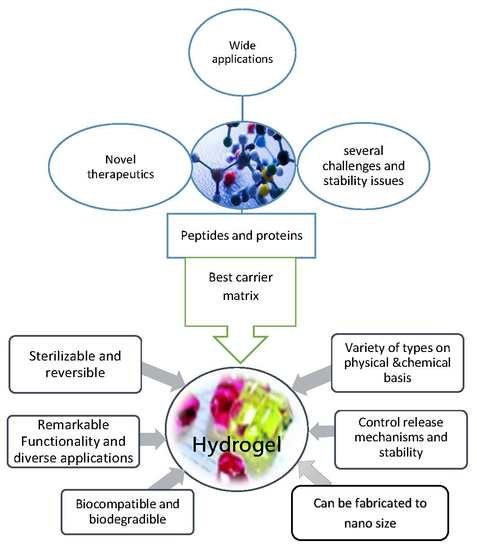
A Review on Recent Advances in Stabilizing Peptides/Proteins upon Fabrication in Hydrogels from Biodegradable Polymers
Abstract
1. Introduction
2. Pharmaceutical Peptides and Proteins
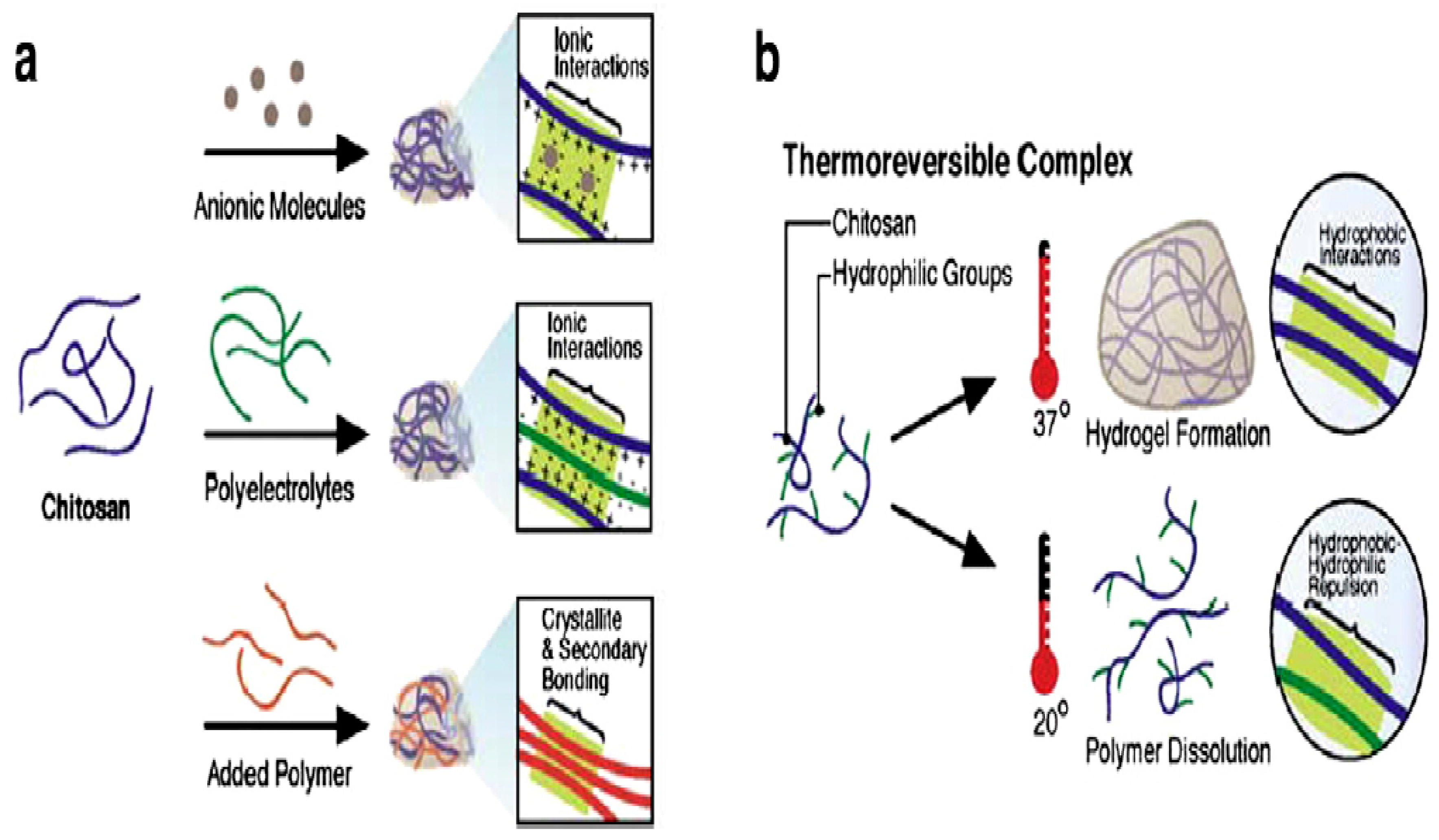
3. Hydrogels
3.1. Smart Hydrogels
3.2. pH Sensitive Hydrogels
3.3. Temperature Sensitive Hydrogels
3.3.1. Positive Temperature Hydrogels
3.3.2. Negative Temperature Hydrogel
