Spray Drying of a Subcritical Extract Using Marrubium vulgare as a Method of Choice for Obtaining High Quality Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Liquid Extract and Liquid Feed Preparations
2.4. Spray Drying Process and its Efficiency
2.5. Analysis of MVPs Stability Properties
2.5.1. Moisture Content
2.5.2. Hygroscopicity
2.6. Analysis of Mvps Solubility and Wettability Properties
Water Solubility (WSI) and Water Absorption (WAI) Indexes
2.7. Analysis of Mvps Flow Behavior Properties
2.7.1. Bulk Density
2.7.2. Powder Characterization
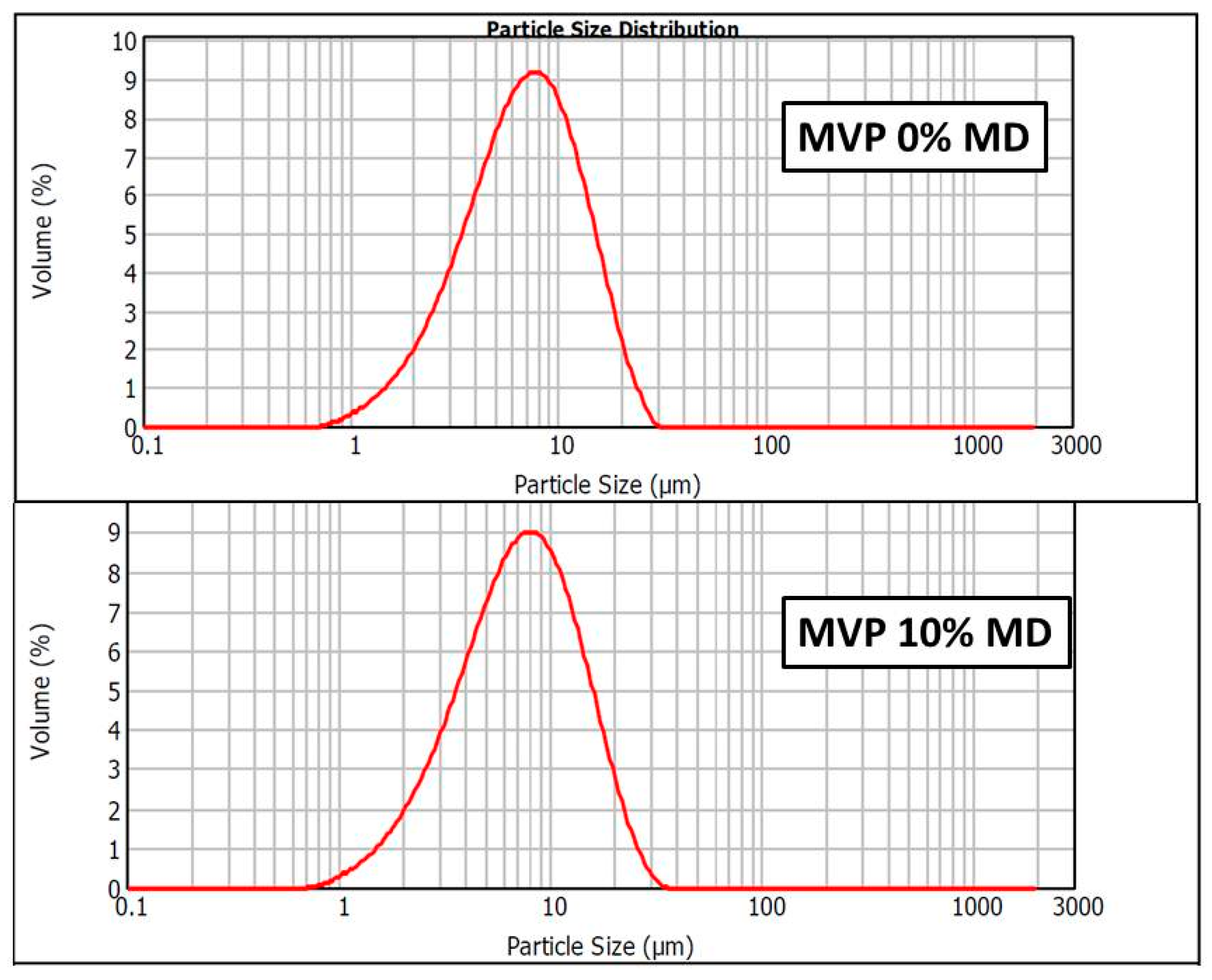
2.7.3. Particle Size Analysis
2.7.4. Morphology-Scanning Electron Microscopy (SEM)
2.8. Analysis of Mvps Crystallographic and Thermal Properties
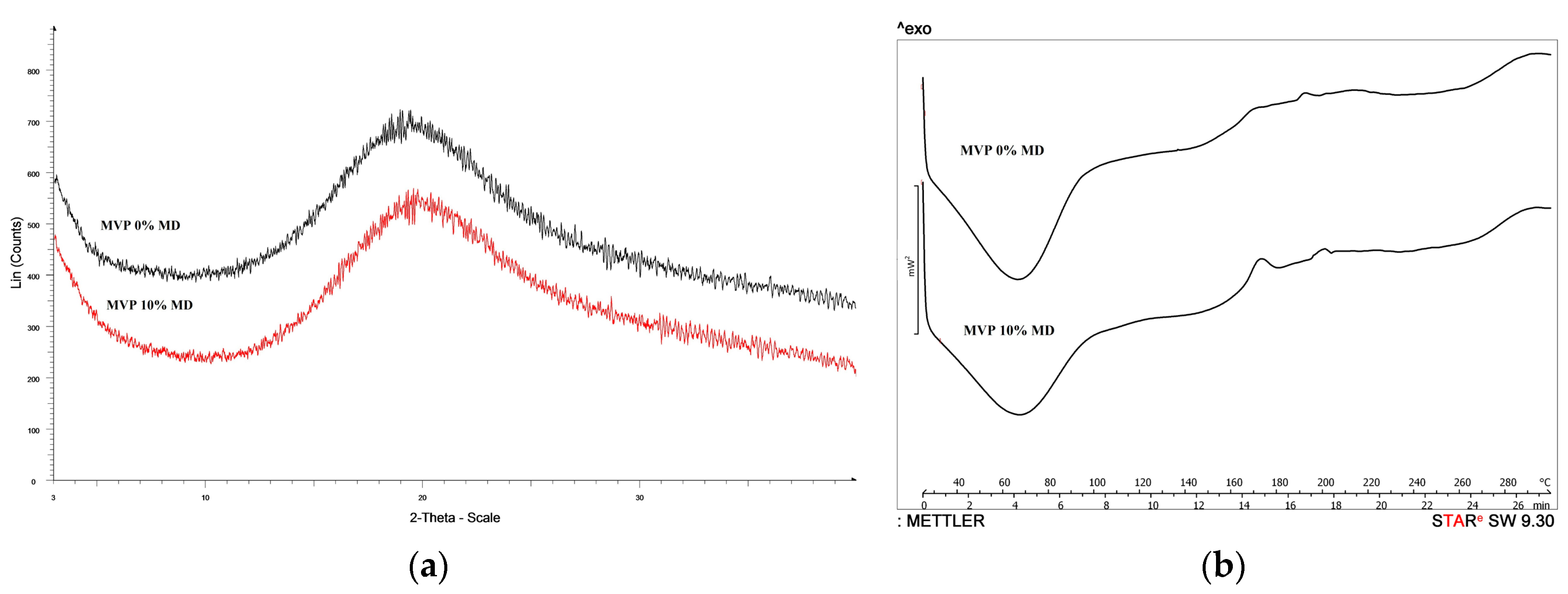
2.8.1. Differential Scanning Calorimetry Analysis (DSC)
2.8.2. X-ray Powder Diffraction Analysis (XRDP)
2.9. Analysis of Mvps Bioactive Compounds
2.9.1. Total Phenol Content
2.9.2. Total Flavonoids Content
2.9.3. DPPH Assay
2.9.4. FRAP Assay
2.9.5. HPLC Analysis
3. Results
3.1. Process Efficiency
3.2. Evaluation of Micrometric Properties and Structure of the Mvps
3.3. Moisture Content and Hygroscopicity
3.4. Water Solubility (WSI) and Water Absorbtion (WAI) Indexes
3.5. MVPs Flow Behavior Properties
3.6. Polyphenol Content in MVPs
