Development of A New Delivery System Based on Drug-Loadable Electrospun Nanofibers for Psoriasis Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Polymers, Solvents, Drugs, and Common Materials
2.2. Electrospinning
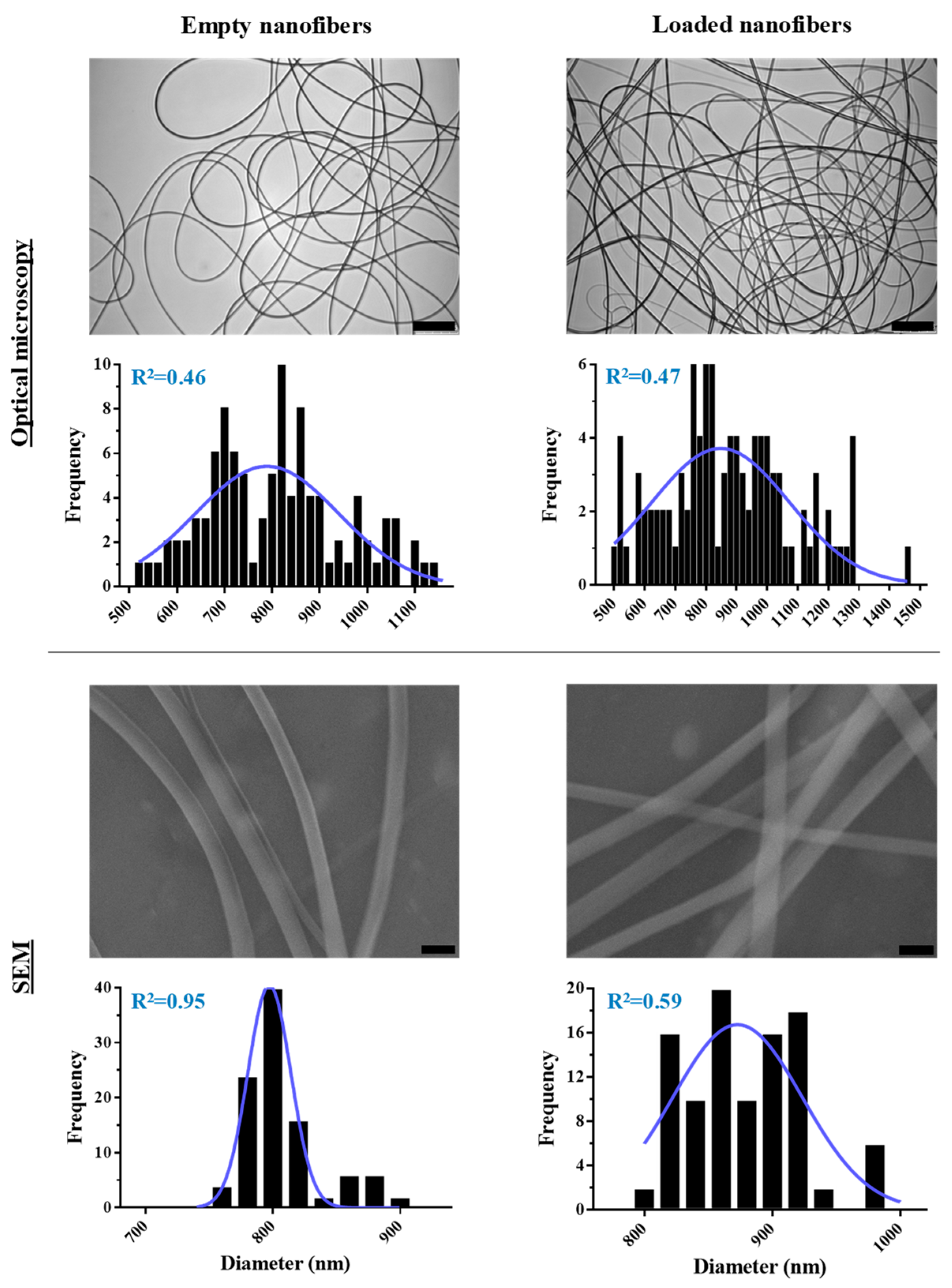
2.3. Microscopy
2.4. Gas Chromatography and Mass Spectrometry (GC-MS)
2.5. Cell Culture
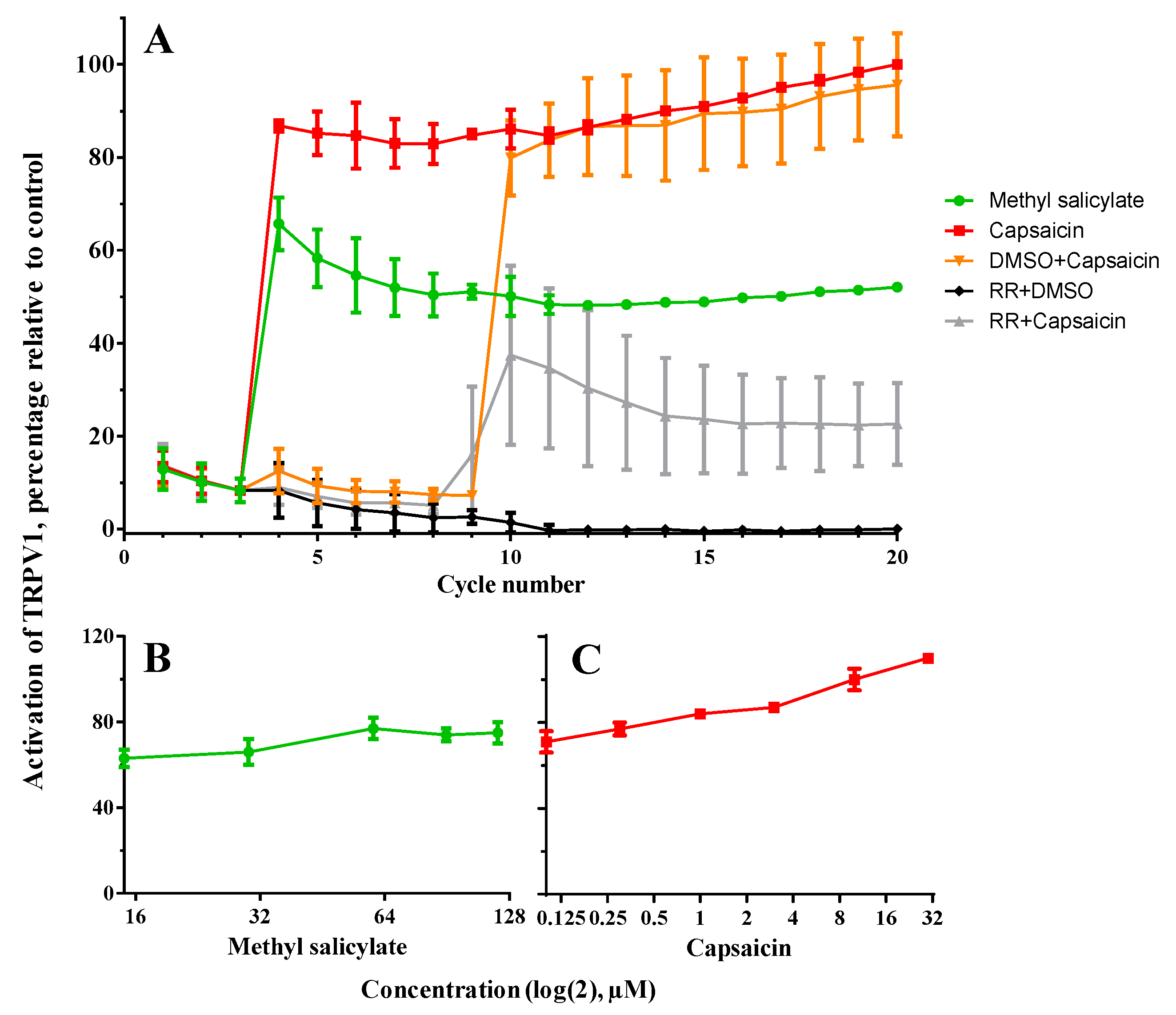
2.6. TRPV1 Channel Activity Assays
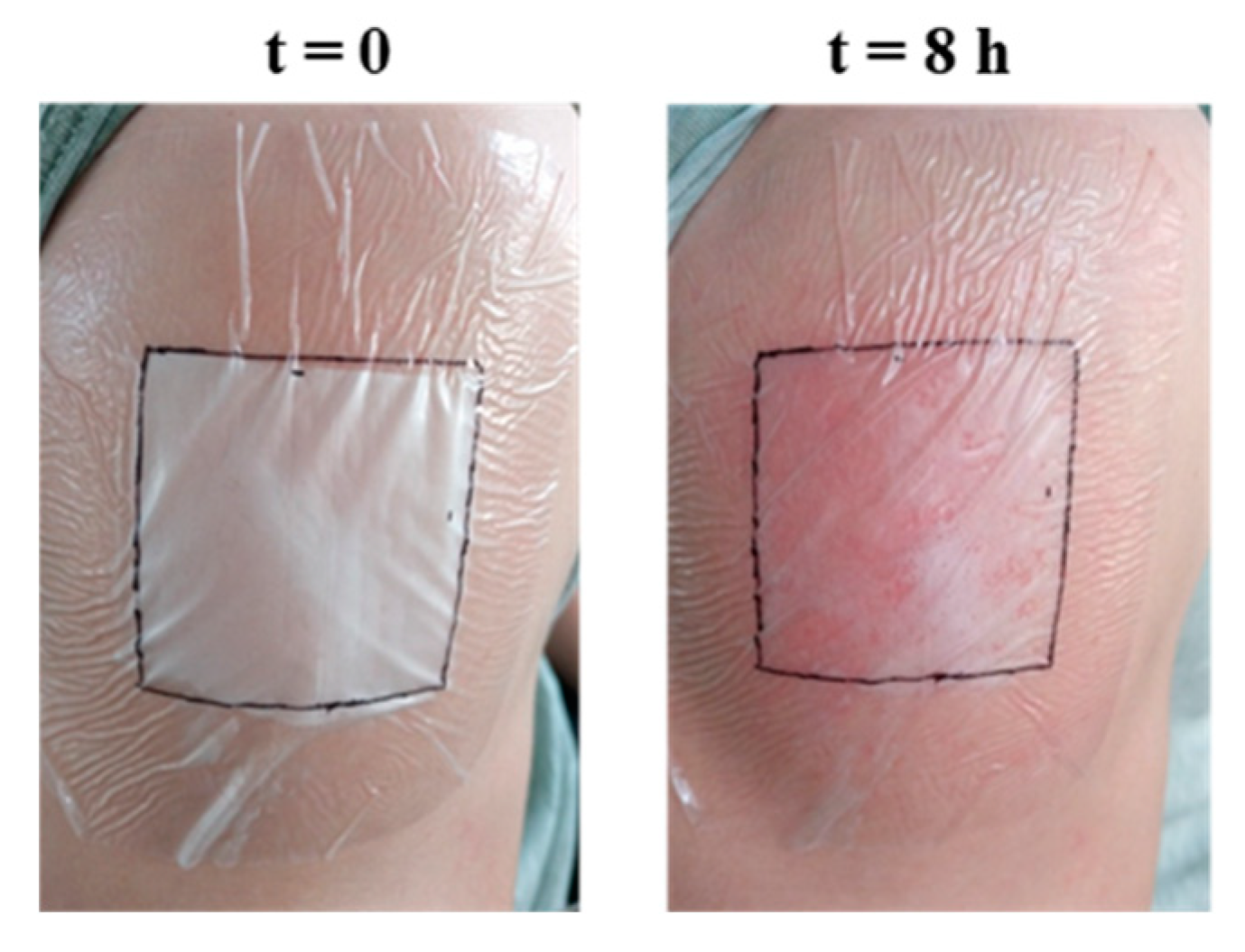
2.7. Preliminary In Vivo Test of Topical Application
2.8. Data Analysis and Graphics
3. Results and Discussion
3.1. Optimization of PMVE/MA-ES Nanofibers Encapsulating Three Therapeutic Agents
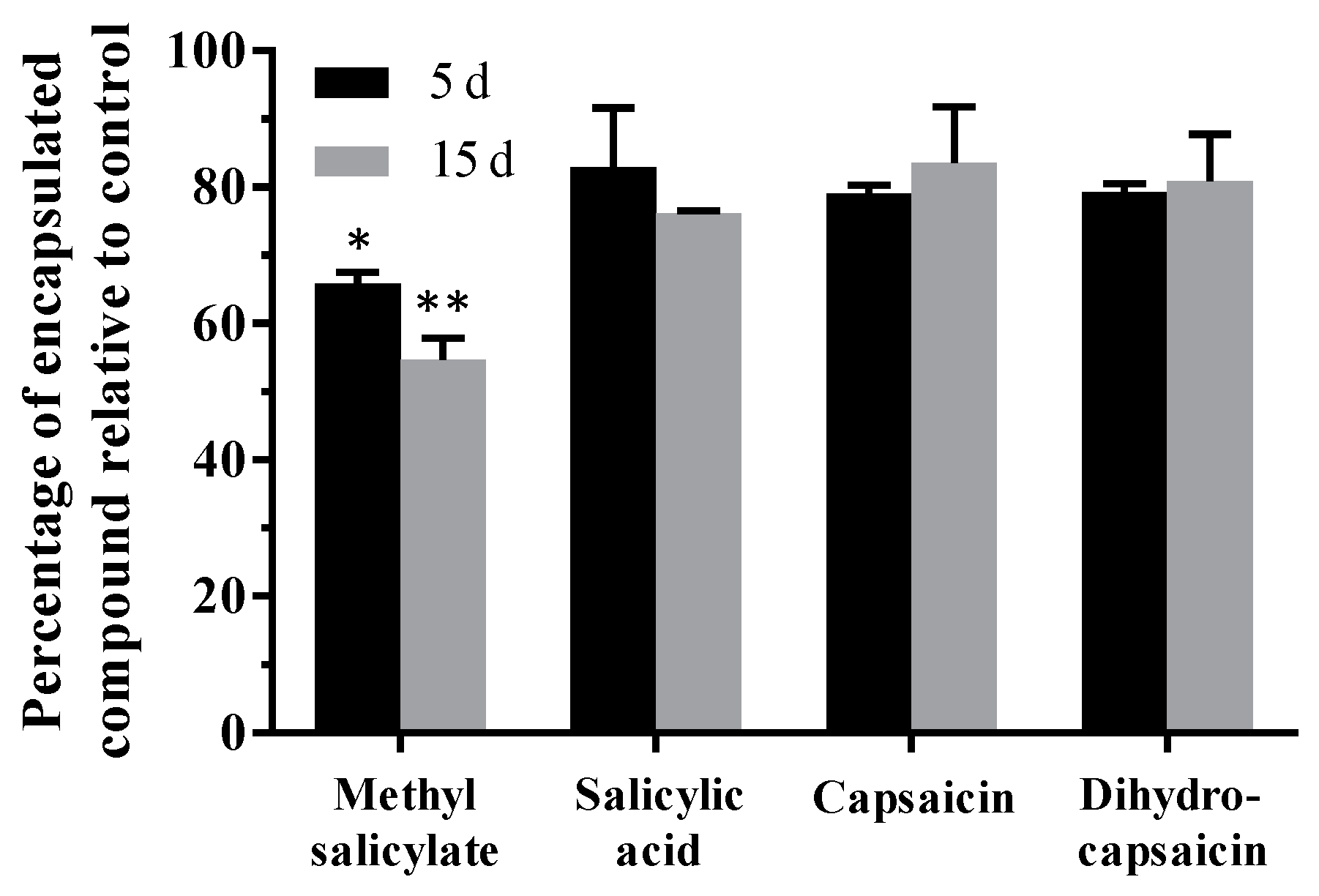
3.2. Determination of the Encapsulated Content and Its Stability over Time
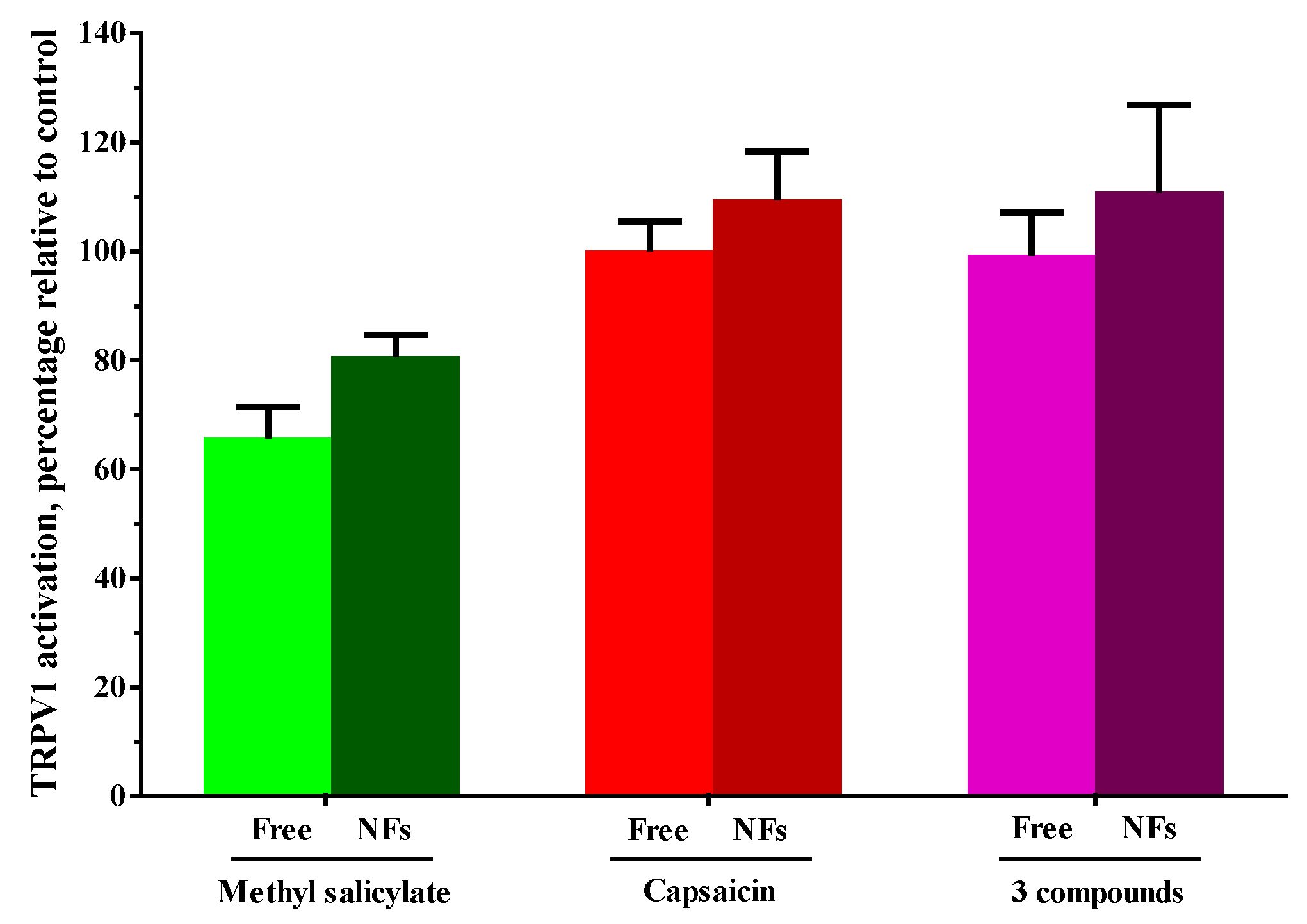
3.3. Assessment of the Activation Capacity of TRPV1 by the Encapsulated Agents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boehncke, W.H.; Schon, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Christophers, E. Psoriasis—Epidemiology and clinical spectrum. Clin. Exp. Dermatol. 2001, 26, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, B.J.; Qin, J.Z.; Nestle, F.O. Immunopathogenesis of psoriasis. Clin. Rev. Allergy Immunol. 2007, 33, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, B.J. Cracking the cytokine code in psoriasis. Nat. Med. 2007, 13, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, A.; Di Meglio, P.; Nestle, F.O. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J. Investig. Dermatol. 2009, 129, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.; Burden, A.D. Psoriasis: Advances in pathophysiology and management. Postgrad. Med. J. 2007, 83, 690–697. [Google Scholar] [CrossRef]
