Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems
Abstract
1. Introduction
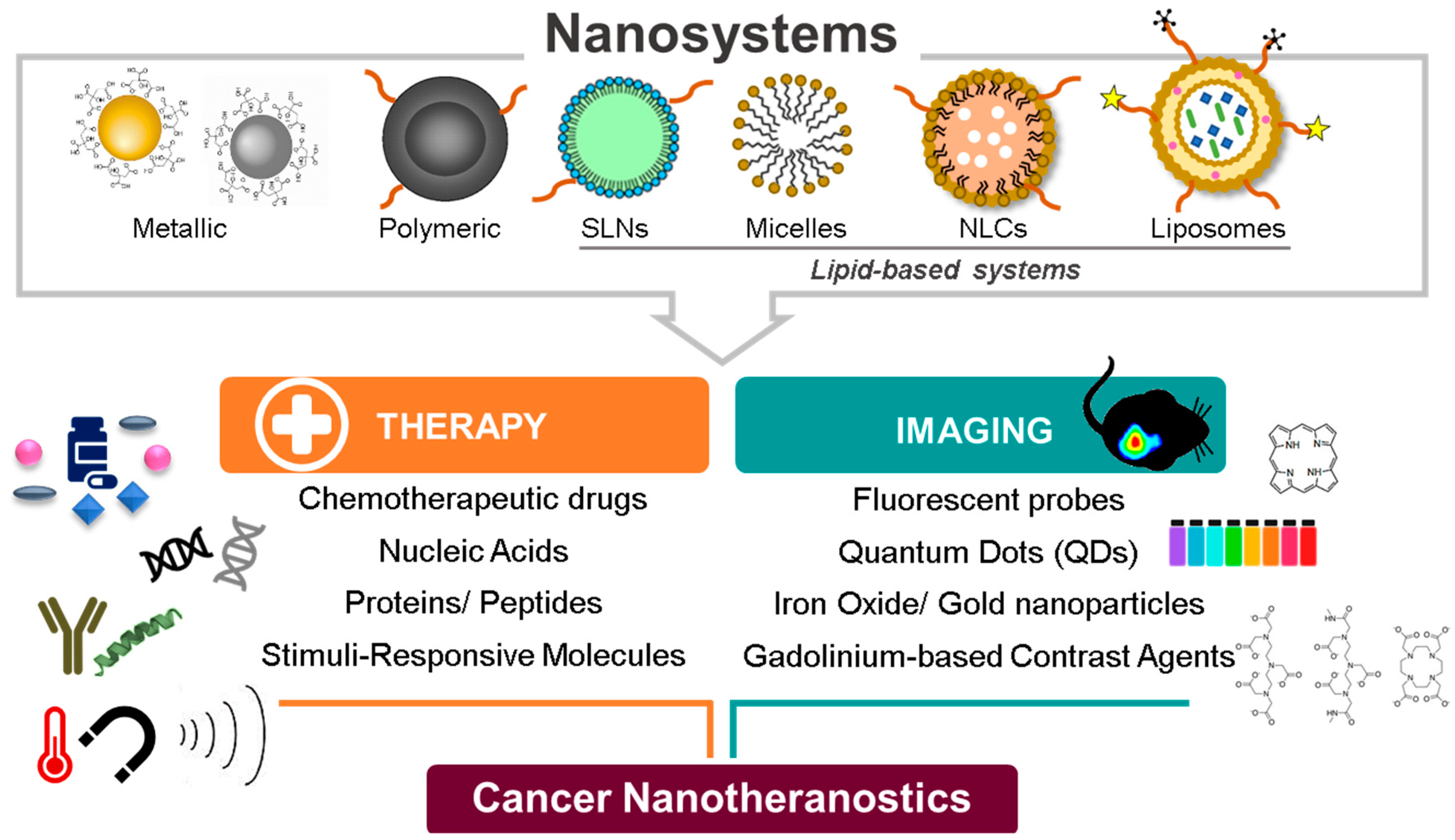
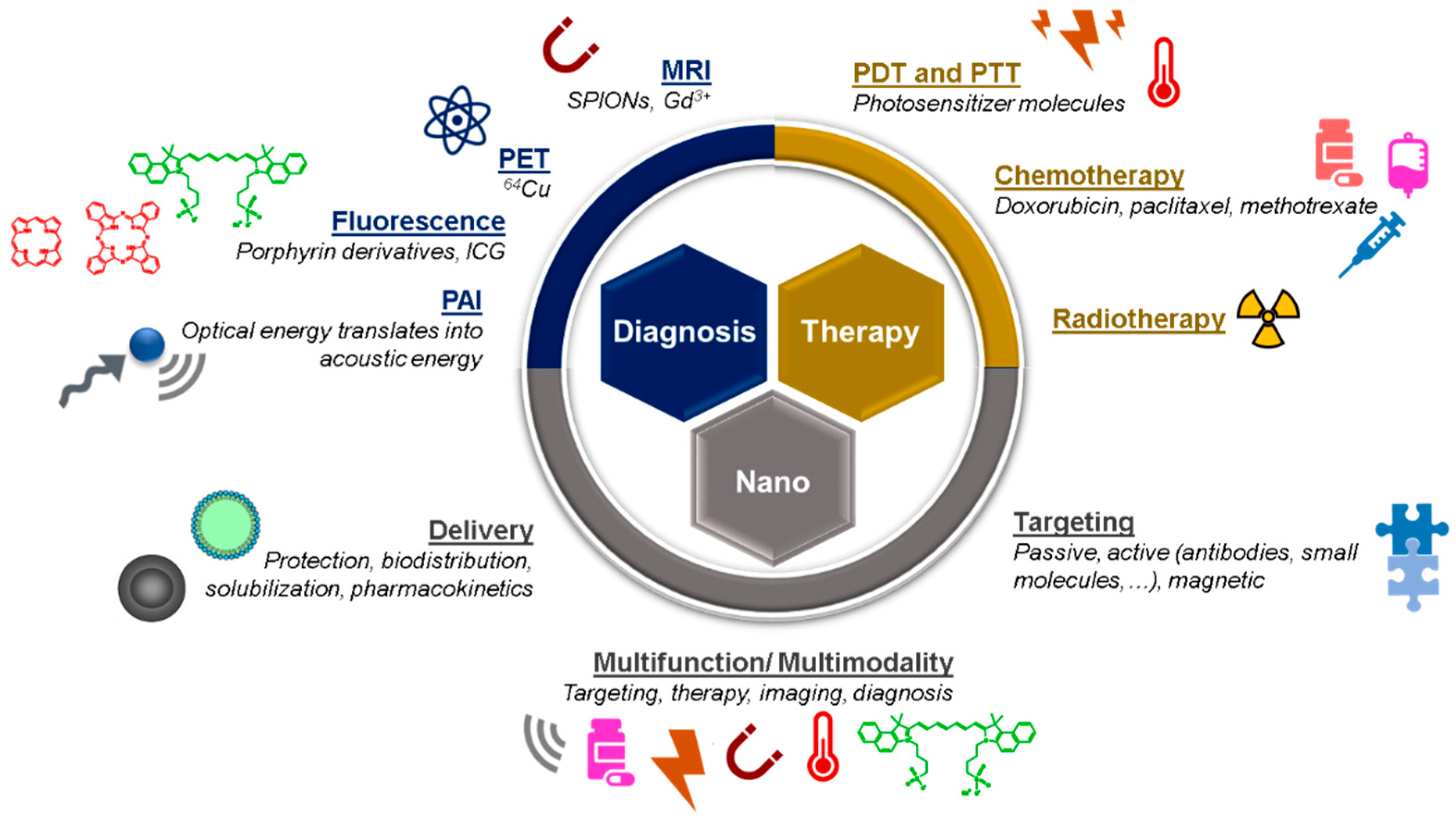
2. Theranostic Nanosystems: From Single to Multimodality
2.1. The Evolution of the Design and Composition of Nanotheranostic Systems
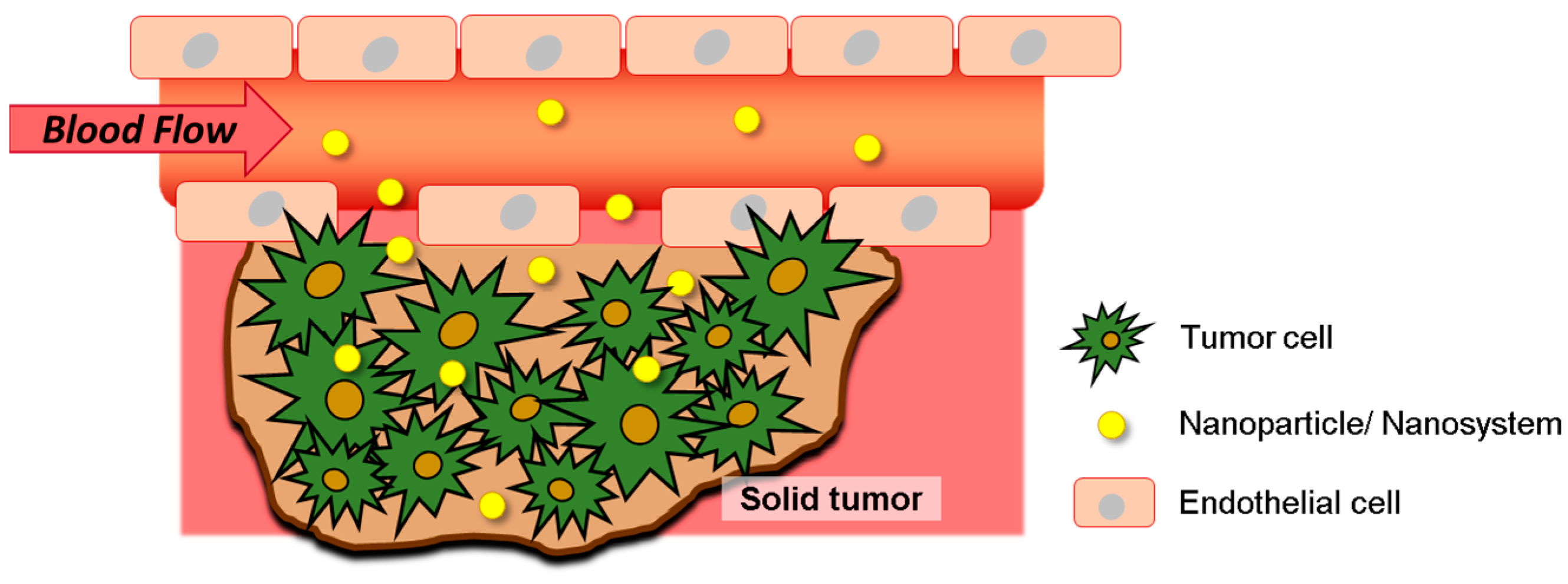
2.2. Exploring Tumor Microenvironment for Improved Nanotheranostics Targeting
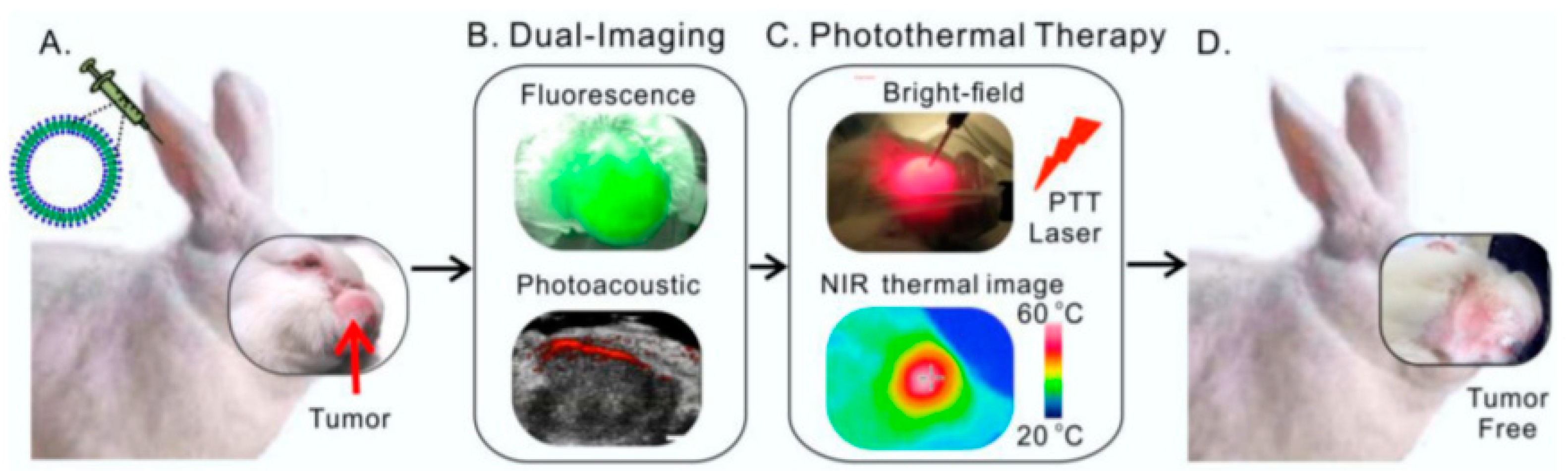
2.3. Nanotheranostics as an Efficient and Safe Alternative in Clinic
3. Nanotheranostics in Diagnosis and Treatment of Cancer
3.1. Polymeric and Metallic-Based Nanoparticles
3.1.1. Non-Melanoma Skin Cancer and Cutaneous Melanoma
3.1.2. Head and Neck Cancers
3.1.3. Thyroid Cancer
3.1.4. Breast Cancer
3.1.5. Prostate Cancer
3.2. Lipid-Based Nanosystems
3.3. Liposomes
3.3.1. Melanoma and Non-Melanoma Skin Cancer
3.3.2. Breast Cancer
3.3.3. Gynecologic Cancer
3.3.4. Colon Cancer
3.3.5. Hepatocellular Carcinoma
3.3.6. Brain-Related Cancer
3.4. Liposomes in Clinical Studies
3.5. Others
3.5.1. Lipid Nanoparticles
3.5.2. Solid Lipid Nanoparticles
3.5.3. Nanostructured Lipid Carriers
3.5.4. Lipid Nanocapsules
3.5.5. Lipid-Based Micelles
4. Meeting the Criteria for a Successful Translation of Nanotheranostics into Clinic Settings
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nabil, G.; Bhise, K.; Sau, S.; Atef, M.; El-Banna, H.A.; Iyer, A.K. Nanoengineered delivery systems for cancer imaging and therapy: Recent advances, future directions and patent evaluation. Drug Discov. Today 2018. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H.; Deng, Y.; Sun, H.; Ke, X.; Ci, T. Near-infrared light triggered drug delivery system for higher efficacy of combined chemo-photothermal treatment. Acta Biomater. 2017, 51, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Nanotheranostics and image-guided drug delivery: Current concepts and future directions. Mol. Pharm. 2010, 7, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Dreifuss, T.; Betzer, O.; Motiei, M.; Popovtzer, R.; Barnoy, E. The effect of nanoparticle size on theranostic systems: The optimal particle size for imaging is not necessarily optimal for drug delivery. In Nanoscale Imaging, Sensing, and Actuation for Biomedical Applications XV; Cartwright, A.N., Nicolau, D.V., Fixler, D., Eds.; SPIE: Bellingham, WA, USA, 2018; Volume 10506, p. 39. [Google Scholar]
- Cruz, M.E.M.; Simões, S.I.; Corvo, M.L.; Martins, M.B.; Gaspar, M.M. Formulation of NPDDS for Macromolecules. In Drug Delivery Nanoparticles: Formulation and Characterization; Pathak, Y., Thassu, D., Eds.; Informa Healthcare: New York, NY, USA, 2009; pp. 35–49. [Google Scholar]
- Dai, Z. Multifunctional liposomes for imaging-guided therapy. In Advances in Nanotheranostics I: Design and Fabrication of Theranosic Nanoparticles; Dai, Z., Ed.; Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2016; Volume 6, ISBN 978-3-662-48542-2. [Google Scholar]
- Perrie, Y.; Ramsay, E. Nanomedicines: Exploring the past, present and future. Drug Discov. World 2017, 18, 17–22. [Google Scholar]
- Zhang, P.; Hu, C.; Ran, W.; Meng, J.; Yin, Q.; Li, Y. Recent progress in light-triggered nanotheranostics for cancer treatment. Theranostics 2016, 6, 948–968. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A.M. Design of Nanostructures for Theranostics Applications; Andrew, W., Ed.; Pharmaceutical Nanotechnology; Elsevier Science: Amsterdam, The Netherlands, 2017; ISBN 9780128136706. [Google Scholar]
- Dykman, L.A.; Khlebtsov, N.G. Multifunctional gold-based nanocomposites for theranostics. Biomaterials 2016, 108, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Kostevsek, N.; Locatelli, E.; Garrovo, C.; Arena, F.; Monaco, I.; Nikolov, I.P.; Sturm, S.; Rozman, K.Z.; Lorusso, V.; Giustetto, P.; et al. The one-step synthesis and surface functionalization of dumbbell-like gold-iron oxide nanoparticles: A chitosan-based nanotheranostic system. Chem. Commun. 2016, 52, 378–381. [Google Scholar] [CrossRef]
- Li, H.; Harriss, B.I.; Phinikaridou, A.; Lacerda, S.; Ramniceanu, G.; Doan, B.-T.; Ho, K.-L.; Chan, C.-F.; Lo, W.-S.; Botnar, R.M.; et al. Gadolinium and Platinum in Tandem: Real-time Multi-Modal Monitoring of Drug Delivery by MRI and Fluorescence Imaging. Nanotheranostics 2017, 1, 186–195. [Google Scholar] [CrossRef]
- Willerding, L.; Limmer, S.; Hossann, M.; Zengerle, A.; Wachholz, K.; ten Hagen, T.L.M.; Koning, G.A.; Sroka, R.; Lindner, L.H.; Peller, M. Method of hyperthermia and tumor size influence effectiveness of doxorubicin release from thermosensitive liposomes in experimental tumors. J. Control. Release 2016, 222, 47–55. [Google Scholar] [CrossRef]
- Murphy, C.J.; Vartanian, A.M.; Geiger, F.M.; Hamers, R.J.; Pedersen, J.; Cui, Q.; Haynes, C.L.; Carlson, E.E.; Hernandez, R.; Klaper, R.D.; et al. Biological responses to engineered nanomaterials: Needs for the next decade. ACS Cent. Sci. 2015, 1, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.O.; Petersen, S.B.; Pinto Reis, C.; Rijo, P.; Molpeceres, J.; Vorum, H.; Neves-Petersen, M.T. Lysozyme photochemistry as a function of temperature. the protective effect of nanoparticles on lysozyme photostability. PLoS ONE 2015, 10, e0144454. [Google Scholar] [CrossRef]
- Silva, C.O.; Petersen, S.B.; Reis, C.P.; Rijo, P.; Molpeceres, J.; Fernandes, A.S.; Gonçalves, O.; Gomes, A.C.; Correia, I.; Vorum, H.; et al. EGF functionalized polymer-coated gold nanoparticles promote EGF photostability and EGFR internalization for p therapy. PLoS ONE 2016, 11, e0165419. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.O.; Molpeceres, J.; Batanero, B.; Fernandes, A.S.; Saraiva, N.; Costa, J.G.; Rijo, P.; Figueiredo, I.V.; Faísca, P.; Reis, C.P. Functionalized diterpene parvifloron D-loaded hybrid nanoparticles for targeted delivery in melanoma therapy. Ther. Deliv. 2016, 7, 521–544. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.D.; Klippstein, R.; Wang, J.T.-W.; Hodgins, N.; Mei, K.-C.; Sosabowski, J.; Hider, R.C.; Abbate, V.; Gupta, P.N.; Al-Jamal, K.T. Novel hyaluronic acid conjugates for dual nuclear imaging and therapy in CD44-expressing tumors in mice in vivo. Nanotheranostics 2017, 1, 59–79. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; Rehman, A. ur Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef]
- Yang, C.; Vu-Quang, H.; Husum, D.M.U.; Tingskov, S.J.; Vinding, M.S.; Nielsen, T.; Song, P.; Nielsen, N.C.; Nørregaard, R.; Kjems, J. Theranostic poly(lactic-co-glycolic acid) nanoparticle for magnetic resonance/infrared fluorescence bimodal imaging and efficient siRNA delivery to macrophages and its evaluation in a kidney injury model. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2451–2462. [Google Scholar] [CrossRef]
- Zhao, Z.; Lou, S.; Hu, Y.; Zhu, J.; Zhang, C. A nano-in-nano polymer–dendrimer nanoparticle-based nanosystem for controlled multidrug delivery. Mol. Pharm. 2017, 14, 2697–2710. [Google Scholar] [CrossRef]
- Lou, S.; Zhao, Z.; Dezort, M.; Lohneis, T.; Zhang, C. Multifunctional nanosystem for targeted and controlled delivery of multiple chemotherapeutic agents for the treatment of drug-resistant breast cancer. ACS Omega 2018, 3, 9210–9219. [Google Scholar] [CrossRef]