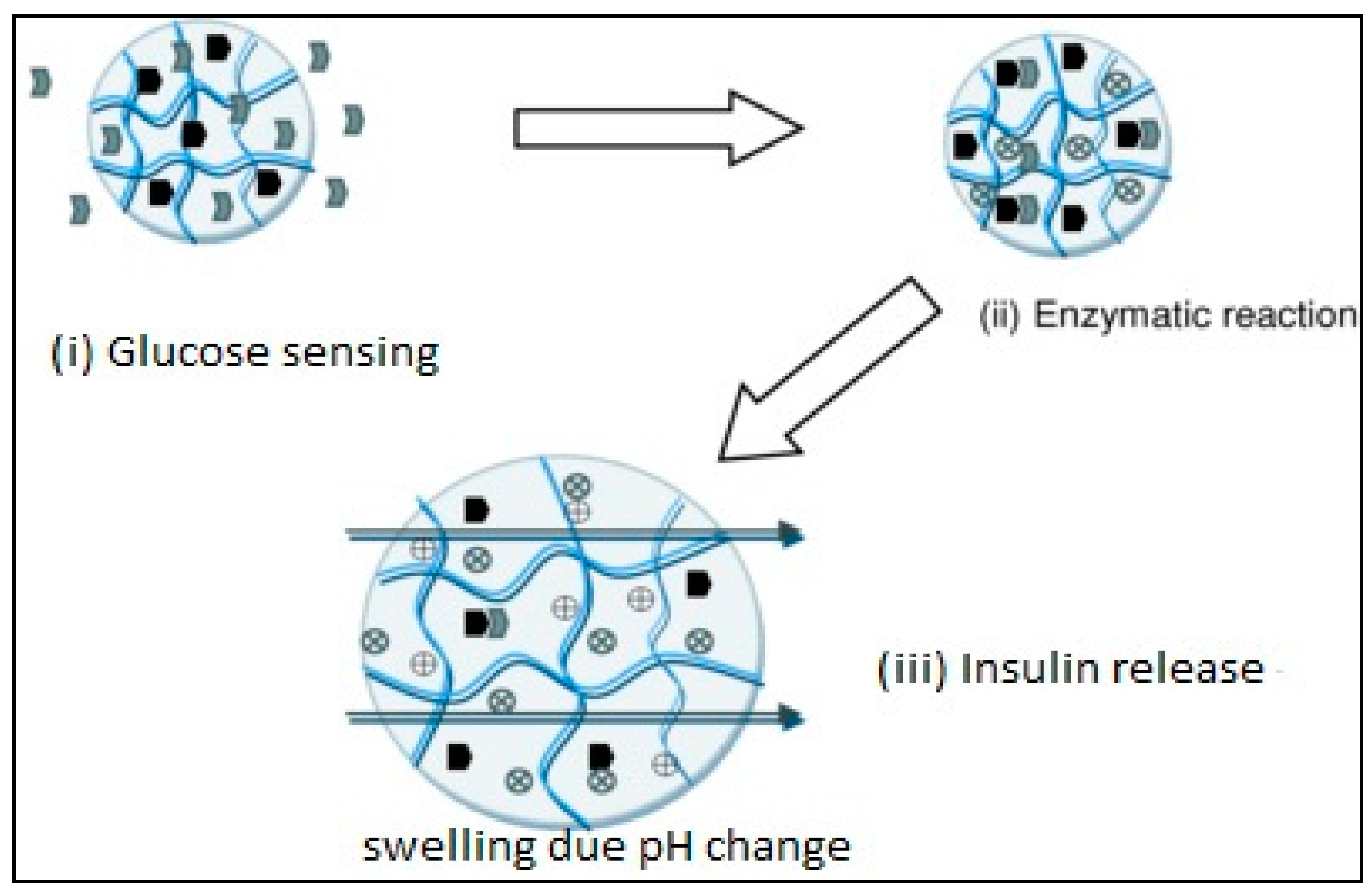
3.4. Glucose-Sensitive Hydrogels
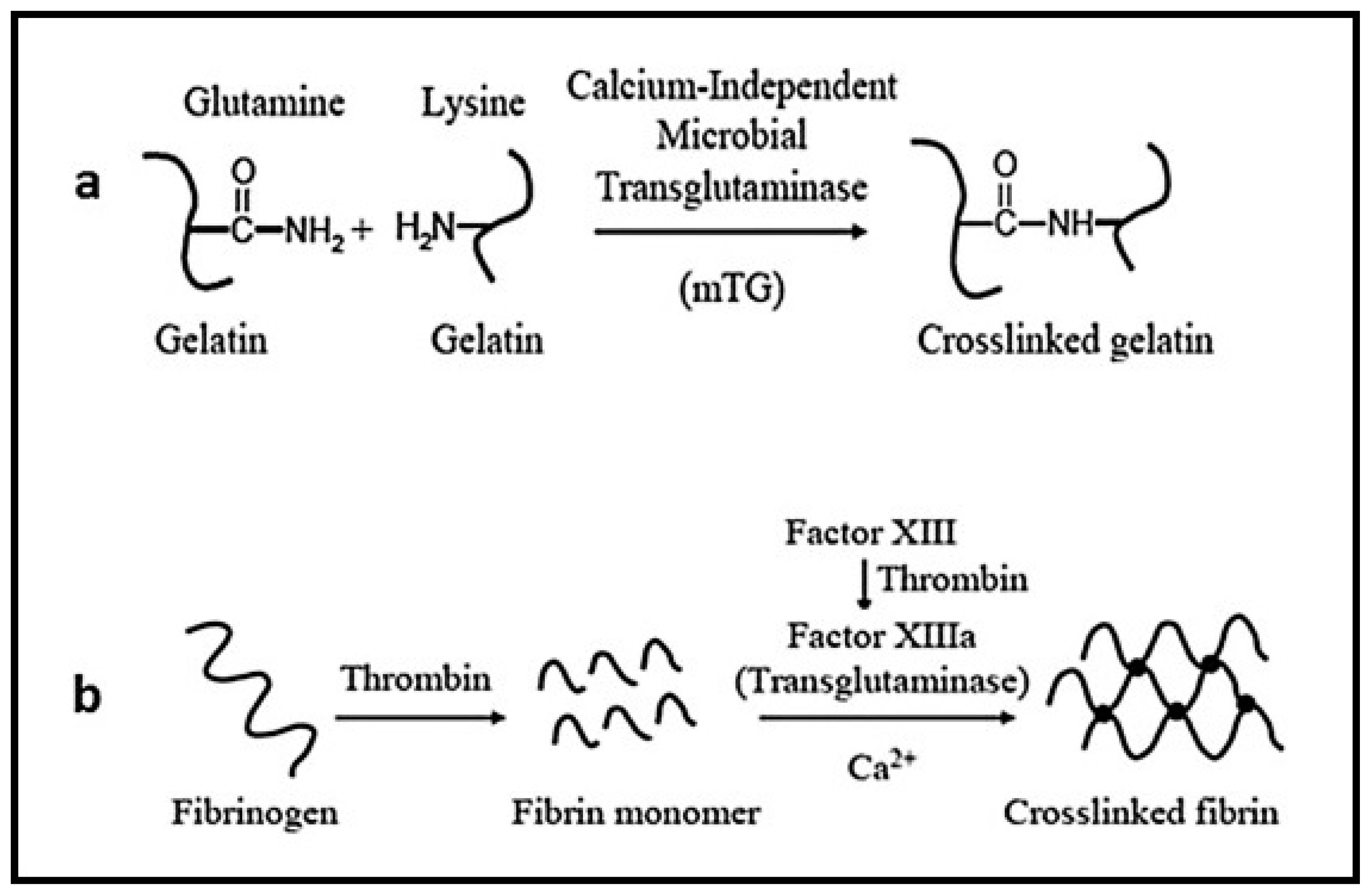
3.5. Protein-Based Hydrogels
3.6. Antigen-Responsive Hydrogels
3.7. Monomers Used for Fabrication of Hydrogels
3.8. Loading of Drugs in Hydrogel
- (a)
- Post-loading
- (b)
- In situ loading
3.9. Release Mechanism of Drug from Hydrogel Matrices
- Diffusion-controlled
- Swelling-controlled and
- Chemically-controlled.