- Wozel, G. Psoriasis treatment in difficult locations: Scalp, nails, and intertriginous areas. Clin. Dermatol. 2008, 26, 448–459. [Google Scholar] [CrossRef]
- Raut, A.S.; Prabhu, R.H.; Patravale, V.B. Psoriasis clinical implications and treatment: A review. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 183–216. [Google Scholar] [CrossRef]
- Hsu, L.; Snodgrass, B.T.; Armstrong, A.W. Antidrug antibodies in psoriasis: A systematic review. Br. J. Dermatol. 2014, 170, 261–273. [Google Scholar] [CrossRef]
- Ryan, C.; Korman, N.J.; Gelfand, J.M.; Lim, H.W.; Elmets, C.A.; Feldman, S.R.; Gottlieb, A.B.; Koo, J.Y.; Lebwohl, M.; Leonardi, C.L.; et al. Research gaps in psoriasis: Opportunities for future studies. J. Am. Acad. Dermatol. 2014, 70, 146–167. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Cavallotti, C.; Berardesca, E. Emollients, moisturizers, and keratolytic agents in psoriasis. Clin. Dermatol. 2008, 26, 380–386. [Google Scholar] [CrossRef]
- Dawn, A.; Yosipovitch, G. Treating itch in psoriasis. Dermatol. Nurs. 2006, 18, 227. [Google Scholar]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [PubMed]
- Ferrer-Montiel, A.; García-Martínez, C.; Morenilla-Palao, C.; García-Sanz, N.; Fernández-Carvajal, A.; Fernández-Ballester, G.; Planells-Cases, R. Molecular architecture of the vanilloid receptor. FEBS J. 2004, 271, 1820–1826. [Google Scholar]
- Baumann, T.K.; Martenson, M.E. Extracellular protons both increase the activity and reduce the conductance of capsaicin-gated channels. J. Neurosci. 2000, 20, RC80. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Bley, K. Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br. J. Anaesth. 