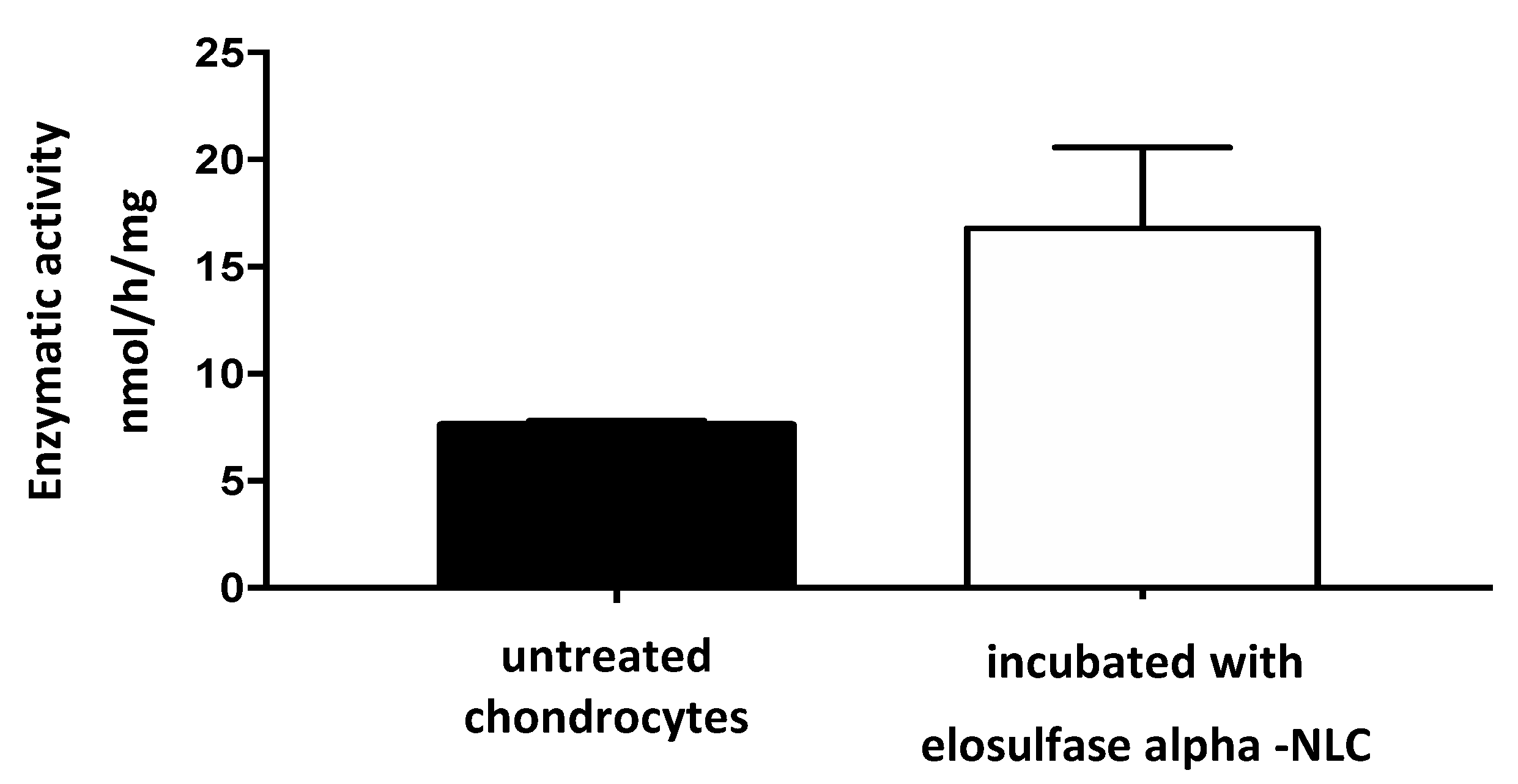
2.2.9. In Vitro Biological Activity
Cell Culture Conditions
Primary Chondrocytes from Healthy Patients
Cytocompatibility Assay
Internalization Studies of NLC in TC28a2 Chondrocytes and Pathological Fibroblasts from MPS IVA Patients
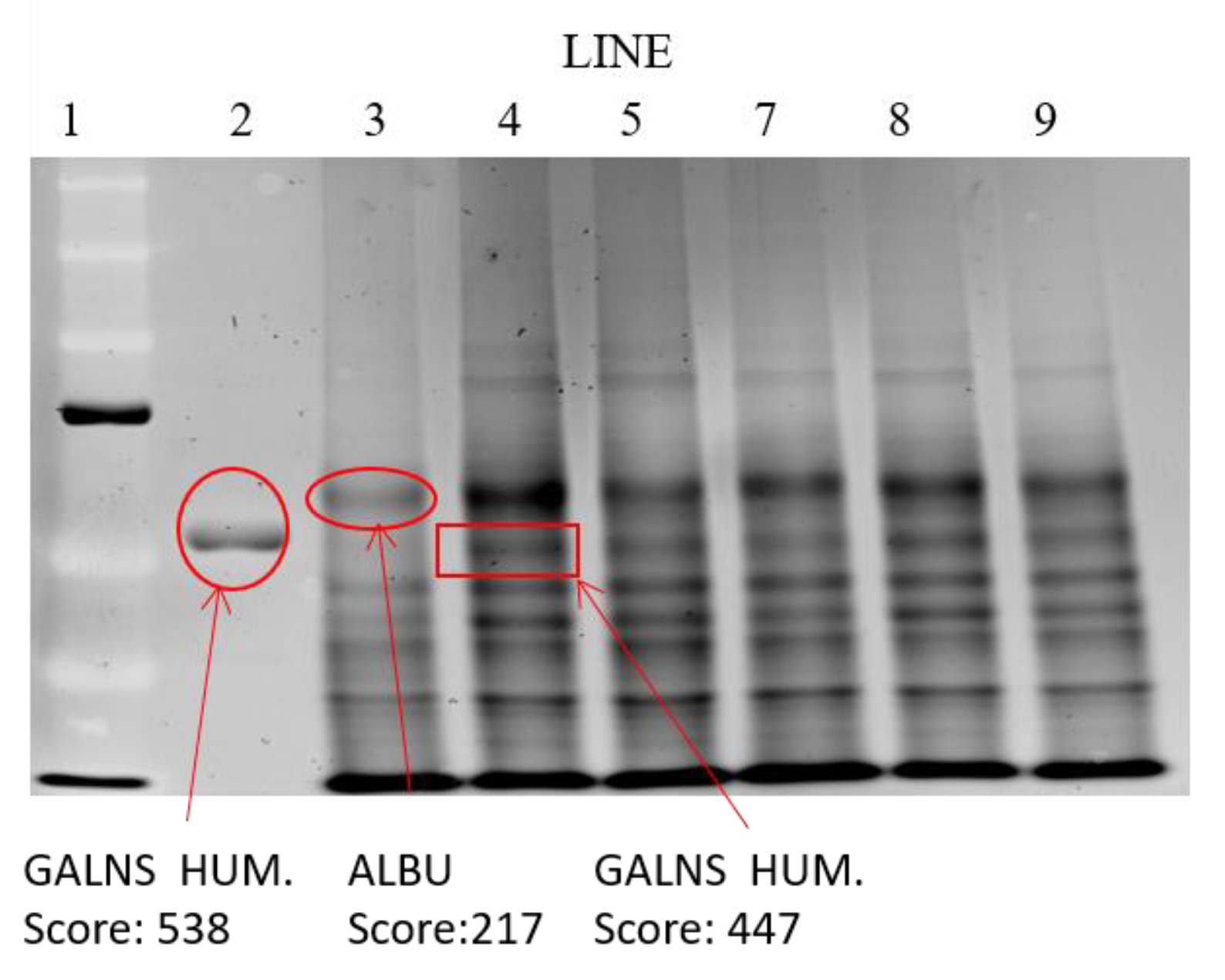
Electrophoretic Identification of Elosulfase Alpha in Cells and Quantification by MALDI-TOF Analysis
2.2.10. In Vivo Biodistribution Study
3. Results and Discussion
3.1. Physical Characterization of a Mixture of Lipid Components—Crystallinity
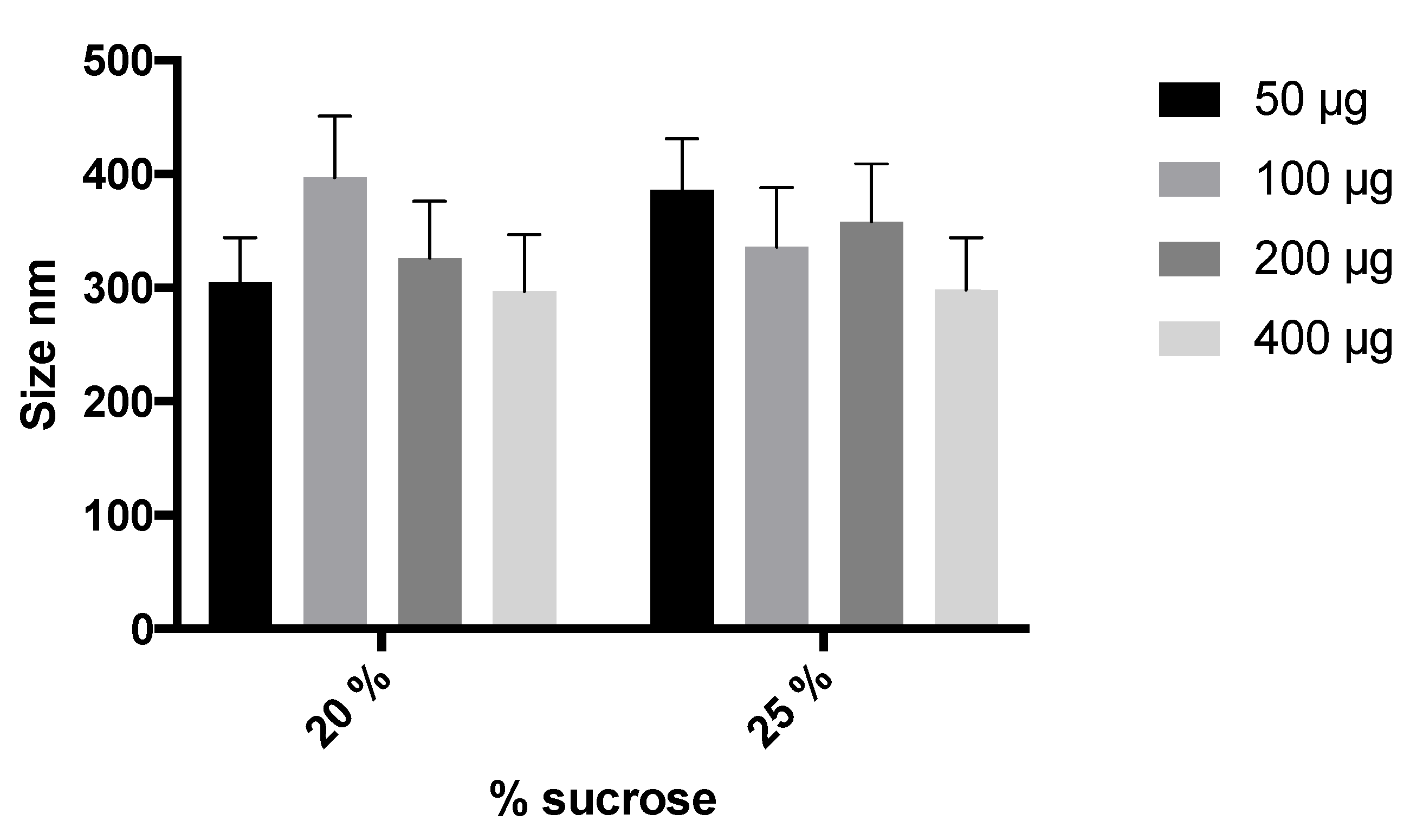
3.2. Preparation and Characterization of Elosulfase Alfa-Loaded NLCs
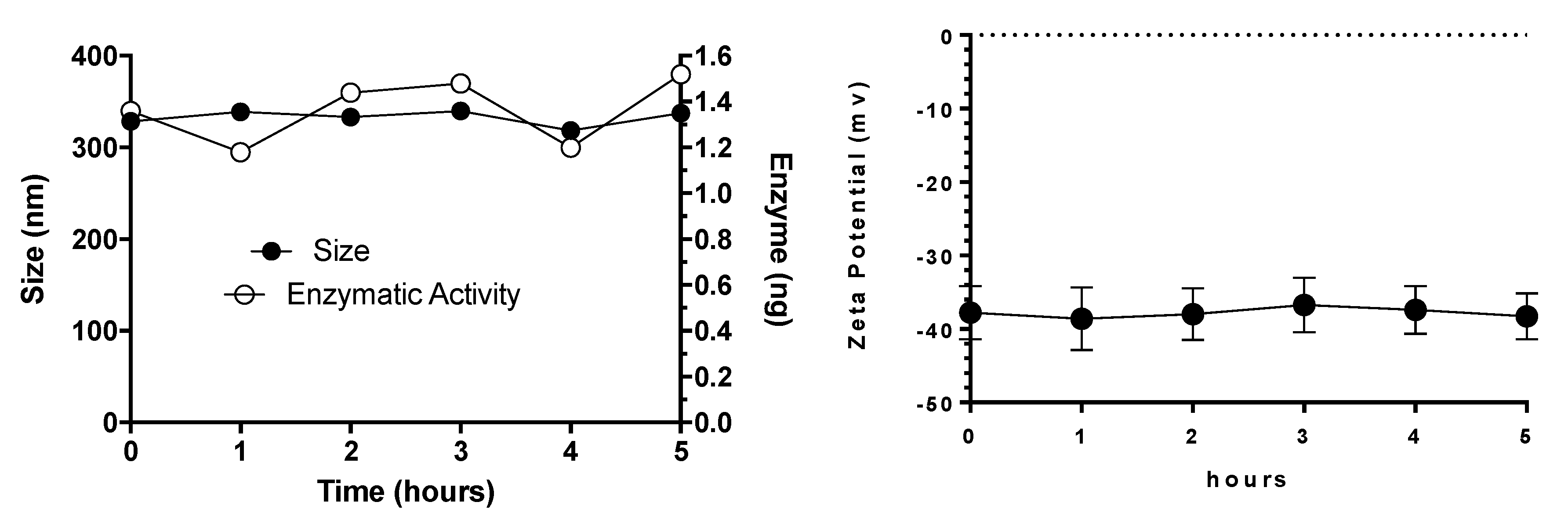
3.3. Plasma Stability of Elosulfase Alfa-NLC
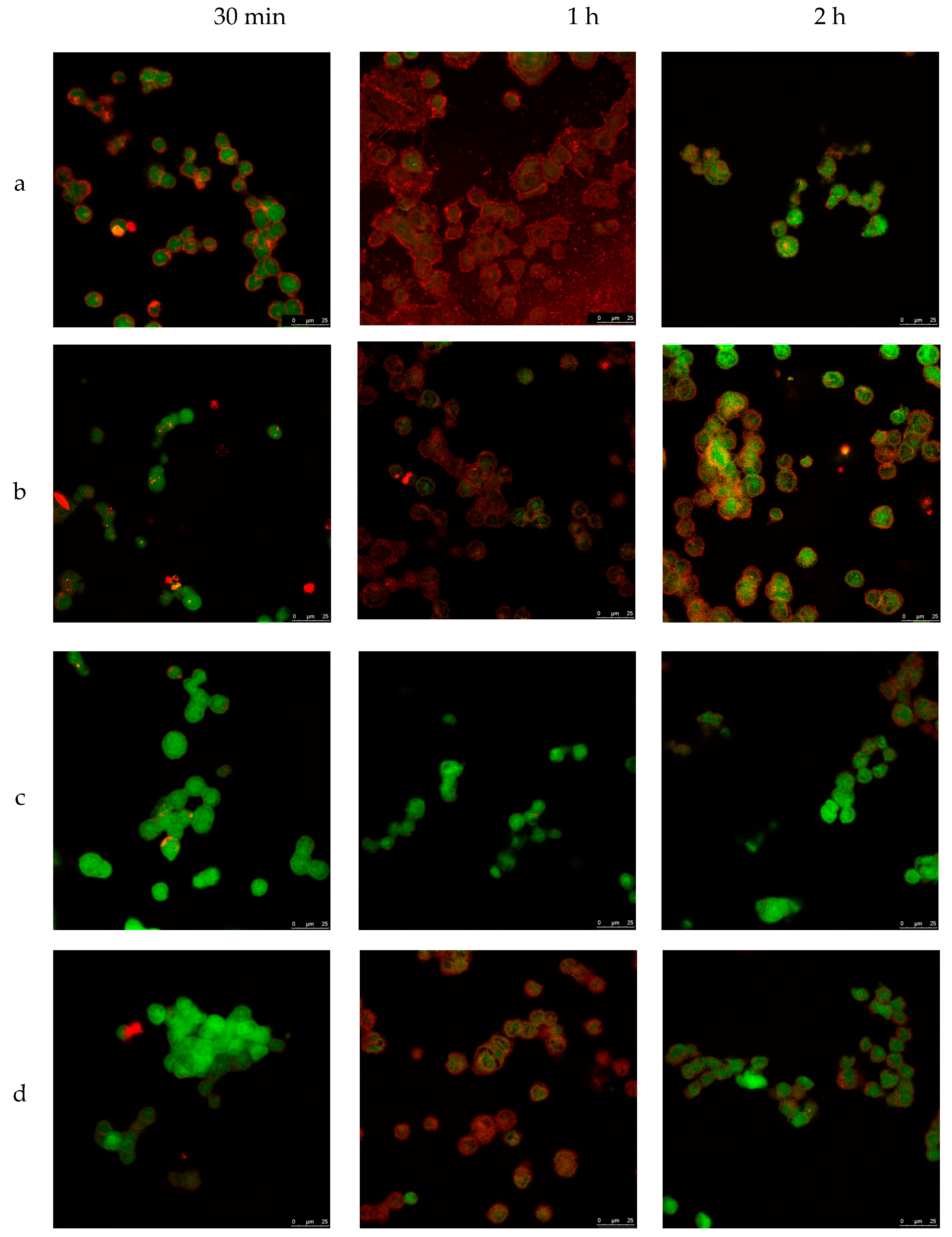
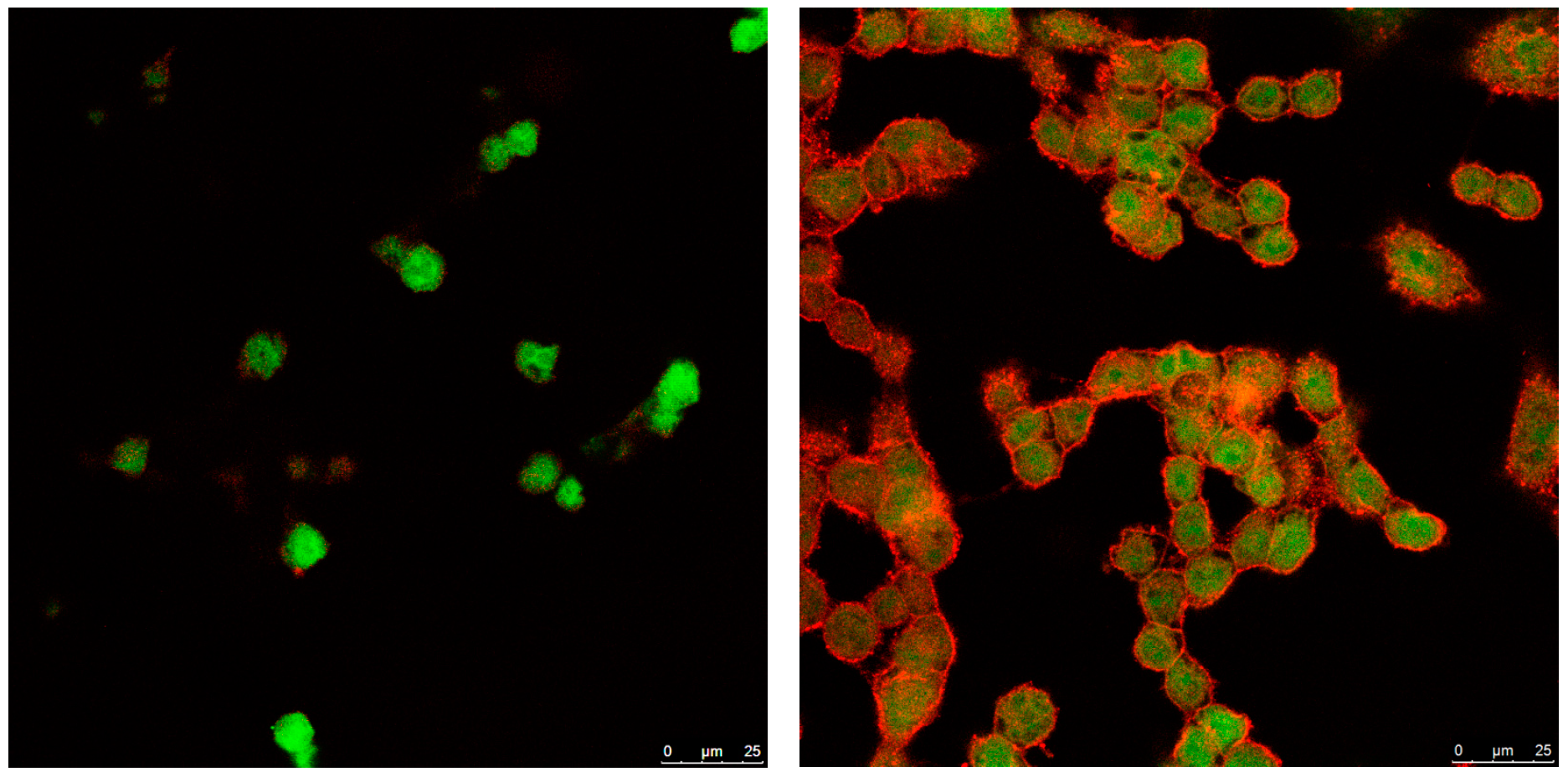
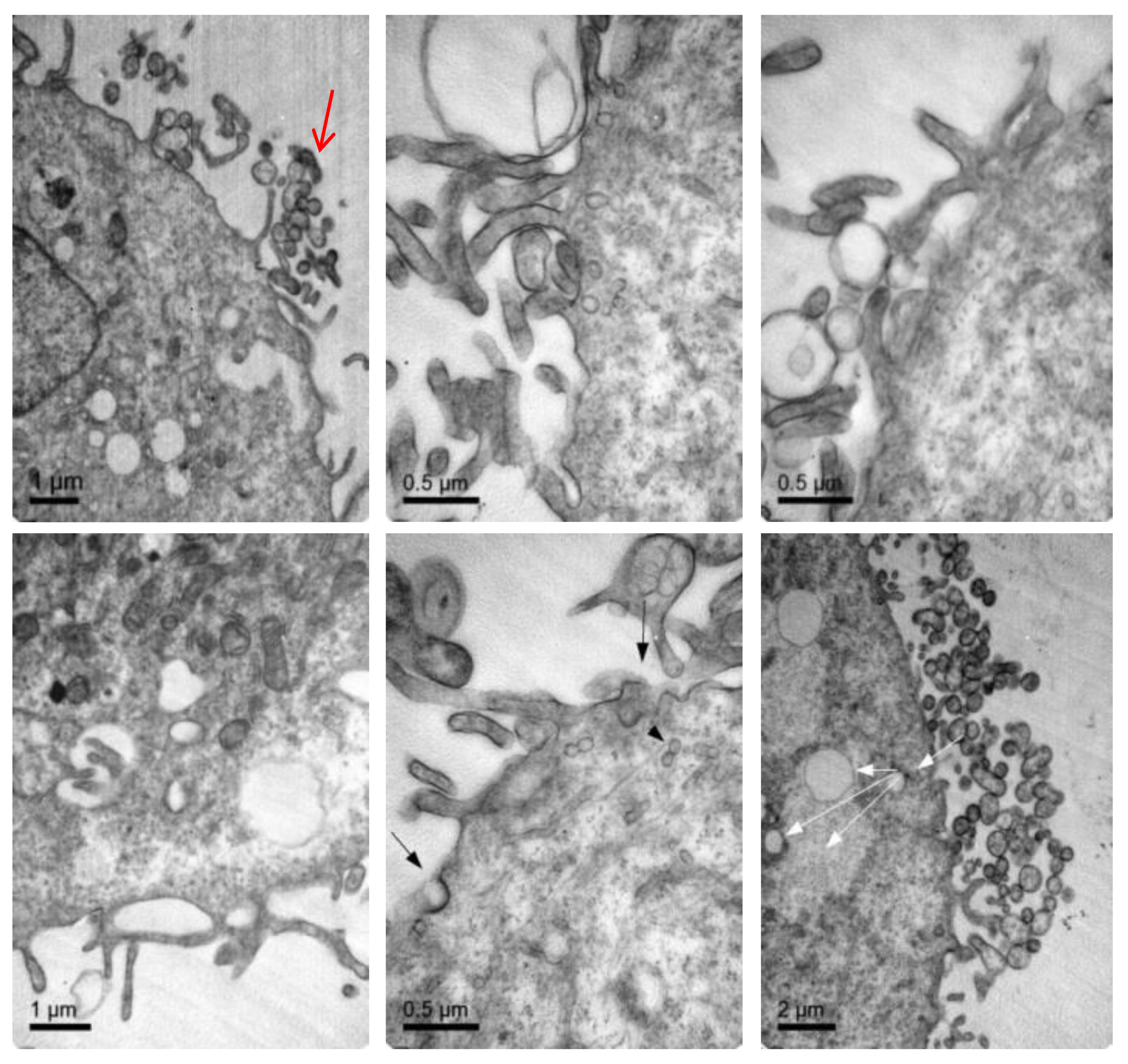
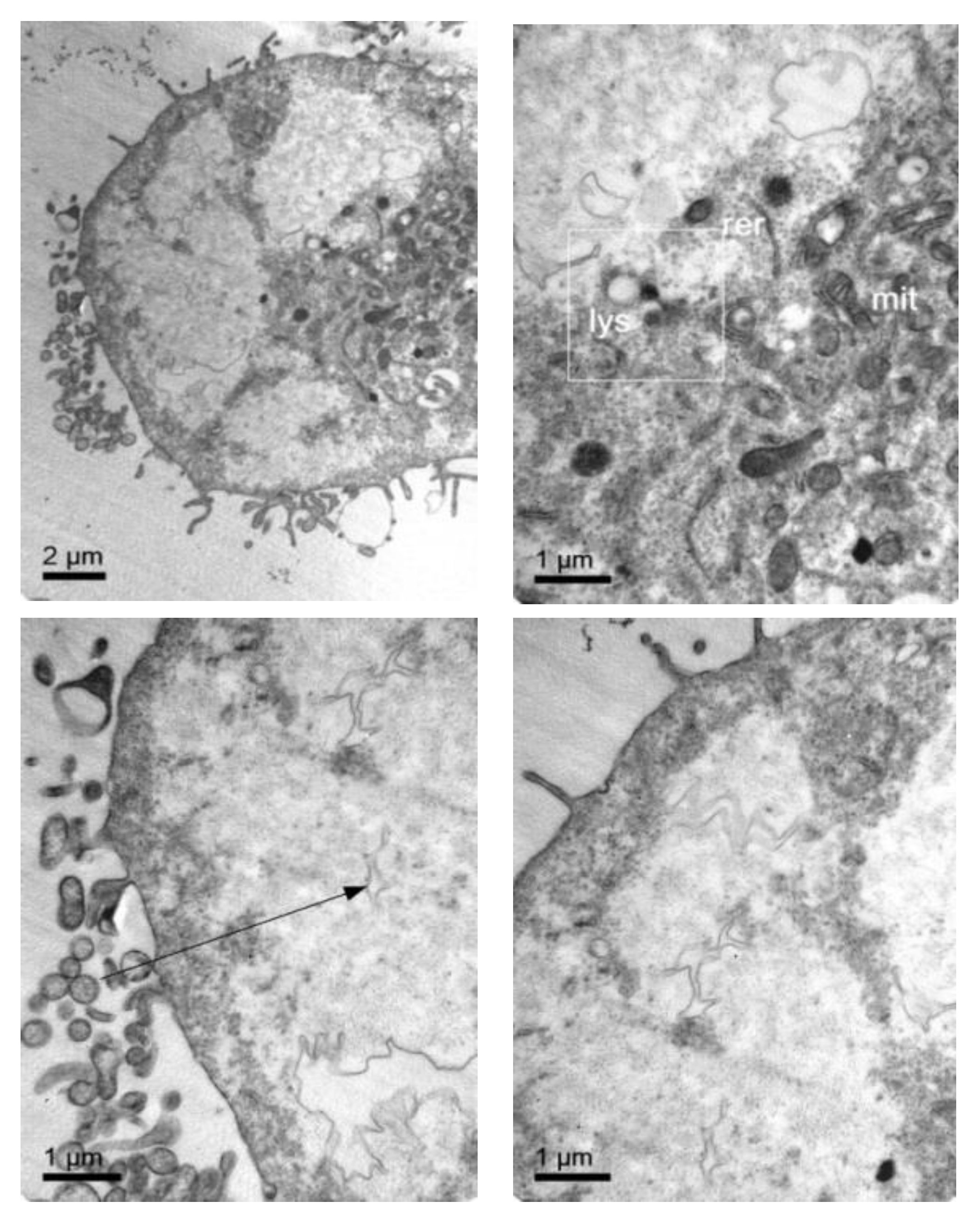
3.4. Cellular Internalization of Elosulfase Alfa-NLC (TC28a2 Chondrocytes)
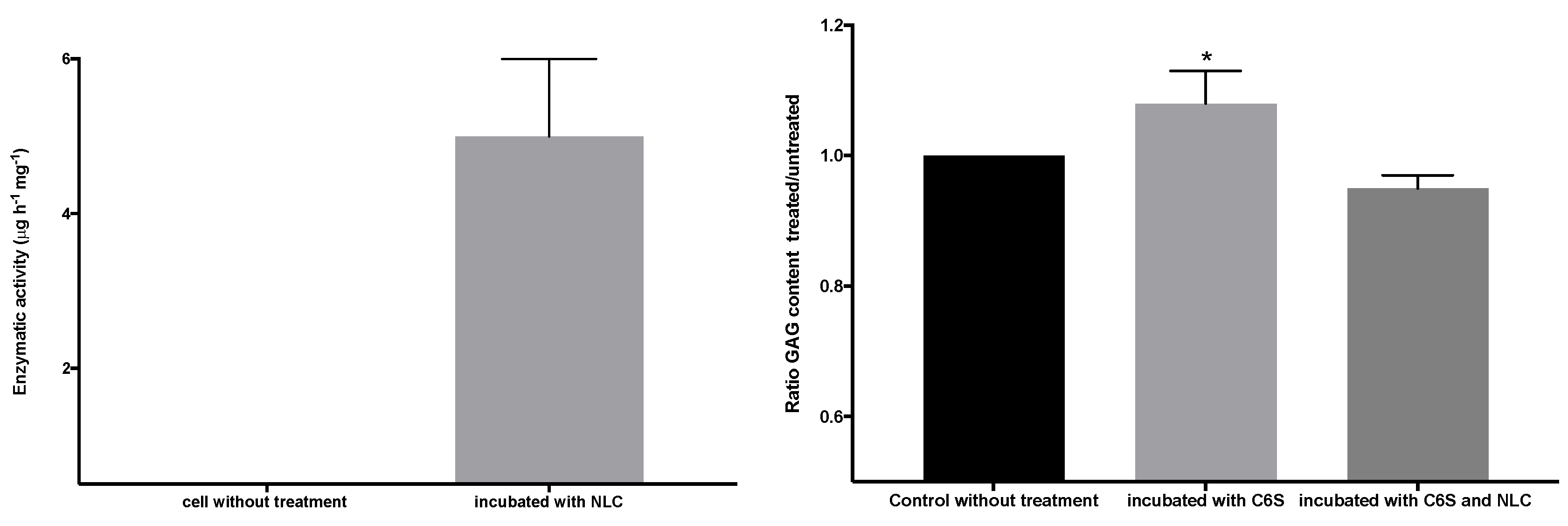
3.5. Cellular Internalization of Elosulfase Alfa-NLC and Enzyme Cellular Release in Pathological Fibroblasts from MPS IVA Patients
3.6. Cell Viability Test upon Co-Incubation with NLC
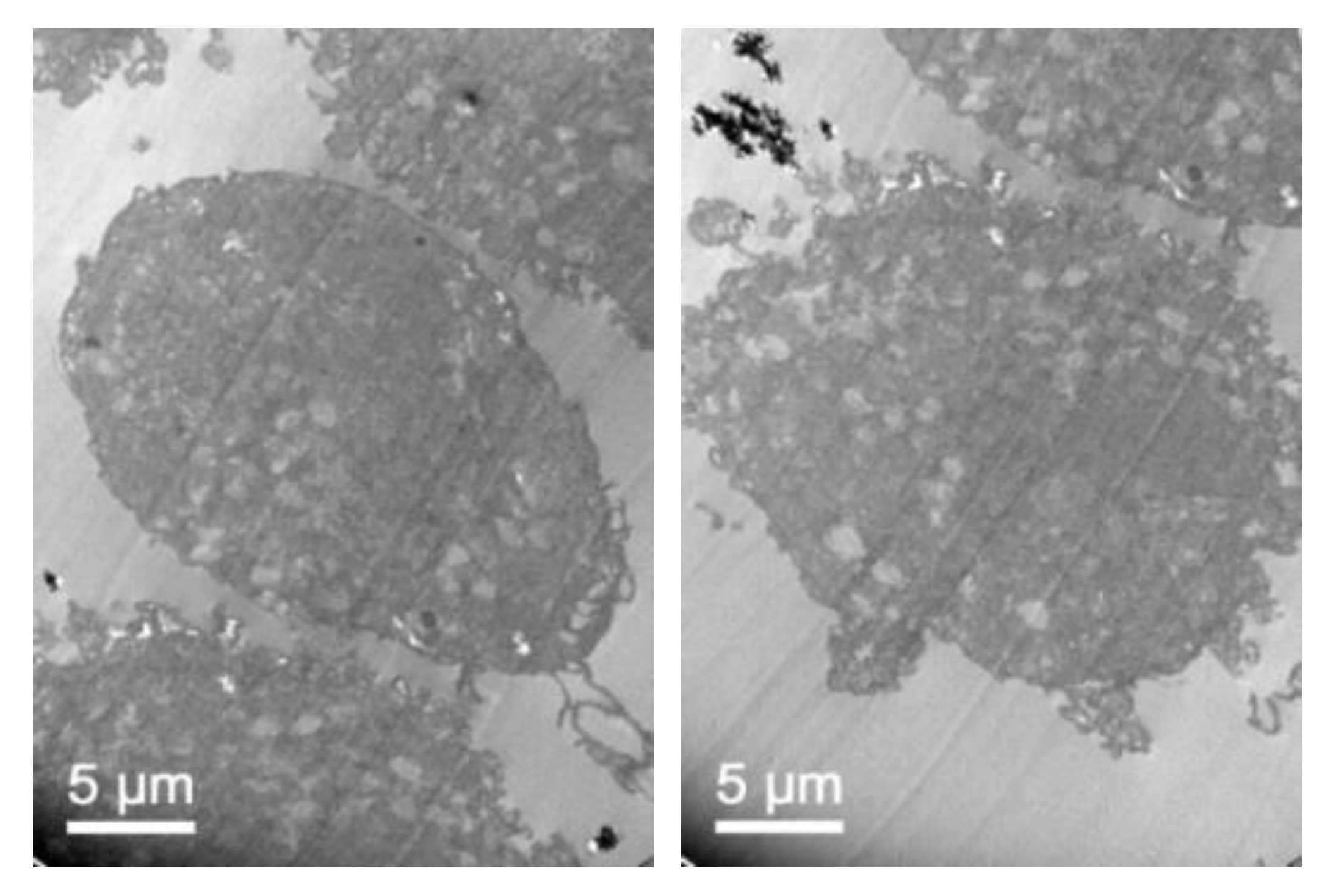
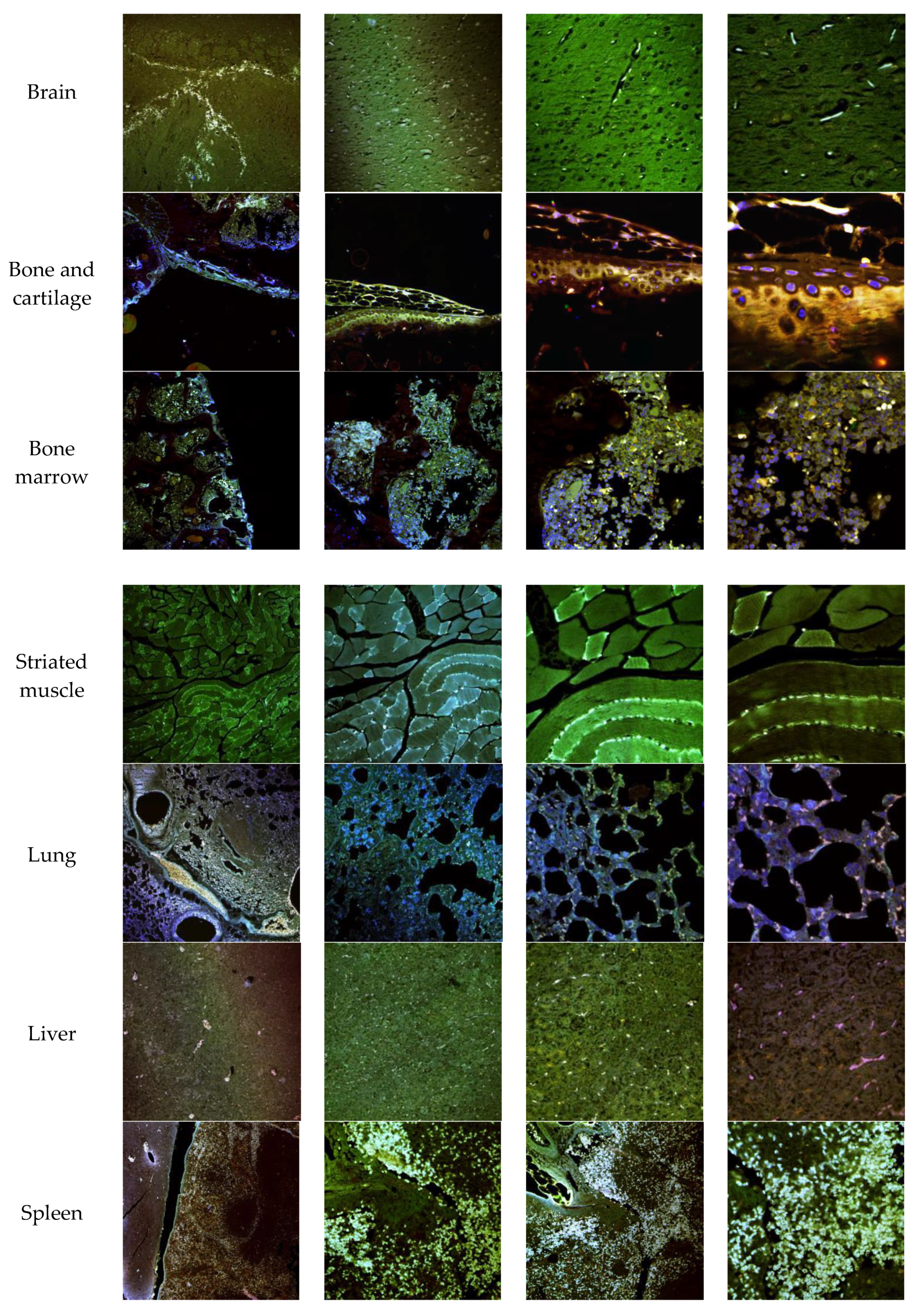
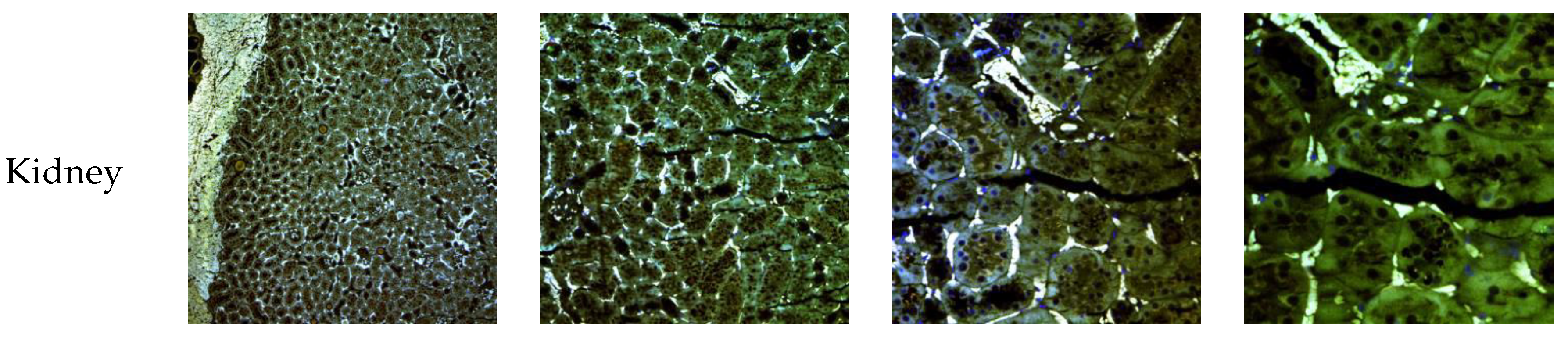
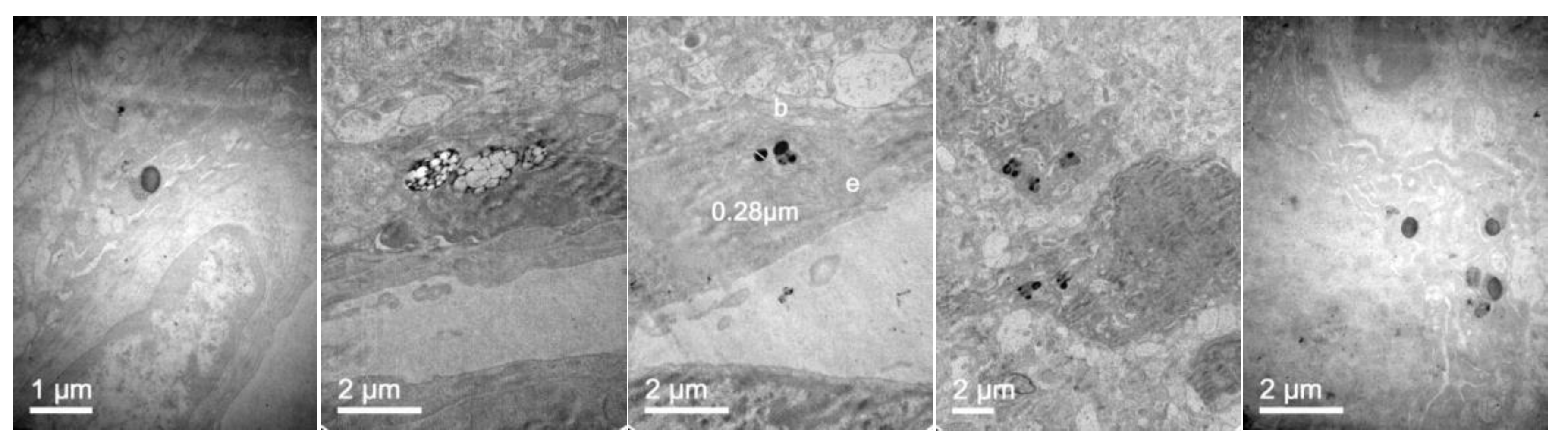
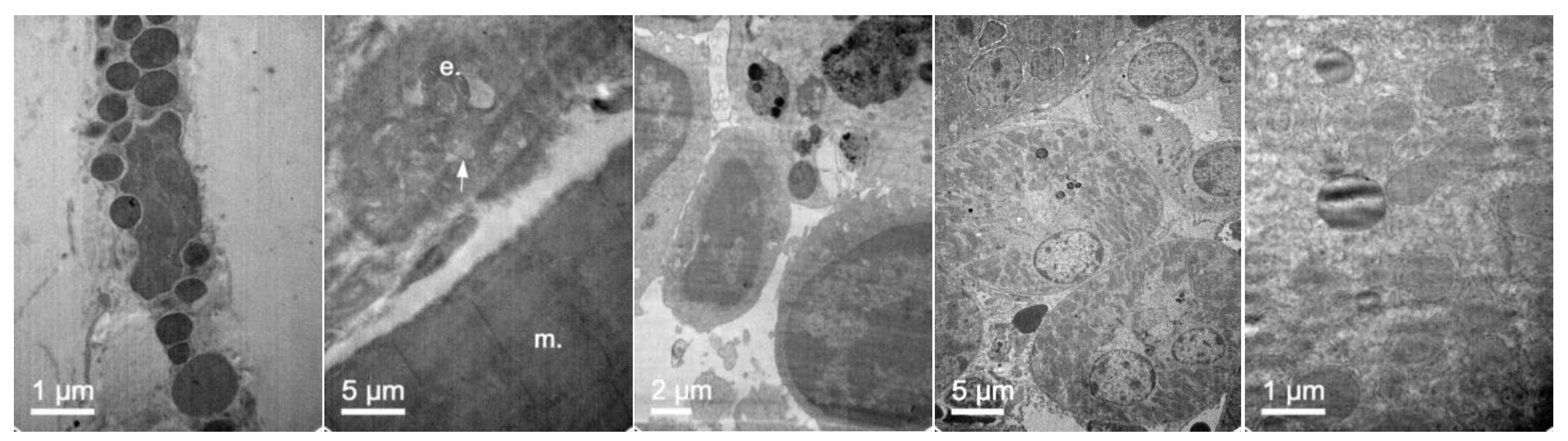
3.7. In Vivo NLC Distribution Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ballabio, A.; Gieselmann, V. Lysosomal disorders: From storage to cellular damage. Biochim. Biophys. Acta 2009, 1793, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Muro, S. New biotechnological and nanomedicine strategies for treatment of lysosomal storage disorders. