Magnetic Mixed Micelles Composed of a Non-Ionic Surfactant and Nitroxide Radicals Containing a d-Glucosamine Unit: Preparation, Stability, and Biomedical Application
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation, Stability and In Vitro MRI Contrast Ability of 2/4n
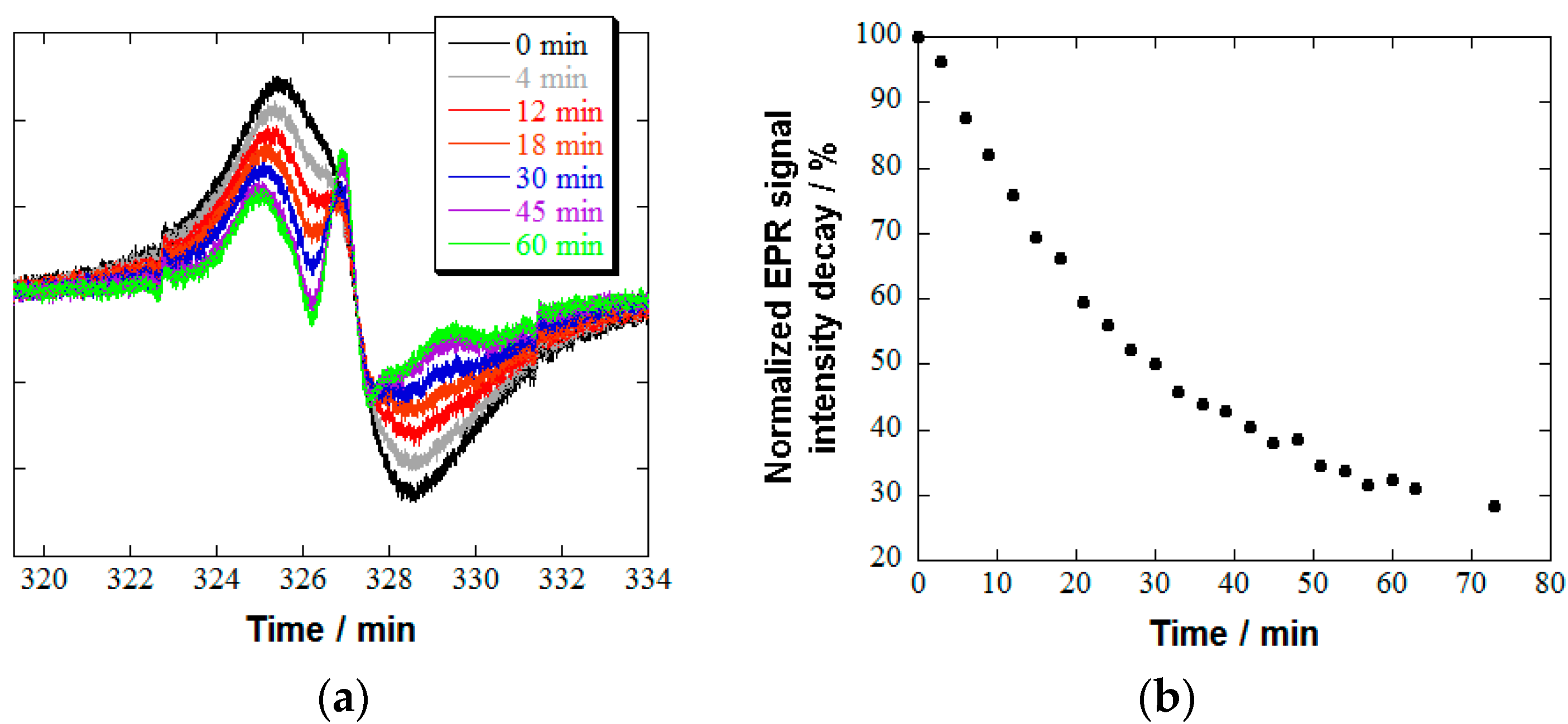
2.2. Reduction Resistivity of 2/414 in the Presence of Ascorbic Acid
