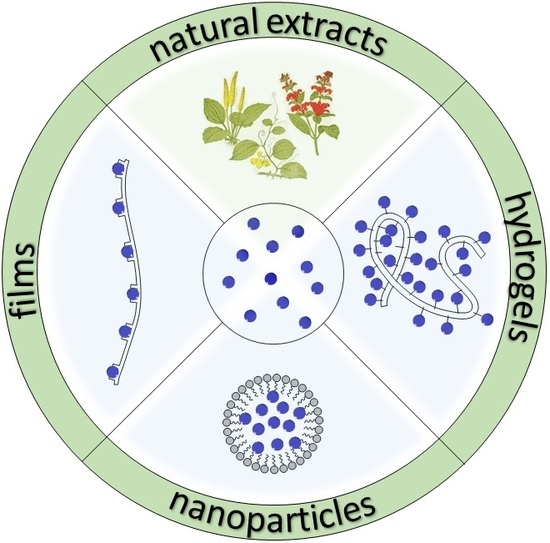
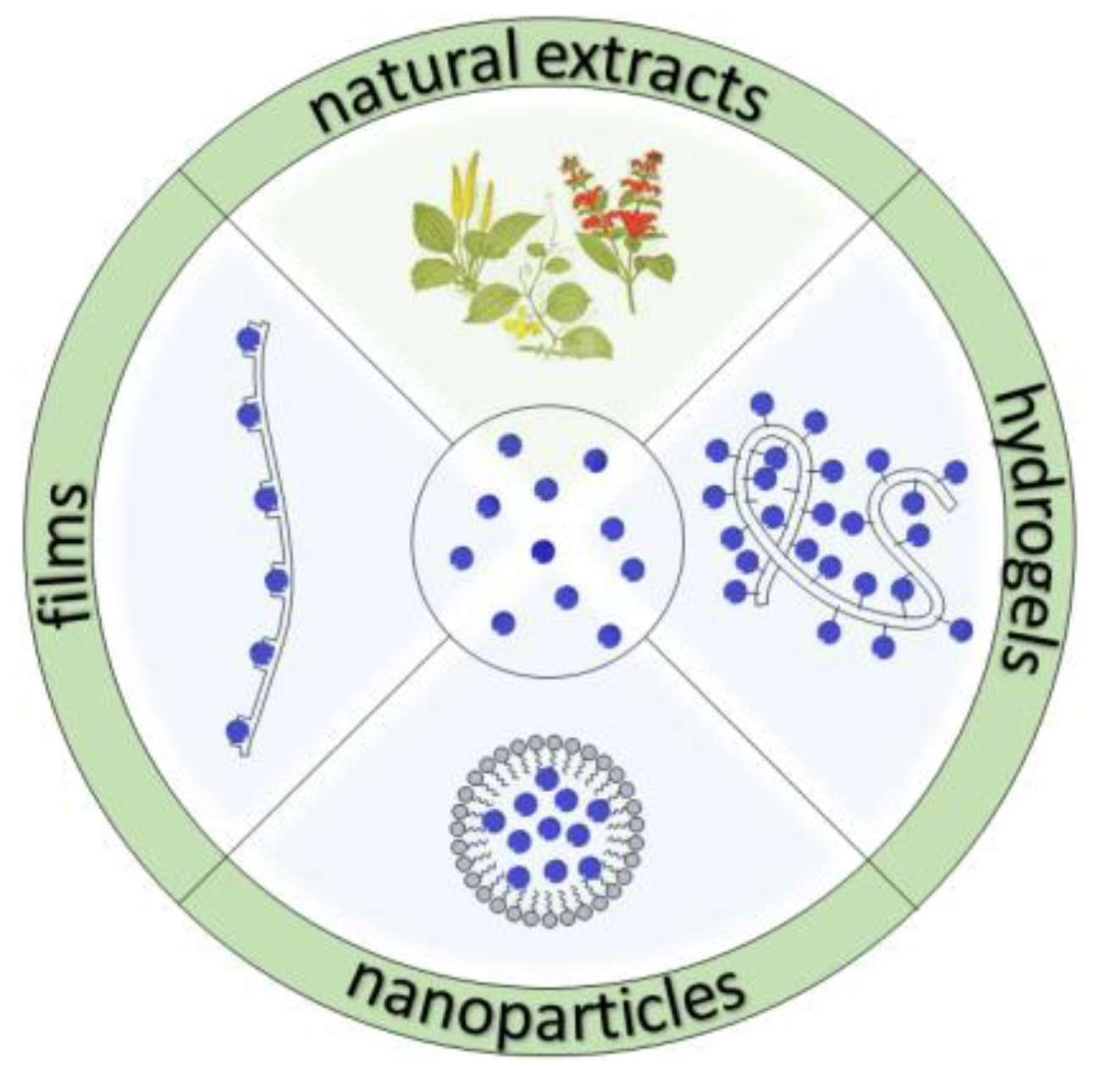
Recent Developments in the Reduction of Oxidative Stress through Antioxidant Polymeric Formulations
Abstract
1. Introduction
2. Polymeric Formulations
2.1. Natural Extracts
2.2. Films
2.3. Hydrogels
2.4. Nanoparticles
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Venkataraman, K.; Khurana, S.; Tai, T.C. Oxidative stress in aging—Matters of the heart and mind. Int. J. Mol. Sci. 2013, 14, 17897–17925. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal. Transduct. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, S.; Sheikholeslami-Vatani, D. Oxidative stress and anti-oxidant responses to regular resistance training in young and older adult women. Geriatr. Gerontol. Int. 2019, 19, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 2014, 42, S125–S152. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Khandrika, L.; Kumar, B.; Koul, S.; Maroni, P.; Koul, H.K. Oxidative stress in prostate cancer. Cancer Lett. 2009, 282, 125–136. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M.; Schmidt, A.M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.-K.; Ju, W.; Cho, B.; Oh, S.-W.; Park, S.M.; Koo, B.-K.; Park, B.-J. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: Systematic review and meta-analysis of randomised controlled trials. J. Cancer Prev. 2013, 18, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, C. Antioxidant supplements to prevent mortality. JAMA 2013, 310, 1178–1179. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Li, J.; Yuan, Z. Effect of antioxidant vitamin supplementation on cardiovascular outcomes: A meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e56803. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Vari, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Zaki, N.M. Strategies for oral delivery and mitochondrial targeting of CoQ10. Drug Deliv. 2016, 23, 1868–1881. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Jung, H.; Li, X. Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges. AAPS J. 2015, 17, 1327–1340. [Google Scholar] [CrossRef]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Ghosh, T.; Sinha, S.; Chattopadhyay, K.; Karmakar, P.; Ray, B. Polysaccharides from Turbinaria conoides: Structural features and antioxidant capacity. Food Chem. 2010, 118, 823–829. [Google Scholar] [CrossRef]
- Li, M.; Xu, Y.; Yang, W.; Li, J.; Xu, X.; Zhang, X.; Chen, F.; Li, D. In vitro synergistic anti-oxidant activities of solvent-extracted fractions from Astragalus membranaceus and Glycyrrhiza uralensis. LWT-Food Sci. Technol. 2011, 44, 1745–1751. [Google Scholar] [CrossRef]
- Chatterjee, U.R.; Bandyopadhyay, S.S.; Ghosh, D.; Ghosal, P.K.; Ray, B. In vitro anti-oxidant activity, fluorescence quenching study and structural features of carbohydrate polymers from Phyllanthus emblica. Int. J. Biol. Macromol. 2011, 49, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Das, A.; Spieldenner, J.; Rink, C.; Roy, S. Supplementation of a standardized extract from Phyllanthus emblica improves cardiovascular risk factors and platelet aggregation in overweight/class-1 obese adults. J. Med. Food. 2015, 18, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Jahanbin, K. Structural characterization of a new water-soluble polysaccharide isolated from Acanthophyllum acerosum roots and its antioxidant activity. Int. J. Biol. Macromol. 2018, 107, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Guan, X.Y.; Yang, W.Z.; Liu, K.D.; Ye, M.; Sun, C. Antioxidant flavonoids from Epimedium wushanense. Fitoterapia 2012, 83, 44–48. [Google Scholar] [CrossRef]
- Cheng, H.; Feng, S.; Jia, X.; Li, Q.; Zhou, Y.; Ding, C. Structural characterization and antioxidant activities of polysaccharides extracted from Epimedium acuminatum. Carbohydr. Polym. 2013, 92, 63–68. [Google Scholar] [CrossRef]
- Bensky, D.; Gamble, A. Chinese Herbal Medicine: Materia Medica, Revised ed.; Eastland Press: Seattle, WA, USA, 1993. [Google Scholar]
- Wang, Q.; Sun, Y.; Yang, B.; Wang, Z.; Liu, Y.; Cao, Q.; Sun, X.; Kuang, H. Optimization of polysaccharides extraction from seeds of Pharbitis nil and its anti-oxidant activity. Carbohydr. Polym. 2014, 102, 460–466. [Google Scholar] [CrossRef]
- Shu, G.W.; He, Y.X.; Lei, N.; Cao, J.L.; Chen, H.; Chen, L. Cellulase-Assisted Extraction of Polysaccharides from White Hyacinth Bean: Characterization of Antioxidant Activity and Promotion for Probiotics Proliferation. Molecules 2017, 22, 1764. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta Glucan: Supplement or Grug? From Laboratory to Clinical Trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef]
