Analysis of the Prevalence of HTLV-1 Proviral DNA in Cervical Smears and Carcinomas from HIV Positive and Negative Kenyan Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. LBC Samples
2.3. HIV Testing
2.4. Extraction of Genomic DNA
2.5. Papillocheck® HPV Genotyping
2.6. PCR
2.7. DNA Sequencing
2.8. Statistical Analysis
3. Results
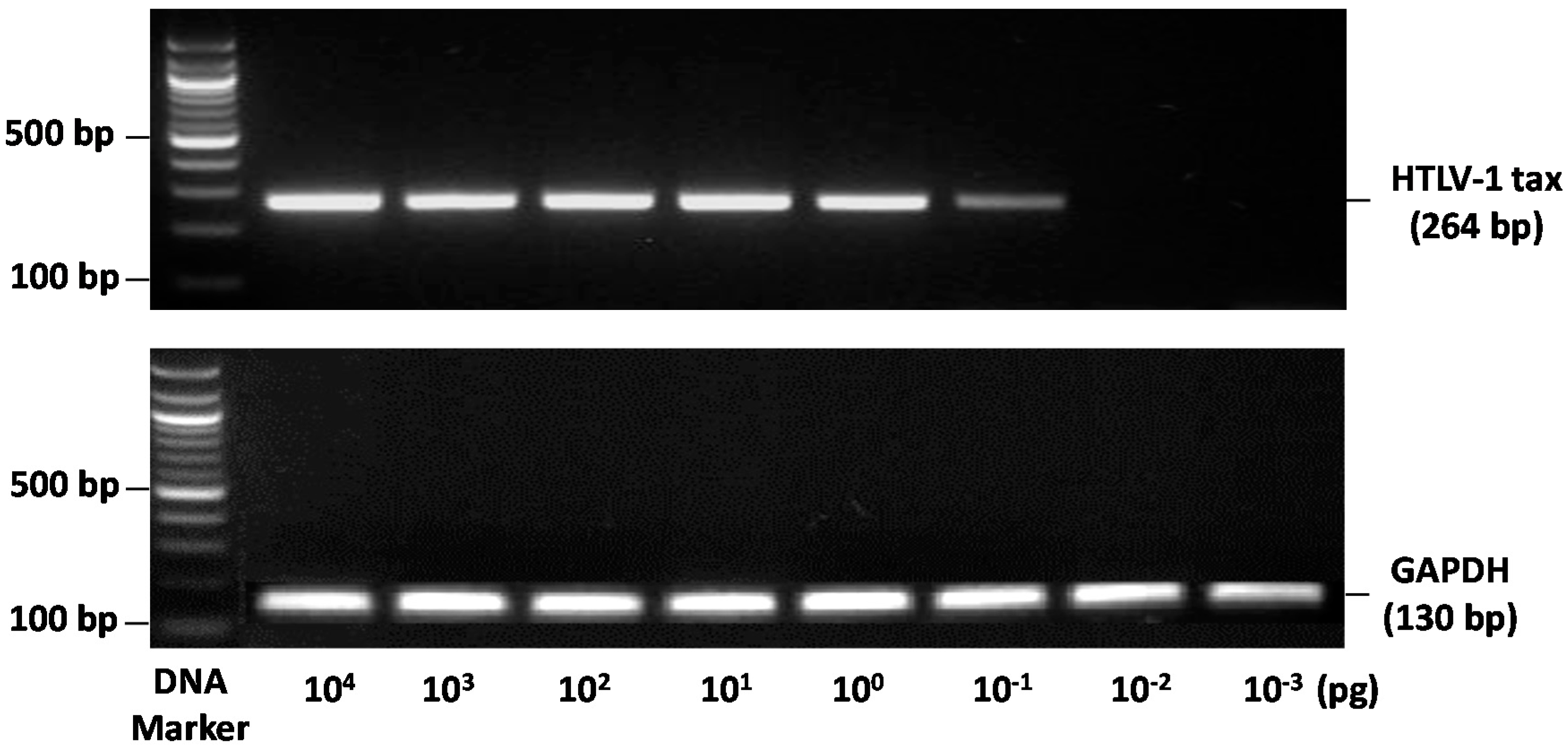
3.1. DNA Quality
3.2. PCR Detection of Proviral HTLV-1 Tax DNA
3.3. Analysis of the Association between HTLV-1 Infection and HIV Infection in LBC DNA
3.4. Analysis of the Association of HTLV-1 Infection with Abnormal Cervical Cytology
3.5. Analysis of the Association between HTLV-1 Infection, Stage of HIV and Use of Highly Active Antiretroviral Therapy (HAART)
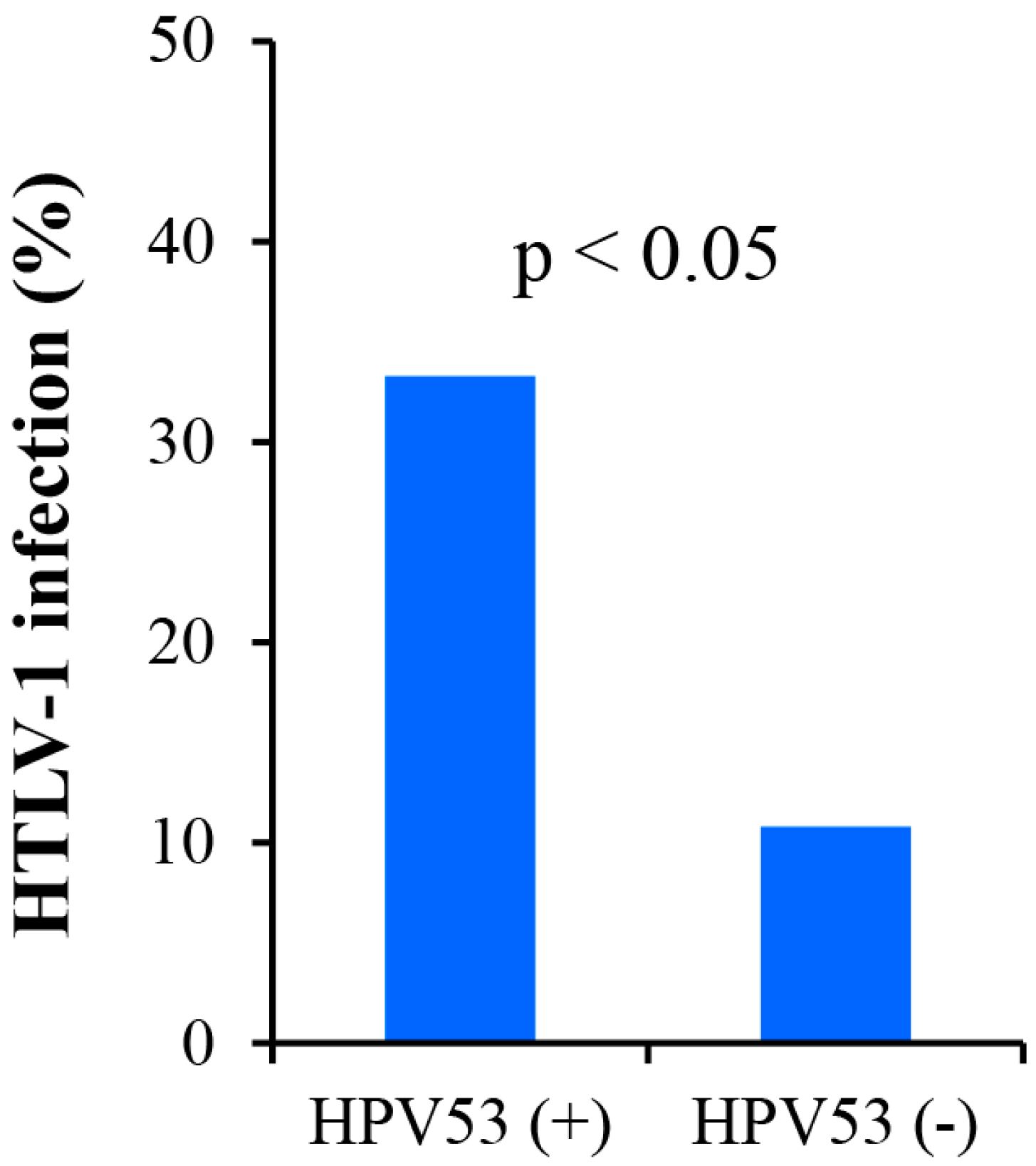
3.6. Analysis of the Association between HTLV-1 Infection and HPV in LBC DNAs
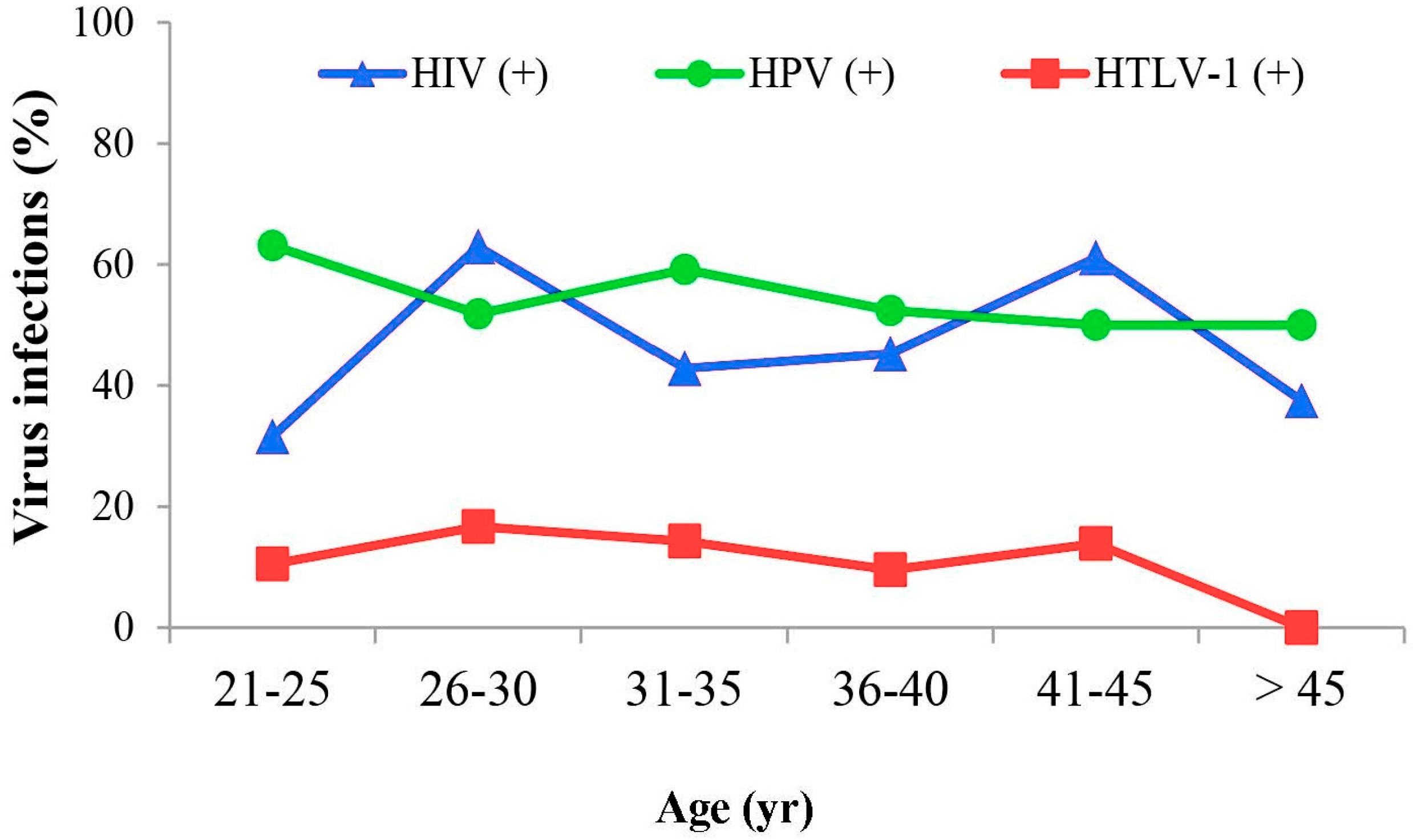
3.7. Analysis of the Relationship between HTLV-1 Infection and Patient Age
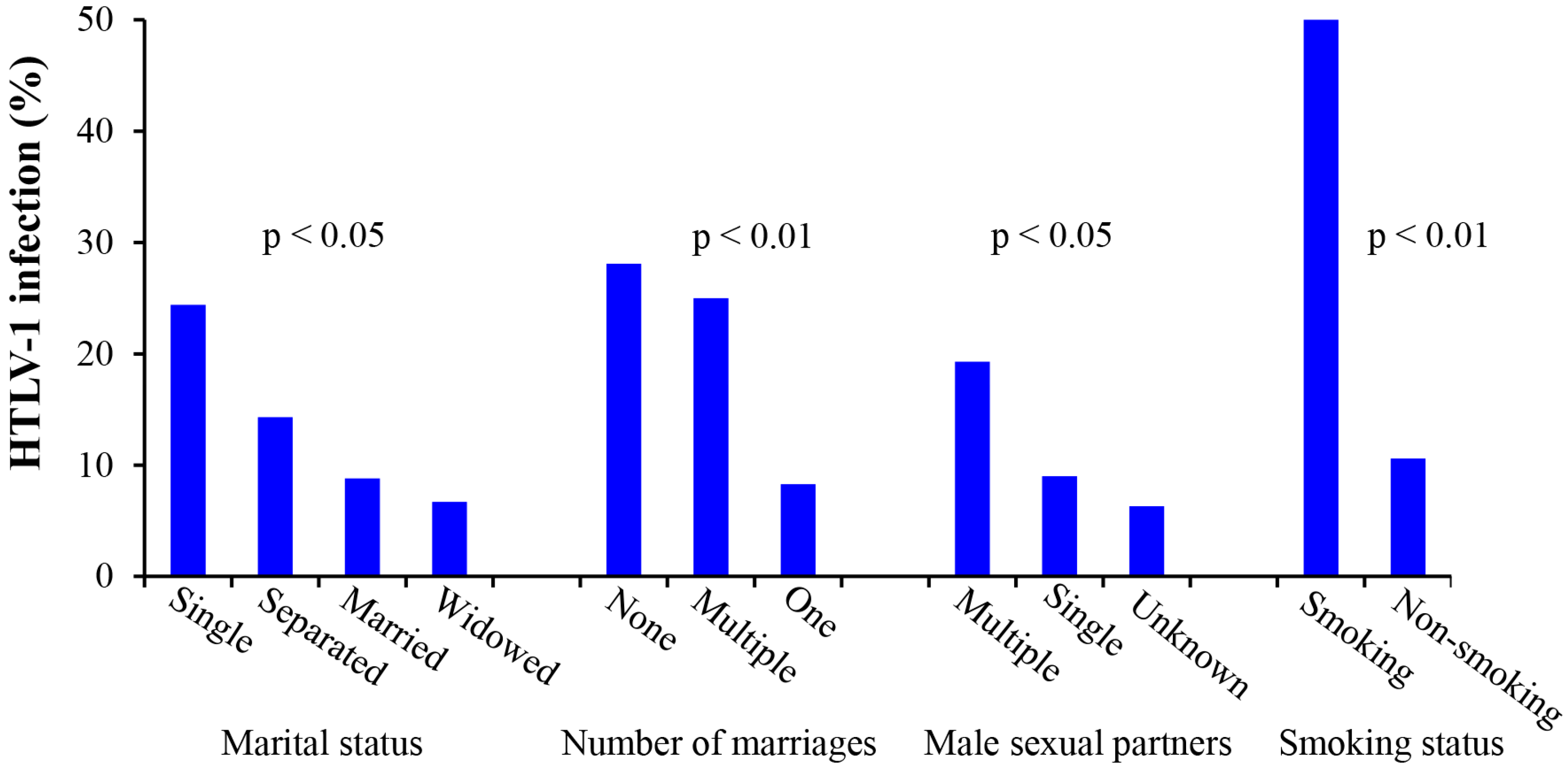
3.8. Analysis of the Relationship between HTLV-1 Infection and Patient Sociodemographics
3.9. Analysis of HTLV-1 DNA in ICC DNA’s
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moens, B.; Lopez, G.; Adaui, V.; Gonzalez, E.; Kerremans, L.; Clark, D.; Verdonck, K.; Gotuzzo, E.; Vanham, G.; Cassar, O.; et al. Development and validation of a multiplex real-time PCR assay for simultaneous genotyping and human T-lymphotropic virus type 1, 2, and 3 proviral load determination. J. Clin. Microbiol. 