Hepatitis E Seroprevalence in Europe: A Meta-Analysis
Abstract
:1. Introduction
2. Methods
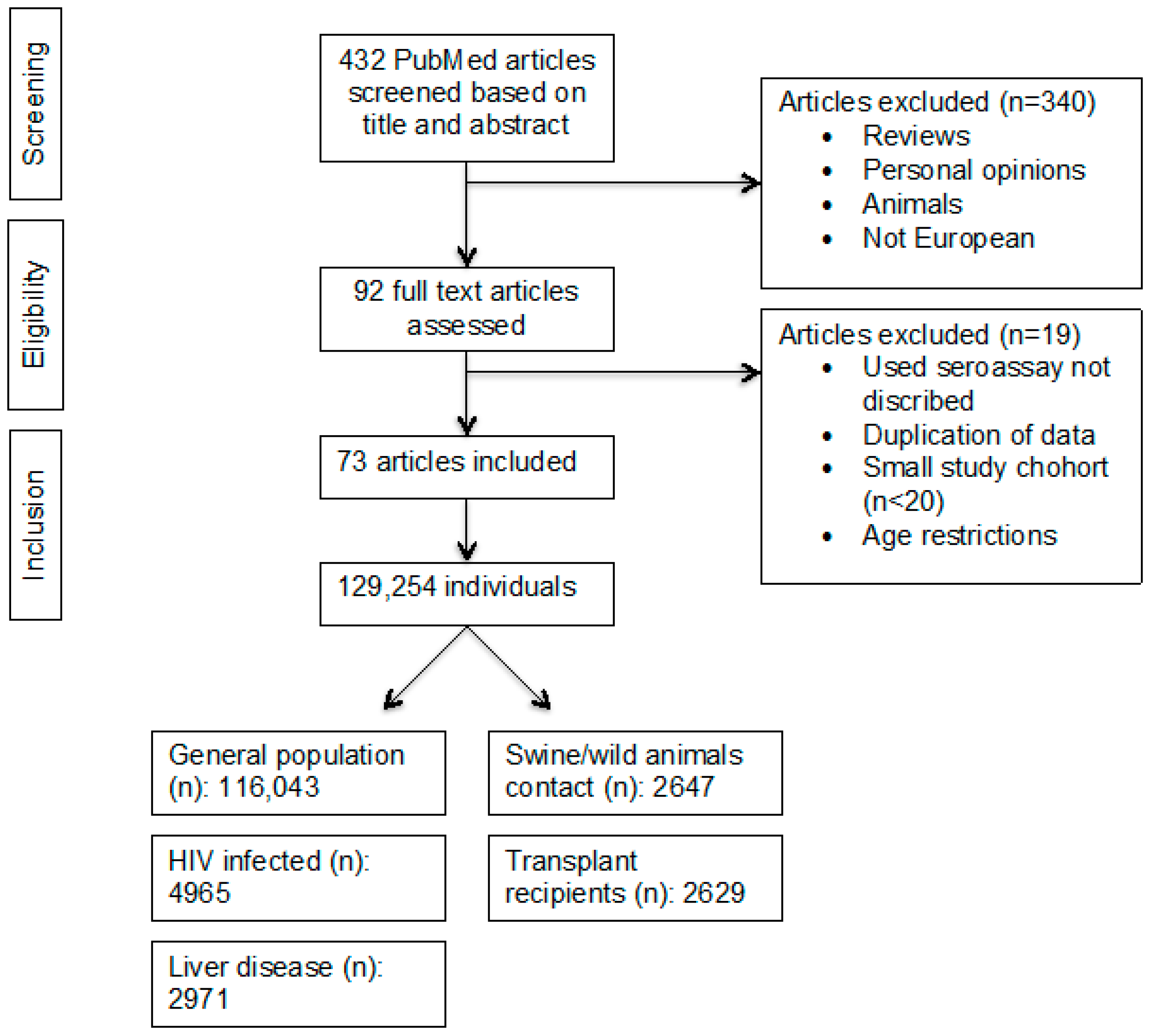
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction
2.3. Study Quality
2.4. Statistical Analysis
3. Results
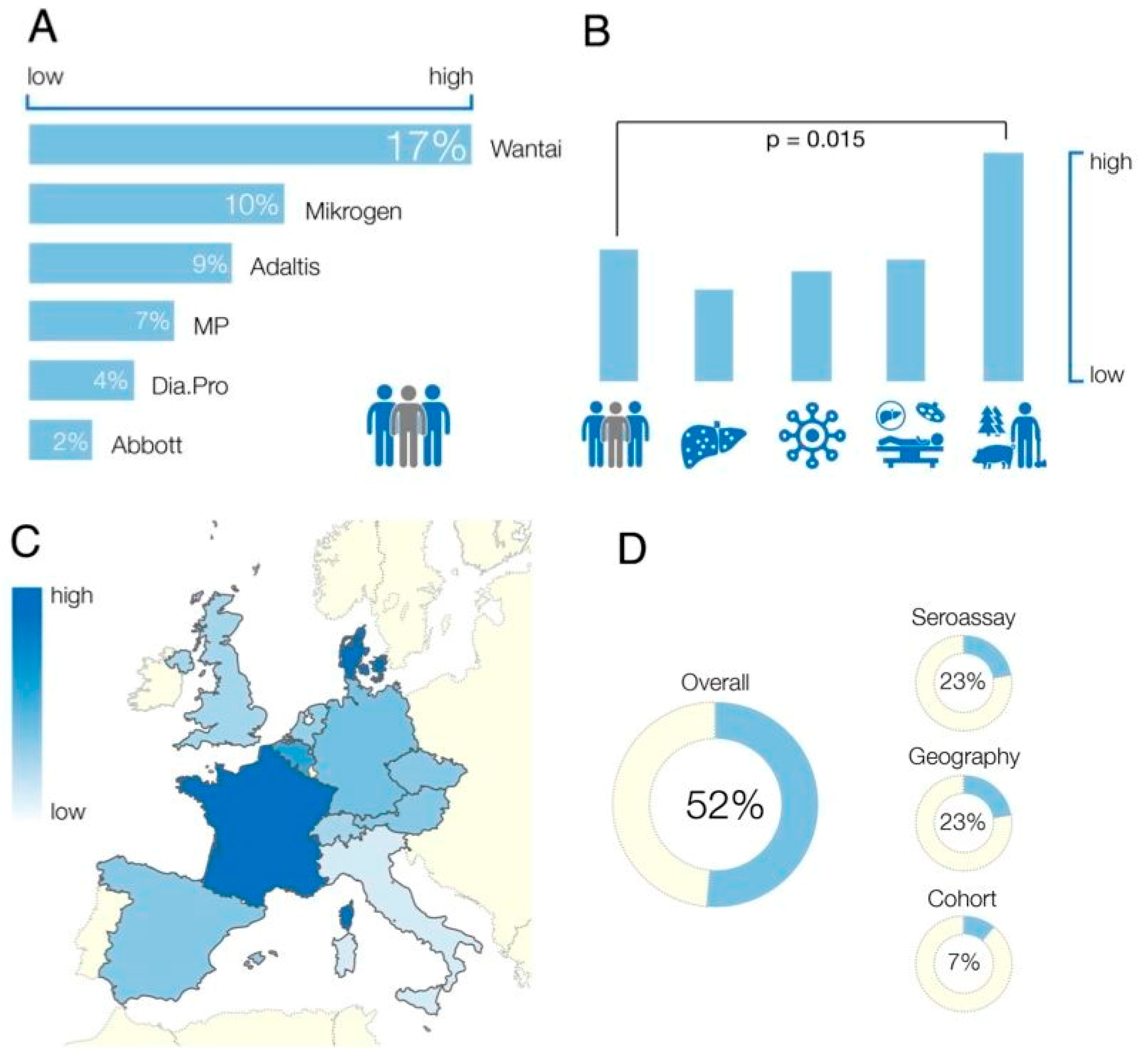
3.1. Anti-HEV IgG Assays Employed
3.2. Study Cohort
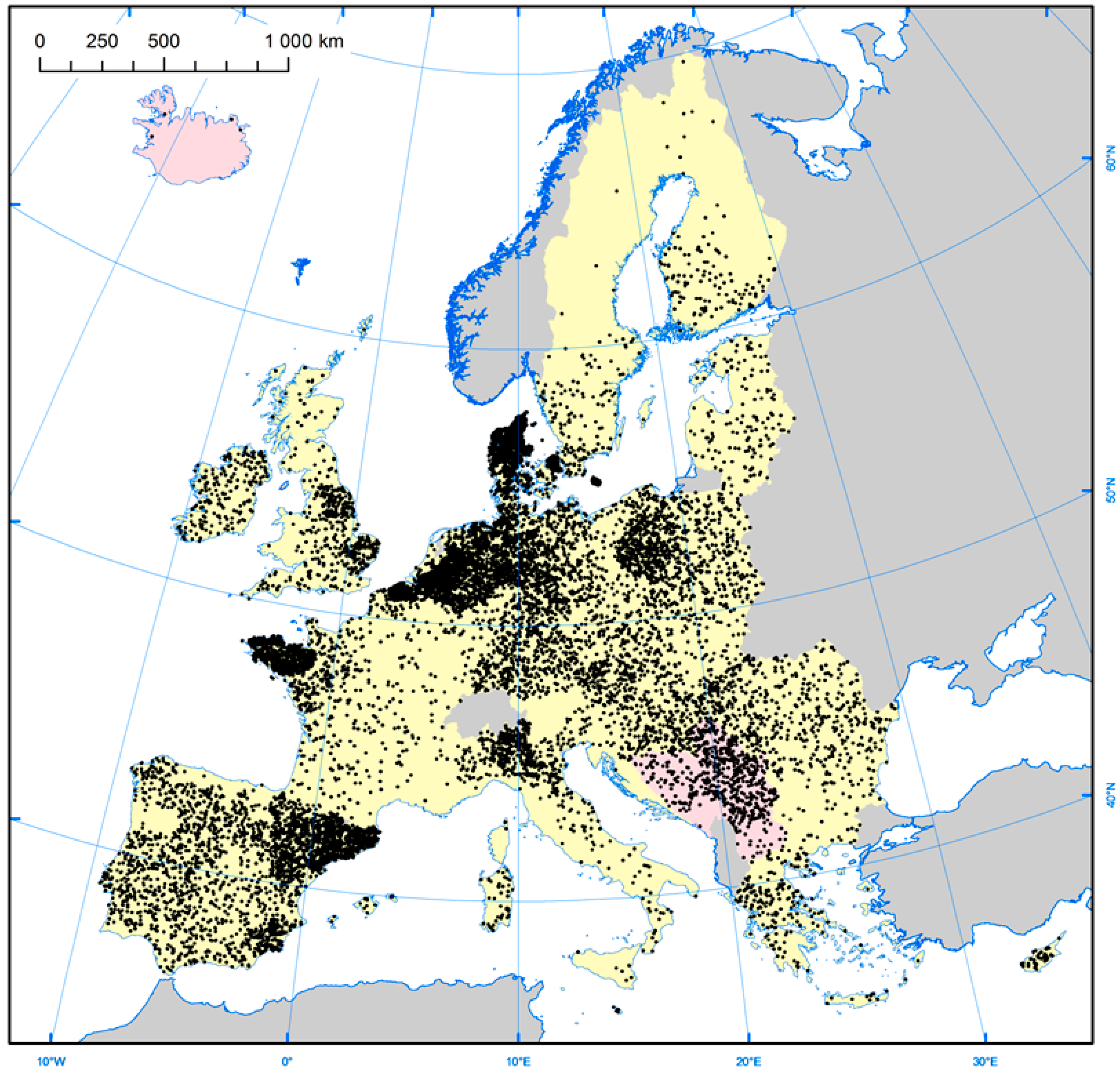
3.3. Geographical Location
3.4. Age and Gender
3.5. Study Heterogeneity
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kamar, N.; Bendall, R.; Legrand-Abravanel, F.; Xia, N.S.; Ijaz, S.; Izopet, J.; Dalton, H.R. Hepatitis E. Lancet 2012, 379, 2477–2488. [Google Scholar] [PubMed]
- Rein, D.B.; Stevens, G.A.; Theaker, J.; Wittenborn, J.S.; Wiersma, S.T. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 2012, 55, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.R.; Bendall, R.; Ijaz, S.; Banks, M. Hepatitis E: An emerging infection in developed countries. Lancet Infect. Dis. 2008, 8, 698–709. [Google Scholar] [CrossRef]
- Dalton, H.R.; Stableforth, W.; Hazeldine, S.; Thurairajah, P.; Ramnarace, R.; Warshow, U.; Ijaz, S.; Ellis, V.; Bendall, R. Autochthonous hepatitis E in Southwest England: A comparison with hepatitis A. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Selves, J.; Mansuy, J.M.; Ouezzani, L.; Peron, J.M.; Guitard, J.; Cointault, O.; Esposito, L.; Abravanel, F.; Danjoux, M.; Durand, D.; Vinel, J.P.; Izopet, J.; Rostaing, L. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N. Engl. J. Med. 2008, 358, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Pischke, S.; Stiefel, P.; Franz, B.; Bremer, B.; Suneetha, P.V.; Heim, A.; Ganzenmueller, T.; Schlue, J.; Horn-Wichmann, R.; Raupach, R.; et al. Chronic hepatitis e in heart transplant recipients. Am. J. Transplant. 2012, 12, 3128–3133. [Google Scholar] [CrossRef] [PubMed]