3.7. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.-M.; Ştefănescu, R.; Bild, V.; Melnic, S.; et al. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, S.; Vadnere, G.P.; Sharma, V.K.; Usman, M.D.R. Marrubium vulgare L.: A review on phytochemical and pharmacological aspects. J. Intercult. Ethnopharmacol. 2017, 6, 429–452. [Google Scholar] [CrossRef]
- Popoola, O.; Elbagory, A.; Ameer, F.; Hussein, A. Marrubiin. Molecules 2013, 18, 9049–9060. [Google Scholar] [CrossRef] [PubMed]
- Bardai, S.; Morel, N.; Wibo, M.; Fabre, N.; Llabres, G.; Lyoussi, B.; Quetin-Leclercq, J. The vasorelaxant activity of marrubenol and marrubiin from Marrubium vulgare. Planta. Med. 2003, 69, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Pukalskas, A.; Venskutonis, P.R.; Salido, S.; de Waard, P.; van Beek, T.A. Isolation, identification and activity of natural antioxidants from horehound (Marrubium vulgare L.) cultivated in Lithuania. Food Chem. 2012, 130, 695–701. [Google Scholar] [CrossRef]
- Schlemper, V.; Ribas, A.; Nicolau, M.; Cechinel Filho, V. Antispasmodic effects of hydroalcoholic extract of Marrubium vulgare on isolated tissues. Phytomedicine 1996, 3, 211–216. [Google Scholar] [CrossRef]
- De Souza, M.M.; De Jesus, R.A.P.; Cechinel-Filho, V.; Schlemper, V. Analgesic profile of hydroalcoholic extract obtained from Marrubium vulgare. Phytomedicine 1998, 5, 103–107. [Google Scholar] [CrossRef]