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, E.F.; Muenzer, J. The mucopolysaccharidoses. In The Metabolic and Molecular Bases of Inherited Disease, 8th ed.; Scriver, C.R., Beaudet, A.L., Sly, W.S., Valle, D., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 3421–3452. [Google Scholar]
- Tomatsu, S.; Orii, K.O.; Vogler, C.; Nakayama, J.; Levy, B.; Grubb, J.H.; Gutierrez, M.A.; Shim, S.; Yamaguchi, S.; Nishioka, T. Mouse model of N-acetylgalactosamine-6-sulfate sulfatase deficiency (Galns-/-) produced by targeted disruption of the gene defective in Morquio A disease. Hum. Mol. Genet. 2003, 12, 3349–3358. [Google Scholar] [CrossRef] [PubMed]
- Sukegawa, K.; Nakamura, H.; Kato, Z.; Tomatsu, S.; Montaño, A.M.; Fukao, T.; Toietta, G.; Tortora, P.; Orii, T.; Kondo, N. Biochemical and structural analysis of missense mutations in N-acetylgalactosamine-6-sulfate sulfatase causing mucopolysaccharidosis IVA phenotypes. Hum. Mol. Genet. 2000, 9, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Sawamoto, K.; Alméciga-Díaz, C.J.; Mason, R.W.; Orii, T.; Tomatsu, S. Mucopolysaccharidosis type IVA: Clinical features, biochemistry, diagnosis, genetics, and treatment. In Mucopolysaccharidoses Update; Tomatsu, S., Ed.; Nova Science Publishers: New York, NY, USA, 2018; pp. 235–272. [Google Scholar]
- Montaño, A.M.; Tomatsu, S.; Gottesman, G.S.; Smith, M.; Orii, T. International Morquio a Registry: Clinical manifestation and natural course of Morquio A disease. J. Inherit. Metab. Dis. 2007, 30, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Peracha, H.; Sawamoto, K.; Averill, L.; Kecskemethy, H.; Theroux, M.; Thacker, M.; Nagao, K.; Pizarro, C.; Mackenzie, W.; Kobayashi, H.; et al. Diagnosis and prognosis of Mucopolysaccharidosis IVA. Mol. Genet. Metab. 2018, 125, 18–37. [Google Scholar] [CrossRef]