- Pischke, S.; Greer, M.; Hardtke, S.; Bremer, B.; Gisa, A.; Lehmann, P.; Haverich, A.; Welte, T.; Manns, M.P.; Wedemeyer, H.; et al. Course and treatment of chronic hepatitis E virus infection in lung transplant recipients. Transpl. Infect. Dis. 2014, 16, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Tavitian, S.; Peron, J.M.; Huynh, A.; Mansuy, J.M.; Ysebaert, L.; Huguet, F.; Vinel, J.P.; Attal, M.; Izopet, J.; Recher, C. Hepatitis E virus excretion can be prolonged in patients with hematological malignancies. J. Clin. Virol. 2010, 49, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.R.; Bendall, R.P.; Keane, F.E.; Tedder, R.S.; Ijaz, S. Persistent carriage of hepatitis E virus in patients with HIV infection. N. Engl. J. Med. 2009, 361, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Perrin, H.; Madden, R.G.; Stanley, A.; Crossan, C.; Hunter, J.G.; Vine, L.; Lane, K.; Devooght-Johnson, N.; McLaughlin, C.; Petrik, J.; et al. Hepatitis E virus in patients with decompensated chronic liver disease: A prospective UK/French study. Aliment. Pharmacol. Ther. 2015, 42, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Said, B.; Ijaz, S.; Kafatos, G.; Booth, L.; Thomas, H.L.; Walsh, A.; Ramsay, M.; Morgan, D.; Hepatitis, E. Incident Investigation Team. Hepatitis E outbreak on cruise ship. Emerg. Infect. Dis. 2009, 15, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Shata, M.T.; Navaneethan, U. The mystery of hepatitis E seroprevalence in developed countries: Is there subclinical infection due to hepatitis E virus? Clin. Infect. Dis. 2008, 47, 1032–1034. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, P.E.; Ijaz, S.; Brailsford, S.R.; Brett, R.; Dicks, S.; Haywood, B.; Kennedy, I.T.; Kitchen, A.; Patel, P.; Poh, J.; et al. Hepatitis E virus in blood components: A prevalence and transmission study in southeast England. Lancet 2014, 384, 1766–1773. [Google Scholar] [CrossRef]
- Zaaijer, H.L. No artifact, hepatitis E is emerging. Hepatology 2015, 62, 654. [Google Scholar] [CrossRef] [PubMed]
- Mallet, V.; Sberro-Soussan, R.; Vallet-Pichard, A.; Roque-Alfonso, A.M.; Pol, S. Transmission of Hepatitis E Virus by Plasma Exchange: A Case Report. Ann. Intern. Med. 2016, 164, 851–852. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, T.; Diekmann, J.; Johne, R.; Eberhardt, M.; Knabbe, C.; Dreier, J. Novel approach for detection of hepatitis E virus infection in German blood donors. J. Clin. Microbiol. 2012, 50, 2708–2713. [Google Scholar] [CrossRef] [PubMed]