- Boudjelal, A.; Henchiri, C.; Siracusa, L.; Sari, M.; Ruberto, G. Compositional analysis and in vivo anti-diabetic activity of wild Algerian Marrubium vulgare L. infusion. Fitoterapia 2012, 83, 286–292. [Google Scholar] [CrossRef]
- Masoodi, M.H.; Ahmed, B.; Zargar, I.M.; Khan, S.A.; Khan, S.; Singh, P. Antibacterial activity of whole plant extract of Marrubium vulgare. Afr. J. Biotechnol. 2008, 7, 86–87. [Google Scholar]
- Quave, C.L.; Pieroni, A.; Bennett, B.C. Dermatological remedies in the traditional pharmacopoeia of Vulture-Alto Bradano, inland southern Italy. J. Ethnobiol. Ethnomed. 2008, 4, 5. [Google Scholar] [CrossRef]
- Vincenzi, M.; Maialetti, F.; Dessi, M.R. Monographs on botanical flavouring substances used in foods. Fitoterapia 1995, 66, 203–210. [Google Scholar]
- Weel, K.G.; Venskutonis, P.R.; Pukalskas, A.; Gruzdiene, D.; Linssen, J.P. Antioxidant activity of horehound (Marrubium vulgare L) grown in Lithuania. Eur. J. Lipid Sci. Technol. 1999, 101, 395–399. [Google Scholar] [CrossRef]
- Smith, T.; Lynch, M.E.; Johnson, J.; Kawa, K.; Bauman, H.; Blumenthal, M. Herbal and dietary supplement sales in the US increase 6/8%. Available online: http://cms.herbalgram.org/press/2015/HerbalDietarySupplementSalesinUSRisein2014.html?ts=1565174083&signature=733188db45b6e9f353c16f5df00ec28a (accessed on 7 August 2019).