2011, 107, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Hu, Y.W.; Huang, C.; Ma, X.; Kang, C.M.; Zhang, Y.; Guo, F.X.; Lu, J.B.; Xiu, J.C.; Qiu, Y.R.; et al. Dihydrocapsaicin suppresses proinflammatory cytokines expression by enhancing nuclear factor ia in a nf-kappab-dependent manner. Arch. Biochem. Biophys. 2016, 604, 27–35. [Google Scholar] [CrossRef]
- Tang, J.; Luo, K.; Li, Y.; Chen, Q.; Tang, D.; Wang, D.; Xiao, J. Capsaicin attenuates LPS-induced inflammatory cytokine production by upregulation of LXR alpha. Int. Immunopharmacol. 2015, 28, 264–269. [Google Scholar] [CrossRef]
- Reyes-Escogido, M.d.L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and pharmacological aspects of capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z. The two faces of capsaicin. Cancer Res. 2011, 71, 2809–2814. [Google Scholar] [CrossRef]
- Ohta, T.; Imagawa, T.; Ito, S. Involvement of transient receptor potential vanilloid subtype 1 in analgesic action of methylsalicylate. Mol. Pharmacol. 2009, 75, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.K.-Y. Biochemical pharmacology of nonsteroidal anti-inflammatory drugs. Biochem. Pharmacol. 1998, 55, 543–547. [Google Scholar] [PubMed]
- Xie, J.; Li, X.; Xia, Y. Putting electrospun nanofibers to work for biomedical research. Macromol. Rapid Commun. 2008, 29, 1775–1792. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Sun, Y.; Yang, F.; van den Beucken, J.J.; Fan, M.; Chen, Z.; Jansen, J.A. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 2011, 28, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Mira, A.; Mateo, C.R.; Mallavia, R.; Falco, A. Poly (methyl vinyl ether-alt-maleic acid) and ethyl monoester as building polymers for drug-loadable electrospun nanofibers. Sci. Rep. 2017, 7, 17205. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, T.; de Cerain, A.L.; Irache, J.; Martín-Arbella, N.; Wilcox, M.; Pearson, J.; Azqueta, A. Evaluation of the cytotoxicity, genotoxicity and mucus permeation capacity of several surface modified poly (anhydride) nanoparticles designed for oral drug delivery. Int. J. Pharm. 2017, 517, 67–79. [Google Scholar] [CrossRef]
- Iglesias, T.; Dusinska, M.; El Yamani, N.; Irache, J.M.; Azqueta, A.; Lopez de Cerain, A. In vitro evaluation of the genotoxicity of poly(anhydride) nanoparticles designed for oral drug delivery. Int. J. Pharm. 2017, 523, 418–426. [Google Scholar] [CrossRef]
- Prieto, E.; Puente, B.; Uixera, A.; Garcia de Jalon, J.A.; Perez, S.; Pablo, L.; Irache, J.M.; Garcia, M.A.; Bregante, M.A. Gantrez an nanoparticles for ocular delivery of memantine: In vitro release evaluation in albino rabbits. Ophthalmic Res. 2012, 48, 109–117. [Google Scholar] [CrossRef]
- Gardner, C.M.; Burke, N.A.; Chu, T.; Shen, F.; Potter, M.A.; Stover, H.D. Poly(methyl vinyl ether-alt-maleic acid) polymers for cell encapsulation. J. Biomater. Sci. Polym. Ed. 2011, 22, 2127–2145. [Google Scholar] [CrossRef]
- Kahveci, Z.; Vazquez-Guillo, R.; Mira, A.; Martinez, L.; Falco, A.; Mallavia, R.; Mateo, C.R. Selective recognition and imaging of bacterial model membranes over mammalian ones by using cationic conjugated polyelectrolytes. Analyst 2016, 141, 6287–6296. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Guilló, R.; Falco, A.; Martínez-Tomé, M.J.; Mateo, C.R.; Herrero, M.A.; Vázquez, E.; Mallavia, R. Advantageous microwave-assisted suzuki polycondensation for the synthesis of aniline-fluorene alternate copolymers as molecular model with solvent sensing properties. Polymers 2018, 10, 215. [Google Scholar] [CrossRef]
