Treatment of HEV Infection in Patients with a Solid-Organ Transplant and Chronic Hepatitis
Abstract
:1. Introduction
2. Reducing Immunosuppressive Therapy
3. Antiviral Therapies
3.1. Pegylated Interferon
3.2. Ribavirin
3.3. Predictive Factors for a SVR in Patients Receiving Ribavirin
3.4. Alternative Antiviral Therapy
4. Treatment of HEV Infection with Additional Complications
4.1. Treatment in Hematology Patients
4.2. Treatment of HEV Infection in HIV-Positive Patients
4.3. Treatment of Acute-Phase HEV
4.4. Treatment of HEV-Induced Extra-Hepatic Manifestations
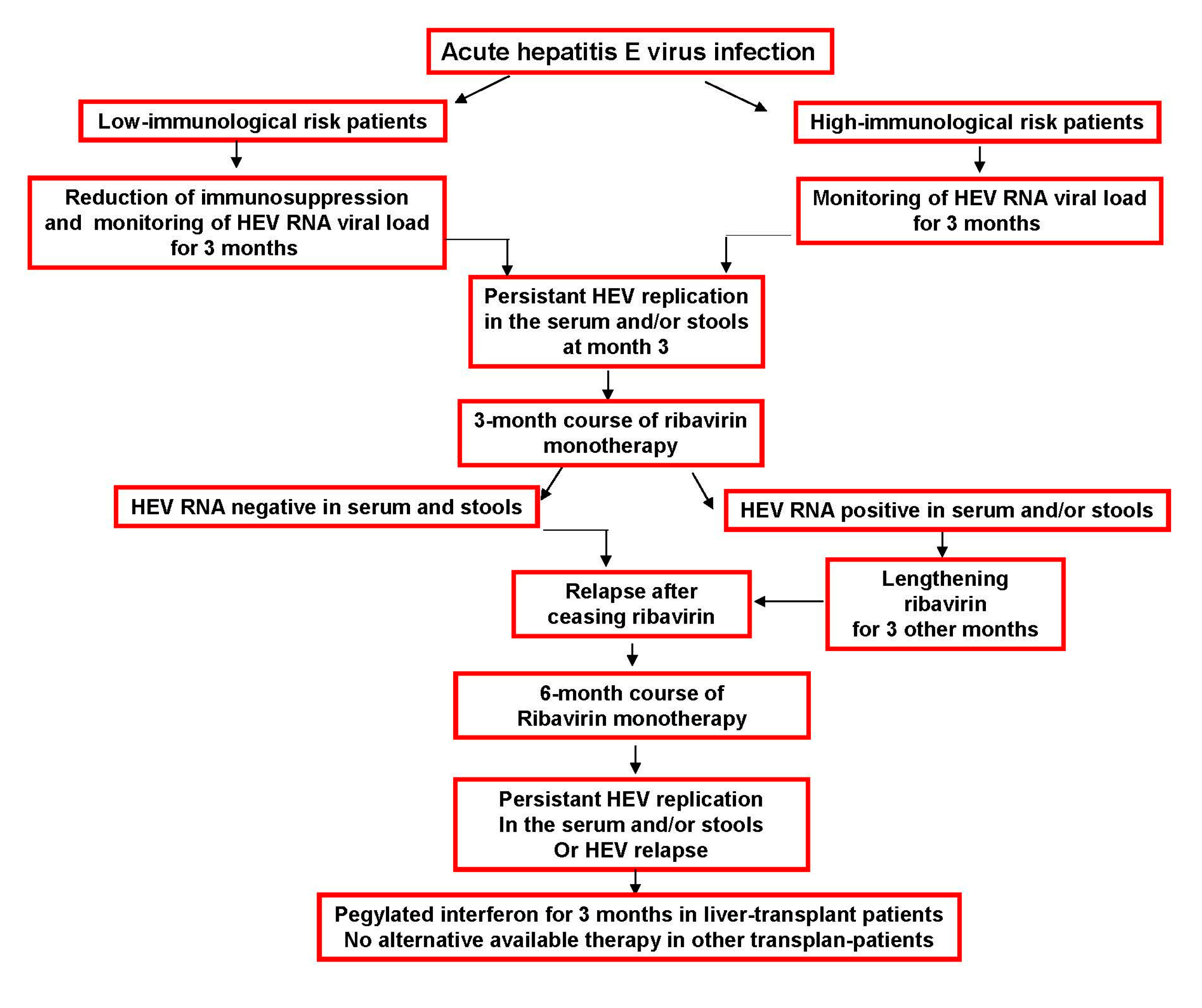
5. Suggested HEV Infection Therapy Algorithm
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Kamar, N.; Bendall, R.; Legrand-Abravanel, F.; Xia, N.S.; Ijaz, S.; Izopet, J.; Dalton, H.R. Hepatitis E. Lancet 2012, 379, 2477–2488. [Google Scholar] [CrossRef]
- Kamar, N.; Selves, J.; Mansuy, J.M.; Ouezzani, L.; Peron, J.M.; Guitard, J.; Cointault, O.; Esposito, L.; Abravanel, F.; Danjoux, M.; et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N. Engl. J. Med. 2008, 358, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Koenecke, C.; Pischke, S.; Beutel, G.; Ritter, U.; Ganser, A.; Wedemeyer, H.; Eder, M. Hepatitis E virus infection in a hematopoietic stem cell donor. Bone Marrow Transplant. 2014, 49, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Ollier, L.; Tieulie, N.; Sanderson, F.; Heudier, P.; Giordanengo, V.; Fuzibet, J.; Nicand, E. Chronic hepatitis after hepatitis E virus infection in a patient with non-hodgkin lymphoma taking rituximab. Ann. Intern. Med. 2009, 150, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H.; Luxembourger, C.; Gottenberg, J.E.; Fournier, S.; Abravanel, F.; Cantagrel, A.; Chatelus, E.; Claudepierre, P.; Hudry, C.; Izopet, J.; et al. Outcome of hepatitis E virus infection in patients with inflammatory arthritides treated with immunosuppressants: A French retrospective multicenter study. Medicine (Baltimore) 2015, 94, e675. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.R.; Bendall, R.; Keane, F.; Tedder, R.; Ijaz, S. Persistent carriage of hepatitis E virus in patients with HIV infection. N. Engl. J. Med. 2009, 361, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Garrouste, C.; Haagsma, E.B.; Garrigue, V.; Pischke, S.; Chauvet, C.; Dumortier, J.; Cannesson, A.; Cassuto-Viguier, E.; Thervet, E.; et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 2011, 140, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Marion, O.; Abravanel, F.; Izopet, J.; Dalton, H.R. Extrahepatic manifestations of hepatitis E virus. Liver Int. 2016, 36, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.R.; Kamar, N.; van Eijk, J.J.; McLean, B.N.; Cintas, P.; Bendall, R.P.; Jacobs, B.C. Hepatitis E virus and neurological injury. Nat. Rev. Neurol. 