3.10. Proteins/Peptides Stability in Hydrogels
3.11. Recent Advancement in Hydrogel
3.11.1. Supramolecular Hydrogelators and Hydrogels
3.11.2. Antibacterial Hydrogelators/Hydrogels
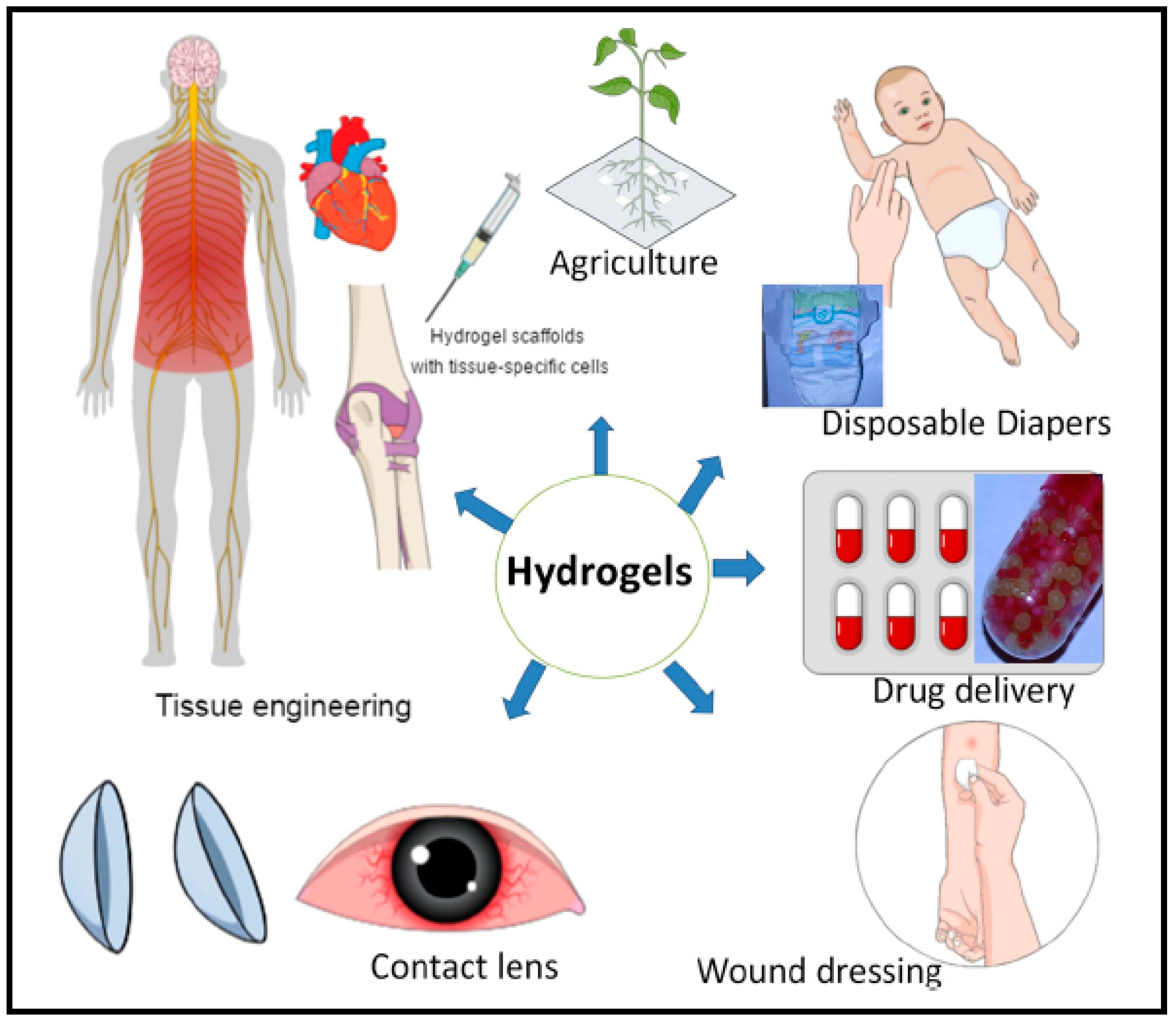
3.12. Hydrogels for Pharmaceutical Applications
- (1)
- Special hydrogels are composed of superabsorbent materials and they are used as hygienic materials for various purposes such as in disposable diapers and lady napkins and absorb the secreted fluids [118].
- (2)
- Superabsorbent hydrogels are mainly used as targeted drug delivery system and Nano/controlled drug delivery systems.
- (3)
- Polysaccharides-based hydrogels for bioactive coatings (e.g., catheter, stent), replacement of nucleus pulpous and cellular scaffold (artificial organs)
- (4)
- (5)
- Acidic cellulose–chitin hybrid gel that is a novel electrode which is used for electric double layer capacitor.
- (6)
- Hydrogels that are prepared from silk fibers prepared excellent interstitial fluid support capacity and they are employed for articular cartilage repair [122].
- (7)
- Elastin-like protein hydrogels are found to improve neuriteout-growth in neuronal cultures. These hydrogels contain engineered polymers that produce elastin-like proteins recombinant protein containing amino acids.
- (8)
- Elastin-based polymeric hydrogels are mainly utilized for advanced engineering of elastic tissues, such as skin, lung, and vasculature. They are composed of expandable polymeric fibers which facilitate the blood vessels to stretch and relax billion times or more during their lifetime period [123].
- (9)
- Poly (hydroxyethyl methacrylate)-based hydrogels are used to ensures good wound-healing therefore mostly applied in wound dressings, specifically burn dressings applications. It is used for contact lenses, drug delivery and tissue engineering purposes for bone marrow and spinal cord cell regeneration. It also promotes cell adhesion in artificial skin and cartilage manufacturing method [124].