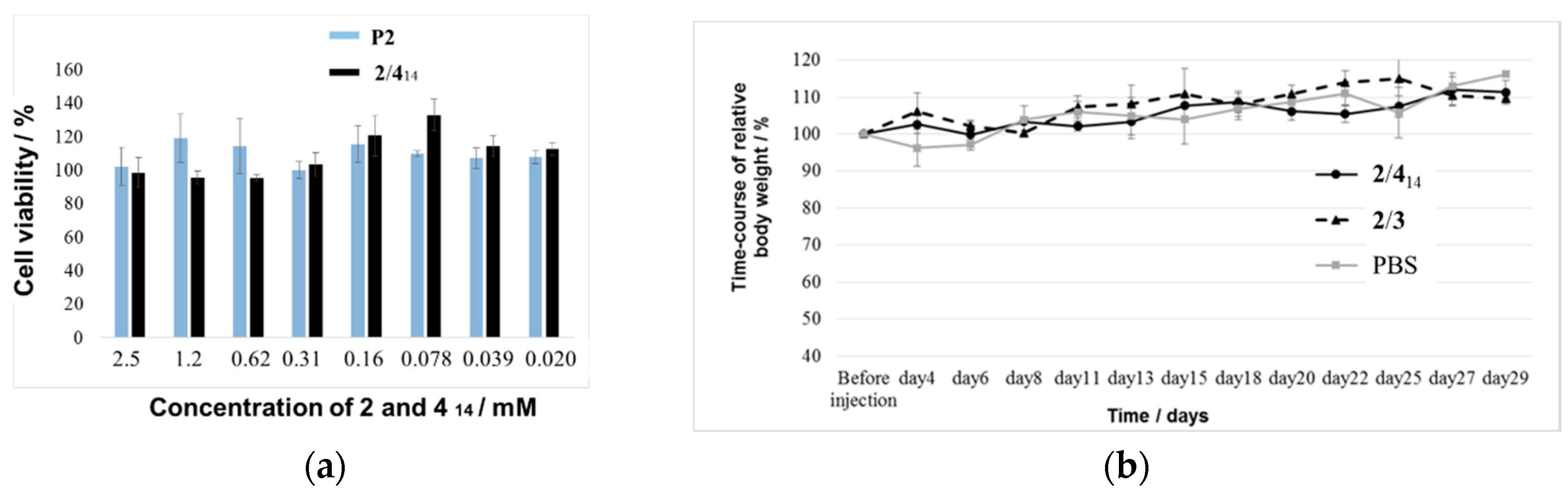
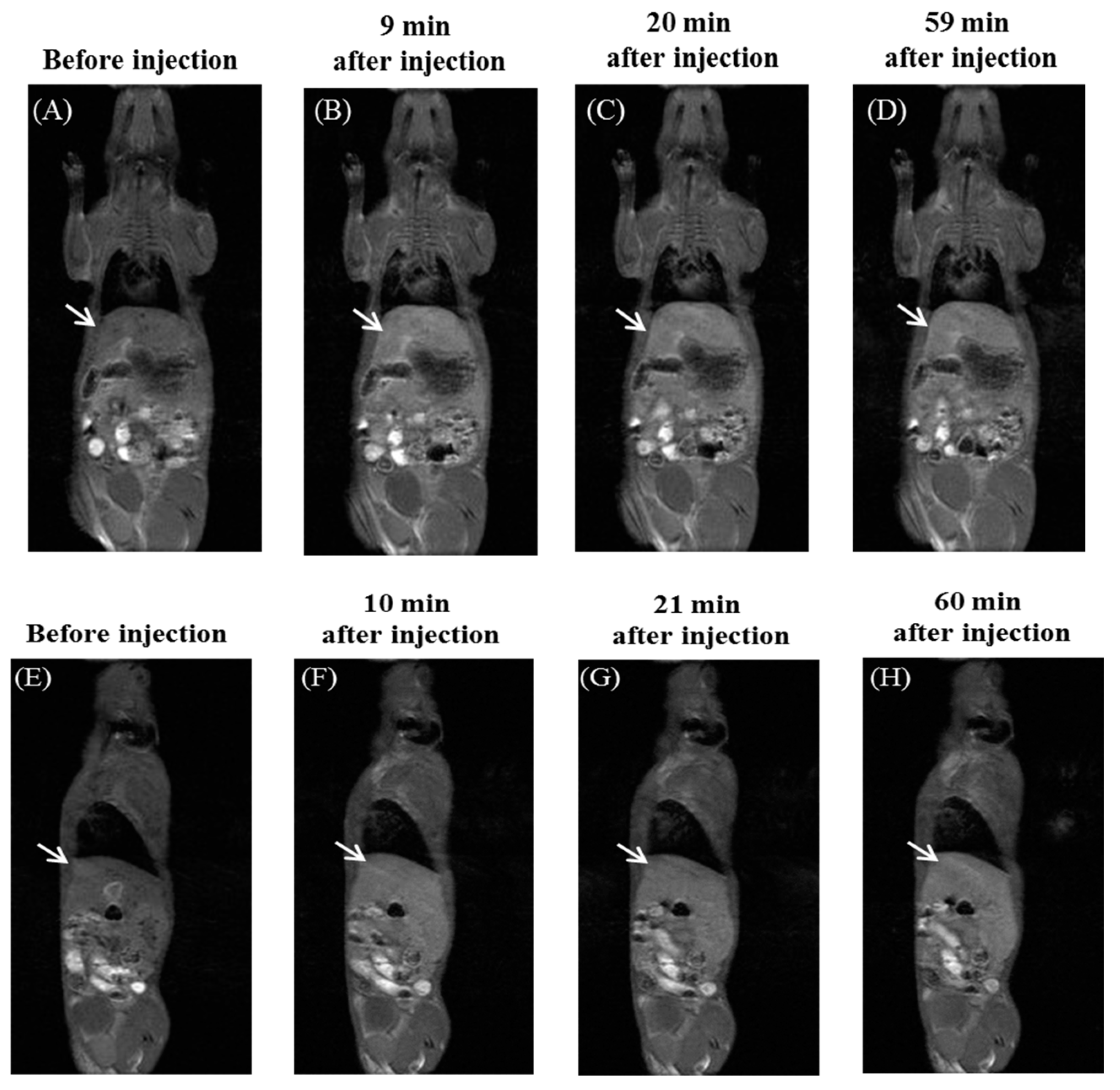
2.3. Biomedical Application of 2/414
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Molecular Imaging and Contrast Agent Database (MICAD) Internet, Bethesda (MD), National Center for Biotechnology Information (US), 2004–2013. Available online: http://www.ncbi.nlm.nih.gov/books/NBK5330/ (accessed on 1 March 2018).