- Vladić, J.; Ambrus, R.; Szabó-Révész, P.; Vasić, A.; Cvejin, A.; Pavlić, B.; Vidović, S. Recycling of filter tea industry by-products: Production of A. millefolium powder using spray drying technique. Ind. Crops Prod. 2016, 80, 197–206. [Google Scholar] [CrossRef]
- Naffati, A.; Vladić, J.; Pavlić, B.; Radosavljević, R.; Gavarić, A.; Vidović, S. Recycling of filter tea industry by-products: Application of subcritical water extraction for recovery of bioactive compounds from A. uva-ursi herbal dust. J. Supercrit. Fluids 2017, 121, 1–9. [Google Scholar] [CrossRef]
- Ju, Y.H.; Huynh, L.H.; Kasim, N.S.; Guo, T.J.; Wang, J.H.; Fazary, A.E. Analysis of soluble and insoluble fractions of alkali and subcritical water treated sugarcane bagasse. Carbohydr. Polym. 2011, 83, 591–599. [Google Scholar] [CrossRef]
- Zeković, Z.; Vidović, S.; Vladić, J.; Radosavljević, R.; Cvejin, A.; Elgndi, M.A.; Pavlić, B. Optimization of subcritical water extraction of antioxidants from Coriandrum sativum seeds by response surface methodology. J. Supercrit. Fluids 2014, 95, 560–566. [Google Scholar] [CrossRef]
- Phoungchandang, S.; Sertwasana, A. Spray-drying of ginger juice and physicochemical properties of ginger powders. Science Asia 2010, 36, 40–45. [Google Scholar] [CrossRef]
- Carr, R.L. Evaluating flow properties of solids. Chem. Eng. 1965, 72, 163–168. [Google Scholar]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Markham, K.R. Techniques of Flavonoid Identification; Academic Press: London, UK, 1982; Volume 31. [Google Scholar]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2, 2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Zhou, J.H.; Zeng, Y.L.; Ouyang, X.L. The enhancement and encapsulation of Agaricus bisporus flavor. J. Food Eng. 2004, 65, 391–396. [Google Scholar] [CrossRef]
- Keshani, S.; Daud, W.R.W.; Nourouzi, M.M.; Namvar, F.; Ghasemi, M. Spray drying: An overview on wall deposition, process and modeling. J. Food Eng. 2015, 146, 152–162. [Google Scholar] [CrossRef]
- Roos, Y.H. Glass transition temperature and its relevance in food processing. Annu. Rev. Food Sci. Technol. 2010, 1, 469–496. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.K.; Ua-Arak, T.; Adhikari, B.P.; Howes, T.; Bhandari, B.R. Glass transition behavior of spray dried orange juice powder measured by differential scanning calorimetry (DSC) and thermal mechanical compression test (TMCT). Int. J. Food Prop. 2007, 10, 661–673. [Google Scholar] [CrossRef]
- Ozmen, L.T.A.G.; Langrish, T.A.G. A study of the limitations to spray dryer outlet performance. Drying Technol. 2003, 21, 895–917. [Google Scholar] [CrossRef]
- Moyler, D.A. Extraction of Flavours and Fragrances. In Extraction of Natural Products Using Near-Critical Solvents; King, M.B., Bott, T.R., Eds.; Blackie Academic and Professional: Glasgow, UK, 1993. [Google Scholar]