- Chhabra, H.; Deshpande, R.; Kanitkar, M.; Jaiswal, A.; Kale, V.P.; Bellare, J.R. A nano zinc oxide doped electrospun scaffold improves wound healing in a rodent model. RSC Adv. 2016, 6, 1428–1439. [Google Scholar] [CrossRef]
- Xavier, P.; Jain, S.; Chatterjee, K.; Bose, S. Designer porous antibacterial membranes derived from thermally induced phase separation of PS/PVME blends decorated with an electrospun nanofiber scaffold. RSC Adv. 2016, 6, 10865–10872. [Google Scholar] [CrossRef]
- Carrillo-Arcos, U.A.; Rojas-Ocampo, J.; Porcel, S. Oxidative cyclization of alkenoic acids promoted by agoac. Dalton Trans. 2016, 45, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Penas, E.; Lopez-Alvarez, M.; de Narvajas, F.M.; Ursua, A. Simultaneous gc determination of turpentine, camphor, menthol and methyl salicylate in a topical analgesic formulation (dologex®). Chromatographia 2000, 52, 245–248. [Google Scholar] [CrossRef]
- Serafini, M.; Griglio, A.; Aprile, S.; Seiti, F.; Travelli, C.; Pattarino, F.; Grosa, G.; Sorba, G.; Genazzani, A.A.; Gonzalez-Rodriguez, S.; et al. Targeting transient receptor potential vanilloid 1 (TRPV1) channel softly: The discovery of passerini adducts as a topical treatment for inflammatory skin disorders. J. Med. Chem. 2018, 61, 4436–4455. [Google Scholar] [CrossRef]
- Kim, S.; Kang, C.; Shin, C.Y.; Hwang, S.W.; Yang, Y.D.; Shim, W.S.; Park, M.-Y.; Kim, E.; Kim, M.; Kim, B.-M. TRPV1 recapitulates native capsaicin receptor in sensory neurons in association with Fas-associated factor 1. J. Neurosci. 2006, 26, 2403–2412. [Google Scholar] [CrossRef]