2009, 47, 3682–3691. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.C. Human retroviruses after 20 years: A perspective from the past and prospects for their future control. Immunol. Rev. 2002, 185, 236–265. [Google Scholar] [CrossRef] [PubMed]
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef] [PubMed]
- Hlela, C.; Shepperd, S.; Khumalo, N.P.; Taylor, G.P. The prevalence of human T-cell lymphotropic virus type 1 in the general population is unknown. AIDS Rev. 2009, 11, 205–214. [Google Scholar] [PubMed]
- LaGrenade, L.; Hanchard, B.; Fletcher, V.; Cranston, B.; Blattner, W. Infective dermatitis of Jamaican children: A marker for HTLV-I infection. Lancet 1990, 336, 1345–1347. [Google Scholar] [PubMed]
- Mochizuki, M.; Watanabe, T.; Yamaguchi, K.; Takatsuki, K.; Yoshimura, K.; Shirao, M.; Nakashima, S.; Mori, S.; Araki, S.; Miyata, N. HTLV-I uveitis: A distinct clinical entity caused by HTLV-I. Jpn. J. Cancer Res. 1992, 83, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Verdonck, K.; Gonzalez, E.; Van Dooren, S.; Vandamme, A.M.; Vanham, G.; Gotuzzo, E. Human T-lymphotropic virus 1: Recent knowledge about an ancient infection. Lancet Infect. Dis. 2007, 7, 266–281. [Google Scholar] [CrossRef]
- Casoli, C.; Pilotti, E.; Bertazzoni, U. Molecular and cellular interactions of HIV-1/HTLV coinfection and impact on AIDS progression. AIDS Rev. 2007, 9, 140–149. [Google Scholar] [PubMed]