- Leeke, G.; Gaspar, F.; Santos, R. Influence of water on the extraction of essential oils from a model herb using supercritical carbon dioxide. Ind. Eng. Chem. Res. 2002, 41, 2033–2039. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Ramos, L.; Kristenson, E.M.; Brinkman, U.T. Current use of pressurized liquid extraction and subcritical water extraction in environmental analysis. J. Chromatogr. A 2002, 975, 3–29. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Datta, N.; Howes, T. Problems associated with spray drying of sugar-rich foods. Drying Technol. 1997, 15, 671–684. [Google Scholar] [CrossRef]
- Young, S.L.; Sarda, X.; Rosenberg, M. Microencapsulating properties of whey proteins. 1. Microencapsulation of anhydrous milk fat. J. Dairy Sci. 1993, 76, 2868–2877. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation efficiency of food flavours and oils during spray drying. Drying Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Phisut, N. Spray drying technique of fruit juice powder: Some factors influencing the properties of product. Int. Food Res. J. 2012, 19, 1297–1306. [Google Scholar]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Hartel, R.W. Phase transitions during food powder production and powder stability. In Encapsulated and Powdered Foods; CRC Press: Boca Raton, FL, USA, 2005; pp. 273–304. [Google Scholar]
- Levine, H.; Slade, L. A polymer physico-chemical approach to the study of commercial starch hydrolysis products (SHPs). Carbohyd. Polym. 1986, 6, 213–244. [Google Scholar] [CrossRef]
- Roos, Y. Characterization of food polymers using state diagrams. J. Food Eng. 1995, 24, 339–360. [Google Scholar] [CrossRef]
- Mani, S.; Jaya, S.; Das, H. Sticky issues on spray drying of fruit juices. In Proceedings of the ASAE/CSAE North-Central Intersectional Meeting, Saskatoon, SK, Canada, 27–28 September 2002; pp. 1–18. [Google Scholar]
- Vidović, S.S.; Vladić, J.Z.; Vaštag, Ž.G.; Zeković, Z.P.; Popović, L.M. Maltodextrin as a carrier of health benefit compounds in Satureja montana dry powder extract obtained by spray drying technique. Powder Technol. 2014, 258, 209–215. [Google Scholar] [CrossRef]
- Sinija, V.R.; Mishra, H.N.; Bal, S. Process technology for production of soluble tea powder. J. Food Eng. 2007, 82, 276–283. [Google Scholar] [CrossRef]
- Chang, Y.X.; Yang, J.J.; Pan, R.L.; Chang, Q.; Liao, Y.H. Anti-hygroscopic effect of leucine on spray-dried herbal extract powders. Powder Technol. 2014, 266, 388–395. [Google Scholar] [CrossRef]
- Angel, R.C.M.; Espinosa-Muñoz, L.C.; Aviles-Aviles, C.; González-García, R.; Moscosa-Santillán, M.; Grajales-Lagunes, A.; Abud-Archila, M. Spray-drying of passion fruit juice using lactose-maltodextrin blends as the support material. Braz. Arch. Boil. Technol. 2009, 52, 1011–1018. [Google Scholar] [CrossRef]