- Malayeri, A.A.; Brooks, K.; Bryant, L.H.; Evers, R.; Kumar, P.; Reich, D.S.; Bluemke, D.A. NIH perspective on reports of gadolinium deposition in the brain. J. Am. Coll. Radiol. 2016, 13, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Gale, E.M.; Caravan, P.; Rao, A.G.; McDonald, R.J.; Winfeld, M.; Fleck, R.J.; Gee, M.S. Gadolinium-based contrast agents in pediatric magnetic resonance imaging. Pediatr. Radiol. 2017, 47, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Radbruch, A.; Weberling, L.D.; Kieslich, P.J.; Eidel, O.; Burth, S.; Kickingereder, P.; Heiland, S.; Wick, W.; Schlemmer, H.P.; Bendszus, M. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 2015, 275, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Fukusato, T.; Matsuda, M.; Toyoda, K.; Oba, H.; Kotoku, J.; Haruyama, T.; Kitajima, K.; Furui, S. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: Evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015, 276, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Gulani, V.; Calamante, F.; Shellock, F.G.; Kanal, E.; Reeder, S.B. Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol. 2017, 16, 564–570. [Google Scholar] [CrossRef]
- Thomsen, H.S.; Morcos, S.K.; Almen, T.; Bellin, M.F.; Bertolotto, M.; Bongartz, G.; Clement, O.; Leander, P.; Heinz-Peer, G.; Reimer, P.; et al. Nephrogenic systemic fibrosis and gadolinium-based contrastmedia: Updated ESUR contrast medium safety committee guidelines. Eur. Radiol. 2013, 23, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Fraum, T.J.; Ludwig, D.R.; Bashir, M.R.; Fowler, K.J. Gadolinium-based contrast agents: A comprehensive risk assessment. J. Magn. Reson. Imaging 2017, 46, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Afzal, V.; Brasch, R.C.; Nitecki, D.E.; Wolff, S. Nitroxyl spin label contrast enhancers for magnetic resonance imaging studies of acute toxicity and mutagenesis. Investig. Radiol. 1984, 19, 549–552. [Google Scholar] [CrossRef]