- Lavery, C.; Hendriksz, C. Mortality in patients with morquio syndrome A. JIMD Rep. 2015, 15, 59–66. [Google Scholar]
- Yasuda, E.; Fushimi, K.; Suzuki, Y.; Shimizu, K.; Takami, T.; Zustin, J.; Patel, P.; Ruhnke, K.; Shimada, T.; Boyce, B.; et al. Pathogenesis of Morquio A syndrome: An autopsied case reveals systemic storage disorder. Mol. Genet. Metab. 2013, 109, 301–311. [Google Scholar] [CrossRef]
- Khan, S.; Alméciga-Díaz, C.J.; Sawamoto, K.; Mackenzie, W.G.; Theroux, M.C.; Pizarro, C.; Mason, R.W.; Orii, T.; Tomatsu, S. Mucopolysaccharidosis IVA and glycosaminoglycans. Mol. Genet. Metab. 2017, 120, 78–95. [Google Scholar] [CrossRef]
- Khan, S.A.; Mason, R.W.; Giugliani, R.; Orii, K.; Fukao, T.; Suzuki, Y.; Yamaguchi, S.; Kobayashi, H.; Orii, T.; Tomatsu, S. Glycosaminoglycans analysis in blood and urine of mucopolysaccharidoses by tandem mass spectrometry. Mol. Genet. Metab. 2018, 125, 44–52. [Google Scholar] [CrossRef]
- Tomatsu, S.; Montano, A.M.; Dung, V.C.; Ohashi, A.; Oikawa, H.; Oguma, T.; Orii, T.; Barretra, L.; Sly, W.S. Enhancement of drug delivery: Enzyme-replacement therapy for murine Morquio A syndrome. Mol. Ther. 2010, 18, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Millan, J.L.; Narisawa, S.; Lemire, I.; Loisel, T.P.; Boileau, G.; Leonard, P.; Gramatikova, S.; Terkeltaub, R.; Camacho, N.P.; McKee, M.D. Enzyme replacement therapy for murine hypophosphatasia. J. Bone Miner. Res. 2008, 23, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Barton, N.W.; Brady, R.O.; Dambrosia, J.M.; Di Bisceglie, A.M.; Doppelt, S.H.; Hill, S.C.; Mankin, H.J.; Murray, G.J.; Parker, R.I.; Argoff, C.E.; et al. Replacement therapy for inherited enzyme deficiency--macrophage-targeted glucocerebrosidase for Gaucher’s disease. N. Engl. J. Med. 1991, 324, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Musson, D.G.; Schweighardt, B.; Tompkins, T.; Jesaitis, L.; Shaywitz, A.J.; Yang, K.; O’Neill, C.A. Pharmacokinetic and Pharmacodynamic Evaluation of Elosulfase Alfa, an Enzyme Replacement Therapy in Patients with Morquio a Syndrome. Clin. Pharmacokinet. 2014, 53, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, S.; Sawamoto, K.; Shimada, T.; Bober, M.B.; Kubaski, F.; Yasuda, E.; Mason, R.W.; Khan, S.; Alméciga-Díaz, C.J.; Barrera, L.A. Enzyme replacement therapy for treating mucopolysaccharidosis type IVA (Morquio A syndrome): Effect and limitations. Expert Opin. Orphan Drugs 2015, 3, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Tompkins, T.; Decker, C.; Jesaitis, L.; Khan, S.; Slasor, P.; Harmatz, P.; O’Neill, C.A.; Schweighardt, B. Long-term Immunogenicity of Elosulfase Alfa in the Treatment of Morquio a Syndrome: Results From MOR-005, a Phase III Extension Study. Clin. Ther. 2017, 39, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Ponder, K.P. Immune response hinders therapy for lysosomal storage diseases. J. Clin. Investig. 2008, 118, 2686–2689. [Google Scholar] [CrossRef] [PubMed]