- Pischke, S.; Suneetha, P.V.; Baechlein, C.; Barg-Hock, H.; Heim, A.; Kamar, N.; Schlue, J.; Strassburg, C.P.; Lehner, F.; Raupach, R.; et al. Hepatitis E virus infection as a cause of graft hepatitis in liver transplant recipients. Liver Transplant. 2010, 16, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Mansuy, J.M.; Bendall, R.; Legrand-Abravanel, F.; Saune, K.; Miedouge, M.; Ellis, V.; Rech, H.; Destruel, F.; Kamar, N.; Dalton, H.R.; et al. Hepatitis E virus antibodies in blood donors, France. Emerg. Infect. Dis. 2011, 17, 2309–2312. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-analysis of prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Conducting Meta-Analyses in R with The metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansuy, J.M.; Sauna, K.; Rech, H.; Abravanel, F.; Mengelle, C.; Homme, S.L.; Destruel, F.; Kamar, N.; Izopet, J. Seroprevalence in blood donors reveals widespread, multi-source exposure to hepatitis E virus, southern France, October 2011. Euro Surveill. 2015, 20, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Renou, C.; Lafeuillade, A.; Cadranel, J.F.; Pavio, N.; Pariente, A.; Allegre, T.; Poggi, C.; Penaranda, G.; Cordier, F.; Nicand, E. Hepatitis E virus in HIV-infected patients. Aids 2010, 24, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Cleland, A.; Smith, L.; Crossan, C.; Blatchford, O.; Dalton, H.R.; Scobie, L.; Petrik, J. Hepatitis E virus in Scottish blood donors. Vox Sang. 2013, 105, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.J.; Sichler, M.; Schemmerer, M.; Behrens, G.; Leitzmann, M.F.; Jilg, W. Decline in hepatitis E virus antibody prevalence in southeastern Germany, 1996–2011. Hepatology 2014, 60, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Hartl, J.; Kreuels, B.; Polywka, S.; Addo, M.; Luethgehetmann, M.; Dandri, M.; Dammermann, W.; Sterneck, M.; Lohse, A.W.; Pischke, S. Comparison of autochthonous and imported cases of hepatitis A or hepatitis E. Z. Gastroenterol. 2015, 53, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Tamisier, M.; Moal, V.; Gerolami, R.; Colson, P. Discrepancy between anti-hepatitis E virus immunoglobulin G prevalence assessed by two assays in kidney and liver transplant recipients. J. Clin. Virol. 