- Keana, J.F.W.; Pou, S.; Rosen, G.M. Nitroxides as potential contrast enhancing agents for MRI application: Influence of structure on the rate of reduction by rat hepatocytes, whole liver homogenate, subcellular fractions, and ascorbate. Magn. Reson. Med. 1987, 3, 83–88. [Google Scholar] [CrossRef]
- Saphier, O.; Silberstein, T.; Shames, A.I.; Likhtenshtein, G.I.; Maimon, E.; Mankuta, D.; Mazor, M.; Katz, M.; Meyerstein, D.; Meyerstein, N. The reduction of a nitroxide spin label as a probe of human blood antioxidant properties. Free Radic. Res. 2003, 37, 301–308. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiao, C.; Oyaizu, K.; Chikushi, N.; Chen, X.; Nishide, H. Synthesis of amphiphilic block copolymers bearing stable nitroxyl radicals. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5404–5414. [Google Scholar] [CrossRef]
- Dobrynin, S.A.; Glazachev, Y.I.; Gatilov, Y.V.; Chernyak, E.I.; Salnikov, G.E.; Kirilyuk, I.A. Synthesis of 3,4-bis(hydroxymethyl)-2,2,5,5-tetraethylpyrrolidin-1-oxyl via 1,3-dipolar cycloaddition of azomethine ylide to activated alkene. J. Org. Chem. 2018, 83, 5392–5397. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, T.; Mikyamoto, D.; Nagasaki, Y. Design of core-shell-type nanoparticles carrying stable radicals in the core. Biomacromolecules 2009, 10, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Marx, L.; Chiarelli, R.; Guiberteau, T.; Rassat, A. A comparative study of the reduction by ascorbate of 1,1,3,3-tetraethylisoindolin-2-yloxyl and of 1,1,3,3-tetramethylisoindolin-2-yloxyl. J. Chem. Soc. Perkin Trans. 1 2000, 1181–1182. [Google Scholar] [CrossRef]
- Paletta, J.T.; Pink, M.; Foley, B.; Rajca, S.; Rajca, A. Synthesis and reduction kinetics of sterically shielded pyrrolidine nitroxides. Org. Lett. 2012, 14, 5322–5325. [Google Scholar] [CrossRef] [PubMed]
- Kirilyuk, I.A.; Bobko, A.A.; Semenov, S.V.; Komarov, D.A.; Irtegova, I.G.; Grigor’ev, I.A.; Bagryanskaya, E. Effect of sterical shielding on the redox properties of imidazoline and imidazolidine nitroxides. J. Org. Chem. 2015, 80, 9118–9125. [Google Scholar] [CrossRef]