- Dray, A.; Forbes, C.; Burgess, G. Ruthenium red blocks the capsaicin-induced increase in intracellular calcium and activation of membrane currents in sensory neurones as well as the activation of peripheral nociceptors in vitro. Neurosci. Lett. 1990, 110, 52–59. [Google Scholar] [CrossRef]
- Shen, X.; Yu, D.; Zhu, L.; Branford-White, C.; White, K.; Chatterton, N.P. Electrospun diclofenac sodium loaded Eudragit® L 100-55 nanofibers for colon-targeted drug delivery. Int. J. Pharm. 2011, 408, 200–207. [Google Scholar] [CrossRef]
- Samprasit, W.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Rojanarata, T.; Kaomongkolgit, R.; Opanasopit, P. Fast releasing oral electrospun PVP/CD nanofiber mats of taste-masked meloxicam. Int. J. Pharm. 2015, 487, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Moghe, A.; Gupta, B. Co-axial electrospinning for nanofiber structures: Preparation and applications. Polym. Rev. 2008, 48, 353–377. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, G.; Fang, D.; Xu, J.; Zhang, H.; Nie, J. Effects of solution properties and electric field on the electrospinning of hyaluronic acid. Carbohydr. Polym. 2011, 83, 1011–1015. [Google Scholar] [CrossRef]
- Zeng, J.; Xu, X.; Chen, X.; Liang, Q.; Bian, X.; Yang, L.; Jing, X. Biodegradable electrospun fibers for drug delivery. J. Control. Release 2003, 92, 227–231. [Google Scholar] [CrossRef]
- Nezarati, R.M.; Eifert, M.B.; Cosgriff-Hernandez, E. Effects of humidity and solution viscosity on electrospun fiber morphology. Tissue Eng. Part C Methods 2013, 19, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Fong, H.; Chun, I.; Reneker, D. Beaded nanofibers formed during electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Reda, R.I.; Wen, M.M.; El-Kamel, A.H. Ketoprofen-loaded eudragit electrospun nanofibers for the treatment of oral mucositis. Int. J. Nanomed. 2017, 12, 2335–2351. [Google Scholar] [CrossRef] [PubMed]