- Tang, H.; Guo, Y.; Peng, L.; Fang, H.; Wang, Z.; Zheng, Y.; Ran, H.; Chen, Y. In vivo targeted, responsive, and synergistic cancer nanotheranostics by magnetic resonance imaging-guided synergistic high-intensity focused ultrasound ablation and chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 15428–15441. [Google Scholar] [CrossRef] [PubMed]
- Perlman, O.; Weitz, I.S.; Sivan, S.S.; Abu-Khalla, H.; Benguigui, M.; Shaked, Y.; Azhari, H. Copper oxide loaded PLGA nanospheres: Towards a multifunctional nanoscale platform for ultrasound-based imaging and therapy. Nanotechnology 2018, 29, 185102. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Chen, Q.; Liu, Z. Recent advances in the development of organic photothermal nano-agents. Nano Res. 2015, 8, 340–354. [Google Scholar] [CrossRef]
- Spring, B.Q.; Bryan Sears, R.; Zheng, L.Z.; Mai, Z.; Watanabe, R.; Sherwood, M.E.; Schoenfeld, D.A.; Pogue, B.W.; Pereira, S.P.; Villa, E.; et al. A photoactivable multi-inhibitor nanoliposome for tumour control and simultaneous inhibition of treatment escape pathways. Nat. Nanotechnol. 2016, 11, 378–387. [Google Scholar] [CrossRef]
- Maeda, H.; Sawa, T.; Konno, T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J. Control. Release 2001, 74, 47–61. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Jin, J.; Ovais, M.; Chen, C. Stimulus-responsive gold nanotheranostic platforms for targeting the tumor microenvironment. Nano Today 2018, 22, 83–99. [Google Scholar] [CrossRef]
- Maeda, H. Polymer therapeutics and the EPR effect. J. Drug Target. 2017, 25, 781–785. [Google Scholar] [CrossRef]
- Khatun, Z.; Nurunnabi, M.; Nafiujjaman, M.; Reeck, G.R.; Khan, H.A.; Cho, K.J.; Lee, Y. A hyaluronic acid nanogel for photo-chemo theranostics of lung cancer with simultaneous light-responsive controlled release of doxorubicin. Nanoscale 2015, 7, 10680–10689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, K.; Li, S.; Xin, X.; Yuan, S.; Ma, G.; Yan, X. Self-assembled minimalist multifunctional theranostic nanoplatform for magnetic resonance imaging-guided tumor photodynamic therapy. ACS Nano 2018, 12, 8266–8276. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Huang, X.; Yu, G.; Wang, S.; Zhou, Z.; Shen, Z.; Fan, W.; Liu, Y.; Davisson, M.; et al. Glutathione-responsive self-assembled magnetic gold nanowreath for enhanced tumor imaging and imaging-guided photothermal therapy. ACS Nano 2018, 12, 8129–8137. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hu, M.; Yu, X.; Li, Y.; Fu, Y.; Zhou, X.; Zhang, D.; Li, J. Design and evaluation of pH-sensitive liposomes constructed by poly(2-ethyl-2-oxazoline)-cholesterol hemisuccinate for doxorubicin delivery. Eur. J. Pharm. Biopharm. 2015, 91, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, C.; Huang, Y.; Zhang, F.; Lin, G. Study of the pH-sensitive mechanism of tumor-targeting liposomes. Colloid Surf. B Biointerfaces 2017, 151, 19–25. [Google Scholar] [CrossRef]
- Li, B.; Tang, J.; Chen, W.; Hao, G.; Kurniawan, N.; Gu, Z.; Xu, Z.P. Novel theranostic nanoplatform for complete mice tumor elimination via MR imaging-guided acid-enhanced photothermo-/chemo-therapy. Biomaterials 2018, 177, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Atallah, I.; Milet, C.; Coll, J.L.; Reyt, E.; Righini, C.A.; Hurbin, A. Role of near-infrared fluorescence imaging in head and neck cancer surgery: From animal models to humans. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-S.; Jang, S.J.; Yoon, Y.I.; Cho, H.-S.; Lee, H.J.; Cho, Y.-S.; Shin, H.S.; Yoon, T.-J. Magnetic liposomal particles for magnetic imaging, sensing, and the pH-sensitive delivery of therapeutics. Part. Part. Syst. Charact. 2016, 33, 242–247. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Blau, R.; Epshtein, Y.; Pisarevsky, E.; Tiram, G.; Israeli Dangoor, S.; Yeini, E.; Krivitsky, A.; Eldar-Boock, A.; Ben-Shushan, D.; Gibori, H.; et al. Image-guided surgery using near-infrared Turn-ON fluorescent nanoprobes for precise detection of tumor margins. Theranostics 2018, 8, 3437–3460. [Google Scholar] [CrossRef] [PubMed]
- Pizzimenti, S.; Dianzani, C.; Zara, G.P.; Ferretti, C.; Rossi, F.; Gigliotti, C.; Daga, M.; Ciamporcero, E.; Maina, G.; Barrera, G. Challenges and opportunities of nanoparticle-based theranostics in skin cancer. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 177–188. ISBN 9780128029268. [Google Scholar]
- Youl, P.H.; Janda, M.; Aitken, J.F.; Del Mar, C.B.; Whiteman, D.C.; Baade, P.D. Body-site distribution of skin cancer, pre-malignant and common benign pigmented lesions excised in general practice. Br. J. Dermatol. 2011, 165, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, S.; Lee, D.; Kim, G.; Lee, B.; Cho, Y.; Zheng, B.-Y.; Ke, M.-R.; Huang, J.-D.; Nam, K.T.; et al. Facile Supramolecular Approach to Nucleic-Acid-Driven Activatable Nanotheranostics That Overcome Drawbacks of Photodynamic Therapy. ACS Nano 2018, 12, 681–688. [Google Scholar] [CrossRef]
- Yang, W.; Guo, W.; Le, W.; Lv, G.; Zhang, F.; Shi, L.; Wang, X.; Wang, J.; Wang, S.; Chang, J.; et al. Albumin-Bioinspired Gd:CuS nanotheranostic agent for in vivo photoacoustic/magnetic resonance imaging-guided tumor-targeted photothermal therapy. ACS Nano 2016, 10, 10245–10257. [Google Scholar] [CrossRef] [PubMed]
- Gray-Schopfer, V.; Wellbrock, C.; Marais, R. Melanoma biology and new targeted therapy. Nature 2007, 445, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Pautu, V.; Leonetti, D.; Lepeltier, E.; Clere, N.; Passirani, C. Nanomedicine as a potent strategy in melanoma tumor microenvironment. Pharmacol. Res. 2017, 126, 31–53. [Google Scholar] [CrossRef]
- Oliveira Silva, C.; Martinho, N.; Aniceto, N.; Pinto Reis, C. Challenges and progresses in nanotechnology for melanoma prevention and treatment. In CRC Concise Encyclopedia of Nanotechnology; Ildusovich Kharisov, B., Vasilievna Kharissova, O., Ortiz-Mendez, U., Eds.; CRC Press, Taylor & Francis Publishing Group: Boca Raton, FL, USA, 2015; pp. 453–470. [Google Scholar]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, I.; Buzea, C.; Tron, V. Towards new therapeutic approaches for malignant melanoma. Expert Rev. Mol. Med. 2011, 13, e33. [Google Scholar] [CrossRef] [PubMed]