- Tanaka, G.; Okayama, A.; Watanabe, T.; Aizawa, S.; Stuver, S.; Mueller, N.; Hsieh, C.C.; Tsubouchi, H. The clonal expansion of human T lymphotropic virus type 1-infected T cells: A comparison between seroconverters and long-term carriers. J. Infect. Dis. 2005, 191, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Proietti, F.A.; Carneiro-Proietti, A.B.; Catalan-Soares, B.C.; Murphy, E.L. Global epidemiology of HTLV-I infection and associated diseases. Oncogene 2005, 24, 6058–6068. [Google Scholar] [CrossRef] [PubMed]
- Gessain, A.; Cassar, O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.C. Human retroviruses in the second decade: A personal perspective. Nat. Med. 1995, 1, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, B.; da Silva, Z.; Larsen, O.; Vastrup, P.; Andersson, S.; Aaby, P. Dual infections with HIV-1, HIV-2 and HTLV-I are more common in older women than in men in Guinea-Bissau. AIDS 2003, 17, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Wignall, F.S.; Hyams, K.C.; Phillips, I.A.; Escamilla, J.; Tejada, A.; Li, O.; Lopez, F.; Chauca, G.; Sanchez, S.; Roberts, C.R. Sexual transmission of human T-lymphotropic virus type I in Peruvian prostitutes. J. Med. Virol. 1992, 38, 44–48. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, A.C.; Casseb, J.S.; Neitzert, E.; de Souza, M.L.; Mammano, F.; Del Mistro, A.; De Rossi, A.; Chieco-Bianchi, L. HTLV-I and HTLV-II infections among HIV-1 seropositive patients in Sao Paulo, Brazil. Eur. J. Epidemiol. 