- Kofuji, K.; Aoki, A.; Tsubaki, K.; Konishi, M.; Isobe, T.; Murata, Y. Antioxidant Activity of β-Glucan. ISRN Pharm. 2011, 2012, 5. [Google Scholar] [CrossRef]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, antibacterial and in vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.B.; Madyastha, K.M. C-Phycocyanin: A Potent Peroxyl Radical Scavenger in Vivo and in Vitro. Biochem. Biophys. Res. Commun. 2000, 275, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, S.; Benvenuti, F.; Pagliarani, S.; Francogli, S.; Scoglio, S.; Canestrari, F. Antioxidant properties of a novel phycocyanin extract from the blue-green alga Aphanizomenon flos-aquae. Life Sci. 2004, 75, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Mishra, S.; Ghosh, P.K. Antioxidant potential of C-phycocyanin isolated from cyanobacterial species Lyngbya, Phormidium and Spirulina spp. Indian J. Biochem. Biophys 2006, 43, 25–31. [Google Scholar]
- Pleonsil, P.; Soogarun, S.; Suwanwong, Y. Anti-oxidant activity of holo- and apo-c-phycocyanin and their protective effects on human erythrocytes. Int. J. Biol. Macromol. 2013, 60, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; Yang, H.; Chen, Y.T.; Li, P.P. Biosynthesis of Fluorescent β Subunits of C-Phycocyanin from Spirulina subsalsa in Escherichia coli, and Their Antioxidant Properties. Molecules 2018, 23, 1369. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.J.; Zhou, W.; Guo, J.; Nie, Z.W.; Shin, K.T.; Kim, N.H.; Lv, W.F.; Cui, X.S. C-Phycocyanin protects against mitochondrial dysfunction and oxidative stress in parthenogenetic porcine embryos. Sci. Rep. 2017, 7, 16992. [Google Scholar] [CrossRef] [PubMed]
- Park, W.S.; Kim, H.J.; Li, M.; Lim, D.H.; Kim, J.; Kwak, S.S.; Kang, C.M.; Ferruzzi, M.G.; Ahn, M.J. Two Classes of Pigments, Carotenoids and C-Phycocyanin, in Spirulina Powder and Their Antioxidant Activities. Molecules 2018, 23, 2065. [Google Scholar] [CrossRef]
- Frankel, E.N. Antioxidants in lipid foods and their impact on food quality. Food Chem. 1996, 57, 51–55. [Google Scholar] [CrossRef]
- Tomida, H.; Fujii, T.; Furutani, N.; Michihara, A.; Yasufuku, T.; Akasaki, K.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Anraku, M. Antioxidant properties of some different molecular weight chitosans. Carbohydr. Res. 2009, 344, 1690–1696. [Google Scholar] [CrossRef]
- Alsharabasy, A.M. Semi-synthesis of chitosan with high molecular weight and enhanced deacetylation degree. Polym. Sci. 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Hromis, N.; Lazic, V.; Popovic, S.; Markov, S.; Vastag, Z.; Suput, D.; Tomovic, V. Investigation of a product-specific active packaging material based on chitosan biofilm with spice oleoresins. J. Food Nutr. Res. 2016, 55, 78–88. [Google Scholar]
- Qin, Y.Y.; Zhang, Z.H.; Li, L.; Yuan, M.L.; Fan, J.; Zhao, T.R. Physio-mechanical properties of an active chitosan film incorporated with montmorillonite and natural antioxidants extracted from pomegranate rind. J. Food Sci. Technol. 2015, 52, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Lv, H.; Yang, B.; Chen, X.; Sun, H. Physical Properties, Antioxidant and Antimicrobial Activity of Chitosan Films Containing Carvacrol and Pomegranate Peel Extract. Molecules 2015, 20, 11034–11045. [Google Scholar] [CrossRef]
- Bozic, M.; Gorgieva, S.; Kokol, V. Laccase-mediated functionalization of chitosan by caffeic and gallic acids for modulating antioxidant and antimicrobial properties. Carbohydr. Polym. 2012, 87, 2388–2398. [Google Scholar] [CrossRef]
- Joana, T.M.; Miguel, A.C.; Antonio, A.V. Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocoll. 2012, 27, 220–227. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crops Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, P.F.; Duarte, M.P.; Fernando, A.L. Antioxidant Migration Studies in Chitosan Films Incorporated with Plant Extracts. J. Renew. Mater. 2018, 6, 548–558. [Google Scholar] [CrossRef]
- Cao, T.L.; Yang, S.-Y.; Song, K.B. Development of Burdock Root Inulin/Chitosan Blend Films Containing Oregano and Thyme Essential Oils. Int. J. Mol. Sci. 2018, 19, 131. [Google Scholar] [CrossRef]
- Shahid-ul-Islam; Rather, L.J.; Mohammad, F. Phytochemistry, biological activities and potential of annatto in natural colorant production for industrial applications—A review. J. Adv. Res. 2016, 7, 499–514. [Google Scholar] [CrossRef]
- Afonso, C.R.; Hirano, R.S.; Gaspar, A.L.; Chagas, E.G.L.; Carvalho, R.A.; Silva, F.V.; Leonardi, G.R.; Lopes, P.S.; Silva, C.F.; Yoshida, C.M.P. Biodegradable antioxidant chitosan films useful as an anti-aging skin mask. Int. J. Biol. Macromol. 2019, 132, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Angellier, H.; Molina, B.S.; Dole, P.; Dufresne, A. Thermoplastic starch–waxy maize starch nanocrystals nanocomposites. Biomacromolecules 2006, 7, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Talón, E.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of the incorporation of antioxidants on physicochemical and antioxidant properties of wheat starch–chitosan films. J. Food Eng. 2013, 118, 271–278. [Google Scholar] [CrossRef]