- McKean, M.A.; Amaria, R.N. Challenges and opportunities of neoadjuvant treatment in locally advanced melanoma. Am. J. Clin. Dermatol. 2018, 19, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Van de Wiel, M.; Dockx, Y.; Van den Wyngaert, T.; Stroobants, S.; Tjalma, W.A.A.; Huizing, M.T. Neoadjuvant systemic therapy in breast cancer: Challenges and uncertainties. Eur. J. Obstet. Gynecol. 2017, 210, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Liang, R.; An, X.; Wang, K.; Shen, G.; Tu, Y.; Zhu, J.; Tao, J. Recent advances in targeted nanoparticles drug delivery to melanoma. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 769–794. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Yasemi, M.; Safaie-Qamsari, E.; Rashidiani, J.; Abkar, M.; Hassani, M.; Mirhosseini, S.A.; Kooshki, H. Using gold nanoparticles in diagnosis and treatment of melanoma cancer. Artif. Cells Nanomed. Biotechnol. 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Labala, S.; Jose, A.; Venuganti, V.V.K. Transcutaneous iontophoretic delivery of STAT3 siRNA using layer-by-layer chitosan coated gold nanoparticles to treat melanoma. Colloids Surf. B Biointerfaces 2016, 146, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Silva, C.O.; Monteiro, C.M.; Nicolai, M.; Viana, A.; Andrade, J.M.; Barasoain, I.; Stankovic, T.; Quintana, J.; Hernández, I. Anticancer properties of the abietane diterpene 6,7-dehydroroyleanone obtained by optimized extraction. Future Med. Chem. 2018, 10, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liang, W.; Zhu, Y.; Guo, Z.; Jian, J.; Jiang, B.-P.; Liang, H.; Shen, X.-C. Supercharged fluorescent protein functionalized water-soluble poly(N-phenylglycine) nanoparticles for highly effective imaging-guided photothermal therapy. Chem. Commun. 2018, 54, 10292–10295. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Johnson, N.W.; Kumar, N. Global epidemiology of head and neck cancers: A continuing challenge. Oncology 2016, 91, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Jefferson, G. Chapter 64: Head and Neck Cancer. In Genomic and Personalized Medicine; Version 2; Academic Press: New York, NY, USA, 2013; Volume 1–2, pp. 742–748. [Google Scholar]
- Gregoire, V.; Lefebvre, J.-L.; Licitra, L.; Felip, E. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21, v184–v186. [Google Scholar] [CrossRef]
- Younes, M.N.; Yazici, Y.D.; Kim, S.; Jasser, S.A.; El-Naggar, A.K.; Myers, J.N. Dual epidermal growth factor receptor and vascular endothelial growth factor receptor inhibition with NVP-AEE788 for the treatment of aggressive follicular thyroid cancer. Clin. Cancer Res. 2006, 12, 3425–3434. [Google Scholar] [CrossRef]
- Maiti, G.P.; Mondal, P.; Mukherjee, N.; Ghosh, A.; Ghosh, S.; Dey, S.; Chakrabarty, J.; Roy, A.; Biswas, J.; Roychoudhury, S.; et al. Overexpression of EGFR in Head and Neck Squamous Cell Carcinoma Is Associated with Inactivation of SH3GL2 and CDC25A Genes. PLoS ONE 2013, 8, e63440. [Google Scholar] [CrossRef]
- Shao, H.; Cheng, H.Y.; Cook, R.G.; Tweardy, D.J. Identification and characterization of signal transducer and activator of transcription 3 recruitment sites within the epidermal growth factor receptor. Cancer Res. 2003, 63, 3923–3930. [Google Scholar] [CrossRef]
- Song, J.; Qu, J.; Swihart, M.T.; Prasad, P.N. Near-IR responsive nanostructures for nanobiophotonics: Emerging impacts on nanomedicine. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 771–788. [Google Scholar] [CrossRef]
- Alvarez, M.M.; Aizenberg, J.; Analoui, M.; Andrews, A.M.; Bisker, G.; Boyden, E.S.; Kamm, R.D.; Karp, J.M.; Mooney, D.J.; Oklu, R.; et al. Emerging Trends in Micro- and Nanoscale Technologies in Medicine: From Basic Discoveries to Translation. ACS Nano 2017, 11, 5195–5214. [Google Scholar] [CrossRef]
- Lux, F.; Tran, V.L.; Thomas, E.; Dufort, S.; Rossetti, F.; Martini, M.; Truillet, C.; Doussineau, T.; Bort, G.; Denat, F.; et al. AGuIX® from bench to bedside—Transfer of an ultrasmall theranostic gadolinium-based nanoparticle to clinical medicine. Br. J. Radiol. 2018, 91, 20180365. [Google Scholar] [CrossRef] [PubMed]
- Abadeer, N.S.; Murphy, C.J. Recent progress in cancer thermal therapy using gold nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Zhang, S.; Gupta, S.; Fitzgerald, T.J.; Bogdanov, A.A. Dual radiosensitization and anti-STAT3 anti-proliferative strategy based on delivery of gold nanoparticle - oligonucleotide nanoconstructs to head and neck cancer cells. Nanotheranostics 2018, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dreifuss, T.; Davidi, E.S.; Motiei, M.; Barnoy, E.; Popovtzer, A.; Popovtzer, R.; Bragilovski, D.; Lubimov, L.; Kindler, M.J.J. All-in-one theranostic nanoagent for head and neck cancer treatment. In Nanoscale Imaging, Sensing, and Actuation for Biomedical Applications XV; Cartwright, A.N., Nicolau, D.V., Fixler, D., Eds.; SPIE: Bellingham, WA, USA, 2018; Volume 10506, p. 40. [Google Scholar]
- Davidi, E.S.; Dreifuss, T.; Motiei, M.; Shai, E.; Bragilovski, D.; Lubimov, L.; Kindler, M.J.J.; Popovtzer, A.; Don, J.; Popovtzer, R. Cisplatin-conjugated gold nanoparticles as a theranostic agent for head and neck cancer. Head Neck 2018, 40, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W.Y.; McMahon, M.T.; Kato, Y.; Liu, G.; Bulte, J.W.M.; Bhujwalla, Z.M.; Artemov, D.; Van Zijl, P.C.M. Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn. Reson. Med. 2012, 68, 1764–1773. [Google Scholar] [CrossRef] [PubMed]
- Muhanna, N.; Jin, C.S.; Huynh, E.; Chan, H.; Qiu, Y.; Jiang, W.; Cui, L.; Burgess, L.; Akens, M.K.; Chen, J.; et al. Phototheranostic porphyrin nanoparticles enable visualization and targeted treatment of head and neck cancer in clinically relevant models. Theranostics 2015, 5, 1428–1443. [Google Scholar] [CrossRef] [PubMed]
- Rich, L.J.; Miller, A.; Singh, A.K.; Seshadri, M. Photoacoustic imaging as an early biomarker of radio therapeutic efficacy in head and neck cancer. Theranostics 2018, 8, 2064–2078. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lin, X.; Shao, Y.; Xiang, F.; Samir, A.E. Imaging and screening of thyroid cancer. Radiol. Clin. N. Am. 2017, 55, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- James, B.C.; Aschebrook-Kilfoy, B.; White, M.G.; Applewhite, M.K.; Kaplan, S.P.; Angelos, P.; Kaplan, E.L.; Grogan, R.H. Quality of life in thyroid cancer - assessment of physician perceptions. J. Surg. Res. 2018, 226, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Parry, Z.; Macnab, R. Thyroid disease and thyroid surgery. Anaesth. Intensive Care Med. 2017, 18, 488–495. [Google Scholar] [CrossRef]
- Ahn, B.C. Personalized Medicine Based on Theranostic Radioiodine Molecular Imaging for Differentiated Thyroid Cancer. Biomed Res. Int. 2016, 2016, 1680464. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Cao, X.; Xu, G.; Song, Y.; Li, G.; Zheng, H.; Zhang, N. Potential role for carbon nanoparticles to guide central neck dissection in patients with papillary thyroid cancer. Surgery 2016, 160, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Yu, J.; Fan, Y.-X.; Lu, X.-B. Carbon nanoparticle lymph node tracer improves the outcomes of surgical treatment in papillary thyroid cancer. Cancer Biomarkers Sect. A Dis. Markers 2018, 23, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Du, Z.P.; Qiu, N.C.; Liu, M.E.; Liu, S.; Jiang, D.Z.; Zhang, W.; Qiu, M. Application of carbon nanoparticles accelerates the rapid recovery of parathyroid function during thyroid carcinoma surgery with central lymph node dissection: A retrospective cohort study. Int. J. Surg. 2016, 36, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, X.; Zhang, H.; Chen, L.; Zhou, S.; Wu, K.; Wang, Z.; Kong, L.; Zhuang, H. Carbon nanoparticle-guided central lymph node dissection in clinically node-negative patients with papillary thyroid carcinoma. Head Neck 2016, 38, 840–845. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, B.; Li, T.; Li, J. Thyroid Cancer Detection by Ultrasound Molecular Imaging with SHP2-Targeted Perfluorocarbon Nanoparticles. Contrast Media Mol. Imaging 2018, 2018. [Google Scholar] [CrossRef]