2016, 12, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Weclawiack, H.; Guilbeaud-Frugier, C.; Legrand-Abravanel, F.; Cointault, O.; Ribes, D.; Esposito, L.; Cardeau, I.; Guitard, J.; Sallusto, F.; et al. Hepatitis E virus and the kidney in solid-organ-transplant patients. Transplantation 2012, 93, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Abravanel, F.; Selves, J.; Garrouste, C.; Esposito, L.; Lavayssiere, L.; Cointault, O.; Ribes, D.; Cardeau, I.; Nogier, M.B.; et al. Influence of immunosuppressive therapy on the natural history of genotype 3 hepatitis-E virus infection after organ transplantation. Transplantation 2010, 89, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Suneetha, P.V.; Pischke, S.; Schlaphoff, V.; Grabowski, J.; Fytili, P.; Gronert, A.A.; Bremer, B.; Markova, A.; Jaroszewicz, J.; Bara, C.; et al. HEV-specific T- cell responses are associated with control of HEV infection. Hepatology 2012, 55, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Lhomme, S.; Abravanel, F.; Dubois, M.; Sandres Saune, K.; Rostaing, L.; Kamar, N.; Izopet, J. HEV quasispecies and the outcome of acute hepatitis E in solid-organ transplant patients. J. Virol. 2012, 86, 100006–100014. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, X.; Debing, Y.; Chen, K.; Van der Laan, L.J.; Neyts, J.; Janssen, H.L.; Metselaar, H.J.; Peppelenbosch, M.P.; Pan, Q. Calcineurin inhibitors stimulate and mycophenolic acid inhibits replication of hepatitis E virus. Gastroenterology 2014, 146, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, L.; Wang, W.; Watashi, K.; Wang, Y.; Sprengers, D.; de Ruiter, P.E.; van der Laan, L.J.; Metselaar, H.J.; Kamar, N.; et al. Disparity of basal and therapeutically activated interferon signalling in constraining hepatitis E virus infection. J. Viral Hepat. 2016, 23, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Todt, D.; Francois, C.; Anggakusuma; Behrendt, P.; Engelmann, M.; Knegendorf, L.; Vieyres, G.; Wedemeyer, H.; Hartmann, R.; Pietschmann, T.; et al. Antiviral activities of different interferon types and subtypes against hepatitis E virus replication. Antimicrob. Agents Chemother. 2016, 60, 2132–2139. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Rostaing, L.; Abravanel, F.; Garrouste, C.; Esposito, L.; Cardeau-Desangles, I.; Mansuy, J.M.; Selves, J.; Peron, J.M.; Otal, P.; et al. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation. Clin. Infect. Dis. 2010, 50, e30–e33. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Abravanel, F.; Garrouste, C.; Cardeau-Desangles, I.; Mansuy, J.M.; Weclawiak, H.; Izopet, J.; Rostaing, L. Three-month pegylated interferon-alpha-2a therapy for chronic hepatitis E virus infection in a haemodialysis patient. Nephrol. Dial. Transplant. 2010, 25, 2792–2795. [Google Scholar] [CrossRef] [PubMed]