- (10)
- PVA-based hydrogels are used widely in biomedical applications including drug delivery, artificial tears, contact lenses, artificial cell encapsulation and as nerve cuffs [125]. PVA hydrogel also found application in injectable implants, soft tissue fillers, cartilage reconstructive, aesthetic surgery, artificial organs, drug delivery systems and wound dressings. It provides ahumid environment that is useful for wound healing [126].
- (11)
- Poly (ethylene glycol)-based have high biocompatibility, lack of toxic effects on the surrounding tissues and high solubility in water which serve as good candidates for drug delivery systems [124]. They are employed for cell delivery to improve tissue regeneration.
- (12)
- Thermosensitive tri-block poly (ethylene glycol)-poly (ε-caprolactone)-poly (ethylene glycol)-based hydrogels which are formed in situ, can be easily utilized in various biomedical fields such as cell encapsulation, controlled drug delivery, and tissue repair. Poly (imide) (PI) hydrogels and PVA hydrogels are used in plastic and reconstructive surgeries [124].
- (13)
- Polyacrylate (PA)-based polymeric hydrogels play important role in advanced aesthetic corrections, soft tissue fillers and enhancement materials [124].
- (14)
- Poly (urethane) hydrogels serve as drug matrices, artificial kidney membranes, and catheter coating materials [127].
4. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martinho, N.; Damgé, C.; Reis, C.P. Recent advances in drug delivery systems. J. Biomater. Nanobiotechnol. 2011, 2, 510–526. [Google Scholar] [CrossRef]
- Graham, N. Hydrogels in controlled drug delivery. In Polymeric Biomaterials; Springer: Berlin, Germany, 1986; pp. 170–194. [Google Scholar]
- Bajpai, S.; Sonkusley, J. Hydrogels for oral drug delivery of peptides: Synthesis and characterization. J. Appl. Polym. Sci. 2002, 83, 1717–1729. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Park, J.H.; Lee, D.S. Injectable polymeric hydrogels for the delivery of therapeutic agents: A review. Eur. Polym. J. 2015, 72, 602–619. [Google Scholar] [CrossRef]
- Buchholz, F.L.; Graham, A.T. Modern Superabsorbent Polymer Technology; John Wiley Sons, Inc.: New York, NY, USA, 1998. [Google Scholar]
- Brannon-Peppas, L.; Harland, R.S. Absorbent Polymer Technology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 8. [Google Scholar]
- Li, Y.; Huang, G.; Zhang, X.; Li, B.; Chen, Y.; Lu, T.; Lu, T.J.; Xu, F. Magnetic hydrogels and their potential biomedical applications. Adv. Funct. Mater. 2013, 23, 660–672. [Google Scholar] [CrossRef]
- Iizawa, T.; Taketa, H.; Maruta, M.; Ishido, T.; Gotoh, T.; Sakohara, S. Synthesis of porous poly (N-isopropylacrylamide) gel beads by sedimentation polymerization and their morphology. J. Appl. Polym. Sci. 2007, 104, 842–850. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Yoshii, F. Hydrogels and their medical applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1999, 151, 56–64. [Google Scholar] [CrossRef]
- El-Hafian, E.A.; Elgannodi, E.; Mainal, A.; Yahaya, A.H.B. Characterization of chitosan in acetic acid: Rheological and thermal studies. Turk. J. Chem. 2010, 34, 47–56. [Google Scholar]
- Khan, A.; Othman, M.; Razak, K.; MdAkil, H. Synthesis and physicochemical investigation of chitosan-PMAA-based dual-responsive hydrogels. J. Polym. Res. 2013, 20, 273. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Suri, S.; Roy, K. In situ crosslinking hydrogels for combinatorial delivery of chemokines and siRNA–DNA carrying microparticles to dendritic cells. Biomaterials 2009, 30, 5187–5200. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.A.; Tabata, Y.; Mikos, A.G. Dual growth factor delivery from degradable oligo (poly (ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J. Control. Release 2005, 101, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, K. Biocompatibility issues of implantable drug delivery systems. Pharm. Res. 1996, 13, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, H.J.; Herber, S.; Olthuis, W.; Bergveld, P. Stimulus-sensitive hydrogels and their applications in chemical (micro) analysis. Analyst 2003, 128, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Jen, A.C.; Wake, M.C.; Mikos, A.G. Hydrogels for cell immobilization. Biotechnol. Bioeng. 1996, 50, 357–364. [Google Scholar] [CrossRef]
- Wang, K.; Burban, J.; Cussler, E. Hydrogels as separation agents. In Responsive Gels: Volume Transitions II; Springer: Berlin, Germany, 1993; pp. 67–79. [Google Scholar]
- Bennett, S.L.; Melanson, D.A.; Torchiana, D.F.; Wiseman, D.M.; Sawhney, A.S. Next-generation hydrogel films as tissue sealants and adhesion barriers. J. Card. Surg. 2003, 18, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Peppas, N.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular hydrogelators and hydrogels: From soft matter to molecular biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Black, K.A.; Lin, B.F.; Wonder, E.A.; Desai, S.S.; Chung, E.J.; Ulery, B.D.; Katari, R.S.; Tirrell, M.V. Biocompatibility and characterization of a Peptide amphiphile hydrogel for applications in peripheral nerve regeneration. Tissue Eng. Part A 2015, 21, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Seow, W.Y.; Hauser, C.A. Short to ultrashort peptide hydrogels for biomedical uses. Mater. Today 2014, 17, 381–388. [Google Scholar] [CrossRef]