- Nagura, K.; Takemoto, Y.; Moronaga, S.; Uchida, Y.; Shimono, S.; Shiino, A.; Tanigaki, K.; Amano, T.; Yoshino, F.; Noda, Y.; et al. Preparation of robust metal-free magnetic nanoemulsions encapsulating low-molecular-weight nitroxide radicals and hydrophobic drugs directed toward MRI-visible targeted delivery. Chem. Eur. J. 2017, 23, 15713–15720. [Google Scholar] [CrossRef]
- Nagura, K.; Bogdanov, A.; Chumakova, N.; Vorobiev, A.K.; Moronaga, S.; Imai, H.; Matsuda, T.; Noda, Y.; Maeda, T.; Koizumi, S.; et al. Size-tunable MRI-visible nitroxide-based magnetic mixed micelles: Preparation, stability and theranostic application. Submitted for publication.
- Jiang, X.; Xin, H.; Ren, Q.; Gu, J.; Zhu, L.; Du, F.; Feng, C.; Xie, Y.; Sha, X.; Fang, X. Nanoparticles of 2-deoxy-d-glucose functionalized poly(ethyleneglycol)-co-poly(trimethylene carbonate) for dual-targeted drug delivery in glioma treatment. Biomaterials 2014, 35, 518–529. [Google Scholar] [CrossRef]
- Xiong, F.; Xiong, C.; Hua, X.; Shan, X.; Zhang, Y.; Gu, N. Preparation, characterization of 2-deoxy-d-glucose functionalized dimercaptosuccinic acid-coated maghemite nanoparticles for targeting tumor cells. Pham. Res. 2012, 29, 1087–1097. [Google Scholar] [CrossRef]
- Ranjbar-Navazi, Z.; Eskandani, M.; Johari-Ahar, M.; Nemati, A.; Akbari, H.; Davaran, S.; Omidi, Y. Doxorubicin-conjugated d-glucosamine- and folate- bi-functionalised InP/ZnS quantum dots for cancer cells imaging and therapy. J. Drug Target. 2018, 3, 267–277. [Google Scholar] [CrossRef]
- Korotcov, A.V.; Ye, Y.; Chen, Y.; Zhang, F.; Huang, S.; Lin, S.; Sridhar, R.; Achilefu, S.; Wang, P.C. Glucosamine-linked near-infrared fluorescent probes for imaging of solid tumor xenografts. Mol. Imaging Biol. 2012, 14, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Uematsu, T.; Nakayama, Y.; Takahashi, H.; Tsue, H.; Tanaka, K.; Tamura, R. Partial