1994, 10, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Manns, A.; Hisada, M.; La Grenade, L. Human T-lymphotropic virus type I infection. Lancet 1999, 353, 1951–1958. [Google Scholar] [CrossRef]
- Feigal, E.; Murphy, E.; Vranizan, K.; Bacchetti, P.; Chaisson, R.; Drummond, J.E.; Blattner, W.; McGrath, M.; Greenspan, J.; Moss, A. Human T cell lymphotropic virus types I and II in intravenous drug users in San Francisco: Risk factors associated with seropositivity. J. Infect. Dis. 1991, 164, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Percher, F.; Jeannin, P.; Martin-Latil, S.; Gessain, A.; Afonso, P.V.; Vidy-Roche, A.; Ceccaldi, P.E. Mother-to-Child Transmission of HTLV-1 Epidemiological Aspects, Mechanisms and Determinants of Mother-to-Child Transmission. Viruses 2016, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Ambroziak, J.A.; Blackbourn, D.J.; Herndier, B.G.; Glogau, R.G.; Gullett, J.H.; McDonald, A.R.; Lennette, E.T.; Levy, J.A. Herpes-like sequences in HIV-infected and uninfected Kaposi's sarcoma patients. Science 1995, 268, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Schalling, M.; Ekman, M.; Kaaya, E.E.; Linde, A.; Biberfeld, P. A role for a new herpes virus (KSHV) in different forms of Kaposi's sarcoma. Nat. Med. 1995, 1, 707–708. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Tosato, G. Neoplastic conditions in the context of HIV-1 infection. Curr. HIV Res. 2004, 2, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.; Chetty, R. Postmodern cancer: The role of human immunodeficiency virus in uterine cervical cancer. Mol. Pathol. 2002, 55, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Mbanya, D.N.; Takam, D.; Ndumbe, P.M. Serological findings amongst first-time blood donors in Yaounde, Cameroon: Is safe donation a reality or a myth? Transfus. Med. 2003, 13, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Carneiro-Proietti, A.B.; Catalan-Soares, B.C.; Castro-Costa, C.M.; Murphy, E.L.; Sabino, E.C.; Hisada, M.; Galvao-Castro, B.; Alcantara, L.C.; Remondegui, C.; Verdonck, K.; et al. HTLV in the Americas: Challenges and perspectives. Rev. Panam. Salud Publica 2006, 19, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Góngora-Biachi, R.; González-Martínez, P.; Castro-Sansores, C.; Bastarrachea-Ortiz, J. Infection with HTLV virus type I-II in patients with cervico-uterine cancer in the Yucatan peninsula Mexico. J.Ginecol. Obstet. Mex. 1997, 65, 141–144. [Google Scholar]
- Miyazaki, K.; Yamaguchi, K.; Tohya, T.; Ohba, T.; Takatsuki, K.; Okamura, H. Human T-cell leukemia virus type I infection as an oncogenic and prognostic risk factor in cervical and vaginal carcinoma. Obstet. Gynecol. 1991, 77, 107–110. [Google Scholar] [CrossRef]