- Mishra, A.; Panda, J.; Basu, A.; Chauhan, V. Nanovesicles based on self-assembly of conformationally constrained aromatic residue containing amphiphilic dipeptides. Langmuir 2008, 24, 4571–4576. [Google Scholar] [CrossRef] [PubMed]
- Panda, J.J.; Kaul, A.; Kumar, S.; Alam, S.; Mishra, A.K.; Kundu, G.C.; Chauhan, V.S. Modified dipeptide-based nanoparticles: Vehicles for targeted tumor drug delivery. Nanomedicine 2013, 8, 1927–1942. [Google Scholar] [CrossRef] [PubMed]
- Melchionna, M.; Styan, K.E.; Marchesan, S. The unexpected advantages of using D-amino acids for peptide self-assembly into nanostructured hydrogels for medicine. Curr. Top. Med. Chem. 2016, 16, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Vargiu, A.; Iglesias, D.; Styan, K.E.; Waddington, L.J.; Easton, C.D.; Marchesan, S. Design of a hydrophobic tripeptide that self-assembles into amphiphilic superstructures forming a hydrogel biomaterial. Chem. Commun. 2016, 52, 5912–5915. [Google Scholar] [CrossRef] [PubMed]
- Kurbasic, M.; Romanno, C.; Garcia, A.; Kralj, S.; Marchesan, S. Assembly of a Tripeptide and anti-inflammatory drugs into supramolecular hydrogels for sustained release. Gels 2017, 3, 29. [Google Scholar] [CrossRef]
- Ratnaparkhi, M.; Chaudhari, S.; Pandya, V. Peptides and proteins in pharmaceuticals. Int. J. Curr. Pharm. Res. 2011, 3, 1–9. [Google Scholar]
- Vyas, S.P.; Khar, R.K. Targeted Controlled Drug Delivery: Novel Carrier Systems; CBS Publishers Distributors: Delhi, India, 2004. [Google Scholar]
- Perez, C.; Castellanos, I.J.; Costantino, H.R.; Al-Azzam, W.; Griebenow, K. Recent trends in stabilizing protein structure upon encapsulation and release from bioerodible polymers. J. Pharm. Pharmacol. 2002, 54, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Rawat, A.; Bhatt, N.; Chauhan, M. Structural modification of proteins and peptides. Indian J. Pharm. Educ. Res. 2014, 48, 34–47. [Google Scholar] [CrossRef]
- Irie, T.; Uekama, K. Cyclodextrins in peptide and protein delivery. Adv. Drug Deliv. Rev. 1999, 36, 101–123. [Google Scholar] [CrossRef]
- Schiffter, H.A. 5.46-The Delivery of Drugs–Peptides and Proteins, 2nd ed.; Academic Press: Burlington, ON, Canada, 2011; pp. 587–604. [Google Scholar]
- Pitt, C. The controlled parenteral delivery of polypeptides and proteins. Int. J. Pharm. 1990, 59, 173–196. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Lee, D.S. Injectable biodegradable hydrogels. Macromol. Biosci. 2010, 10, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Mequanint, K. Hydrogel biomaterials. In Biomedical Engineering-Frontiers and Challenges; InTech: London, UK, 2011. [Google Scholar]
- Jabbari, E.; Nozari, S. Synthesis of acrylic acid hydrogel by g-irradiation crosslinking of poly (acrylic acid) in aqueous solution. Iran. Polym. J. 1999, 8, 1026–1265199. [Google Scholar]
- Jianqi, F.; Lixia, G. PVA/PAA thermo-crosslinking hydrogel fiber: Preparation and pH-sensitive properties in electrolyte solution. Eur. Polym. J. 2002, 38, 1653–1658. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Ni, X.; Wang, X.; Li, H.; Leong, K.W. Self-assembled supramolecular hydrogels formed by biodegradable PEO–PHB–PEO triblock copolymers and α-cyclodextrin for controlled drug delivery. Biomaterials 2006, 27, 4132–4140. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Baker, J.P.; Stephens, D.; Blanch, H.; Prausnitz, J. Swelling equilibria for acrylamide-based polyampholyte hydrogels. Macromolecules 1992, 25, 1955–1958. [Google Scholar] [CrossRef]
- Laftah, W.A.; Hashim, S.; Ibrahim, A.N. Polymer hydrogels: A review. Polym. Plast. Technol. Eng. 2011, 50, 1475–1486. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M.Q. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–89. [Google Scholar] [CrossRef]
- Serra, L.; Doménech, J.; Peppas, N.A. Drug transport mechanisms and release kinetics from molecularly designed poly (acrylic acid-g-ethylene glycol) hydrogels. Biomaterials 2006, 27, 5440–5451. [Google Scholar] [CrossRef] [PubMed]
- Behl, M.; Zotzmann, J.; Lendlein, A. Shape-memory polymers and shape-changing polymers. Adv. Polym. Sci. 2010, 226, 97–145. [Google Scholar]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Podual, K.; Doyle, F.; Peppas, N. Preparation and dynamic response of cationic copolymer hydrogels containing glucose oxidase. Polymer 2000, 41, 3975–3983. [Google Scholar] [CrossRef]
- Brahim, S.; Narinesingh, D.; Guiseppi-Elie, A. Bio-smart hydrogels: Co-joined molecular recognition and signal transduction in biosensor fabrication and drug delivery. Biosens. Bioelectron. 2002, 17, 973–981. [Google Scholar] [CrossRef]
- Miyata, T.; Uragami, T.; Nakamae, K. Biomolecule-sensitive hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 79–98. [Google Scholar] [CrossRef]
- Kopeček, J. Smart and genetically engineered biomaterials and drug delivery systems. Eur. J. Pharm. Sci. 2003, 20, 1–16. [Google Scholar] [CrossRef]