- Canbolat, M.F.; Celebioglu, A.; Uyar, T. Drug delivery system based on cyclodextrin-naproxen inclusion complex incorporated in electrospun polycaprolactone nanofibers. Colloids Surf. B 2014, 115, 15–21. [Google Scholar] [CrossRef]
- Krogstad, E.A.; Woodrow, K.A. Manufacturing scale-up of electrospun poly (vinyl alcohol) fibers containing tenofovir for vaginal drug delivery. Int. J. Pharm. 2014, 475, 282–291. [Google Scholar] [CrossRef]
- Ball, C.; Woodrow, K.A. Electrospun solid dispersions of maraviroc for rapid intravaginal preexposure prophylaxis of hiv. Antimicrob. Agents Chemother. 2014, 58, 4855–4865. [Google Scholar] [CrossRef]
- Lebwohl, M. The role of salicylic acid in the treatment of psoriasis. Int. J. Dermatol. 1999, 38, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wan, M.; Chen, H.; Ye, C.; Zhao, Y.; Yi, J.; Xia, Y.; Lai, W. Clinical evidence on the efficacy and safety of an antioxidant optimized 1.5% salicylic acid (SA) cream in the treatment of facial acne: An open, baseline-controlled clinical study. Skin Res. Technol. 2013, 19, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Opanasopit, P.; Sila-on, W.; Rojanarata, T.; Ngawhirunpat, T. Fabrication and properties of capsicum extract-loaded PVA and CA nanofiber patches. Pharm. Dev. Technol. 2013, 18, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gupta, M.; Mangal, S.; Agrawal, U.; Vyas, S.P. Capsaicin-loaded vesicular systems designed for enhancing localized delivery for psoriasis therapy. Artif. Cells Nanomed. Biotechnol. 2016, 44, 825–834. [Google Scholar] [CrossRef]