- Li, S.; Zhang, D.; Sheng, S.; Sun, H. Targeting thyroid cancer with acid-triggered release of doxorubicin from silicon dioxide nanoparticles. Int. J. Nanomed. 2017, 12, 5993–6003. [Google Scholar] [CrossRef]
- Sun, T.; Dou, J.H.; Liu, S.; Wang, X.; Zheng, X.; Wang, Y.; Pei, J.; Xie, Z. Second Near-Infrared Conjugated Polymer Nanoparticles for Photoacoustic Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 7919–7926. [Google Scholar] [CrossRef]
- Godone, R.L.N.; Leitão, G.M.; Araújo, N.B.; Castelletti, C.H.M.; Lima-Filho, J.L.; Martins, D.B.G. Clinical and molecular aspects of breast cancer: Targets and therapies. Biomed. Pharmacother. 2018, 106, 14–34. [Google Scholar] [CrossRef]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Ju, J.; Zhu, A.-J.; Yuan, P. Progress in targeted therapy for breast cancer. Chronic Dis. Transl. Med. 2018, 4, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Semkina, A.S.; Abakumov, M.A.; Skorikov, A.S.; Abakumova, T.O.; Melnikov, P.A.; Grinenko, N.F.; Cherepanov, S.A.; Vishnevskiy, D.A.; Naumenko, V.A.; Ionova, K.P.; et al. Multimodal doxorubicin loaded magnetic nanoparticles for VEGF targeted theranostics of breast cancer. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Kayani, Z.; Bordbar, A.K.; Firuzi, O. Novel folic acid-conjugated doxorubicin loaded β-lactoglobulin nanoparticles induce apoptosis in breast cancer cells. Biomed. Pharmacother. 2018, 107, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, J.; Yang, X.; Yang, Y.; Quan, K.; Xie, N.; Wu, Y.; Ma, C.; Wang, K. Gold nanoparticle-based 2′-O-methyl modified DNA probes for breast cancerous theranostics. Talanta 2018, 183, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Yuvakkumar, R.; Vijayakumar, S.; Vaseeharan, B. Cytotoxicity of phloroglucinol engineered silver (Ag) nanoparticles against MCF-7 breast cancer cell lines. Mater. Chem. Phys. 2018, 220, 402–408. [Google Scholar] [CrossRef]
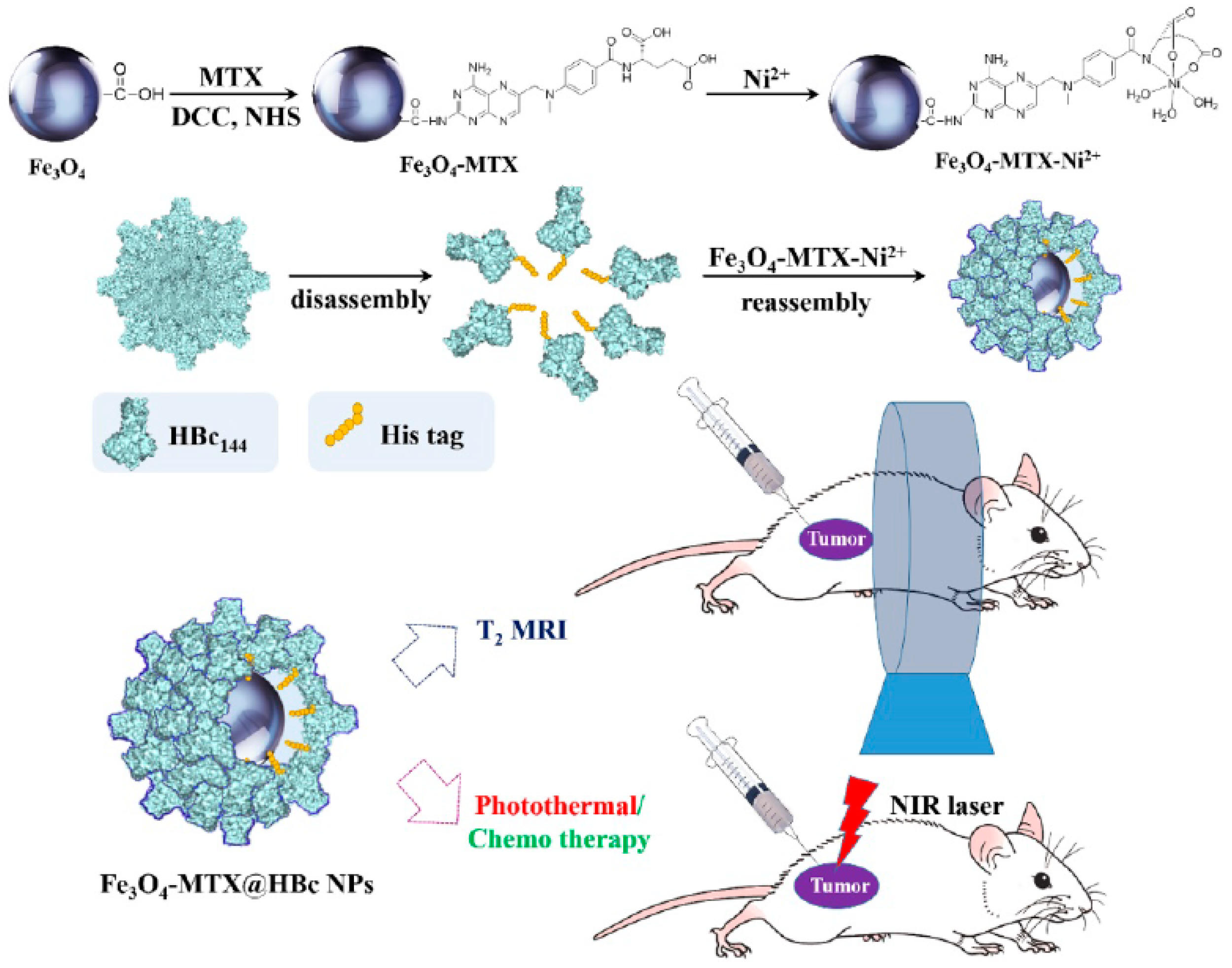
- Zhang, Q.; Shan, W.; Ai, C.; Chen, Z.; Zhou, T.; Lv, X.; Zhou, X.; Ye, S.; Ren, L.; Wang, X. Construction of multifunctional Fe3O4-MTX@HBc nanoparticles for MR Imaging and Photothermal Therapy/Chemotherapy. Nanotheranostics 2018, 2, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, X.-Y.; Chen, Y.-L.; Liu, F.-Q.; Tan, M.-X.; Ao, M.; Yu, J.-H.; Ran, H.; Wang, Z.-X. A light-controllable specific drug delivery nanoplatform for targeted bimodal imaging-guided photothermal/chemo synergistic cancer therapy. Acta Biomater. 2018, 80, 308–326. [Google Scholar] [CrossRef]
- Kiess, A.P.; Banerjee, S.R.; Mease, R.C.; Rowe, S.P.; Rao, A.; Foss, C.A.; Chen, Y.; Yang, X.; Cho, S.Y.; Nimmagadda, S.; et al. Prostate-specific membrane antigen as a target for cancer imaging and therapy. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 241–268. [Google Scholar]
- Emmett, L.; Willowson, K.; Violet, J.; Shin, J.; Blanksby, A.; Lee, J. Lutetium 177 PSMA radionuclide therapy for men with prostate cancer: A review of the current literature and discussion of practical aspects of therapy. J. Med. Radiat. Sci. 2017, 64, 52–60. [Google Scholar] [CrossRef]
- Baratto, L.; Jadvar, H.; Iagaru, A. Prostate Cancer Theranostics Targeting Gastrin-Releasing Peptide Receptors. Mol. Imaging Biol. 2018, 20, 501–509. [Google Scholar] [CrossRef]
- Nock, B.A.; Kaloudi, A.; Lymperis, E.; Giarika, A.; Kulkarni, H.R.; Klette, I.; Singh, A.; Krenning, E.P.; de Jong, M.; Maina, T.; et al. Theranostic Perspectives in Prostate Cancer with the Gastrin-Releasing Peptide Receptor Antagonist NeoBOMB1: Preclinical and First Clinical Results. J. Nucl. Med. 2017, 58, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Belz, J.; Castilla-Ojo, N.; Sridhar, S.; Kumar, R. Radiosensitizing silica nanoparticles encapsulating docetaxel for treatment of prostate cancer. In Methods in Molecular Biology; 2017; Volume 1530, pp. 403–409. [Google Scholar] [CrossRef]
- Loiseau, A.; Boudon, J.; Mirjolet, C.; Créhange, G.; Millot, N. Taxane-Grafted Metal-Oxide Nanoparticles as a New Theranostic Tool against Cancer: The Promising Example of Docetaxel-Functionalized Titanate Nanotubes on Prostate Tumors. Adv. Healthc. Mater. 2017, 6, 1700245. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, Y.; Wang, Y.; Zhang, M.; Luo, Y.; Tang, J.; Wang, Z.; Wang, D.; Hao, L.; Wang, Z. Paclitaxel-loaded and A10-3.2 aptamer-targeted poly(lactide-co-glycolic acid) nanobubbles for ultrasound imaging and therapy of prostate cancer. Int. J. Nanomed. 2017, 12, 5313–5330. [Google Scholar] [CrossRef] [PubMed]
- Elgqvist, J. Nanoparticles as theranostic vehicles in experimental and clinical applications-focus on prostate and breast cancer. Int. J. Mol. Sci. 2017, 18, 1102. [Google Scholar] [CrossRef] [PubMed]
- Leandro, F.Z.; Martins, J.; Fontes, A.M.; Tedesco, A.C. Evaluation of theranostic nanocarriers for near-infrared imaging and photodynamic therapy on human prostate cancer cells. Colloids Surf. B Biointerfaces 2017, 154, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, D.; Kim, J.P.; Yang, J. Highly photostable nanogels for fluorescence-based theranostics. Bioact. Mater. 2018, 3, 39–47. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, S.; Agarwal, P.; Dumbleton, J.; Yu, J.; Lu, X.; He, X. Multi-layered polymeric nanoparticles for pH-responsive and sequenced release of theranostic agents. Chem. Commun. 2015, 51, 7733–7736. [Google Scholar] [CrossRef] [PubMed]
- Flores, O.; Santra, S.; Kaittanis, C.; Bassiouni, R.; Khaled, A.S.; Khaled, A.R.; Grimm, J.; Perez, J.M. PSMA-targeted theranostic nanocarrier for prostate cancer. Theranostics 2017, 7, 2477–2494. [Google Scholar] [CrossRef] [PubMed]
- Mangadlao, J.D.; Wang, X.; McCleese, C.; Escamilla, M.; Ramamurthy, G.; Wang, Z.; Govande, M.; Basilion, J.P.; Burda, C. Prostate-Specific Membrane Antigen Targeted Gold Nanoparticles for Theranostics of Prostate Cancer. ACS Nano 2018, 12, 3714–3725. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.; Mukherjee, S.; Chakraborty, I.; Dash, T.K.; Senapati, S.; Bhattacharyya, R.; Shunmugam, R. Iron(III) Coordinated Polymeric Nanomaterial: A Next-Generation Theranostic Agent for High-Resolution T1-Weighted Magnetic Resonance Imaging and Anticancer Drug Delivery. ACS Biomater. Sci. Eng. 2018, 4, 1738–1749. [Google Scholar] [CrossRef]
- Moeendarbari, S.; Tekade, R.; Mulgaonkar, A.; Christensen, P.; Ramezani, S.; Hassan, G.; Jiang, R.; Öz, O.K.; Hao, Y.; Sun, X. Theranostic Nanoseeds for Efficacious Internal Radiation Therapy of Unresectable Solid Tumors. Sci. Rep. 2016, 6, 20614. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, K.T.; Nicol, J.R.; Ghita, M.; Rosa, S.; Chaudhary, P.; McGarry, C.K.; McCarthy, H.O.; Jimenez-Sanchez, G.; Bazzi, R.; Roux, S.; et al. Preclinical evaluation of gold-DTDTPA nanoparticles as theranostic agents in prostate cancer radiotherapy. Nanomedicine 2016, 11, 2035–2047. [Google Scholar] [CrossRef] [PubMed]