- Kim, S.; Baek, S.-K.; Go, E.; Song, K.B. Application of Adzuki Bean Starch in Antioxidant Films Containing Cocoa Nibs Extract. Polymers 2018, 10, 1210. [Google Scholar] [CrossRef] [PubMed]
- Witzler, M.; Alzagameem, A.; Bergs, M.; Khaldi-Hansen, B.; Klein, S.; Hielscher, D.; Kamm, B.; Kreyenschmidt, J.; Tobiasch, E.; Schulze, M. Lignin-Derived Biomaterials for Drug Release and Tissue Engineering. Molecules 2018, 23, 1885. [Google Scholar] [CrossRef] [PubMed]
- Arshanitsa, A.; Ponomarenko, J.; Dizhbite, T.; Andersone, A.; Richard, J.A.G.; Jacinta, V.D.P.; Lauberts, M.; Telysheva, G. Fractionation of technical lignins as a tool for improvement of their antioxidant properties. J. Anal. Appl. Pyrol. 2013, 103, 78–85. [Google Scholar] [CrossRef]
- Li, P.; Sirviö, J.A.; Haapala, A.; Khakalo, A.; Liimatainen, H. Anti-oxidative and UV-absorbing biohybrid film of cellulose nanofibrils and tannin extract. Food Hydrocoll. 2019, 92, 208–217. [Google Scholar] [CrossRef]
- Ruan, C.; Zhang, Y.; Wang, J.; Sun, Y.; Gao, X.; Xiong, G.; Liang, J. Preparation and antioxidant activity of sodium alginate and carboxymethyl cellulose edible films with epigallocatechin gallate. Int. J. Biol. Macromol. 2019, 134, 1038–1044. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, J.; Wang, H.; Song, T.; Hu, W.; Li, S. Preparation and Characterization of Antioxidative and UV-Protective Larch Bark Tannin/PVA Composite Membranes. Molecules 2018, 23, 2073. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Arrigo, R.; Baiamonte, M.; Rizzarelli, P.; Curcuruto, G. Concentration-dependent anti-/pro-oxidant activity of natural phenolic compounds in bio-polyesters. Polym. Degrad. Stab. 2017, 142, 21–28. [Google Scholar] [CrossRef]
- Ge, L.; Zhu, M.; Li, X.; Xu, Y.; Ma, X.; Shi, R.; Li, D.; Mu, C. Development of active rosmarinic acid-gelatin biodegradable films with antioxidant and long-term antibacterial activities. Food Hydrocoll. 2018, 83, 308–316. [Google Scholar] [CrossRef]
- Garcia-Orue, I.; Santos-Vizcaino, E.; Etxabide, A.; Uranga, J.; Bayat, A.; Guerrero, P.; Igartua, M.; de la Caba, K.; Hernandez, R.M. Development of Bioinspired Gelatin and Gelatin/Chitosan Bilayer Hydrofilms for Wound Healing. Pharmaceutics 2019, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, L. A Natural Antibacterial-Antioxidant Film from Soy Protein Isolate Incorporated with Cortex Phellodendron Extract. Polymers 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, N.; Kumar, N.; Kumar, M. Hydrogels for pharmaceutical and biomedical applications. Crit. Rev. Ther. Drug Carr. Syst. 2005, 22, 107–149. [Google Scholar] [CrossRef]
- Wattamwar, P.P.; Biswal, D.; Cochran, D.B.; Lyvers, A.C.; Eitel, R.E.; Anderson, K.W.; Hilt, J.Z.; Dziubla, T.D. Synthesis and characterization of poly(antioxidant β-amino esters) for controlled release of polyphenolic antioxidants. Acta Biomater. 2012, 8, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Authimoolam, S.P.; Hilt, J.Z.; Dziubla, T.D. Quercetin conjugated poly(β-amino esters) nanogels for the treatment of cellular oxidative stress. Acta Biomater. 2015, 27, 194–204. [Google Scholar] [CrossRef]
- Gupta, P.; Jordan, C.T.; Mitov, M.I.; Butterfield, D.A.; Hilt, J.Z.; Dziubla, T.D. Controlled curcumin release via conjugation into PBAE nanogels enhances mitochondrial protection against oxidative stress. Int. J. Pharm. 2016, 511, 1012–1021. [Google Scholar] [CrossRef]
- Lakes, A.L.; Jordan, C.T.; Gupta, P.; Puleo, D.A.; Hilt, J.Z.; Dziubla, T.D. Reducible disulfide poly(beta-amino ester) hydrogels for antioxidant delivery. Acta Biomater. 2018, 68, 178–189. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C.; Aktas, N.; Turk, M. Biocompatible and biodegradable poly(Tannic Acid) hydrogel with antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2016, 82, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Hwang, C.H.; Kim, H.E.; Jeong, S.H. Enhancement of bio-stability and mechanical properties of hyaluronic acid hydrogels by tannic acid treatment. Carbohydr. Polym. 2018, 186, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Vales, T.P.; Cho, B.K.; Kim, J.K.; Kim, H.J. Development of Gallic Acid-Modified Hydrogels Using Interpenetrating Chitosan Network and Evaluation of Their Antioxidant Activity. Molecules 2017, 22, 1976. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kang, B.; Vales, T.P.; Yang, S.K.; Lee, J.; Kim, H. Polyphenol-functionalized hydrogels using an interpenetrating chitosan network and investigation of their antioxidant activity. Macromol. Res. 2018, 26, 35–39. [Google Scholar] [CrossRef]