- Haagsma, E.B.; Riezebos-Brilman, A.; van den Berg, A.P.; Porte, R.J.; Niesters, H.G. Treatment of chronic hepatitis E in liver transplant recipients with pegylated interferon alpha-2b. Liver Transplant. 2010, 16, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Rostaing, L.; Izopet, J.; Baron, E.; Duffaut, M.; Puel, J.; Durand, D. Treatment of chronic hepatitis C with recombinant interferon alpha in kidney transplant recipients. Transplantation 1995, 59, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Rostaing, L.; Abravanel, F.; Garrouste, C.; Lhomme, S.; Esposito, L.; Basse, G.; Cointault, O.; Ribes, D.; Nogier, M.B.; et al. Ribavirin therapy inhibits viral replication in patients with chronic hepatitis E virus infection. Gastroenterology 2010, 139, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Mallet, V.; Nicand, E.; Sultanik, P.; Chakvetadze, C.; Tesse, S.; Thervet, E.; Mouthon, L.; Sogni, P.; Pol, S. Brief communication: Case reports of ribavirin treatment for chronic hepatitis E. Ann. Intern. Med. 2010, 153, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Pischke, S.; Hardkte, S.; Bode, U.; Birkner, S.; Chatzikyrkou, C.; Kauffmann, W.; Bara, C.; Glottlieb, J.; Wenzel, J.J.; Manns, M.; et al. Ribavirin treatment of acute and chronic hepatitis E: A single-centre experience. Liver Int. 2013, 33, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Izopet, J.; Tripon, S.; Bismuth, M.; Hillaire, S.; Dumortier, J.; Radenne, S.; Coilly, A.; Garrigue, V.; D’Alteroche, L.; et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N. Engl. J. Med. 2014, 370, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Debing, Y.; Emerson, S.U.; Wang, Y.; Pan, Q.; Balzarini, J.; Dallmeier, K.; Neyts, J. Ribavirin inhibits in vitro hepatitis E virus replication through depletion of cellular GTP pools and is moderately synergistic with alpha interferon. Antimicrob. Agents Chemother. 2014, 58, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Lhomme, S.; Abravanel, F.; Cointault, O.; Esposito, L.; Cardeau-Desangles, I.; Del Bello, A.; Dorr, G.; Lavayssiere, L.; Nogier, M.B.; et al. An early viral response predicts the virological response to ribavirin in hepatitis E virus organ transplant patients. Transplantation 2015, 99, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Todt, D.; Gisa, A.; Radonic, A.; Nitsche, A.; Behrendt, P.; Suneetha, P.V.; Pischke, S.; Bremer, B.; Brown, R.J.; Manns, M.P.; et al. In vivo evidence for ribavirin-induced mutagenesis of the hepatitis E virus genome. Gut 2016. [Google Scholar] [CrossRef] [PubMed]