- Avvakumova, S.; Galbiati, E.; Sironi, L.; Locarno, S.A.; Gambini, L.; Macchi, C.; Pandolfi, L.; Ruscica, M.; Magni, P.; Collini, M.; et al. Theranostic Nanocages for Imaging and Photothermal Therapy of Prostate Cancer Cells by Active Targeting of Neuropeptide-Y Receptor. Bioconjug. Chem. 2016, 27, 2911–2922. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Guo, W.; Long, Q.; Ma, A.; Liu, Q.; Zhang, H.; Huang, Y.; Chandrasekaran, S.; Pan, C.; Lam, K.S.; et al. HSP90 inhibitor encapsulated photo-theranostic nanoparticles for synergistic combination cancer therapy. Theranostics 2016, 6, 1324–1335. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine (Lond) 2013, 8, 1509–1528. [Google Scholar] [CrossRef] [PubMed]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Lamichhane, N.; Udayakumar, T.S.; D’Souza, W.D.; Simone, C.B.; Raghavan, S.R.; Polf, J.; Mahmood, J. Liposomes: Clinical applications and potential for image-guided drug delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef]
- Petersen, A.L.; Hansen, A.E.; Gabizon, A.; Andresen, T.L. Liposome imaging agents in personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1417–1435. [Google Scholar] [CrossRef]
- Al-Jamal, W.T.; Al-Jamal, K.T.; Tian, B.; Cakebread, A.; Halket, J.M.; Kostarelos, K. Tumor targeting of functionalized quantum dot-liposome hybrids by intravenous administration. Mol. Pharm. 2009, 6, 520–530. [Google Scholar] [CrossRef]
- Wang, Q.; Chao, Y.-M. Multifunctional quantum dots and liposome complexes in drug delivery. J. Biomed. Res. 2018, 32, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, R.; Estelrich, J.; Busquets, M.A. Liposomes loaded with hydrophobic iron oxide nanoparticles: Suitable T(2) contrast agents for MRI. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, E.; Nizri, E.; Golenser, J.; Shmuel, M.; Magdassi, S.; Eyal, S. Imaging the urinary pathways in mice by liposomal indocyanine green. Nanomedicine 2015, 11, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Liu, D.; Zhou, G.; Li, Y.; Wang, P.; Hu, K.; Gu, N.; Ji, M. Liposomally formulated phospholipid-conjugated novel near-infrared fluorescence probe for particle size effect on cellular uptake and biodistribution in vivo. Colloids Surf. B. Biointerfaces 2018, 161, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Kim, H.-C.; Wolfram, J.; Mu, C.; Zhang, W.; Liu, H.; Xie, Y.; Mai, J.; Zhang, H.; Li, Z.; et al. A liposome encapsulated ruthenium polypyridine complex as a theranostic platform for triple-negative breast cancer. Nano Lett. 2017, 17, 2913–2920. [Google Scholar] [CrossRef]
- Cittadino, E.; Ferraretto, M.; Torres, E.; Maiocchi, A.; Crielaard, B.J.; Lammers, T.; Storm, G.; Aime, S.; Terreno, E. MRI evaluation of the antitumor activity of paramagnetic liposomes loaded with prednisolone phosphate. Eur. J. Pharm. Sci. 2012, 45, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Rizzitelli, S.; Giustetto, P.; Boffa, C.; Delli Castelli, D.; Cutrin, J.C.; Aime, S.; Terreno, E. In vivo MRI visualization of release from liposomes triggered by local application of pulsed low-intensity non-focused ultrasound. Nanomedicine 2014, 10, 901–904. [Google Scholar] [CrossRef]
- Beztsinna, N.; Tsvetkova, Y.; Jose, J.; Rhourri-Frih, B.; Al Rawashdeh, W.; Lammers, T.; Kiessling, F.; Bestel, I. Photoacoustic imaging of tumor targeting with riboflavin-functionalized theranostic nanocarriers. Int. J. Nanomed. 2017, 12, 3813–3825. [Google Scholar] [CrossRef]
- Lozano, N.; Al-Ahmady, Z.S.; Beziere, N.S.; Ntziachristos, V.; Kostarelos, K. Monoclonal antibody-targeted PEGylated liposome-ICG encapsulating doxorubicin as a potential theranostic agent. Int. J. Pharm. 2015, 482, 2–10. [Google Scholar] [CrossRef]
- Shemesh, C.S.; Moshkelani, D.; Zhang, H. Thermosensitive liposome formulated indocyanine green for near-infrared triggered photodynamic therapy: In vivo evaluation for triple-negative breast cancer. Pharm. Res. 2015, 32, 1604–1614. [Google Scholar] [CrossRef]
- Mikhaylov, G.; Mikac, U.; Magaeva, A.A.; Itin, V.I.; Naiden, E.P.; Psakhye, I.; Babes, L.; Reinheckel, T.; Peters, C.; Zeiser, R.; et al. Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nat. Nanotechnol. 2011, 6, 594–602. [Google Scholar] [CrossRef]
- Feng, L.; Cheng, L.; Dong, Z.; Tao, D.; Barnhart, T.E.; Cai, W.; Chen, M.; Liu, Z. Theranostic liposomes with hypoxia-activated prodrug to effectively destruct hypoxic tumors post-photodynamic therapy. ACS Nano 2017, 11, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Rizzitelli, S.; Giustetto, P.; Cutrin, J.C.; Delli Castelli, D.; Boffa, C.; Ruzza, M.; Menchise, V.; Molinari, F.; Aime, S.; Terreno, E. Sonosensitive theranostic liposomes for preclinical in vivo MRI-guided visualization of doxorubicin release stimulated by pulsed low intensity non-focused ultrasound. J. Control. Release 2015, 202, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Rizzitelli, S.; Giustetto, P.; Faletto, D.; Delli Castelli, D.; Aime, S.; Terreno, E. The release of Doxorubicin from liposomes monitored by MRI and triggered by a combination of US stimuli led to a complete tumor regression in a breast cancer mouse model. J. Control. Release 2016, 230, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Lei, M.; Tan, X.; Tan, F.; Li, N. Theranostic liposomes containing conjugated polymer dots and doxorubicin for bio-imaging and targeted therapeutic delivery. RSC Adv. 2016, 6, 1945–1957. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Zhu, D.; Song, C. Design of multifunctional magnetic iron oxide nanoparticles/mitoxantrone-loaded liposomes for both magnetic resonance imaging and targeted cancer therapy. Int. J. Nanomed. 2014, 9, 4055–4066. [Google Scholar] [CrossRef]
- Dai, W.; Yang, F.; Ma, L.; Fan, Y.; He, B.; He, Q.; Wang, X.; Zhang, H.; Zhang, Q. Combined mTOR inhibitor rapamycin and doxorubicin-loaded cyclic octapeptide modified liposomes for targeting integrin α3 in triple-negative breast cancer. Biomaterials 2014, 35, 5347–5358. [Google Scholar] [CrossRef]
- Yaari, Z.; da Silva, D.; Zinger, A.; Goldman, E.; Kajal, A.; Tshuva, R.; Barak, E.; Dahan, N.; Hershkovitz, D.; Goldfeder, M.; et al. Theranostic barcoded nanoparticles for personalized cancer medicine. Nat. Commun. 2016, 7, 13325. [Google Scholar] [CrossRef]
- Zhao, P.; Zheng, M.; Luo, Z.; Gong, P.; Gao, G.; Sheng, Z.; Zheng, C.; Ma, Y.; Cai, L. NIR-driven smart theranostic nanomedicine for on-demand drug release and synergistic antitumour therapy. Sci. Rep. 2015, 5, 14258. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, C.; Sun, X.; Chen, J.; Yang, Z.; Zhao, H.; Feng, L.; Liu, Z. H2O2-responsive liposomal nanoprobe for photoacoustic inflammation imaging and tumor theranostics via in vivo chromogenic assay. Proc. Natl. Acad. Sci. USA 2017, 114, 5343–5348. [Google Scholar] [CrossRef]