2013, 56, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.J.; Preiss, J.; Schemmerer, M.; Huber, B.; Jilg, W. Test performance characteristics of Anti-HEV IgG assays strongly influence hepatitis E seroprevalence estimates. J. Infect. Dis. 2013, 207, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Schnegg, A.; Bürgisser, P.; André, C.; Kenfak-Foguena, A.; Canellini, G.; Moradpour, D.; Abravanel, F.; Izopet, J.; Cavassini, M.; Darling, K.E. An Analysis of the Benefit of Using HEV Genotype 3 Antigens in Detecting Anti-HEV IgG in a European Population. PLoS ONE 2013, 8, e62980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kmush, B.L.; Labrique, A.B.; Dalton, H.R.; Ahmed, Z.B.; Ticehurst, J.; Heaney, C.D.; Nelson, K.E.; Zaman, K. Two Generations of ”Gold Standards”: The Impact of a Decade in Hepatitis E Virus Testing Innovation on Population Seroprevalence. Am. J. Trop. Med. Hyg. 2015, 93, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Bendall, R.; Ellis, V.; Ijaz, S.; Ali, R.; Dalton, H. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J. Med. Virol. 2010, 82, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Halliday, J.S.; Harrison, G.L.; Brown, A.; Hunter, J.G.; Bendall, R.; Penny, D.; Toatu, T.; Abdad, M.Y.; Klenerman, P.; Barnes, E.; et al. Hepatitis E virus infection, Papua New Guinea, Fiji, and Kiribati, 2003–2005. Emerg. Infect. Dis. 2014, 20, 1057–1058. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.R.; Fellows, H.J.; Gane, E.J.; Wong, P.; Gerred, S.; Schroeder, B.; Croxson, M.C.; Garkavenko, O. Hepatitis E in new zealand. J. Gastroenterol. Hepatol. 2007, 22, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Baylis, S.A.; Gartner, T.; Nick, S.; Ovemyr, J.; Blumel, J. Occurrence of hepatitis E virus RNA in plasma donations from Sweden, Germany and the United States. Vox Sang. 2012, 103, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Slot, E.; Hogema, B.M.; Riezebos-Brilman, A.; Kok, T.M.; Molier, M.; Zaaijer, H.L. Silent hepatitis E virus infection in Dutch blood donors, 2011 to 2012. Euro Surveill. 2013, 18, 20550. [Google Scholar] [CrossRef]