- Gupta, M.K.; Martin, J.R.; Werfel, T.A.; Shen, T.; Page, J.M.; Duvall, C.L. Cell protective, ABC triblock polymer-based thermoresponsive hydrogels with ROS-triggered degradation and drug release. J. Am. Chem. Soc. 2014, 136, 14896–14902. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Martin, J.R.; Dollinger, B.R.; Hattaway, M.E.; Duvall, C.L. Thermogelling, ABC Triblock Copolymer Platform for Resorbable Hydrogels with Tunable, Degradation-Mediated Drug Release. Adv. Funct. Mater. 2017, 27, 1704107. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; He, C.; Ren, K.; Xiao, C.; Chen, X. Thermosensitive Polypeptide Hydrogels as a Platform for ROS-Triggered Cargo Release with Innate Cytoprotective Ability under Oxidative Stress. Adv. Healthc. Mater. 2016, 5, 1979–1990. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Patil, S.; Bhargava, N.; Kang, J.F.; Riedel, L.M.; Seal, S.; Hickman, J.J. Auto-catalytic Ceria Nanoparticles Offer Neuroprotection to Adult Rat Spinal Cord Neurons. Biomaterials 2007, 28, 1918–1925. [Google Scholar] [CrossRef]
- Weaver, J.D.; Stabler, C.L. Antioxidant cerium oxide nanoparticle hydrogels for cellular encapsulation. Acta Biomater. 2015, 16, 136–144. [Google Scholar] [CrossRef]
- Quarta, A.; Di Corato, R.; Manna, L.; Ragusa, A.; Pellegrino, T. Fluorescent-Magnetic Hybrid Nanostructures: Preparation, Properties, and Applications in Biology. IEEE T. Nanobiosci. 2007, 6, 298–308. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Ragusa, A.; García, I.; Penadés, S. Nanoparticles as Nonviral Gene Delivery Vectors. IEEE T. Nanobiosci. 2007, 6, 319–330. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds—A Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.Y.; Lee, Y.; Lee, S.; Park, S.B.; Lee, J.K. Bioinspired Polymerization of Dopamine to Generate Melanin-Like Nanoparticles Having an Excellent Free-Radical-Scavenging Property. Biomacromolecules 2011, 12, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zeng, Z.; Liu, L.; Chen, J.; Zhou, H.; Huang, L.; Huang, J.; Xu, H.; Xu, Y.; Chen, Z.; et al. Polydopamine nanoparticles for the treatment of acute inflammation-induced injury. Nanoscale 2018, 10, 6981–6991. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, R.; Lu, M.; You, G.; Wang, Y.; Chen, G.; Zhao, C.; Wang, Z.; Song, X.; Wu, Y.; et al. Bioinspired Polydopamine-Coated Hemoglobin as Potential Oxygen Carrier with Antioxidant Properties. Biomacromolecules 2017, 18, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Zhao, J.; Sun, J.; Hu, M.; Yang, X. Polydopamine Nanoparticles as Efficient Scavengers for Reactive Oxygen Species in Periodontal Disease. ACS Nano 2018, 12, 8882–8892. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Ji, X.; Askhatova, D.; Du, R.; Lu, L.; Shi, J. Comprehensive Insights into the Multi-Antioxidative Mechanisms of Melanin Nanoparticles and Their Application to Protect Brain from Injury in Ischemic Stroke. J. Am. Chem. Soc. 2017, 139, 856–862. [Google Scholar] [CrossRef]
- Malvindi, M.A.; Di Corato, R.; Curcio, A.; Melisi, D.; Rimoli, M.G.; Tortiglione, C.; Tino, A.; Chandramohan, G.; Brunetti, V.; Cingolani, R.; et al. Multiple functionalization of fluorescent nanoparticles for specific biolabeling and drug delivery of dopamine. Nanoscale 2011, 3, 5110–5119. [Google Scholar] [CrossRef]
- Donghyuck, Y.; Kyeonghye, G.; Hyungmin, K.; Gilson, K.; Dongmei, W.; Dongwon, L. Antioxidant polymeric nanoparticles as novel therapeutics for airway inflammatory diseases. Int. J. Pharm. 2013, 450, 87–94. [Google Scholar] [CrossRef]
- Jeong, D.; Kang, C.; Jung, E.; Yoo, D.; Wu, D.; Lee, D. Porous antioxidant polymer microparticles as therapeutic systems for the airway inflammatory diseases. J. Control. Release 2016, 233, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Larrañaga, A.; Isa, I.L.M.; Patil, V.; Thamboo, S.; Lomora, M.; Yague, M.A.F.; Sarasua, J.R.; Palivan, C.G.; Pandit, A. Antioxidant functionalized polymer capsules to prevent oxidative stress. Acta Biomater. 2018, 67, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Martín-Saldaña, S.; Palao-Suay, R.; Aguilar, M.R.; García-Fernández, L.; Arévalo, H.; Trinidad, A.; Ramírez-Camacho, R.; San Román, J. pH-sensitive polymeric nanoparticles with antioxidant and anti-inflammatory properties against cisplatin-induced hearing loss. J. Control. Release 2018, 270, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Priore, P.; Giudetti, A.M.; Ciccarella, G.; Gaballo, A. Neuroprotective Investigation of Chitosan Nanoparticles for Dopamine Delivery. Appl. Sci. 2018, 8, 474. [Google Scholar] [CrossRef]