- Hogekamp, S.; Schubert, H. Rehydration of food powders. Food Sci. Technol. Int. 2003, 9, 223–235. [Google Scholar] [CrossRef]
- Schubert, H. Instantization of powdered food products. Int. Chem. Eng. 1993, 33, 28–45. [Google Scholar]
- Legako, J.; Dunford, N.T. Effect of spray nozzle design on fish oil–whey protein microcapsule properties. J. Food Sci. 2010, 75, E394–E400. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Chen, W. Trends of spray drying: A critical review on drying of fruit and vegetable juices. Trends Food Sci. Technol. 2017, 65, 49–67. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Corke, H. Production and properties of spray-dried amaranthus betacyanin pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Tze, N.L.; Han, C.P.; Yusof, Y.A.; Ling, C.N.; Talib, R.A.; Taip, F.S.; Aziz, M.G. Physicochemical and nutritional properties of spray-dried pitaya fruit powder as natural colorant. Food Sci. Biotechnol. 2012, 21, 675–682. [Google Scholar] [CrossRef]
- Yousefi, S.; Emam-Djomeh, Z.; Mousavi, S.M. Effect of carrier type and spray drying on the physicochemical properties of powdered and reconstituted pomegranate juice (Punica Granatum L.). J. Food Sci. Technol. 2011, 48, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Peleg, M. Physical characteristics of food powders. In Physical Properties of Foods; Peleg, M., Bagley, E., Eds.; AVI: New York, NY, USA, 1983; pp. 293–323. [Google Scholar]
- Bennick, A. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral. Biol. Med. 2002, 13, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768. [Google Scholar] [CrossRef] [PubMed]
- Couto, R.O.; Conceição, E.C.; Chaul, L.T.; Oliveira, E.M.; Martins, F.S.; Bara, M.T.F.; Rezende, K.R.; Alves, S.F.; Paula, J.R. Spray-dried rosemary extracts: Physicochemical and antioxidant properties. Food Chem. 2012, 131, 99–105. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Vidaković, A.; Vidović, S.; Velićanski, A.; Versari, A.; Radosavljević, R.; Zeković, Z. Sage processing from by-product to high quality powder: I. Bioactive potential. Ind. Crops Prod. 2017, 107, 81–89. [Google Scholar] [CrossRef]
- Donovan, J.L.; Bell, J.R.; Kasim-Karakas, S.; German, J.B.; Walzem, R.L.; Hansen, R.J.; Waterhouse, A.L. Catechin is present as metabolites in human plasma after consumption of red wine. J. Nutr. 1999, 129, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Kosaraju, S.L.; Labbett, D.; Emin, M.; Konczak, I.; Lundin, L. Delivering polyphenols for healthy ageing. Nutr. Diet. 2008, 65, S48–S52. [Google Scholar] [CrossRef]

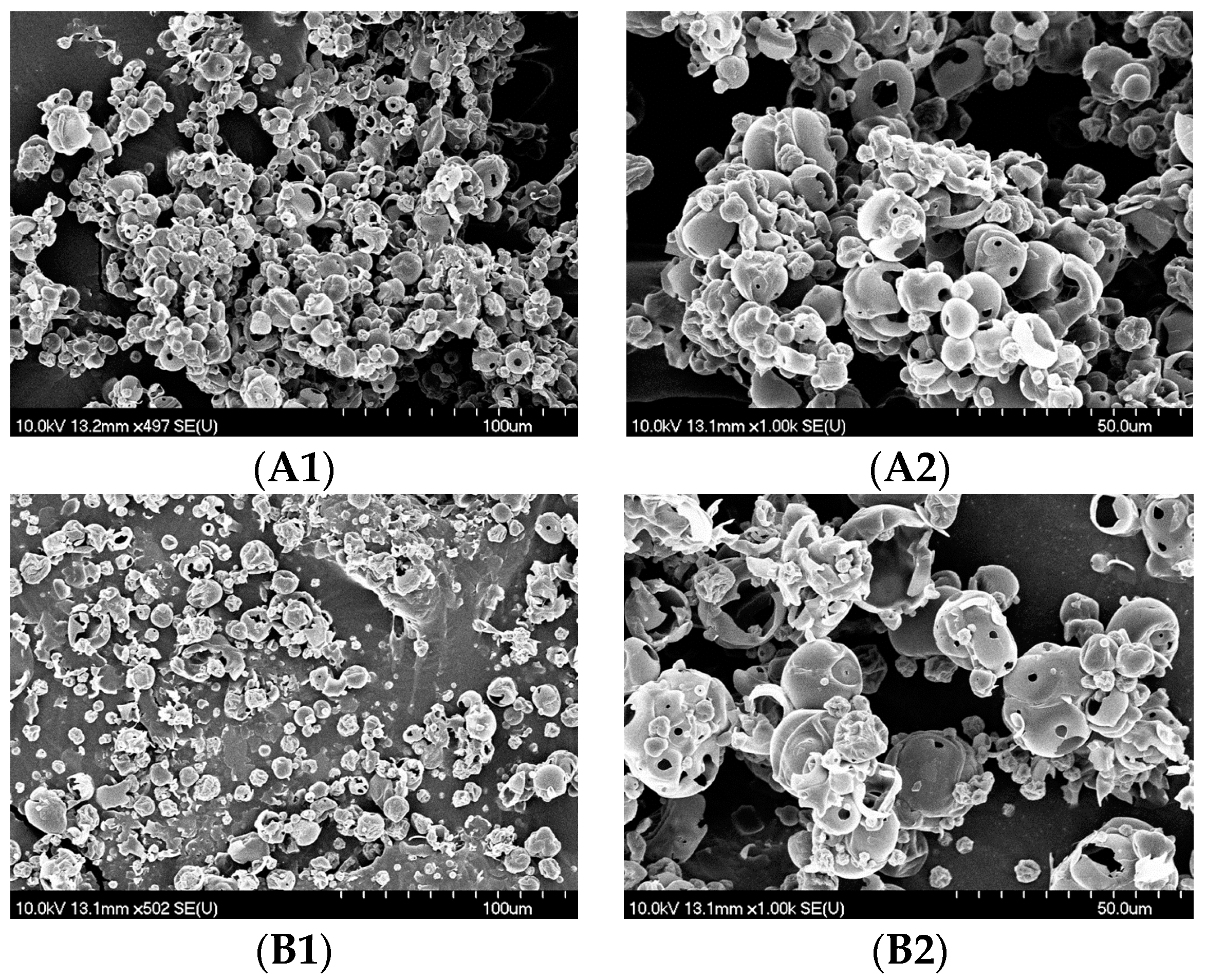
| Sample | Total Solids [mg/mL] | Amount of Added Maltodextrin [g] | Volume of Spray Dried Liquid Feed [L] | Tinlet [°C] | Toutlet [°C] |