- Strickler, H.D.; Rattray, C.; Escoffery, C.; Manns, A.; Schiffman, M.H.; Brown, C.; Cranston, B.; Hanchard, B.; Palefsky, J.M.; Blattner, W.A. Human T-cell lymphotropic virus type I and severe neoplasia of the cervix in Jamaica. Int. J. Cancer 1995, 61, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Castle, P.E.; Escoffery, C.; Schachter, J.; Rattray, C.; Schiffman, M.; Moncada, J.; Sugai, K.; Brown, C.; Cranston, B.; Hanchard, B.; et al. Chlamydia trachomatis, herpes simplex virus 2, and human T-cell lymphotrophic virus type 1 are not associated with grade of cervical neoplasia in Jamaican colposcopy patients. Sex. Transm. Dis. 2003, 30, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Raab, S.S.; Hart, A.R.; D’Antonio, J.A.; Grzybicki, D.M. Clinical perception of disease probability associated with Bethesda System diagnoses. Am. J. Clin. Pathol. 2001, 115, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Ohtani, K.; Nakamura, M.; Sugamura, K. Activation of endogenous c-fos proto-oncogene expression by human T-cell leukemia virus type I-encoded p40tax protein in the human T-cell line, Jurkat. J. Virol. 1989, 63, 3220–3226. [Google Scholar] [PubMed]
- Maranga, I.O.; Hampson, L.; Oliver, A.W.; He, X.; Gichangi, P.; Rana, F.; Opiyo, A.; Hampson, I.N. HIV Infection Alters the Spectrum of HPV Subtypes Found in Cervical Smears and Carcinomas from Kenyan Women. Open Virol. J. 2013, 7, 19–27. [Google Scholar] [CrossRef] [PubMed]
- AETC HIV Classification: CDC and WHO Staging Systems. Available online: http://aidsetc.org/guide/hiv-classification-cdc-and-who-staging-systems (accessed on April 2014).