- Zheng, X.-C.; Ren, W.; Zhang, S.; Zhong, T.; Duan, X.-C.; Yin, Y.-F.; Xu, M.-Q.; Hao, Y.-L.; Li, Z.-T.; Li, H.; et al. The theranostic efficiency of tumor-specific, pH-responsive, peptide-modified, liposome-containing paclitaxel and superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 2018, 13, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Kenny, G.D.; Kamaly, N.; Kalber, T.L.; Brody, L.P.; Sahuri, M.; Shamsaei, E.; Miller, A.D.; Bell, J.D. Novel multifunctional nanoparticle mediates siRNA tumour delivery, visualisation and therapeutic tumour reduction in vivo. J. Control. Release 2011, 149, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ma, J.; Li, Y.; Li, Q.; Tan, C.; Song, H.; Cai, S.; Chen, D.; Hou, Z.; Chen, Q.; et al. Core-interlayer-shell Fe3O4@mSiO2@lipid-PEG-methotrexate nanoparticle for multimodal imaging and multistage targeted chemo-photodynamic therapy. Int. J. Pharm. 2017, 521, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Y.; Ma, J.; Li, Q.; Li, Y.; Zhou, X.; Zhao, D.; Song, H.; Chen, Q.; Zhu, X. Light/magnetic hyperthermia triggered drug released from multi-functional thermo-sensitive magnetoliposomes for precise cancer synergetic theranostics. J. Control. Release 2018, 272, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.; Carter, K.; Luo, D.; Shao, S.; Geng, J.; Li, C.; Chitgupi, U.; Turowski, S.G.; Li, N.; Atilla-Gokcumen, G.E.; et al. Multifunctional liposomes for image-guided intratumoral chemo-phototherapy. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Li, J.; Pan, Y.; Zhang, X.; Zheng, X.; Wang, Z.; Zhang, M.; Zhang, H.; Chen, L. Noninvasive theranostic imaging of HSV-TK/GCV suicide gene therapy in liver cancer by folate-targeted quantum dot-based liposomes. Biomater. Sci. 2015, 3, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, F.; Yuan, C.; Li, M.; Wang, T.; Chen, B.; Jin, J.; Zhao, P.; Tong, J.; Luo, S.; et al. Magnetic nanoliposomes as in situ microbubble bombers for multimodality image-guided cancer theranostics. ACS Nano 2017, 11, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, E.-J.; Lee, H.; Kim, H.M.; Chang, M.J.; Park, S.Y.; Hong, K.S.; Kim, J.S.; Sessler, J.L. Liposomal texaphyrin theranostics for metastatic liver cancer. J. Am. Chem. Soc. 2016, 138, 16380–16387. [Google Scholar] [CrossRef]
- Wu, B.; Wan, B.; Lu, S.-T.; Deng, K.; Li, X.-Q.; Wu, B.-L.; Li, Y.-S.; Liao, R.-F.; Huang, S.-W.; Xu, H.-B. Near-infrared light-triggered theranostics for tumor-specific enhanced multimodal imaging and photothermal therapy. Int. J. Nanomed. 2017, 12, 4467–4478. [Google Scholar] [CrossRef]
- Xu, H.-L.; Yang, J.-J.; ZhuGe, D.-L.; Lin, M.-T.; Zhu, Q.-Y.; Jin, B.-H.; Tong, M.-Q.; Shen, B.-X.; Xiao, J.; Zhao, Y.-Z. Glioma-targeted delivery of a theranostic liposome integrated with quantum dots, superparamagnetic iron oxide, and cilengitide for dual-imaging guiding cancer surgery. Adv. Healthc. Mater. 2018, 7, e1701130. [Google Scholar] [CrossRef]
- de Smet, M.; Heijman, E.; Langereis, S.; Hijnen, N.M.; Grüll, H. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: An in vivo proof-of-concept study. J. Control. Release 2011, 150, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Saesoo, S.; Sathornsumetee, S.; Anekwiang, P.; Treetidnipa, C.; Thuwajit, P.; Bunthot, S.; Maneeprakorn, W.; Maurizi, L.; Hofmann, H.; Rungsardthong, R.U.; et al. Characterization of liposome-containing SPIONs conjugated with anti-CD20 developed as a novel theranostic agent for central nervous system lymphoma. Colloids Surf. B Biointerfaces 2018, 161, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Rajora, M.A.; Ding, L.; Valic, M.; Jiang, W.; Overchuk, M.; Chen, J.; Zheng, G. Tailored theranostic apolipoprotein E3 porphyrin-lipid nanoparticles target glioblastoma. Chem. Sci. 2017, 8, 5371–5384. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Zhang, K.; Cao, Y.; Chen, X.; Wang, K.; Liu, M.; Pei, R. Hydrophobic IR-780 dye encapsulated in cRGD-conjugated solid lipid nanoparticles for NIR imaging-guided photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 12217–12226. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, K.; Yang, X.; Zhou, Y.; Ping, Q.; Oupicky, D.; Sun, M. Dual-function nanostructured lipid carriers to deliver IR780 for breast cancer treatment: Anti-metastatic and photothermal anti-tumor therapy. Acta Biomater. 2017, 53, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Olerile, L.D.; Liu, Y.; Zhang, B.; Wang, T.; Mu, S.; Zhang, J.; Selotlegeng, L.; Zhang, N. Near-infrared mediated quantum dots and paclitaxel co-loaded nanostructured lipid carriers for cancer theragnostic. Colloids Surf. B Biointerfaces 2017, 150, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Balzeau, J.; Pinier, M.; Berges, R.; Saulnier, P.; Benoit, J.-P.; Eyer, J. The effect of functionalizing lipid nanocapsules with NFL-TBS.40-63 peptide on their uptake by glioblastoma cells. Biomaterials 2013, 34, 3381–3389. [Google Scholar] [CrossRef]
- Ma, M.; Hao, Y.; Liu, N.; Yin, Z.; Wang, L.; Liang, X.; Zhang, X. A novel lipid-based nanomicelle of docetaxel: Evaluation of antitumor activity and biodistribution. Int. J. Nanomed. 2012, 7, 3389–3398. [Google Scholar] [CrossRef]
- Volkov, Y. Quantum dots in nanomedicine: Recent trends, advances and unresolved issues. Biochem. Biophys. Res. Commun. 2015, 468, 419–427. [Google Scholar] [CrossRef]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomed. 2017, 12, 5421–5431. [Google Scholar] [CrossRef]
- Maurice-Dror, C.; Perets, R.; Bar-Sela, G. Glucocorticoids as an adjunct to oncologic treatment in solid malignancies—not an innocent bystander. Crit. Rev. Oncol. Hematol. 2018, 126, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Herr, I.; Pfitzenmaier, J. Glucocorticoid use in prostate cancer and other solid tumours: Implications for effectiveness of cytotoxic treatment and metastases. Lancet. Oncol. 2006, 7, 425–430. [Google Scholar] [CrossRef]
- Lemaster, J.E.; Jokerst, J. V What is new in nanoparticle-based photoacoustic imaging? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Porcu, E.P.; Salis, A.; Gavini, E.; Rassu, G.; Maestri, M.; Giunchedi, P. Indocyanine green delivery systems for tumour detection and treatments. Biotechnol. Adv. 2016, 34, 768–789. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Ouhtit, A.; Gaur, R.; Fernando, A.; Schwarzenberger, P.; Su, J.; Ismail, M.F.; El-Sayyad, H.I.; Karande, A.; Elmageed, Z.A.; et al. Biochemical characterization of riboflavin carrier protein (RCP) in prostate cancer. Front. Biosci. 2009, 14, 3634–3640. [Google Scholar] [CrossRef]
- Jayapaul, J.; Arns, S.; Bunker, M.; Weiler, M.; Rutherford, S.; Comba, P.; Kiessling, F. In vivo evaluation of riboflavin receptor targeted fluorescent USPIO in mice with prostate cancer xenografts. Nano Res. 2016, 9, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.C.; Ho, R.J.Y. Interactions of indocyanine green and lipid in enhancing near-infrared fluorescence properties: The basis for near-infrared imaging in vivo. Biochemistry 2014, 53, 1275–1283. [Google Scholar] [CrossRef]
- Anja, P.; Anahid, J.; Janko, K. Cysteine cathepsins: Their biological and molecular significance in cancer stem cells. Semin. Cancer Biol. 2018. [Google Scholar] [CrossRef]
- Maurer, A.H.; Elsinga, P.; Fanti, S.; Nguyen, B.; Oyen, W.J.G.; Weber, W.A. Imaging the folate receptor on cancer cells with 99mTc-etarfolatide: Properties, clinical use, and future potential of Folate receptor imaging. J. Nucl. Med. 2014, 55, 701–704. [Google Scholar] [CrossRef]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef]
- Li, K.; Liu, B. Polymer encapsulated conjugated polymer nanoparticles for fluorescence bioimaging. J. Mater. Chem. 2012, 22, 1257–1264. [Google Scholar] [CrossRef]