- Rassu, G.; Porcu, E.P.; Fancello, S.; Obinu, A.; Senes, N.; Galleri, G.; Migheli, R.; Gavini, E.; Giunchedi, P. Intranasal Delivery of Genistein-Loaded Nanoparticles as a Potential Preventive System against Neurodegenerative Disorders. Pharmaceutics 2018, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Ray, L.; Pal, M.K.; Ray, R.S. Synergism of co-delivered nanosized antioxidants displayed enhanced anticancer efficacy in human colon cancer cell lines. Bioact. Mater. 2017, 2, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.B.; Kann, B.; Coradini, K.; Offerhaus, H.L.; Beck, R.C.R.; Windbergs, M. Skin penetration behavior of lipid-core nanocapsules for simultaneous delivery of resveratrol and curcumin. Eur. J. Pharm. Sci. 2015, 78, 204–213. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B.; Xie, D.; Hu, Y.; Li, H.L.; Zhong, L.L.; Wang, H.W.; Jiang, W.; Ke, Z.P.; Zheng, D.H. Nanoparticle-mediated dual delivery of resveratrol and DAP5 ameliorates kidney ischemia/reperfusion injury by inhibiting cell apoptosis and inflammation. Oncotarget 2017, 8, 39547–39558. [Google Scholar] [CrossRef][Green Version]
- Caddeo, C.; Pucci, L.; Gabriele, M.; Carbone, C.; Fernàndez-Busquets, X.; Valenti, D.; Pons, R.; Vassallo, A.; Fadda, A.M.; Manconi, M. Stability, biocompatibility and antioxidant activity of PEG-modified liposomes containing resveratrol. Int. J. Pharm. 2018, 538, 40–47. [Google Scholar] [CrossRef]
- De Leo, V.; Milano, F.; Mancini, E.; Comparelli, R.; Giotta, L.; Nacci, A.; Longobardi, F.; Garbetta, A.; Agostiano, A.; Catucci, L. Encapsulation of Curcumin-Loaded Liposomes for Colonic Drug Delivery in a pH-Responsive Polymer Cluster Using a pH-Driven and Organic Solvent-Free Process. Molecules 2018, 23, 739. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.S.; Patel, D.K.; et al. Curcumin-Loaded Nanoparticles Potently Induce Adult Neurogenesis and Reverse Cognitive Deficits in Alzheimer’s Disease Model via Canonical Wnt/β-Catenin Pathway. ACS Nano 2014, 8, 76–103. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Fan, D.; Zhao, Z.; Li, Z.; Li, G.; Chen, Y.; He, X.; Chen, A.; Li, J.; Lin, X.; et al. Nano-curcumin prepared via supercritical: Improved anti-bacterial, anti-oxidant and anti-cancer efficacy. Int. J. Pharm. 2015, 496, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, P.E.R.; Ferreira, L.M.; Cargnelutti, L.O.; Denardi, L.; Boligon, A.; Fleck, M.; Brandão, R.; Athayde, M.L.; Cruz, L.; Zanette, R.A.; et al. A new biodegradable polymeric nanoparticle formulation containing Syzygium cumini: Phytochemical profile, antioxidant and antifungal activity and in vivo toxicity. Ind. Crops Prod. 2016, 83, 400–407. [Google Scholar] [CrossRef]
- Santos, L.P.D.; Caon, T.; Battisti, M.A.; Silva, C.H.B.D.; Simões, C.M.O.; Reginatto, F.H.; Campos, A.M.D. Antioxidant polymeric nanoparticles containing standardized extract of Ilex paraguariensis A. St.-Hil. for topical use. Ind. Crops Prod. 2017, 108, 738–747. [Google Scholar] [CrossRef]
- Carella, E.; Ghiazza, M.; Alfè, M.; Gazzano, E.; Ghigo, D.; Gargiulo, V.; Ciajolo, A.; Fubini, B.; Fenoglio, I. Graphenic Nanoparticles from Combustion Sources Scavenge Hydroxyl Radicals Depending Upon Their Structure. BioNanoScience 2013, 3, 112–122. [Google Scholar] [CrossRef]
- Kokalari, I.; Gassino, R.; Giovannozzi, A.M.; Croin, L.; Gazzano, E.; Bergamaschi, E.; Rossi, A.M.; Perrone, G.; Riganti, C.; Ponti, J.; et al. Pro- and anti-oxidant properties of near-infrared (NIR) light responsive carbon nanoparticles. Free Radic. Biol. Med. 2019, 134, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Jiang, D.; Kutyreff, C.J.; Lai, J.; Yan, Y.; Barnhart, T.E.; Yu, B.; Im, H.J.; Kang, L.; Cho, S.Y.; et al. Molybdenum-based nanoclusters act as antioxidants and ameliorate acute kidney injury in mice. Nat. Commun. 2018, 9, 5421. [Google Scholar] [CrossRef]
- Li, F.; Li, T.Y.; Sun, C.X.; Xia, J.H.; Jiao, Y.; Xu, H.P. Selenium-Doped Carbon Quantum Dots for Free-Radical Scavenging. Angew. Chem. Int. Ed. 2017, 56, 9910–9914. [Google Scholar] [CrossRef]
- Zhai, X.N.; Zhang, C.Y.; Zhao, G.H.; Stoll, S.; Ren, F.Z.; Leng, X.J. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J. Nanobiotechnology 2017, 15, 4. [Google Scholar] [CrossRef]
- Menon, S.; Shrudhi, D.K.S.; Agarwal, H.; Shanmugam, V.K. Efficacy of Biogenic Selenium Nanoparticles from an Extract of Ginger towards Evaluation on Anti-Microbial and Anti-Oxidant Activities. Colloid Interface Sci. Commun. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Liu, Z.; Liu, C.Q.; Ju, E.G.; Zhang, Y.; Ren, J.S.; Qu, X.G. Self-Assembly of Multi-Nanozymes to Mimic an Intracellular Antioxidant Defense System. Angew. Chem. Int. Ed. 2016, 55, 6646–6650. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Gordijo, C.R.; Abbasi, A.Z.; Maeda, A.; Ip, A.; Rauth, A.M.; DaCosta, R.S.; Wu, X.Y. Multifunctional Albumin-MnO2 Nanoparticles Modulate Solid Tumor Microenvironment by Attenuating Hypoxia, Acidosis, Vascular Endothelial Growth Factor and Enhance Radiation Response. ACS Nano 2014, 8, 3202–3212. [Google Scholar] [CrossRef] [PubMed]