- Van Tienen, C.; van der Loeff, M.F.; Peterson, I.; Cotten, M.; Holmgren, B.; Andersson, S.; Vincent, T.; Sarge-Njie, R.; Rowland-Jones, S.; Jaye, A.; et al. HTLV-1 in rural Guinea-Bissau: Prevalence, incidence and a continued association with HIV between 1990 and 2007. Retrovirology 2010, 7, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Brites, C.; Sampalo, J.; Oliveira, A. HIV/human T-cell lymphotropic virus coinfection revisited: Impact on AIDS progression. AIDS Rev. 2009, 11, 8–16. [Google Scholar] [PubMed]
- Abdel-Hady, E.S.; Martin-Hirsch, P.; Duggan-Keen, M.; Stern, P.L.; Moore, J.V.; Corbitt, G.; Kitchener, H.C.; Hampson, I.N. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res. 2001, 61, 192–196. [Google Scholar] [PubMed]
- Al-Saleh, W.; Giannini, S.L.; Jacobs, N.; Moutschen, M.; Doyen, J.; Boniver, J.; Delvenne, P. Correlation of T-helper secretory differentiation and types of antigen-presenting cells in squamous intraepithelial lesions of the uterine cervix. J. Pathol. 1998, 184, 283–290. [Google Scholar] [CrossRef]
- Bais, A.G.; Beckmann, I.; Lindemans, J.; Ewing, P.C.; Meijer, C.J.L.M.; Snijders, P.J.F.; Helmerhorst, T.J.M. A shift to a peripheral Th2-type cytokine pattern during the carcinogenesis of cervical cancer becomes manifest in CIN III lesions. J. Clin. Pathol. 2005, 58, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.; Nakagawa, M.; Moscicki, A.B. Cell-mediated response to human papillomavirus infection. Clin. Diagn. Lab. Immunol. 2001, 8, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Abrahao, M.H.; Lima, R.G.; Netto, E.; Brites, C. Short communication: Human lymphotropic virus type 1 coinfection modulates the synthesis of cytokines by peripheral blood mononuclear cells from HIV type 1-infected individuals. AIDS Res. Hum. Retrovir. 2012, 28, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Nakamoto, M.; Nakamura, M.; Kinjo, N.; Hokama, A.; Kinjo, F.; Fujita, J. Low prevalence of human T cell lymphotropic virus type 1 infection in patients with gastric cancer. J. Gastroenterol. Hepatol. 2007, 22, 2238–2241. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health statistics and health information systems: Life tables for WHO Member States; Part III Global health indicators. In World Health Statistics 2012; WHO Press: Geneva, Switzerland, 2012. [Google Scholar]
- Campaner, A.B.; Nadais, R.F.; Galvao, M.A. The effect of cigarette smoking on cervical langerhans cells and T and B lymphocytes in normal uterine cervix epithelium. Int. J. Gynecol. Pathol. 2009, 28, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Dal Maso, L.; Serraino, D.; Franceschi, S. Epidemiology of AIDS-related tumours in developed and developing countries. Eur. J. Cancer 2001, 37, 1188–1201. [Google Scholar] [CrossRef]