|---|---|---|---|---|---|
| MVP 0% MD | 40.90 | 0 | 2 | 130 ± 5 | 75–80 |
| MVP 10% MD | 44.99 | 8.18 | 2 | 130 ± 5 | 75–80 |
| Sample | Average Value | Length [μm] | Width [μm] | Perimeter [μm] | Area [μm2] | Roundness |
|---|---|---|---|---|---|---|
| MVP 0% MD | Average | 4.43 | 3.57 | 15.49 | 14.40 | 1.33 |
| SD± | 0.12 | 0.38 | 0.99 | 1.17 | 0.07 | |
| MVP 10% MD | Average | 6.94 | 4.37 | 21.60 | 23.70 | 1.55 |
| SD± | 2.65 | 1.60 | 6.99 | 12.92 | 0.44 |
| Sample | D 0.1 [μm] | D 0.5 [μm] | D 0.9 [μm] | SSA |
|---|---|---|---|---|
| MVP 0% MD | 2.700 | 6.920 | 14.840 | 1.150 |
| MVP 10% MD | 2.791 | 7.252 | 15.882 | 1.100 |
| Powder Properties | MVP 0% MD | MVP 10% MD |
|---|---|---|
| Moisture content (%) | 4.41 | 3.29 |
| Hygroscopicity after 48 h (%) | 21.12 | 19.83 |
| WSI (%) | 93.18 | 91.19 |
| WAI (%) | 1.80 | 1.97 |
| Sample | Carr Index (%) | Hausner Ratio | Flow Character |
|---|---|---|---|
| MVP 0% MD | 15.01 | 1.18 | Good/free flow |
| MVP 10% MD | 23.23 | 1.30 | Passable/cohesive |
| Sample | Total Solids [mg/mL] | TP [mg GAE/g] | TF [mg CE/g] | IC50 [mg/mL] | EC50 [mg/mL] |
|---|---|---|---|---|---|
| MVP 0% MD | 43.7 | 85.1975 | 31.3668 | 0.0204 | 0.0708 |
| MVP 10% MD | 52.8 | 72.9810 | 26.5851 | 0.0188 | 0.0756 |
| Sample | Ferulic Acid | p-Coumaric Acid | Caffeic Acid | Rutin | Hyperoside |
|---|---|---|---|---|---|
| 0% MD MVP | 48.77 | 26.42 | 14.27 | 134.46 | 17.43 |
| 10% MD MVP | 70.69 | 49.61 | 20.96 | 584.55 | 33.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavarić, A.; Vladić, J.; Ambrus, R.; Jokić, S.; Szabó-Révész, P.; Tomić, M.; Blažić, M.; Vidović, S. Spray Drying of a Subcritical Extract Using Marrubium vulgare as a Method of Choice for Obtaining High Quality Powder. Pharmaceutics 2019, 11, 523. https://doi.org/10.3390/pharmaceutics11100523
Gavarić A, Vladić J, Ambrus R, Jokić S, Szabó-Révész P, Tomić M, Blažić M, Vidović S. Spray Drying of a Subcritical Extract Using Marrubium vulgare as a Method of Choice for Obtaining High Quality Powder. Pharmaceutics. 2019; 11(10):523. https://doi.org/10.3390/pharmaceutics11100523
Chicago/Turabian StyleGavarić, Aleksandra, Jelena Vladić, Rita Ambrus, Stela Jokić, Piroska Szabó-Révész, Milan Tomić, Marijana Blažić, and Senka Vidović. 2019. "Spray Drying of a Subcritical Extract Using Marrubium vulgare as a Method of Choice for Obtaining High Quality Powder" Pharmaceutics 11, no. 10: 523. https://doi.org/10.3390/pharmaceutics11100523
APA StyleGavarić, A., Vladić, J., Ambrus, R., Jokić, S., Szabó-Révész, P., Tomić, M., Blažić, M., & Vidović, S. (2019). Spray Drying of a Subcritical Extract Using Marrubium vulgare as a Method of Choice for Obtaining High Quality Powder. Pharmaceutics, 11(10), 523. https://doi.org/10.3390/pharmaceutics11100523