- Ijaz, S.; Szypulska, R.; Tettmar, K.I.; Kitchen, A.; Tedder, R.S. Detection of hepatitis E virus RNA in plasma mini-pools from blood donors in England. Vox Sang. 2012, 102, 272. [Google Scholar] [CrossRef] [PubMed]
- Beale, M.A.; Tettmar, K.; Szypulska, R.; Tedder, R.S.; Ijaz, S. Is there evidence of recent hepatitis E virus infection in English and North Welsh blood donors? Vox Sang. 2011, 100, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Hofmann, M.; Danzer, M.; Hofer, K.; Kaar, J.; Gabriel, C. Seroprevalence and Incidence of hepatitis E in Blood Donors in Upper Austria. PLoS ONE 2015, 10, e0119576. [Google Scholar] [CrossRef] [PubMed]
- Pavio, N.; Merbah, T.; Thebault, A. Frequent hepatitis E virus contamination in food containing raw pork liver, France. Emerg. Infect. Dis. 2014, 20, 1925–1927. [Google Scholar] [CrossRef] [PubMed]
- Renou, C.; Roque-Afonso, A.M.; Pavio, N. Foodborne transmission of hepatitis E virus from raw pork liver sausage, France. Emerg. Infect. Dis. 2014, 20, 1945–1947. [Google Scholar] [CrossRef] [PubMed]
- Swissinfo.ch. Meat Consumption Hits Ten Years High 2011. Available online: http://www.swissinfo.ch/eng/meat-consumption-hits-ten-year-high/29935790 (accessed on 17 May 2015).
- European Commission, Eurostat, Pig Farmer sector, 2014. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/Pig_farmer_sector_-_statistica_portrait_2014 (accessed on 17 May 2015).
- World Population Data Sheet. Available online: http://www.prb.org/pdf13/2013-population-data-sheet_eng.pdf (accessed on 17 May 2015).
- About the Agriculture and Horticulture Development Board—AHDB 2015. Available online: http://pork.adhb.org.uk/prices-stats/consumption/total-eu-consumption (accessed on 17 May 2015).
- Hunter, J.G.; Madden, R.; Stone, A.; Osborne, N.; Wheeler, B.; Vine, L.; Dickson, A.; Barlow, M.; Lewis, J.; Bendall, R.P.; et al. Coastal clustering of HEV; Cornwall, UK. EJGH 2015, 28, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Hoofnagle, J.H.; Nelson, K.E.; Purcell, R.H. Hepatitis E. N. Engl. J. Med. 2012, 367, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.B.; Engle, R.E.; Hjort, C.; Homburg, K.M.; Vach, W.; Georgsen, J.; Purcell, R.H. Time trend of the prevalence of hepatitis E antibodies among farmers and blood donors: A potential zoonosis in Denmark. Clin. Infect. Dis. 2008, 47, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Holm, D.K.; Moessner, B.K.; Engle, R.E.; Zaaijer, H.L.; Georgsen, J.; Purcell, R.H.; Christensen, P.B. Declining prevalence of hepatitis E antibodies among Danish blood donors. Transfusion 2015, 55, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Pischke, S.; Heim, A.; Bremer, B.; Raupach, R.; Horn-Wichmann, R.; Ganzenmueller, T.; Klose, B.; Goudeva, L.; Wagner, F.; Oehme, A.; et al. Hepatitis E: An emerging infectious disease in Germany? Z. Gastroenterol. 2011, 49, 1255–1257. [Google Scholar] [CrossRef] [PubMed]
- Hogema, B.M.; Molier, M.; Slot, E.; Zaaijer, H.L. Past and present of hepatitis E in The Netherlands. Transfusion 2014, 54, 3092–3096. [Google Scholar] [CrossRef] [PubMed]

| Study cohort | Wantai | Mikrogen | MP | Abbott | Adaltis | Dia.Pro | Others |
|---|---|---|---|---|---|---|---|
| General Population (%) | 16.90 | 10.11 | 6.50 | 2.29 | 8.72 | 4.35 | 12.48 |
| Sample size (n) | 88,204 | 1777 | 14,385 | 1077 | nd * | 5,176 | 3667 |
| Liver Disease (%) | 16.05 | 9.55 | 6.13 | 2.02 | 8.2 | 3.94 | 11.86 |
| Sample size (n) | nd * | 41 | 801 | 129 | nd * | nd * | 2000 |
| Transplant recipients (%) | 18.36 | 11.42 | 7.69 | 2.97 | 9.96 | 5.22 | 13.91 |
| Sample size (n) | 415 | 124 | 1328 | 262 | 64 | 448 | 52 |
| HIV (%) | 15.69 | 9.26 | 5.900 | 1.88 | 7.93 | 3.75 | 11.55 |
| Sample size (n) | 2047 | nd * | 1579 | 123 | 429 | 548 | 238 |
| Swine/Animal Contatct (%) | 28.51 | 20.13 | 15.26 | 8.37 | 18.25 | 11.82 | 23.21 |
| Sample size (n) | 101 | 709 | 1354 | 202 | 43 | nd * | 995 |
| Journal | Year | First Author | Cohort Size | Sero-Prevalence | Assay | Cohort | Country |
|---|---|---|---|---|---|---|---|
| Transfusion | 2015 | Holm | 504 | 10.7 | Other | GP | Denmark |
| Transfusion | 2015 | Holm | 504 | 19.8 | Wantai | GP | Denmark |
| J Clin Virol | 2013 | Rossi-Tamisier | 64 | 10.9 | Adaltis | Tx | France |