- Franceschi, S.; Dal Maso, L.; Arniani, S.; Lo Re, A.; Barchielli, A.; Milandri, C.; Simonato, L.; Vercelli, M.; Zanetti, R.; Rezza, G. Linkage of AIDS and cancer registries in Italy. Int. J. Cancer 1998, 75, 831–834. [Google Scholar] [CrossRef]
- Franceschi, S.; Dal Maso, L.; Pezzotti, P.; Polesel, J.; Braga, C.; Piselli, P.; Serraino, D.; Tagliabue, G.; Federico, M.; Ferretti, S.; et al. Incidence of AIDS-defining cancers after AIDS diagnosis among people with AIDS in Italy, 1986–1998. J. Acquir. Immune Defic. Syndr. 2003, 34, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Odida, M.; Sandin, S.; Mirembe, F.; Kleter, B.; Quint, W.; Weiderpass, E. HPV types, HIV and invasive cervical carcinoma risk in Kampala, Uganda: A case-control study. Infect. Agents Cancer 2011, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Ter Meulen, J.; Eberhardt, H.C.; Luande, J.; Mgaya, H.N.; Chang-Claude, J.; Mtiro, H.; Mhina, M.; Kashaija, P.; Ockert, S.; Yu, X.; et al. Human papillomavirus (HPV) infection, HIV infection and cervical cancer in Tanzania, east Africa. Int. J. Cancer 1992, 51, 515–521. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequence | Conditions | Amplimer Size (bp) |
|---|---|---|---|
| GAPDH-F | 5‘-CATTGACCTCAACTACATGGT-3‘ | 94 °C × 5 min; 33 cycles: 94°C × 25 s; 53 °C × 25 s; 72 °C × 25 s; 72°C × 7 min | 130 |
| GAPDH-R | 5’-TCGCTCCTGGAAGATGGTGAT-3’ | ||
| HTLV-1 Tax-F | 5’-CACCTGTCCAGAGCATCAGA-3’ | 95 °C × 15 min; 45 cycles: 94°C × 30 s; 57 °C × 30s; 75 °C × 30 s; 75°C × 7 min | 264 |
| HTLV-1 Tax-R | 5’-TCTGGAAAAGACAGGGTTGG-3’ |
| HPV (+) | HPV (−) | |
|---|---|---|
| HTLV-1 (+) | 13 | 13 |
| HTLV-1 (−) | 108 | 89 |
| p-value | > 0.05 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Maranga, I.O.; Oliver, A.W.; Gichangi, P.; Hampson, L.; Hampson, I.N. Analysis of the Prevalence of HTLV-1 Proviral DNA in Cervical Smears and Carcinomas from HIV Positive and Negative Kenyan Women. Viruses 2016, 8, 245. https://doi.org/10.3390/v8090245
He X, Maranga IO, Oliver AW, Gichangi P, Hampson L, Hampson IN. Analysis of the Prevalence of HTLV-1 Proviral DNA in Cervical Smears and Carcinomas from HIV Positive and Negative Kenyan Women. Viruses. 2016; 8(9):245. https://doi.org/10.3390/v8090245
Chicago/Turabian StyleHe, Xiaotong, Innocent O. Maranga, Anthony W. Oliver, Peter Gichangi, Lynne Hampson, and Ian N. Hampson. 2016. "Analysis of the Prevalence of HTLV-1 Proviral DNA in Cervical Smears and Carcinomas from HIV Positive and Negative Kenyan Women" Viruses 8, no. 9: 245. https://doi.org/10.3390/v8090245
APA StyleHe, X., Maranga, I. O., Oliver, A. W., Gichangi, P., Hampson, L., & Hampson, I. N. (2016). Analysis of the Prevalence of HTLV-1 Proviral DNA in Cervical Smears and Carcinomas from HIV Positive and Negative Kenyan Women. Viruses, 8(9), 245. https://doi.org/10.3390/v8090245