- de Ruijter, T.C.; Veeck, J.; de Hoon, J.P.J.; van Engeland, M.; Tjan-Heijnen, V.C. Characteristics of triple-negative breast cancer. J. Cancer Res. Clin. Oncol. 2011, 137, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.-P.; Lu, Y.-Y.; Ji, L.-N.; Mao, Z.-W. Metallomics insights into the programmed cell death induced by metal-based anticancer compounds. Met. Integr. Biometal. Sci. 2014, 6, 978–995. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Emadi, A. Ruthenium-based chemotherapeutics: Are they ready for prime time? Cancer Chemother. Pharmacol. 2010, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Rahn, J.; Lichtenfels, R.; Wessjohann, L.A.; Seliger, B. Hydrogen peroxide - production, fate and role in redox signaling of tumor cells. Cell Commun. Signal. CCS 2015, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Damaghi, M.; Tafreshi, N.K.; Lloyd, M.C.; Sprung, R.; Estrella, V.; Wojtkowiak, J.W.; Morse, D.L.; Koomen, J.M.; Bui, M.M.; Gatenby, R.A.; et al. Chronic acidosis in the tumour microenvironment selects for overexpression of LAMP2 in the plasma membrane. Nat. Commun. 2015, 6, 8752. [Google Scholar] [CrossRef]
- Singh, A.; Trivedi, P.; Jain, N.K. Advances in siRNA delivery in cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 274–283. [Google Scholar] [CrossRef]
- Bruix, J.; Gores, G.J.; Mazzaferro, V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut 2014, 63, 844–855. [Google Scholar] [CrossRef]
- Gourlay, J.; Morokoff, A.P.; Luwor, R.B.; Zhu, H.-J.; Kaye, A.H.; Stylli, S.S. The emergent role of exosomes in glioma. J. Clin. Neurosci. 2017, 35, 13–23. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Tran, B.; Rosenthal, M.A. Survival comparison between glioblastoma multiforme and other incurable cancers. J. Clin. Neurosci. 2010, 17, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Man, F.; Lammers, T.; de Rosales, R.T.M. Imaging nanomedicine-based drug delivery: A review of clinical studies. Mol. Imaging Biol. 2018, 20, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Giovinazzo, H.; Kumar, P.; Sheikh, A.; Brooks, K.M.; Ivanovic, M.; Walsh, M.; Caron, W.P.; Kowalsky, R.J.; Song, G.; Whitlow, A.; et al. Technetium Tc 99m sulfur colloid phenotypic probe for the pharmacokinetics and pharmacodynamics of PEGylated liposomal doxorubicin in women with ovarian cancer. Cancer Chemother. Pharmacol. 2016, 77, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shields, A.F.; Siegel, B.A.; Miller, K.D.; Krop, I.; Ma, C.X.; LoRusso, P.M.; Munster, P.N.; Campbell, K.; Gaddy, D.F.; et al. (64)Cu-MM-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin. Cancer Res. 2017, 23, 4190–4202. [Google Scholar] [CrossRef] [PubMed]
- Lyon, P.C.; Gray, M.D.; Mannaris, C.; Folkes, L.K.; Stratford, M.; Campo, L.; Chung, D.Y.F.; Scott, S.; Anderson, M.; Goldin, R.; et al. Safety and feasibility of ultrasound-triggered targeted drug delivery of doxorubicin from thermosensitive liposomes in liver tumours (TARDOX): A single-centre, open-label, phase 1 trial. Lancet. Oncol. 2018, 19, 1027–1039. [Google Scholar] [CrossRef]
- Song, Q.; Song, H.; Xu, J.; Huang, J.; Hu, M.; Gu, X.; Chen, J.; Zheng, G.; Chen, H.; Gao, X. Biomimetic ApoErReconstituted high density lipoprotein nanocarrier for blood-brain barrier penetration and amyloid beta-targeting drug delivery. Mol. Pharm. 2016, 13, 3976–3987. [Google Scholar] [CrossRef]
- Sarkar, G.; Curran, G.L.; Mahlum, E.; Decklever, T.; Wengenack, T.M.; Blahnik, A.; Hoesley, B.; Lowe, V.J.; Poduslo, J.F.; Jenkins, R.B. A carrier for non-covalent delivery of functional beta-galactosidase and antibodies against amyloid plaques and IgM to the brain. PLoS ONE 2011, 6, e28881. [Google Scholar] [CrossRef]
- Lin, Q.; Jin, C.S.; Huang, H.; Ding, L.; Zhang, Z.; Chen, J.; Zheng, G. Nanoparticle-enabled, image-guided treatment planning of target specific RNAi therapeutics in an orthotopic prostate cancer model. Small 2014, 10, 3072–3082. [Google Scholar] [CrossRef]
- Lopes, R.M.; Pereira, J.; Esteves, M.A.; Gaspar, M.M.; Carvalheiro, M.; Eleuterio, C.V.; Goncalves, L.; Jimenez-Ruiz, A.; Almeida, A.J.; Cruz, M.E.M. Lipid-based nanoformulations of trifluralin analogs in the management of Leishmania infantum infections. Nanomedicine (Lond) 2016, 11, 153–170. [Google Scholar] [CrossRef]
- Lopes, R.M.; Gaspar, M.M.; Pereira, J.; Eleutério, C.V.; Carvalheiro, M.; Almeida, A.J.; Cruz, M.E.M. Liposomes versus lipid nanoparticles: Comparative study of lipid-based systems as oryzalin carriers for the treatment of leishmaniasis. J. Biomed. Nanotechnol. 2014, 10, 3647–3657. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Souto, E.B.; Almeida, A.J.; Müller, R.H. Lipid nanoparticles (SLN®, NLC®) for cutaneous drug delivery: Structure, protection and skin effects. J. Biomed. Nanotechnol. 2007, 3, 317–331. [Google Scholar] [CrossRef]
- Mussi, S.V.; Torchilin, V.P. Recent trends in the use of lipidic nanoparticles as pharmaceutical carriers for cancer therapy and diagnostics. J. Mater. Chem. B 2013, 1, 5201. [Google Scholar] [CrossRef]
- Videira, M.A.; Botelho, M.F.; Santos, A.C.; Gouveia, L.F.; de Lima, J.J.P.; Almeida, A.J. Lymphatic uptake of pulmonary delivered radiolabelled solid lipid nanoparticles. J. Drug Target. 2002, 10, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Videira, M.A.; Gano, L.; Santos, C.; Neves, M.; Almeida, A.J. Lymphatic uptake of lipid nanoparticles following endotracheal administration. J. Microencapsul. 2006, 23, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Videira, M.; Almeida, A.J.; Fabra, A. Preclinical evaluation of a pulmonary delivered paclitaxel-loaded lipid nanocarrier antitumor effect. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1208–1215. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
| Product | Company | Clinical Phase | Therapeutic Modality | Diagnostic Modality | Proposed Indication | CT Identifier |
|---|---|---|---|---|---|---|
| CriPec® docetaxel | Cristal Therapeutics | Phase I | Docetaxel | PET (Zirconium-89) | Solid tumors | NCT03712423 |
| AGuIX® | NHTherAguix | Phase I | Radiation therapy | MRI (gadolinium-chelates) | Brain metastases | NCT02820454 |
| AGuIX® | NHTherAguix | Phase I | Radiation therapy or brachytherapy or chemotherapy (cisplatin) | MRI (gadolinium-chelates) | Gynecologic cancer | NCT03308604 |
| Iron oxide nanoparticles (SPIONs) | M.D. Anderson Cancer Center | Early Phase I | - | Ferumoxytol-based MRI | HNSCC | NCT01895829 |
| NBTXR3® | Nanobiotix | Phase I/II | Hafnium oxide nanoparticles (50 nm) | Radiation-stimulated technology (NanoX-Ray) via electron production | Multiple solid cancers, including head and neck cancer, rectal cancer, prostate cancer and breast cancer | NCT02805894 NCT03589339 NCT02901483 NCT02901483 NCT02465593 (Total of 5 active clinical trials) |
| Lipid-Based System/Drug/Imaging Agent | Detection Method | Tumor | Animal Model | Observations | Reference |
|---|---|---|---|---|---|
| Thermosensitive liposomes/doxorubicin/Gd-DTPA-BMA | MRI | Soft Tissue Sarcoma | Brown Norway rat syngeneic model | Liposomal content release was promoted by local application of hyperthermia. | [14] |
| PEG liposomes (14C)/QDs | β Radiation; ICP-MS | Melanoma | C57BL/6 syngeneic model | Biodistribution study. | [119] |
| PEG liposomes/ruthenium polypyridine complex | Fluorescence | TNBC | Athymic nude mice orthotopic model | Ruthenium polypyridine complex was used for imaging and therapy. | [124] |