resolution of racemic trans-4-[5-(4-alkoxyphenyl)-2,5-dimethylpyrrolidine-1-oxyl-2-yl]benzoic acids by the diastereomer method with (R)- or (S)-1-phenylethylamine. Chirality 2008, 20, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Tamura, R.; Ikuma, N.; Shimono, S.; Yamauchi, J.; Aoki, Y.; Nohira, H. Synthesis and characterization of novel all-organic liquid crystalline radicals. Mol. Cryst. Liq. Cryst. 2007, 479, 1251–1259. [Google Scholar] [CrossRef]
- Zhang, Q.; Lebl, T.; Kulczynska, A.; Botting, N.P. The synthesis of novel hexa-13C-labelled glucosinolates from [13C6]-d-glucose. Tetrahedron 2009, 65, 4781–4876. [Google Scholar] [CrossRef]
- Soli, E.D.; Manoso, A.S.; Patterson, M.C.; DeShong, P. Azide and cyanide displacements via hypervalent silicate intermediates. J. Org. Chem. 1999, 64, 3171–3177. [Google Scholar] [CrossRef] [PubMed]
- Muhizi, T.; Grelier, S.; Coma, V. Synthesis and antibacterial activity of aminodeoxyglucose derivatives against Listeria innocua and Salmonella typhimurium. J. Agric. Food Chem. 2009, 57, 8770–8775. [Google Scholar] [CrossRef] [PubMed]
- Livramento, J.B.; Tóth, E.; Sour, A.; Borel, A.; Merbach, A.E.; Ruloff, R. High Relaxivity confined to a small molecular space: A metallostar-based, potential MRI contrast agent. Angew. Chem. Int. Ed. 2005, 44, 1480–1484. [Google Scholar] [CrossRef]
- Caravan, P.; Farrar, C.T.; Frullano, L.; Uppal, R. Influence of molecular parameters and increasing magnetic field strength on relaxivity of gadolinium- and manganese-based T1 contrast agents. Contrast Media Mol. Imaging 2009, 4, 89–100. [Google Scholar] [CrossRef]
- De Sousa, P.L.; Livramento, J.B.; Helm, L.; Merbach, A.E.; Meme, W.; Doan, B.T.; Beloeil, J.C.; Prata, M.I.; Santos, A.C.; Geraldes, C.F.; et al. In vivo MRI assessment of a novel GdIII-based contrast agent designed for high magnetic field applications. Contrast Media Mol. Imaging 2008, 3, 78–85. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the scientific panel on dietetic products, nutrition and allergies on a request from the commission related to the tolerable upper intake level of vitamin C (L-Ascorbic acid, its calcium, potassium and sodium salts and L-Ascorbyl-6-palmitate). EFSA J. 2004, 59, 1–21. [Google Scholar]
- Hyodo, F.; Matsumoto, K.; Matsumoto, A.; Mitchell, J.B.; Krishna, M.C. Probing the intracellular redox status of tumors with magnetic resonance imaging and redox-sensitive contrast agents. Cancer Res. 2006, 66, 9921–9928. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, F.; Chuang, K.H.; Goloshevsky, A.G.; Sulima, A.; Griffiths, G.L.; Mitchell, J.B.; Koretsky, A.P.; Krishna, M.C. Brain redox imaging using blood–brain barrier permeable nitroxide MRI contrast agent. J. Cereb. Blood Flow Metab. 2008, 28, 1165–1174. [Google Scholar] [CrossRef]
- Emoto, M.C.; Yamada, K.; Yamato, M.; Fujii, H.G. Novel ascorbic acid-resistive nitroxide in a lipid emulsion: An efficient brain imaging contrast agent for MRI of small rodents. Neurosci. Lett. 2013, 546, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Mito, F.; Matsuoka, Y.; Yamato, M.; Yamada, K. Nitroxides—Theory, Experiment and Applications; Kokorin, A.I., Ed.; INTECH: Rijeka, Croatia, 2012; Chapter 8. [Google Scholar]
- Ma, Y.; Huang, J.; Song, S.; Chen, H.; Zhang, Z. Cancer-targeted nanotheranostics: Recent advances and perspectives. Small 2016, 12, 4936–4954. [Google Scholar] [CrossRef] [PubMed]
- Sumer, B.; Gao, J. Theranostic nanomedicine for cancer. Nanomedicine 2008, 3, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Movassaghian, S.; Merkel, O.M.; Torchilin, V.P. Applications of polymer micelles for imaging and drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 691–707. [Google Scholar] [CrossRef] [PubMed]
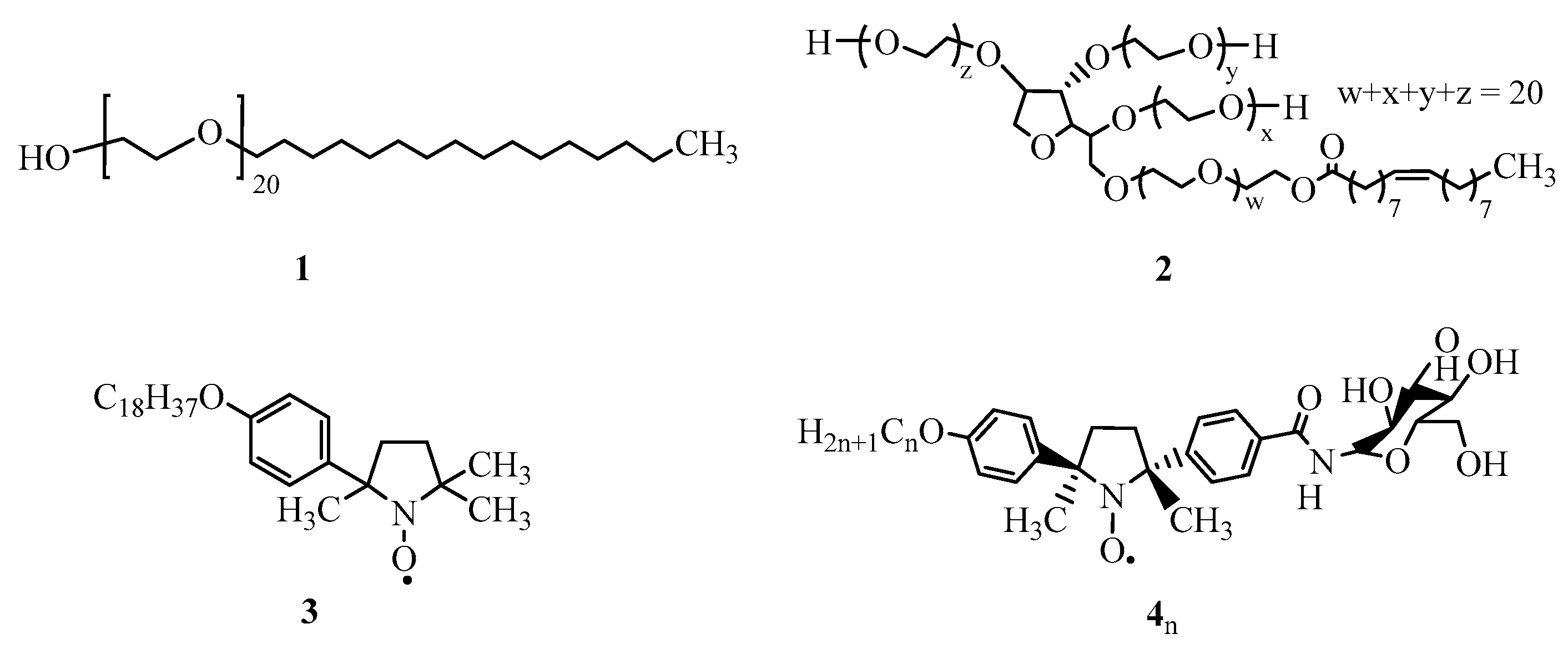
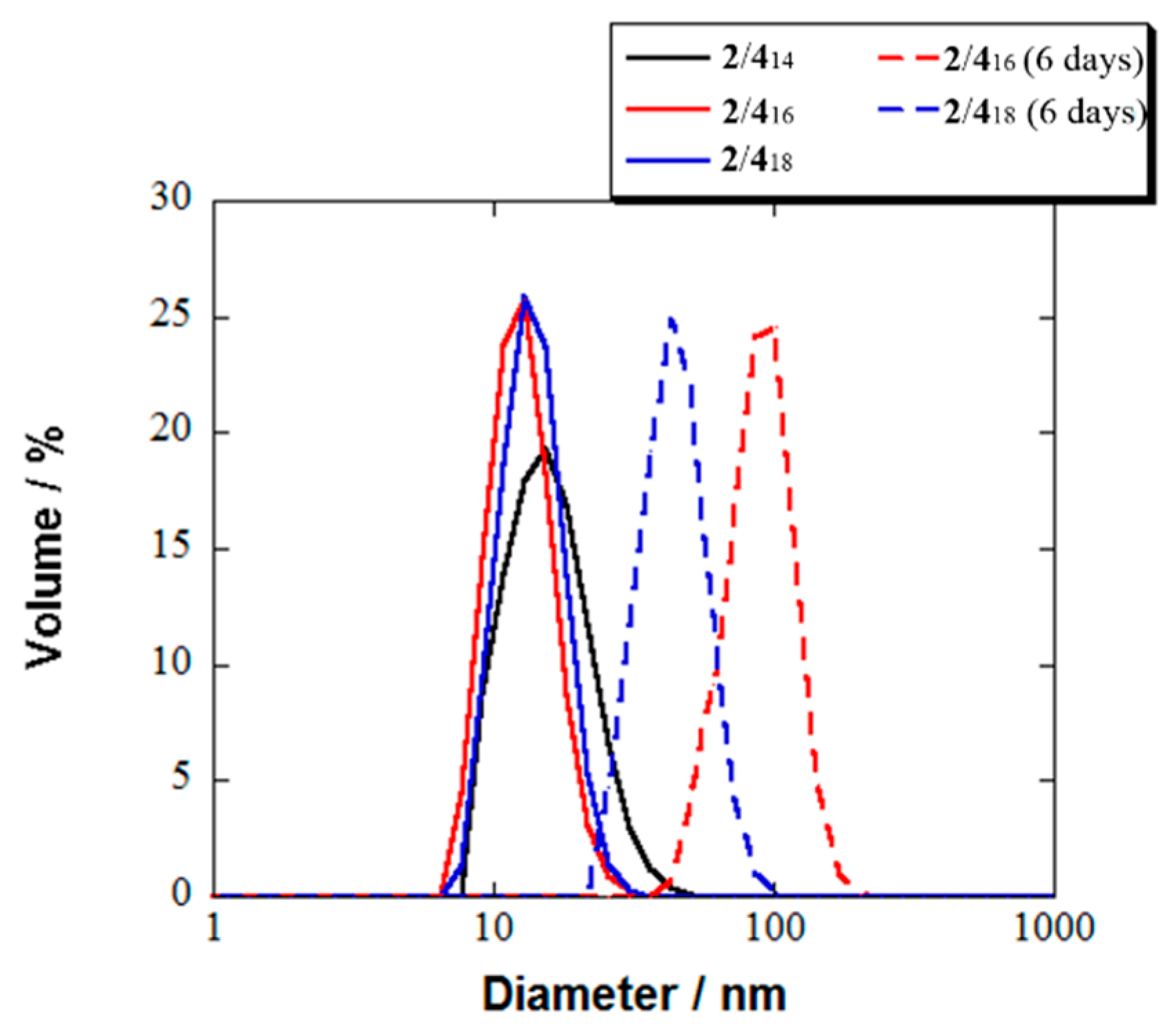
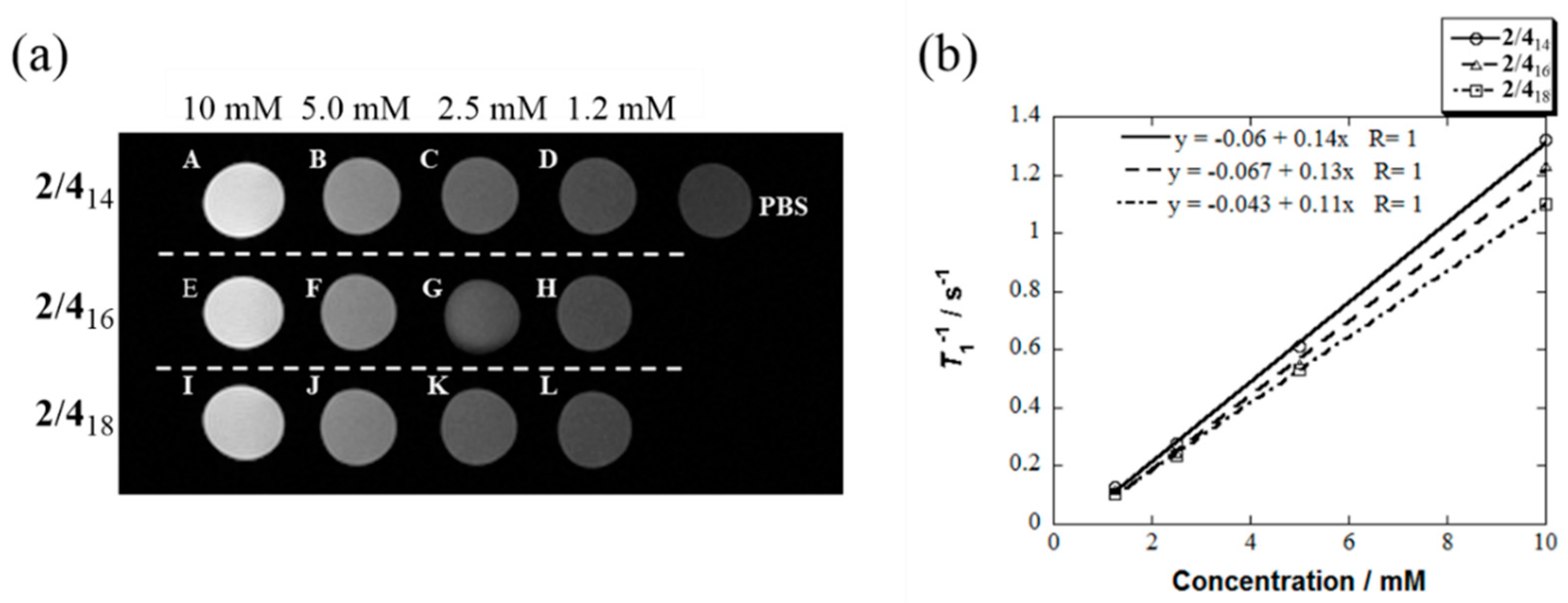
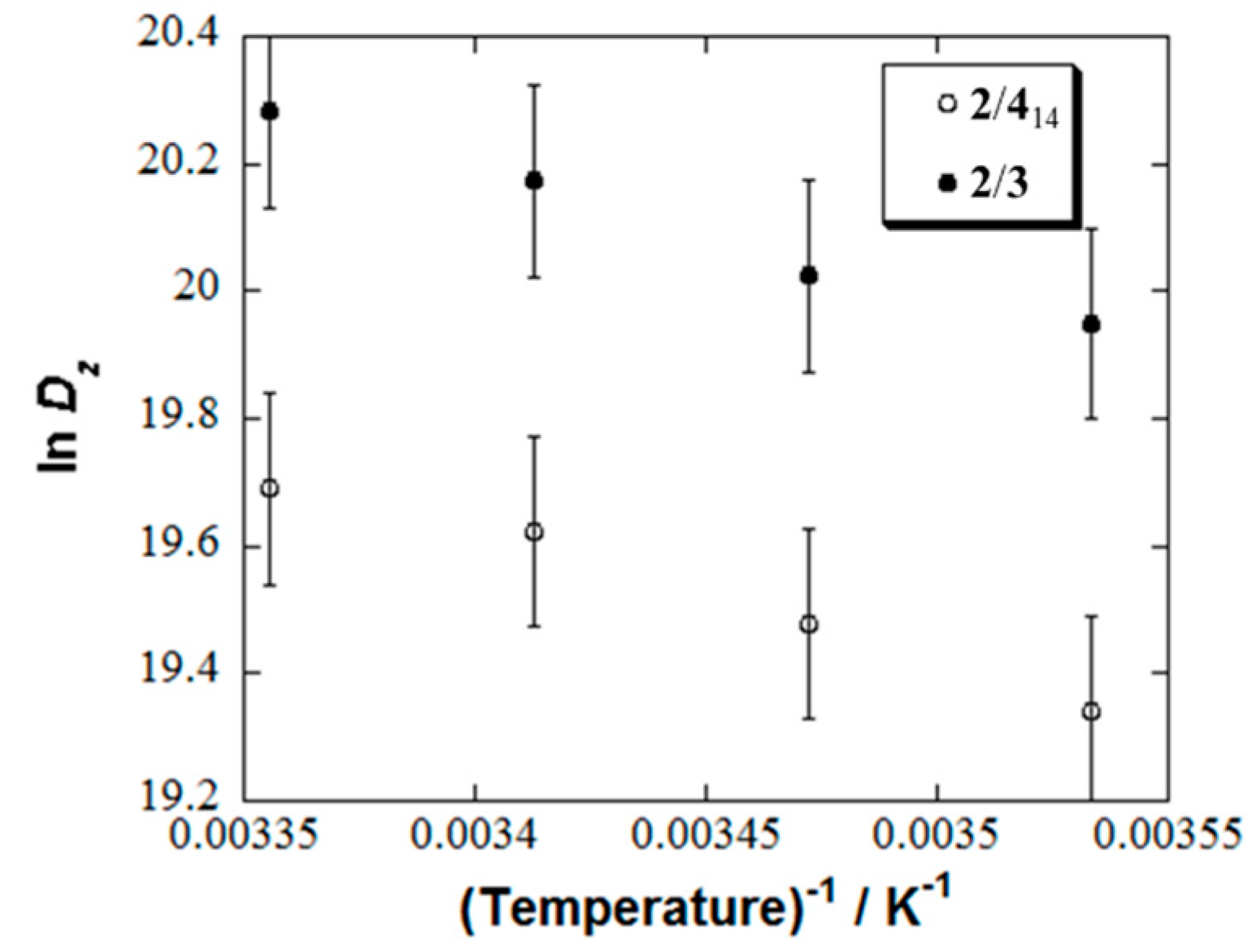