- Debing, Y.; Gisa, A.; Dallmeier, K.; Pischke, S.; Bremer, B.; Manns, M.; Wedemeyer, H.; Suneetha, P.V.; Neyts, J. A mutation in the hepatitis E virus RNA polymerase promotes its replication and associates with ribavirin treatment failure in organ transplant recipients. Gastroenterology 2014, 147, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Lhomme, S.; Kamar, N.; Nicot, F.; Ducos, J.; Bismuth, M.; Garrigue, V.; Petitjean-Lecherbonnier, J.; Ollivier, I.; Alessandri-Gradt, E.; Goria, O.; et al. Mutation in the hepatitis E virus polymerase and outcome of ribavirin therapy. Antimicrob. Agents Chemother. 2015, 60, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Debing, Y.; Ramiere, C.; Dallmeier, K.; Piorkowski, G.; Trabaud, M.A.; Lebosse, F.; Scholtes, C.; Roche, M.; Legras-Lachuer, C.; de Lamballerie, X.; et al. Hepatitis E virus mutations associated with ribavirin treatment failure result in altered viral fitness and ribavirin sensitivity. J. Hepatol 2016. [Google Scholar] [CrossRef] [PubMed]
- Abravanel, F.; Lhomme, S.; Rostaing, L.; Kamar, N.; Izopet, J. Protracted fecal shedding of HEV during ribavirin therapy predicts treatment relapse. Clin. Infect. Dis. 2015, 60, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Dao Thi, V.L.; Debing, Y.; Wu, X.; Rice, C.M.; Neyts, J.; Moradpour, D.; Gouttenoire, J. Sofosbuvir inhibits hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin. Gastroenterology 2016, 150, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Alric, L.; Bonnet, D.; Laurent, G.; Kamar, N.; Izopet, J. Chronic hepatitis E virus infection: Successful virologic response to pegylated interferon-alpha therapy. Ann. Intern. Med. 2010, 153, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Alric, L.; Bonnet, D.; Beynes-Rauzy, O.; Izopet, J.; Kamar, N. Definitive clearance of a chronic hepatitis E virus infection with ribavirin treatment. Am. J. Gastroenterol. 2011, 106, 1562–1563. [Google Scholar] [CrossRef] [PubMed]
- Tavitian, S.; Peron, J.M.; Huguet, F.; Kamar, N.; Abravanel, F.; Beyne-Rauzy, O.; Oberic, L.; Faguer, S.; Alric, L.; Roussel, M.; et al. Ribavirin for chronic hepatitis prevention among patients with hematologic malignancies. Emerg. Infect. Dis. 2015, 21, 1466–1469. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.; Keane, F.; Bendall, R.; Mathew, J.; Ijaz, S. Treatment of chronic hepatitis E in a HIV positive patient. Ann. Intern. Med. 2011, 155, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Neukam, K.; Barreiro, P.; Macias, J.; Avellon, A.; Cifuentes, C.; Martin-Carbonero, L.; Echevarria, J.M.; Vargas, J.; Soriano, V.; Pineda, J.A. Chronic hepatitis E in HIV patients: Rapid progression to cirrhosis and response to oral ribavirin. Clin. Infect. Dis. 2013, 57, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Hajji, H.; Gerolami, R.; Solas, C.; Moreau, J.; Colson, P. Chronic hepatitis E resolution in a human immunodeficiency virus (HIV)-infected patient treated with ribavirin. Int. J. Antimicrob. Agents 2013, 46, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Robbins, A.; Lambert, D.; Ehrhard, F.; Brodard, V.; Hentzien, M.; Lebrun, D.; Nguyen, Y.; Tabary, T.; Peron, J.M.; Izopet, J.; et al. Severe acute hepatitis E in an HIV infected patient: Successful treatment with ribavirin. J. Clin. Virol. 2014, 60, 422–423. [Google Scholar] [CrossRef] [PubMed]