| J Clin Virol | 2013 | Rossi-Tamisier | 64 | 31.3 | Wantai | Tx | France |
| J Infect Dis | 2012 | Wenzel | 200 | 18 | Mikrogen | GP | Germany |
| J Infect Dis | 2012 | Wenzel | 200 | 4.5 | MP | GP | Germany |
| J Infect Dis | 2012 | Wenzel | 200 | 29.5 | Other | GP | Germany |
| Hepatology | 2014 | Wenzel | 1092 | 14.5 | Mikrogen | GP | Germany |
| Hepatology | 2014 | Wenzel | 1092 | 34 | Other | GP | Germany |
| Med Mibrobiol Immun | 2014 | Krumbholz | 235 | 8.5 | Mikrogen | GP | Germany |
| Med Mibrobiol Immun | 2014 | Krumbholz | 235 | 2.6 | MP | GP | Germany |
| Med Mibrobiol Immun | 2014 | Krumbholz | 235 | 7.7 | Other | GP | Germany |
| Med Mibrobiol Immun | 2014 | Krumbholz | 302 | 17.9 | Mikrogen | SW | Germany |
| Med Mibrobiol Immun | 2014 | Krumbholz | 302 | 3.5 | MP | SW | Germany |
| Med Mibrobiol Immun | 2014 | Krumbholz | 302 | 13.2 | Other | SW | Germany |
| Epidemiol Infect | 2008 | Bouwknegt | 644 | 1.7 | Abbott | GP | Netherlands |
| Epidemiol Infect | 2008 | Bouwknegt | 644 | 4.2 | MP | GP | Netherlands |
| Epidemiol Infect | 2008 | Bouwknegt | 49 | 8.1 | Abbott | SW | Netherlands |
| Epidemiol Infect | 2008 | Bouwknegt | 49 | 12.2 | MP | SW | Netherlands |
| Epidemiol Infect | 2008 | Bouwknegt | 153 | 5.2 | Abbott | SW | Netherlands |
| Epidemiol Infect | 2008 | Bouwknegt | 153 | 3.9 | MP | SW | Netherlands |
| Transfusion | 2014 | Sauleda | 10,000 | 10.72 | Mikrogen | GP | Spain |
| Transfusion | 2014 | Sauleda | 10,000 | 19.96 | Wantai | GP | Spain |
| PLoS One | 2013 | Schnegg | 550 | 4.9 | MP | GP | Switzerland |
| PLoS One | 2013 | Schnegg | 550 | 4.2 | Dia.Pro | GP | Switzerland |
| PLoS One | 2013 | Schnegg | 550 | 21.2 | Wantai | GP | Switzerland |
| J Med Virol | 2010 | Bendall | 500 | 3.6 | MP | GP | UK |
| J Med Virol | 2010 | Bendall | 500 | 16.2 | Wantai | GP | UK |
| Title | Abbott | Adaltis | Dia.Pro | Mikrogen | MP | Other | Wantai |
|---|---|---|---|---|---|---|---|
| Austria | 1.9% * | 0.7% * | 6.6% * | 8.9% * | 3.9% * | 9.3% * | 13.9% |
| Belgium | 4.5% * | 2.5% * | 10.9% * | 13.8% * | 7.4% * | 14.3% | 19.7% * |
| Czech Republic | 1.5% * | 0.5% * | 5.9% | 8.1% * | 3.3% * | 8.5% * | 12.9% * |