- Melton, A.C.; Soon, R.K., Jr.; Tompkins, T.; Long, B.; Schweighardt, B.; Qi, Y.; Vitelli, C.; Bagri, A.; Decker, C.; O’Neill, C.A.; et al. Antibodies that neutralize cellular uptake of elosulfase alfa are not associated with reduced efficacy or pharmacodynamic effect in individuals with Morquio A syndrome. J. Immunol. Methods 2017, 440, 41–51. [Google Scholar] [CrossRef]
- Tomatsu, S.; Yasuda, E.; Patel, P.; Ruhnke, K.; Shimada, T.; Mackenzie, W.G.; Mason, R.; Thacker, M.M.; Theroux, M.; Montaño, A.M.; et al. Morquio A syndrome: Diagnosis and current and future therapies. Pediatr. Endocrinol. Rev. 2014, 12, 141–151. [Google Scholar]
- Chen, H.H.; Kazuki, S.; Robert, W.M.; Hironori, K.; Seiji, Y.; Yasuyuki, S.; Kenji, O.; Tadao, O.; Shunji, T. Enzyme replacement therapy for mucopolysaccharidoses; past, present, and future. J. Hum. Genet. 2019, 64, 1153–1171. [Google Scholar] [CrossRef]
- Doherty, C.; Stapleton, M.; Piechnik, M.; Mason, R.W.; Mackenzie, W.G.; Yamaguchi, S.; Kobayashi, H.; Suzuki, Y.; Tomatsu, S. Effect of enzyme replacement therapy on the growth of patients with Morquio A. J. Hum. Genet. 2019, 64, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Harmatz, P. Enzyme Replacement Therapies and Immunogenicity in Lysosomal Storage Diseases: Is There a Pattern? Clin. Ther. 2015, 37, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Hendriksz, C.J.; Burton, B.; Fleming, T.R.; Harmatz, P.; Hughes, D.; Jones, S.A.; Lin, S.-P.; Mengel, E.; Scarpa, M.; Valayannopoulos, V.; et al. Efficacy and safety of enzyme replacement therapy with BMN 110 (elosulfase alfa) for Morquio A syndrome (mucopolysaccharidosis IVA): A phase 3 randomised placebo-controlled study. J. Inherit. Metab. Dis. 2014, 37, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, S.; Montano, A.M.; Ohashi, A.; Gutierrez, M.A.; Oikawa, H.; Oguma, T.; Dung, V.C.; Nishioka, T.; aOrii, T.; Sly, W.S. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum. Mol. Genet. 2007, 17, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Do Cao, J.; Wiedemann, A.; Quinaux, T.; Battaglia-Hsu, S.F.; Mainard, L.; Froissart, R.; Bonnemains, C.; Ragot, S.; Leheup, B.; Journeau, P.; et al. 30 months follow-up of an early enzyme replacement therapy in a severe Morquio A patient: About one case. Mol. Genet. Metab. Rep. 2016, 9, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Sun, J.; Zhai, Y.; He, Z. The endocytosis and intracellular fate of nanomedicines: Implication for rational design. Asian J. Pharmaceut. Sci. 2013, 8, 1–10. [Google Scholar] [CrossRef]
- Qi, C.; Chen, Y.; Jing, Q.Z.; Wang, X.G. Preparation and characterization of catalase-loaded solid lipid nanoparticles protecting enzyme against proteolysis. Int. J. Mol. Sci. 2011, 12, 4282–4293. [Google Scholar] [CrossRef]
- Battaglia, L.; Trotta, M.; Gallarate, M.; Carlotti, M.E.; Zara, G.P.; Bargoni, A. Solvent lipid nanoparticles formed by solvent-in-water emulsion diffusion technique: Development and influence of insulin stability. J. Microencapsul. 2007, 14, 672–684. [Google Scholar] [CrossRef]
- Gallarate, M.; Trotta, M.; Battaglia, L.; Chirio, D. Preparation of solid lipid nanoparticles from W/O/W emulsions: Preliminary studies on insulin encapsulation. J. Microencapsul. 2009, 26, 394–402. [Google Scholar] [CrossRef]
- Araújo, J.; Gonzalez, E.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Nanomedicines for ocular NSAIDs: Safety on drug delivery. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 394–401. [Google Scholar] [CrossRef]