- Peron, J.M.; Dalton, H.; Izopet, J.; Kamar, N. Acute autochthonous hepatitis E in western patients with underlying chronic liver disease: A role for ribavirin? J. Hepatol. 2011, 54, 1323–1324. [Google Scholar] [CrossRef] [PubMed]
- Peron, J.M.; Abravanel, F.; Guillaume, M.; Gerolami, R.; Nana, J.; Anty, R.; Pariente, A.; Renou, C.; Bureau, C.; Robic, M.A.; et al. Treatment of autochthonous acute hepatitis E with short-term ribavirin: A multicenter retrospective study. Liver Int. 2016, 36, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Bendall, R.P.; Peron, J.M.; Cintas, P.; Prudhomme, L.; Mansuy, J.M.; Rostaing, L.; Keane, F.; Ijaz, S.; Izopet, J.; et al. Hepatitis E virus and neurologic disorders. Emerg. Infect. Dis. 2011, 17, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Del Bello, A.; Guilbeau-Frugier, C.; Josse, A.G.; Rostaing, L.; Izopet, J.; Kamar, N. Successful treatment of hepatitis E virus-associated cryoglobulinemic membranoproliferative glomerulonephritis with ribavirin. Transpl. Infect. Dis. 2015, 17, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Guinault, D.; Ribes, D.; Delas, A.; Milongo, D.; Abravanel, F.; Puissant Lubrano, B.; Izopet, J.; Kamar, N. Hepatitis E virus-induced cryglobulinemic glomerulonephritis in a non-immunocompromised person. Am. J. Kidney Dis. 2016, 67, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Chatelut, E.; Manolis, E.; Lafont, T.; Izopet, J.; Rostaing, L. Ribavirin pharmacokinetics in renal and liver transplant patients: Evidence that it depends on renal function. Am. J. Kidney Dis. 2004, 43, 140–146. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamar, N.; Lhomme, S.; Abravanel, F.; Marion, O.; Peron, J.-M.; Alric, L.; Izopet, J. Treatment of HEV Infection in Patients with a Solid-Organ Transplant and Chronic Hepatitis. Viruses 2016, 8, 222. https://doi.org/10.3390/v8080222
Kamar N, Lhomme S, Abravanel F, Marion O, Peron J-M, Alric L, Izopet J. Treatment of HEV Infection in Patients with a Solid-Organ Transplant and Chronic Hepatitis. Viruses. 2016; 8(8):222. https://doi.org/10.3390/v8080222
Chicago/Turabian StyleKamar, Nassim, Sébastien Lhomme, Florence Abravanel, Olivier Marion, Jean-Marie Peron, Laurent Alric, and Jacques Izopet. 2016. "Treatment of HEV Infection in Patients with a Solid-Organ Transplant and Chronic Hepatitis" Viruses 8, no. 8: 222. https://doi.org/10.3390/v8080222
APA StyleKamar, N., Lhomme, S., Abravanel, F., Marion, O., Peron, J.-M., Alric, L., & Izopet, J. (2016). Treatment of HEV Infection in Patients with a Solid-Organ Transplant and Chronic Hepatitis. Viruses, 8(8), 222. https://doi.org/10.3390/v8080222