- Teo, J.Y.; Seo, Y.; Ko, E.; Leong, J.; Hong, Y.T.; Yang, Y.Y.; Kong, H. Surface tethering of stem cells with H2O2-responsive anti-oxidizing colloidal particles for protection against oxidation-induced death. Biomaterials 2019, 201, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Patil, S.; Seal, S.; McGinnis, J.F. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol. 2006, 1, 142–150. [Google Scholar] [CrossRef]
- Lee, S.S.; Song, W.S.; Cho, M.; Puppala, H.L.; Nguyen, P.; Zhu, H.G.; Segatori, L.; Colvin, V.L. Antioxidant Properties of Cerium Oxide Nanocrystals as a Function of Nanocrystal Diameter and Surface Coating. ACS Nano 2013, 7, 9693–9703. [Google Scholar] [CrossRef] [PubMed]
- Soh, M.; Kang, D.W.; Jeong, H.G.; Kim, D.; Kim, D.Y.; Yang, W.; Song, C.; Baik, S.; Choi, I.Y.; Ki, S.K.; et al. Ceria–Zirconia Nanoparticles as an Enhanced Multi-Antioxidant for Sepsis Treatment. Angew. Chem. Int. Ed. 2017, 56, 11399–11403. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Li, J.; Sun, L.; Zhong, L.; Ma, Q. An in vitro comparison of the antioxidant activities of chitosan and green synthesized gold nanoparticles. Carbohydr. Polym. 2019, 211, 161–172. [Google Scholar] [CrossRef]
- Świętek, M.; Lu, Y.C.; Konefał, R.; Ferreira, L.P.; Cruz, M.M.; Ma, Y.H.; Horák, D. Scavenging of reactive oxygen species by phenolic compound-modified maghemite nanoparticles. Beilstein J. Nanotechnol. 2019, 10, 1073–1088. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018, 11, 4955–4984. [Google Scholar] [CrossRef]
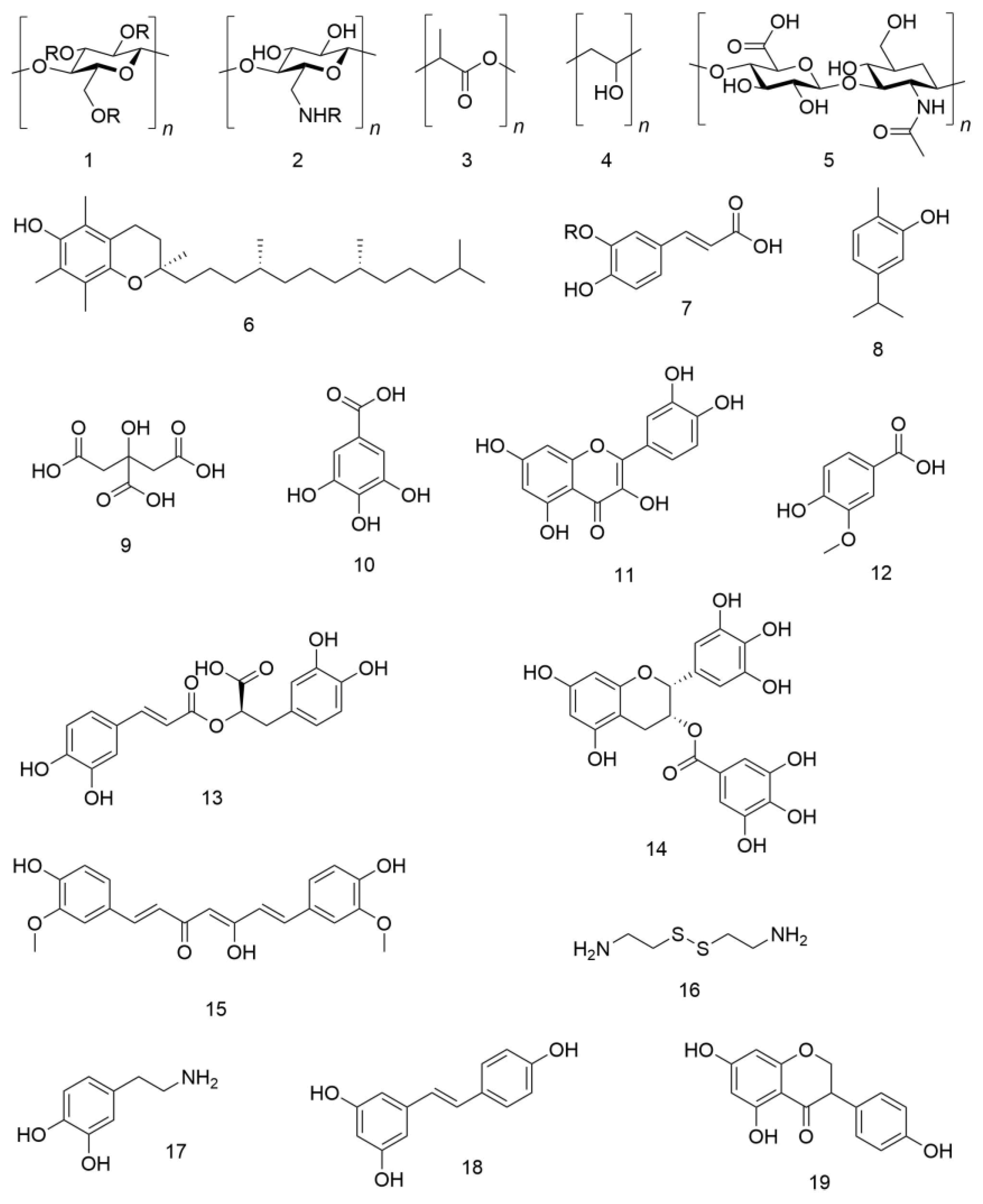
| Source Plant | Antioxidant | Results | Ref. |
|---|---|---|---|
| Astragalus membranaceus and Glycyrrhiza uralensis | phenols and flavonoids | higher antioxidant capacity in vitro than the theoretical sum of two individual herbs, probably because of a synergistic effect | [21] |
| Phyllanthus Emblica | polysaccharides and phenols | antioxidant capacities comparable to BHA and BHT antioxidants proportionally to the phenol content | [22] |
| Phyllanthus Emblica | low MW hydrolysable tannins (emblicanin-A, emblicanin-B, punigluconin, and pedunculagin) | significant decrease of several CVD risk factors | [23] |
| Acanthophyllum acerosum roots | polysaccharide (20.8% d-glucose, 66.2% d-galactose, and 13.0% l-arabinose) | able to scavenge DPPH radicals, but lower activity compared to that of ascorbic acid at the same concentration | [24] |
| Epimedium acuminatum | polysaccharides | higher antioxidant activities by hot water extraction apparently because of a more regular and smoother surface | [26] |
| Pharbitis nil seeds | polysaccharides, with minor percentages of uronic acid and proteins | remarkable ABTS and DPPH radical scavenging activities by ultrasonication extraction | [28] |
| white hyacinth bean | polysaccharides | lower scavenging activity compared to ascorbic acid, but ability to stimulate the growth of several probiotics | [29] |
| barley | β-glucan (mostly β-(1,3-1,4)-d-glucan) | antioxidant activity higher than that of several polymers used as food additives, supposedly because the β-glucan decreased the number of pro-inflammatory cytokines (mostly IL-6 and tumor necrosis factor-alpha, TNF-α) and increased that of the antioxidants | [31] |
| Cystoseira barbata | laminaran polysaccharide | noticeable antimicrobial and antioxidant properties in vitro, as well as wound-healing promotion in vivo | [32] |
| Spirulina sp | apo-c-PC β subunit | antioxidant activity lower than that of the natural extract, confirming the scavenging role of the bilin chromophore | [36] |
| Spirulina subsalsa | PCB-CpcB(C-82) fluorescent phycocyanin β subunit | stronger hydroxyl and DPPH free radicals scavenging activity than apo-CpcB, probably because of the bilin binding | [37] |
| Spirulina platensis | c-PC | reduced apoptosis, DNA damage, and autophagy in oxidatively stressed blastocysts of porcine embryos | [38] |
| commercially available Spirulina powders | carotenoids and c-PC | the c-PC extract had a stronger antioxidant activity compared to the carotenoid fraction | [39] |
| Base Polymer | Additional Antioxidant | Results | Ref. |
|---|---|---|---|
| chitosan | aqueous green tea extract | improved mechanical, water vapor barrier, and antioxidant properties | [43] |
| chitosan | montmorillonite and pomegranate rind powder extract | enhanced water vapor permeability and mechanical properties and excellent antioxidant activities | [44] |
| chitosan | carvacrol and pomegranate peel extract | decreased transparency but improved antioxidant activity | [45] |
| chitosan | caffeic acid or gallic acid | better pH-dependent antioxidant and antimicrobial properties | [46] |