- Sánchez, L.M.D.; Rodríguez, L.; López, M. Electrospinning: La era de las nanofibras. Rev. Iberoam. Polím. 2013, 14, 10–27. [Google Scholar]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef]
- Kantouch, A.; El-Sayed, A.A.; Salama, M.; El-Kheir, A.A.; Mowafi, S. Salicylic acid and some of its derivatives as antibacterial agents for viscose fabric. Int. J. Biol. Macromol. 2013, 62, 603–607. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Ortega, L.; Mira, A.; Fernandez-Carvajal, A.; Mateo, C.R.; Mallavia, R.; Falco, A. Development of A New Delivery System Based on Drug-Loadable Electrospun Nanofibers for Psoriasis Treatment. Pharmaceutics 2019, 11, 14. https://doi.org/10.3390/pharmaceutics11010014
Martínez-Ortega L, Mira A, Fernandez-Carvajal A, Mateo CR, Mallavia R, Falco A. Development of A New Delivery System Based on Drug-Loadable Electrospun Nanofibers for Psoriasis Treatment. Pharmaceutics. 2019; 11(1):14. https://doi.org/10.3390/pharmaceutics11010014
Chicago/Turabian StyleMartínez-Ortega, Leticia, Amalia Mira, Asia Fernandez-Carvajal, C. Reyes Mateo, Ricardo Mallavia, and Alberto Falco. 2019. "Development of A New Delivery System Based on Drug-Loadable Electrospun Nanofibers for Psoriasis Treatment" Pharmaceutics 11, no. 1: 14. https://doi.org/10.3390/pharmaceutics11010014
APA StyleMartínez-Ortega, L., Mira, A., Fernandez-Carvajal, A., Mateo, C. R., Mallavia, R., & Falco, A. (2019). Development of A New Delivery System Based on Drug-Loadable Electrospun Nanofibers for Psoriasis Treatment. Pharmaceutics, 11(1), 14. https://doi.org/10.3390/pharmaceutics11010014