| Micelle | 2/414 | 2/416 | 2/418 |
|---|---|---|---|
| Diameter by DLS | 16 nm a | 13 nm a 92 nm c | 14 nm a 45 nm c |
| Colloidal stability | Dispersion a | Dispersion a | Dispersion a |
Precipitates b | |||
Dispersion d | Dispersion c | Dispersion c |
| Mixed Micelle | Eaz/kJmol−1 |
|---|---|
| 2/414 | 21.1 ± 1.0 |
| 2/3a | 18.4 ± 0.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagura, K.; Takemoto, Y.; Yoshino, F.; Bogdanov, A.; Chumakova, N.; Vorobiev, A.K.; Imai, H.; Matsuda, T.; Shimono, S.; Kato, T.; et al. Magnetic Mixed Micelles Composed of a Non-Ionic Surfactant and Nitroxide Radicals Containing a d-Glucosamine Unit: Preparation, Stability, and Biomedical Application. Pharmaceutics 2019, 11, 42. https://doi.org/10.3390/pharmaceutics11010042
Nagura K, Takemoto Y, Yoshino F, Bogdanov A, Chumakova N, Vorobiev AK, Imai H, Matsuda T, Shimono S, Kato T, et al. Magnetic Mixed Micelles Composed of a Non-Ionic Surfactant and Nitroxide Radicals Containing a d-Glucosamine Unit: Preparation, Stability, and Biomedical Application. Pharmaceutics. 2019; 11(1):42. https://doi.org/10.3390/pharmaceutics11010042
Chicago/Turabian StyleNagura, Kota, Yusa Takemoto, Fumi Yoshino, Alexey Bogdanov, Natalia Chumakova, Andrey Kh. Vorobiev, Hirohiko Imai, Tetsuya Matsuda, Satoshi Shimono, Tatsuhisa Kato, and et al. 2019. "Magnetic Mixed Micelles Composed of a Non-Ionic Surfactant and Nitroxide Radicals Containing a d-Glucosamine Unit: Preparation, Stability, and Biomedical Application" Pharmaceutics 11, no. 1: 42. https://doi.org/10.3390/pharmaceutics11010042
APA StyleNagura, K., Takemoto, Y., Yoshino, F., Bogdanov, A., Chumakova, N., Vorobiev, A. K., Imai, H., Matsuda, T., Shimono, S., Kato, T., Komatsu, N., & Tamura, R. (2019). Magnetic Mixed Micelles Composed of a Non-Ionic Surfactant and Nitroxide Radicals Containing a d-Glucosamine Unit: Preparation, Stability, and Biomedical Application. Pharmaceutics, 11(1), 42. https://doi.org/10.3390/pharmaceutics11010042