- Kopeček, J.; Yang, J. Peptide-directed self-assembly of hydrogels. Acta Biomater. 2009, 5, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Asami, N.; Uragami, T. A reversibly antigen-responsive hydrogel. Nature 1999, 399, 766. [Google Scholar] [CrossRef] [PubMed]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Cardinalx, J.R.; Kim, S.H.; Kim, S.W. Progestin permeation through polymer membranes V: Progesterone release from monolithic hydrogel devices. J. Pharm. Sci. 1981, 70, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Li, H.E.; Luo, E.; Pratt, K. Modeling of environmentally sensitive hydrogels for drug delivery: An overview and recent developments. In Frontiers in Drug Design Discovery; Bentham Science Publishers: Emirate of Sharjah, UAE, 2006; pp. 295–331. [Google Scholar]
- Van Tomme, S.R.; Storm, G.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Barbucci, H. Biological Properties and Applications; Springer: Berlin, Germany, 2009. [Google Scholar]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, Y.B.; Gurny, R.; Jordan, O. A novel thermoresponsive hydrogel based on chitosan. Eur. J. Pharm. Biopharm. 2008, 68, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Amsden, B. Solute diffusion within hydrogels. Mechanisms and models. Macromolecules 1998, 31, 8382–8395. [Google Scholar] [CrossRef]
- Canal, T.; Peppas, N.A. Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J. Biomed. Mater. Res. Part A 1989, 23, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.N.; Metters, A.; Bowman, C.; Anseth, K. Predicting controlled-release behavior of degradable PLA-b-PEG-b-PLA hydrogels. Macromolecules 2001, 34, 4630–4635. [Google Scholar] [CrossRef]
- Cruise, G.M.; Scharp, D.S.; Hubbell, J.A. Characterization of permeability and network structure of interfaciallyphotopolymerized poly (ethylene glycol) diacrylate hydrogels. Biomaterials 1998, 19, 1287–1294. [Google Scholar] [CrossRef]
- Lustig, S.R.; Peppas, N.A. Solute diffusion in swollen membranes. IX. Scaling laws for solute diffusion in gels. J. Appl. Polym. Sci. 1988, 36, 735–747. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N. Modeling of drug release from delivery systems based onhydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef]
- Bettini, R.; Colombo, P.; Massimo, G.; Catellani, P.; Vitali, T. Swelling and drug release in hydrogel matrices: Polymer viscosity and matrix porosity effects. Eur. J. Pharm. Sci. 1994, 2, 213–219. [Google Scholar] [CrossRef]
- Kanjickal, D.G.; Lopina, S.T. Modeling of drug release from polymeric delivery systems—A review. Crit. Rev. Ther. Drug Carrier Syst. 2004, 21, 345–386. [Google Scholar] [PubMed]
- Wang, W. Instability, stabilization, and formulation of liquid protein pharmaceuticals. Int. J. Pharm. 1999, 185, 129–188. [Google Scholar] [CrossRef]
- Schillemans, J.P.; Verheyen, E.; Barendregt, A.; Hennink, W.E.; Van Nostrum, C.F. Anionic and cationic dextran hydrogels for post-loading and release of proteins. J. Control. Release 2011, 150, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Van Beers, M.M.; Jiskoot, W.; Schellekens, H. On the role of aggregates in the immunogenicity of recombinant human interferon beta in patients with multiple sclerosis. J. Interf. Cytokine Res. 2010, 30, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Schwendeman, S.P. Recent advances in the stabilization of proteins encapsulated in injectable PLGA delivery systems. Crit. Rev. Ther. Drug Carrier Syst. 2002, 19, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef] [PubMed]
- Jonker, A.M.; Löwik, D.W.; van Hest, J.C. Peptide- and protein-based hydrogels. Chem. Mater. 2012, 24, 759–773. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Cui, H. Peptide-based supramolecular hydrogels for delivery of biologics. Bioeng. Transl. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xu, B. Nanoscale assemblies of small molecules control the fate of cells. Nano Today 2015, 10, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuang, Y.; Shi, J.; Zhou, J.; Medina, J.E.; Zhou, R.; Yuan, D.; Yang, C.; Wang, H.; Yang, Z.; et al. Enzyme-instructed intracellular molecular self-assembly to boost activity of cisplatin against drug-resistant ovarian cancer cells. Angew. Chem. 2015, 127, 13505–13509. [Google Scholar] [CrossRef]
- Smith, D.J.; Brat, G.; Medina, S.; Tong, D.; Huang, Y.; Grahammer, J.; Furtmüller, G.; Oh, B.; Nagy-Smith, K.; Malczak, P.; et al. A multiphase transitioning peptide hydrogel for suturing ultrasmall vessels. Nat. Nanotechnol. 2016, 11, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ulijn, R.V.; Bibi, N.; Jayawarna, V.; Thornton, P.D.; Todd, S.J.; Mart, R.J.; Smith, A.M.; Gough, J.E. Bioresponsive hydrogels. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Ye, E.; Chee, P.; Prasad, A.; Fang, X.; Owh, C.; Yeo, V.; Loh, X. Supramolecular soft biomaterials for biomedical applications. In In-Situ Gelling Polymers; Springer: Berlin, Germany, 2015; pp. 107–125. [Google Scholar]