| chitosan | α-tocopherol | improved UV protection, higher water vapor permeability, and better antioxidant capacity | [47] |
| chitosan | rosemary, ginger, sage, tea tree, and thyme essential oil (EO); ginger, rosemary, sage, black tea, green tea, and kenaf leaves HAE | improved light barrier and tensile strength | [48] |
| chitosan | rosemary, ginger, sage, tea tree, and thyme EO; ginger, rosemary, sage, black tea, green tea, and kenaf leaves HAE | the highest diffusion and antioxidant activity for the films with ginger, sage, or rosemary EO | [49] |
| chitosan and inulin | oregano and thyme EO | better physicochemical properties and improved antioxidant and antimicrobial activity | [50] |
| reacetylated chitosan | annatto powder and vitamin C | significantly improved ROS scavenging ability | [52] |
| chitosan and wheat starch | citric acid, α-tocopherol, thyme and basil EO | films containing α-tocopherol showed a higher antioxidant ability without affecting the mechanical properties | [54] |
| starch | cocoa nibs extract (CNE) | ability to quench 100% of ABTS and 94% of DPPH produced radicals with the 1% CNE-containing film | [55] |
| polyurethane | lignin fractions | reproducible method for obtaining homogeneous lignin products with reliable physicochemical properties | [57] |
| cellulose nanofibrils | tannin extract | improved antioxidant and UV-adsorbing properties | [58] |
| carboxymethyl cellulose | sodium alginate (SA) and epigallocatechin gallate (EGCG) | edible EGCG-releasing films with strong antioxidant activity in fatty foods | [59] |
| polyvinyl alcohol (PVA) | tannin | good antioxidant activities | [60] |
| polylactic acid (PLA) | ferulic acid (FA), vanillic acid (VA), vitamin E (VE), and quercetin (Q) | highest antioxidant properties when FA and Q were combined at low concentration with PLA | [61] |
| gelatin | rosmarinic acid | excellent ultraviolet barrier capacity, good antioxidant properties, long-term antibacterial activity | [62] |
| gelatin | citric acid and chitosan | allowed the healing process in ex vivo assay in human skin | [63] |
| soybean protein isolate | cortex Phellodendron extract | good rheological properties and additional antioxidant and antimicrobial properties | [64] |
| Base Polymer | Additional Antioxidant | Results | Ref. |
|---|---|---|---|
| PAbAE | curcumin and quercetin | controlled degradation rate and the degradation products suppressed the induced oxidative stress in HUVEC cells | [66] |
| PAbAE | curcumin and quercetin (25–38 wt% loading) | slow release of the antioxidant and inhibition of the oxidative stress | [67] |
| PAbAE | curcumin | ability to protect cells from radicals and increased tolerance to curcumin cytotoxicity | [68] |
| PAbAE | cystamine | environmental redox sensitivity and increased IC50 by an order of magnitude | [69] |
| poly(ethylene glycol)-co-poly(glycerol sebacate) | quaternized chitosan-g-polyaniline | good self-healing, free radical scavenging ability, antibacterial, and antioxidant activities for cutaneous wound healing; enhanced in vivo wound healing process | [70] |
| N-carboxyethyl chitosan | hyaluronic acid-graft-aniline tetramer | high free radical scavenging capacity, high swelling ratio and antimicrobial property; accelerated in vivo healing process | [71] |
| trimethylolpropane triglycidyl ether | tannic acid | good antioxidant ability at slightly acidic pH; robust antimicrobial property | [72] |
| hyaluronic acid (HA) | tannic acid | improved resistance to enzymatic degradation and antioxidant capacity | [73] |
| chitosan | gallic acid and dopamine | stronger antioxidant capacity in the GA-functionalized hydrogels and in those with longer chitosan chains | [74,75] |
| PPS-b-PDMA-b-PNIPAAM | ROS-triggered degradation and drug release | [76] | |
| PPS-b-PDMA-b-PNIPAAM | in vivo differential release kinetics according to the specific degradation mechanism | [77] | |
| methoxy poly(ethylene glycol)-poly(l-methionine) | accelerated release under oxidative stress conditions, both in vitro and in vivo | [78] | |
| alginate | cerium oxide NPs | dose-dependent protection to beta cells from superoxide exposure | [80] |
| Base Polymer | Additional Drug | Results | Ref. |
|---|---|---|---|
| melanin | free radical scavenging capacity similar to that of ascorbic acid in HeLa cells | [85] | |
| polydopamine (pDA) | reduced ROS levels in vivo in murine macrophages; alleviated acute peritonitis and acute lung injury inflammation in murine models | [86] | |
| pDA-coated hemoglobin | reduced the intracellular oxidative stress without affecting the blood constituents | [87] | |
| polydopamine | reduced inflammation by subgingival injection in a murine periodontitis model | [88] | |