| PEG liposomes/PLP/Gd-DOTAMA(C18)2 | MRI | Melanoma | C57BL/6 syngeneic model | ----- | [125] |
| PEG liposomes/Gadoteridol | MRI | Melanoma | C57BL/6 syngeneic model | Liposomal content release was promoted by a local application of pLINFU. | [126] |
| Targeted PEG liposomes/ICG | PAI; US | Epidermoid Carcinoma | CD1 (nu/nu) xenograft model | Biodistribution study Riboflavin was used as a targeting moiety. | [127] |
| Targeted PEG liposomes/doxorubicin/ICG | MSOT | Breast; Colon | Athymic nude-Foxn1 mice xenograft model | Monoclonal antibody hCTM01 was used as a targeting moiety. | [128] |
| Thermosensitive PEG liposomes/ICG | NIR | TNBC | Athymic nude mice (nu/nu) xenograft model | PDT. | [129] |
| PEG liposomes/SPIONs | MRI | Breast | Transgenic mice - MMTV-PyMT | ----- | [130] |
| PEG liposomes/AQ4N/photosensitizer hCe6; 64Cu isotope | Fluorescence, PAI and PET | Breast | BALB/c syngeneic model | AQ4N is a hypoxia-activated prodrug. | [131] |
| PEG liposomes/doxorubicin/Gadoteridol | MRI | Breast | BALB/c syngeneic model | Liposomal content release was promoted by local application of pLINFU and/or sonoporation. | [132,133] |
| Targeted PEG liposomes/doxorubicin/fluorescent probe (PFBT) | Fluorescence | Breast | Nude mice xenograft model | Folate was used as a targeting moiety. | [134] |
| Targeted PEG liposomes/mitoxantrone/SPIONs | MRI | Breast | Athymic nude BALB/c xenograft model | Gonadorelin, a peptide analogue of luteinizing hormone-releasing hormone (LHRH), was used as a targeting moiety. | [135] |
| Targeted PEG liposomes/doxorubicin/NIR probe DiD | NIR | TNBC | Nude BALB/c xenograft model | A cyclic octapeptide was used as a targeting moiety. | [136] |
| PEG liposomes/doxorubicin, gemcitabine, cisplatin or caffeine/DNA barcode and ICG | NIR | TNBC | BALB/c syngeneic model | Specific DNA barcodes were used to screen the therapeutic potency of each anticancer drugs using tumor biopsies. | [137] |
| Temperature-sensitive PEG liposomes/doxorubicin/ICG | NIR | Breast | Nude BALB/c xenograft model | NIR laser-driven chemotherapy and PTT. | [138] |
| PEG liposomes/H2O2-dependent chromogenic reaction | NIR; PAI | Glioma; Breast; Lymph node metastasis | BALB/c orthotopic and syngeneic models | ABTS was used as substrate for HRP for chromogenic reaction; NIR was used for PTT. | [139] |
| Targeted pH-sensitive PEG liposomes/paclitaxel/SPIONs | MRI | Breast | Nude BALB/c xenograft model | The peptide H7K(R2)2 was used as the targeting moiety. | [140] |
| PEG liposomes/siRNA/Gd-DOTA-DSA | MRI | Ovary | Nude BALB/c xenograft model | Functional delivery of anti-survivin. | [141] |
| Targeted PEG liposomes/doxorubicin/ZnPc | Fluorescence; MRI | Cervical | Nude mice xenograft model | Methotrexate was used as an FR-targeting moiety. | [142] |
| Thermosensitive PEG liposomes/methotrexate; doxorubicin/fluorescent dye Cy5.5; iron oxide NPs | NIR; MRI | Cervical | Nude BALB/c xenograft model | Methotrexate used as an anticancer drug and an FR-targeting moiety. | [143] |
| PEG liposomes/oxaliplatin/SRB; Gd-DTPA | Fluorescence; MRI | Colon | BALB/c syngeneic model | Porphyrin-lipid was used for the light-triggered release of liposomal content. | [144] |
| Targeted PEG liposomes/HSV-TK/GCV suicide gene system/QDs | NIR | Liver | BALB/c (nu/nu) xenograft model | Folate was used as a targeting moiety. | [145] |
| PEG liposomes/H2S/SPIONs; DiD | MRI; US; NIR | Liver | Nude BALB/c xenograft model | H2S is a hydrogen sulfide prodrug; ADT was used as an organic H2S donor; SPIONs were used for magnetic targeting and imaging. | [146] |
| Targeted PEG liposomes/doxorubicin/Gd3+ texaphyrin | MRI; Fluorescence | Liver | BALB/c orthotopic model | Folate was used as a targeting moiety; doxorubicin was used as a therapeutic and imaging agent. | [147] |
| Thermosensitive PEG liposomes/IR820; Iohexol; Gd-DTPA | Fluorescence; MIR; CT | Glioma | Nude BALB/c xenograft model | IR820 was used as an imaging and PTT agent. | [148] |
| PEG liposomes/cilengitide/QDs; SPIONs | NIR; MIR | Glioblastoma | Sprague-Dawley rat orthotopic model | ----- | [149] |
| Thermosensitive PEG liposomes/doxorubicin/[Gd(HPDO3A)(H2O)] | MRI | Gliosarcoma | Fisher 344 rat syngeneic model | Liposomal content release was promoted by a local application of HIFU. | [150] |
| Targeted PEG liposomes/Rituximab/SPIONs | MRI | Brain Lymphoma | Athymic nude mice xenograft model | Rituximab was used for targeting and therapy. | [151] |
| Targeted PEG lipid nanoparticles/porphyrin-lipid | Fluorescence | Glioblastoma | Athymic nude mice orthotopic model | apoE3 was used as a targeting moiety and porphyrin-lipid as an imaging modality. | [152] |
| Targeted SLNs/hydrophobic IR780 dye | NIR | Glioblastoma | Athymic nude mice xenograft model | Cycle RGD peptide (cRGD) was used as a targeting moiety and IR780 as an imaging and PTT agent. | [153] |
| Targeted NLCs/NIR dye IR780 | NIR | Breast | BALB/c syngeneic model | NIR dye IR780 was used for imaging and PTT. | [154] |
| NLCs/paclitaxel/QDs | NIR | Liver | Kunming mice syngeneic model | ----- | [155] |
| Targeted nanocapsules/paclitaxel/DiD | Fluorescence | Glioblastoma | C57Bl/6 orthotopic model | NFL-TBS.40-63 (cell-penetrating peptide) was used as a targeting moiety. | [156] |
| Micelles/docetaxel/NIR probe DiR | NIR | Breast | Nude BALB/c xenograft model | ----- | [157] |
| Lipid-Based System/Drug/Imaging Agent | Detection Method | Cancer | Clinical Phase | CT Identifier | Reference |
|---|---|---|---|---|---|
| PEG liposomes/doxorubicin/99mTc | SPECT/CT | Ovary | Early Study | N.A. | [182] |
| HER2-targeted PEG liposomes/doxorubicin/64Cu | PET | Advanced Breast Cancer | Phase I | NCT01304797 | [183] |
| Lyso-thermosensitive liposomes (TARDOX)/doxorubicin/US | US | Liver | Phase I | NCT02181075 | [184] |
| Type | Polymeric | Metallic * | Lipid-based | |
|---|---|---|---|---|
| Features | ||||
| Preparation method (complexity) | ++ | + | ++ | |
| Physico-chemical characterization (easiness) | ++ | + | +++ | |
| Stability | +++ | + | ++ | |
| Multifunctionality (possibility to apply different external stimuli) | ++ | +++ | ++ | |
| Potential toxicity | ++ | +++ | + | |
| In vivo general performance | ++ | + | +++ | |
| Scale-up (easiness) | + | ++ | + | |
| Cost | ++ | + | + | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems. Pharmaceutics 2019, 11, 22. https://doi.org/10.3390/pharmaceutics11010022
Silva CO, Pinho JO, Lopes JM, Almeida AJ, Gaspar MM, Reis C. Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems. Pharmaceutics. 2019; 11(1):22. https://doi.org/10.3390/pharmaceutics11010022
Chicago/Turabian StyleSilva, Catarina Oliveira, Jacinta Oliveira Pinho, Joana Margarida Lopes, António J. Almeida, Maria Manuela Gaspar, and Catarina Reis. 2019. "Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems" Pharmaceutics 11, no. 1: 22. https://doi.org/10.3390/pharmaceutics11010022
APA StyleSilva, C. O., Pinho, J. O., Lopes, J. M., Almeida, A. J., Gaspar, M. M., & Reis, C. (2019). Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems. Pharmaceutics, 11(1), 22. https://doi.org/10.3390/pharmaceutics11010022