- Du, X.; Zhou, J.; Xu, B. Ectoenzyme switches the surface of magnetic nanoparticles for selective binding of cancer cells. J. Colloid Interface Sci. 2015, 447, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Chen, J.; Niu, R. The development of low-molecular weight hydrogels for applications in cancer therapy. Nanoscale 2014, 6, 3474–3482. [Google Scholar] [CrossRef] [PubMed]
- Reithofer, M.R.; Chan, K.; Lakshmanan, A.; Lam, D.; Mishra, A.; Gopalan, B.; Joshi, M.; Wang, S.; Hauser, C. Ligation of anti-cancer drugs to self-assembling ultrashort peptides by click chemistry for localized therapy. Chem. Sci. 2014, 5, 625–630. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Rajan Unnithan, A.; Ramachandra Kurup Sasikala, A.; Samarikhalaj, M.; Thomas, R.G.; Jeong, Y.Y.; Nasseri, S.; Murugesan, P.; Wu, D.; Hee Park, C.; et al. Mussel-inspired electrospunnanofibers functionalized with size-controlled silver nanoparticles for wound dressing application. ACS Appl. Mater. Interfaces 2015, 7, 12176–12183. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Fukuoka, Y.; Morimoto, Y.; Honjo, T.; Koda, D.; Goto, M.; Maruyama, T. Cancer cell death induced by the intracellular self-assembly of an enzyme-responsive supramoleculargelator. J. Am. Chem. Soc. 2015, 137, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, J.; Chen, M.; Yang, Z. Self-assembling small molecules for the detection of important analytes. Chem. Soc. Rev. 2014, 43, 7257–7266. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Jang, D.; Kim, S.; Hong, J. A chemodosimetric gelation system showing fluorescence and sol-to-gel transition for fluoride anions in aqueous media. New J. Chem. 2012, 36, 1145–1148. [Google Scholar] [CrossRef]
- Belay, A.; Collins, A.; Ruzgas, T.; Kissinger, P.T.; Gorton, L.; Csöregi, E. Redox hydrogel-basedbienzyme electrode for L-glutamate monitoring. J. Pharm. Biomed. Anal. 1999, 19, 93–105. [Google Scholar] [CrossRef]
- Cao, C.-Y.; Chen, Y.; Wu, F.; Deng, Y.; Liang, G. Caspase-3 controlled assembly of nanoparticles for fluorescence turn on. Chem. Commun. 2011, 47, 10320–10322. [Google Scholar] [CrossRef] [PubMed]
- Tamaru, S.I.; Kiyonaka, S.; Hamachi, I. Three distinct read-out modes for enzyme activity can operate in a semi-wet supramolecular hydrogel. Chem. Eur. J. 2005, 11, 7294–7304. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Song, A.; Feng, L.; Wei, G.; Dong, S.; Hao, J. Fluorescent hydrogels with tunable nanostructure and viscoelasticity for formaldehyde removal. ACS Appl. Mater. Interfaces 2014, 6, 18319–18328. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, H.; Song, A.; Hao, J. Superhydrogels of nanotubes capable of capturing heavy-metal ions. Chem. Asian J. 2014, 9, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Goswami, A.; Mondal, R. Silver-promoted gelation studies of an unorthodox chelating tripodal pyridine–pyrazole-based ligand: Templated growth of catalytic silver nanoparticles, gas and dye adsorption. New J. Chem. 2014, 38, 2470–2479. [Google Scholar] [CrossRef]
- Pan, S.F.; Luo, S.; Li, S.; Lai, Y.S.; Geng, Y.Y.; He, B.; Gu, Z.W. Ultrasound accelerated gelation of novel L-lysine based hydrogelators. Chem. Comm. 2013, 49, 8045–8047. [Google Scholar] [CrossRef]
- Lau, H.K.; Kiick, K.L. Opportunities for multicomponent hybrid hydrogels in biomedical applications. Biomacromolecules 2014, 16, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.; Chavez, M.; Tan, C. Dynamic biomaterials: Toward engineering autonomous feedback. Curr. Opin. Biotechnol. 2016, 39, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Tanida, T.; Yoshii, T.; Kurotani, K.; Onogi, S.; Urayama, K.; Hamachi, I. Installing logic-gate responses to a variety of biological substances in supramolecular hydrogel–enzyme hybrids. Nat. Chem. 2014, 6, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.J.; Appel, E.A.; Meijer, E.W.; Langer, R. Supramolecular biomaterials. Nat. Mater. 2016, 15, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.W.; Chan, J.M.; Sardon, H.; Ono, R.J.; García, J.M.; Yang, Y.Y.; Hedrick, J.L. Antimicrobial hydrogels: A new weapon in the arsenal against multidrug-resistant infections. Adv. Drug Deliv. Rev. 2014, 78, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, C.; Nagy, K.J.; Schneider, J.P. Design of self-assembling peptide hydrogelators amenable to bacterial expression. Biomaterials 2015, 37, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.; Pavel, M.; Kidd, M.; Gustafsson, B.I. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment. Pharmacol. Ther. 2010, 31, 169–188. [Google Scholar] [PubMed]
- Zhou, J.; Xu, B. Enzyme-instructed self-assembly: A multistep process for potential cancer therapy. Bioconjugate Chem. 2015, 26, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Epstein, I.R.; Vanag, V.; Balazs, A.; Kuksenok, O.; Dayal, P.; Bhattacharya, A. Chemical oscillators in structured media. Accounts Chem. Res. 2011, 45, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Pluta, J.; Karolewicz, B. Hydrogels: Properties and application in the technology of drug form. II. Possibilities of use of hydrogels as active substance carriers. Polim. W Med. 2003, 34, 63–81. [Google Scholar]