| polydopamine | demonstrated activity against multiple RONS; reduced oxidative stress in a rat model of ischemic stroke | [89] | |
| PEG-polymaleic acid (PMA) | dopamine | targeted dopamine delivery through the GLUT-1 transporter | [90] |
| HBA-HPOX | reduced expression of pro-inflammatory mediators in a murine model of asthma | [91] | |
| PVAX | dexamethasone | reduced oxidative stress and suppression of the expression of TNF-a and iNOS in the lung of asthmatic mice | [92] |
| TA | CAT | inhibition of the oxidative stress and prevention of the expression of MMP-3, disintegrin, and ADAMTS-5 in an in vitro inflammation model of degenerative disc disease | [93] |
| vinylimidazole and vinylpyrrolidone | methacrylic derivatives of ibuprofen, α-tocopherol and α-tocopheryl succinate; dexamethasone | lower cisplatin-induced toxicity, downregulation of caspase 3/7 expression, lower IL-1β release, and intracellular ROS accumulation in vitro; reduced hearing loss in vivo | [94] |
| chitosan | dopamine | significant reduction of the oxidative stress in SHSY-5Y cells; increased enzymatic activity of both GPx and SOD | [95] |
| chitosan | genistein | efficient drug delivery to the brain after permeation through the nasal mucosa | [96] |
| chitosan | curcumin, quercetin, aspirin | synergistic effect in inhibiting colon cancer progression in HCT-116 cells | [97] |
| PCL | curcumin and resveratrol | sustained drug release, facilitated skin absorption, deeper penetration of resveratrol | [98] |
| PVP-b-PCL | resveratrol and DAP5 | decreased production of pro-inflammatory cytokines and attenuated renal ischemia reperfusion (I/R) injury in vivo | [99] |
| PEG | resveratrol | optimal protection against oxidative stress in an ex vivo human erythrocytes-based model | [100] |
| Eudragit S100 | curcumin | good ABTS antioxidant activity; inhibition of the drug release until degradation of the NPs | [101] |
| PLGA | curcumin | induced neural stem cells proliferation and neuronal differentiation in adult rats | [102] |
| curcumin | DPPH scavenging efficiency almost comparable to that of ascorbic acid | [103] | |
| PCL | Syzygium cumini seeds extract | high protection against oxidized LDL particles in vitro | [104] |
| PCL | Ilex paraguariensis extract | significant reduction of chlorogenic acid permeated through the skin; increased topical antioxidant effect | [105] |
| graphene-like | reaction with hydroxyl radicals in macrophages | [106,107] | |
| POM | molybdenum NPs | reduction of the clinical symptoms in mice affected by acute kidney injury | [108] |
| selenocysteine-derived | reduced oxidative stress in MDA-MB-231 cells | [109] | |
| low MW chitosan-coated selenium | efficiently penetrated mice tissues and protected GPx activity | [110] | |
| Z. Officinale root extract | good antimicrobial activity and excellent radical scavenging activity when compared to that of ascorbic acid | [111] | |
| pDA | MnO2 NPs and V2O5 nanowires | excellent intracellular ROS removal ability both in vitro and in vivo | [112] |
| Polyelectrolyte–albumin complex | MnO2 NPs | increased tumor oxygenation by 45% in mice | [113] |
| PLGA-HA | MnO2 and EGCG | higher metabolic activity and more elevated secretion of pro-angiogenic factor in vitro in stem cells | [114] |
| Phospholipid–PEG | ceria-zirconia NPs | reduce mortality and systemic inflammation in vivo in sepsis mice model | [117] |
| chitosan | Au NPs | good antioxidant activity which was dependent on the size, shape, and concentration of the NPs | [118] |
| heparin, chitosan + GA/hydroquinone/phloroglucinol | maghemite NPs | highest antioxidant activity observed with CS-GA; an external magnetic field did not increase internalization of the NPs functionalized with the phenols | [119] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, M.S.; Quarta, A.; Marradi, M.; Ragusa, A. Recent Developments in the Reduction of Oxidative Stress through Antioxidant Polymeric Formulations. Pharmaceutics 2019, 11, 505. https://doi.org/10.3390/pharmaceutics11100505
Zafar MS, Quarta A, Marradi M, Ragusa A. Recent Developments in the Reduction of Oxidative Stress through Antioxidant Polymeric Formulations. Pharmaceutics. 2019; 11(10):505. https://doi.org/10.3390/pharmaceutics11100505
Chicago/Turabian StyleZafar, Muhammad Shajih, Alessandra Quarta, Marco Marradi, and Andrea Ragusa. 2019. "Recent Developments in the Reduction of Oxidative Stress through Antioxidant Polymeric Formulations" Pharmaceutics 11, no. 10: 505. https://doi.org/10.3390/pharmaceutics11100505
APA StyleZafar, M. S., Quarta, A., Marradi, M., & Ragusa, A. (2019). Recent Developments in the Reduction of Oxidative Stress through Antioxidant Polymeric Formulations. Pharmaceutics, 11(10), 505. https://doi.org/10.3390/pharmaceutics11100505