- Soares, S.; Fonte, P.; Costa, A.; Andrade, J.; Seabra, V.; Ferreira, D.; Reis, S.; Sarmento, B. Effect of freeze-drying, cryoprotectants and storage conditions on the stability of secondary structure of insulin-loaded solid lipid nanoparticles. Int. J. Pharm. 2013, 456, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef]
- Ullal, A.J.; Millington, D.S.; Bali, D.S. Development of a fluorometric microtiter plate based enzyme assay for MPS IVA (Morquio type A) using dried blood spots. Mol. Genet. Metab. Rep. 2014, 1, 461–464. [Google Scholar] [CrossRef] [PubMed]
- MCamelier, V.; Burin, M.G.; de Mari, J.; Vieira, T.A.; Marasca, G.; Giugliani, R. Practical and reliable enzyme test for the detection of mucopolysaccharidosis IVA (Morquio Syndrome type A) in dried blood samples. Clin. Chim. Acta Int. J. Clin. Chem. 2011, 412, 1805–1808. [Google Scholar] [CrossRef] [PubMed]
- Basson, C. Cell Culture Methods for Molecular and Cell Biology: Methods for Preparation of Media, Supplements, and Substrata for Serum-Free Animal Cell Culture, Volume 1; Methods for Serum-Free Culture of Cells of the Endocrine System, Volume 2; Methods for Serum-Free Culture of Epithelial and Fibroblastic Cells, Volume 3; Methods for Serum-Free Culture of Neuronal and Lymphoid Cells, Volume 4. Yale J. Biol. Med. 1985, 58, 198–199. [Google Scholar]
- Mühlstein, A.; Gelperina, S.; Shipulo, E.; Maksimenko, O.; Kreuter, J. Arylsulfatase A Bound to Poly(Butyl cyanoacrylate) Nanoparticles for Enzyme Replacement Therapy – Physicochemical Evaluation. 2014. Available online: https://www.ingentaconnect.com/content/govi/pharmaz/2014/00000069/00000007/art00006;jsessionid=4etwo62drgeu.x-ic-live-02 (accessed on 14 July 2019).