- Št’astný, M.; Plocová, D.; Etrych, T.; Kovár, M.; Ulbrich, K.; Ríhová, B. HPMA-hydrogels containing cytostatic drugs: Kinetics of the drug release and in vivo efficacy. J. Control. Release 2002, 81, 101–111. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Hydrogen-bonded interpolymer complexes as materials for pharmaceutical applications. Int. J. Pharm. 2007, 334, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Kanjickal, D.; Lopina, S.; Evancho-Chapman, M.M.; Schmidt, S.; Donovan, D. Improving delivery of hydrophobic drugs from hydrogels through cyclodextrins. J. Biomed. Mater. Res. Part A 2005, 74, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, N.; Kumar, N.; Kumar, M.R. Hydrogels for pharmaceutical and biomedical applications. Crit. Rev. Ther. Drug Carrier Syst. 2005, 22, 107–149. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Robinson, J. Bioadhesion in mucosal drug delivery. In Biorelated Polymers and Gels; Academic Press: San Diego, CA, USA, 1998; pp. 135–192. [Google Scholar]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Kikuchi, A.; Okano, T. Pulsatile drug release control using hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 53–77. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 97, 958–971. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Kabiri, K. Superabsorbent polymer materials: A review. Iran. Polym. J. 2008, 17, 451. [Google Scholar]
- Paulino, A.T.; Belfiore, L.; Kubota, L.; Muniz, E.; Tambourgi, E. Efficiency of hydrogels based on natural polysaccharides in the removal of Cd2+ ions from aqueous solutions. Chem. Eng. J. 2011, 168, 68–76. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Raghavendra, G.M.; Varaprasad, K.; Sadiku, R.; Ramam, K.; Raju, K.M. Iota-Carrageenan-based biodegradable Ag0 nanocomposite hydrogels for the inactivation of bacteria. Carbohydr. Polym. 2013, 95, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Mohan, Y.M.; Vimala, K.; Thomas, V.; Varaprasad, K.; Sreedhar, B.; Bajpai, S.K.; Mohana Raju, K. Controlling of silver nanoparticles structure by hydrogel networks. J. Colloid Interface Sci. 2010, 342, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Parkes, M.; Myant, C.; Dini, D.; Cann, P. Tribology-optimised silk protein hydrogels for articular cartilage repair. Tribol. Int. 2015, 89, 9–18. [Google Scholar] [CrossRef]
- Annabi, N.; Mithieux, S.M.; Camci-Unal, G.; Dokmeci, M.R.; Weiss, A.S.; Khademhosseini, A. Elastomeric recombinant protein-based biomaterials. Biochem. Eng. J. 2013, 77, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Gibas, I.; Janik, H. Synthetic polymer hydrogels for biomedical applications. Chem. Chem. Technol. 2010, 4, 297–304. [Google Scholar]
- Das, N. A review on nature and preparation of hydrogel based on starting material. Int. J. Pharm. Pharm. Sci. 2013, 5, 55–58. [Google Scholar]
- Hassan, C.M.; Peppas, N.A. Cellular PVA hydrogels produced by freeze/thawing. J. Appl. Polym. Sci. 2000, 76, 2075–2079. [Google Scholar] [CrossRef]
- Kim, J.H.; Sim, S.; Lee, D.; Kim, D.; Lee, Y.; Chung, D.; Kim, J. Preparation and properties of PHEA/chitosan composite hydrogel. Polym. J. 2004, 36, 943–948. [Google Scholar] [CrossRef]
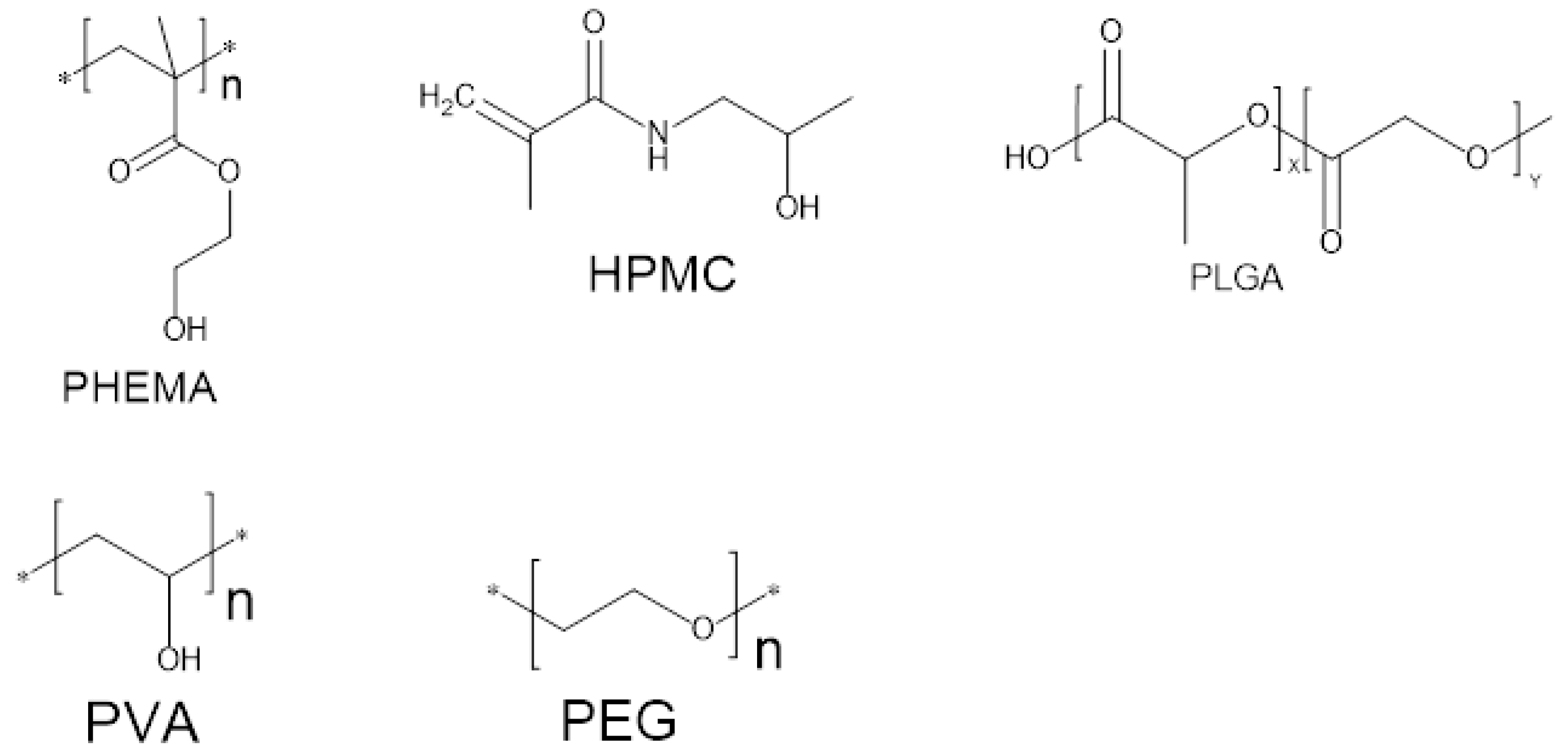
| Monomer Abbreviations | Monomers |
|---|---|
| HPMC | Hydroxypropyl Methylcellulose |
| HEEMA | Hydroxyethoxyethylmethacrylate |
| HDEEMA | Hydroxydiethoxyethyl methacrylate |
| MA | Methacrylate |
| MEEMA | Methoxyethoxyethyl methacrylate |
| PLGA | Polymer of lactic and glycolic acid |
| PHEMA | Poly hydroxyethyl methacrylate |
| PVA | Polyvinyl alcohol |
| PEG | polyethylene glycol |
| PNIPAAm | poly N-isopropyl acrylamide |
| AA | Acrylic acid |
| MAA | Methyl Acrylic acid |
| HPMA | N-(2-hydroxypropyl)metharcrylamide |
| PVA | Polyvinyl alcohol |
| PEGA | PEG-acrylate |
| PEGMA | PEG methacrylate |
| PEGDA | PEG diacrylate |
| PEGDMA | PEG dimethylcrylate |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, F.; Zafar, H.; Zhu, Y.; Ren, Y.; -Ullah, A.; Khan, A.U.; He, X.; Han, H.; Aquib, M.; Boakye-Yiadom, K.O.; et al. A Review on Recent Advances in Stabilizing Peptides/Proteins upon Fabrication in Hydrogels from Biodegradable Polymers. Pharmaceutics 2018, 10, 16. https://doi.org/10.3390/pharmaceutics10010016
Raza F, Zafar H, Zhu Y, Ren Y, -Ullah A, Khan AU, He X, Han H, Aquib M, Boakye-Yiadom KO, et al. A Review on Recent Advances in Stabilizing Peptides/Proteins upon Fabrication in Hydrogels from Biodegradable Polymers. Pharmaceutics. 2018; 10(1):16. https://doi.org/10.3390/pharmaceutics10010016
Chicago/Turabian StyleRaza, Faisal, Hajra Zafar, Ying Zhu, Yuan Ren, Aftab -Ullah, Asif Ullah Khan, Xinyi He, Han Han, Md Aquib, Kofi Oti Boakye-Yiadom, and et al. 2018. "A Review on Recent Advances in Stabilizing Peptides/Proteins upon Fabrication in Hydrogels from Biodegradable Polymers" Pharmaceutics 10, no. 1: 16. https://doi.org/10.3390/pharmaceutics10010016
APA StyleRaza, F., Zafar, H., Zhu, Y., Ren, Y., -Ullah, A., Khan, A. U., He, X., Han, H., Aquib, M., Boakye-Yiadom, K. O., & Ge, L. (2018). A Review on Recent Advances in Stabilizing Peptides/Proteins upon Fabrication in Hydrogels from Biodegradable Polymers. Pharmaceutics, 10(1), 16. https://doi.org/10.3390/pharmaceutics10010016