- Clinical Outcomes in a Subpopulation of Adults with Morquio A syndrome: Results from a long-Term Extension Study of elosulfase Alfa|Orphanet Journal of Rare Diseases|Full Text. Available online: https://ojrd.biomedcentral.com/articles/10.1186/s13023-017-0634-0 (accessed on 14 July 2019).
- Montenegro, L.; Castelli, F.; Sarpietro, M.G. Differential Scanning Calorimetry Analyses of Idebenone-Loaded Solid Lipid Nanoparticles Interactions with a Model of Bio-Membrane: A Comparison with In Vitro Skin Permeation Data. Pharmaceuticals 2018, 11, 138. [Google Scholar] [CrossRef]
- Gaba, B.; Fazil, M.; Khan, S.; Ali, A.; Baboota, S.; Ali, J. Nanostructured lipid carrier system for topical delivery of terbinafine hydrochloride. Bull. Fac. Pharm. Cairo Univ. 2015, 53, 147–159. [Google Scholar] [CrossRef]
- Fernández-Ferreiro, A.; Bargiela, N.F.; Varela, M.S.; Martínez, M.G.; Pardo, M.; Ces, A.P.; Méndez, J.B.; Barcia, M.G.; Lamas, M.J.; Otero-Espina, F.J. Cyclodextrin-polysaccharide-based, in situ-gelled system for ocular antifungal delivery. Beilstein J. Org. Chem. 2014, 10, 2903–2911. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Yang, L.; Wei, Y.; Wang, X.; Wang, Z.; Tao, L. Cytotoxicity study of polyethylene glycol derivatives. RSC Adv. 2017, 7, 18252–18259. [Google Scholar] [CrossRef]
| Components (gel core) | % in Formulation (v/v) |
| Kolliphor® P407 | 60 |
| Elosulfase alfa | 40 |
| Components (lipid coating) | % in Formulation (w/v) |
| Glyceril dibehenate | 8.24 |
| Trymiristin | 8.24 |
| Triestearin | 8.24 |
| Cholesterol | 3.84 |
| Olive oil | 32.96 |
| Caprylic/capric tryglicerides | 27.47 |
| Soy lecitin | 10.98 |
| Lipid | M1 | M2 | M3 | M4 | M5 | M6 |
|---|---|---|---|---|---|---|
| Compritol ATO 888 | 25.0 mg | 75.0 mg | - | - | 91.5 mg | 37.0 mg |
| Dynassan 114 | 25.0 mg | - | 37.5 mg | - | 91.5 mg | 25.0 mg |
| Dynassan 118 | 25.0 mg | - | 37.5 mg | - | - | 25.0 mg |
| Cholesterol | 12.0 mg | 12.0 mg | 12.0 mg | - | - | - |
| Olive Oil | 100.0 mg | 100.0 mg | 100.0 mg | 100.0 mg | - | 100.0 mg |
| Miglyol | 33.0 mg | 33.0 mg | 33.0 mg | 33.0 mg | - | 33.0 mg |
| Enzyme Added (μg) | Mean Size (nm) | PdI |
|---|---|---|
| 50 | 169.0 | 0.241 |
| 100 | 174.7 | 0.306 |
| 200 | 173.0 | 0.270 |
| 400 | 175.7 | 0.150 |
| 500 | 192.1 | 0.207 |
| d-α-tocopheryl-polyethylene Glycol 1000 Succinate Concentration (mg/mL) | Mean Size (nm) | PdI | Potential ζ (mv) |
|---|---|---|---|
| 0.1 | 200.8 ± 15 | 0.236 ± 0.04 | −18.7 ± 0.3 |
| 0.2 | 214.5 ± 12 | 0.158 ± 0.03 | −16.3 ± 0.3 |
| 0.3 | 195.8 ± 10 | 0.164 ± 0.02 | −14.2 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, J.V.; Herrero Filgueira, C.; González, A.d.l.F.; Colón Mejeras, C.; Beiras Iglesias, A.; Tomatsu, S.; Blanco Méndez, J.; Luzardo Álvarez, A.; Couce, M.L.; Otero Espinar, F.J. Enzyme-Loaded Gel Core Nanostructured Lipid Carriers to Improve Treatment of Lysosomal Storage Diseases: Formulation and In Vitro Cellular Studies of Elosulfase Alfa-Loaded Systems. Pharmaceutics 2019, 11, 522. https://doi.org/10.3390/pharmaceutics11100522
Álvarez JV, Herrero Filgueira C, González AdlF, Colón Mejeras C, Beiras Iglesias A, Tomatsu S, Blanco Méndez J, Luzardo Álvarez A, Couce ML, Otero Espinar FJ. Enzyme-Loaded Gel Core Nanostructured Lipid Carriers to Improve Treatment of Lysosomal Storage Diseases: Formulation and In Vitro Cellular Studies of Elosulfase Alfa-Loaded Systems. Pharmaceutics. 2019; 11(10):522. https://doi.org/10.3390/pharmaceutics11100522
Chicago/Turabian StyleÁlvarez, J. Víctor, Carolina Herrero Filgueira, Alexandre de la Fuente González, Cristóbal Colón Mejeras, Andrés Beiras Iglesias, Shunji Tomatsu, José Blanco Méndez, Asteria Luzardo Álvarez, María Luz Couce, and Francisco J. Otero Espinar. 2019. "Enzyme-Loaded Gel Core Nanostructured Lipid Carriers to Improve Treatment of Lysosomal Storage Diseases: Formulation and In Vitro Cellular Studies of Elosulfase Alfa-Loaded Systems" Pharmaceutics 11, no. 10: 522. https://doi.org/10.3390/pharmaceutics11100522
APA StyleÁlvarez, J. V., Herrero Filgueira, C., González, A. d. l. F., Colón Mejeras, C., Beiras Iglesias, A., Tomatsu, S., Blanco Méndez, J., Luzardo Álvarez, A., Couce, M. L., & Otero Espinar, F. J. (2019). Enzyme-Loaded Gel Core Nanostructured Lipid Carriers to Improve Treatment of Lysosomal Storage Diseases: Formulation and In Vitro Cellular Studies of Elosulfase Alfa-Loaded Systems. Pharmaceutics, 11(10), 522. https://doi.org/10.3390/pharmaceutics11100522