| Denmark | 4.8% * | 2.8% * | 11.4% * | 14.3% * | 7.8% * | 15.2% | 19.8% |
| France | 12.0% * | 8.7% | 21.1% * | 24.7% * | 16.3% | 25.4%* | 31.9% |
| Germany | 2.6% | 1.1% * | 7.8% * | 10.3% | 4.8% | 10.8% | 29.5% |
| Italy | 0.1% * | 0.1% * | 2.4% | 3.9% * | 0.9% * | 4.1% | 7.5%* |
| Netherlands | 1.8% | 0.6% * | 6.4% | 8.7% * | 3.7% | 9.1% | 27.0% |
| Spain | 2.2% | 0.9% * | 7.1% | 9.5% * | 4.3% | 10.0%* | 14.7% |
| Switzerland | 1.8% * | 0.6%* | 4.2% | 8.8% | 4.2% | 9.2% | 21,2% |
| UK | 1.4% * | 0.4% * | 5.7% * | 7.9% * | 3.2% | 8.3% * | 12.7% |
| Country | Blood Donors HEV RNA Positive | HEV IgG Seroprevalence | Reference |
|---|---|---|---|
| Midi-Pyrénées, Southwest France * | 1:1438 (1:2200) ** | Gallian et al., 2014 [15] | |
| 52.5% | Mansuy et al., 2011 [18] | ||
| Germany | 1:1200 | Vollmer et al., 2012 [16] | |
| 1:4525 | Baylis et al., 2012 [36] | ||
| 29.5% | Wenzel et al., 2013 [29] | ||
| The Netherlands | 1:2671 | 27.0% | Slot et al., 2013 [37] |
| England | 1:2848 | * | Hewitt et al., 2014 [13] |
| 1:7000 | 12.0% | Ijaz et al., 2012 [38] | |
| 16.0% | Beale et al., 2011 [39] | ||
| 16.0% | Dalton et al., 2008 [4] | ||
| Sweden | 1:7986 | NA | Baylis et al., 2012 [36] |
| Austria | 1:8416 | 13.5% | Fischer et al., 2015 [40] |
| Scotland | 1:14,520 | 4.7% | Cleland et al., 2013 [35] |
| Country | Estimated Human HEV Seroprevalence (WT Assay) | Number of Pigs Slaughtered 2013 (Millions) | Human Population 2013 (Millions) | Pigs/Human Ratio ** | Pork Consumption (Thousand Tons) *** | Pork Consumption (Kg) per Capita *** |
|---|---|---|---|---|---|---|
| France | 31.9 | 23,747 | 63.9 | 0.37 | 1931 | 30.2 |
| Germany | 29.5 | 58,628 | 80.6 | 0.72 | 4358 | 54.1 |
| Denmark | 19.8 | 19,108 | 5.6 | 3.41 | 352 | 62.9 |
| Netherlands | 27.0 | 14,014 | 16.8 | 0.83 | 640 | 38.1 |
| Belgium | 19.7 | 11,915 | 11.2 | 1.06 | 452 | 40.4 |
| Spain | 14.7 | 41,418 | 46.6 | 0.31 | 2363 | 50.7 |
| Switzerland | 13.8 | No comparative data | 8.1 | NA | 201 * | 24.8 |
| Austria | 13.9 | 5417 | 8.5 | 0.64 | 474 | 55.8 |
| Czech Republic | 12.9 | 2652 | 10.5 | 0.25 | 437 | 41.6 |
| UK | 12.7 | 10,299 | 64.1 | 0.16 | 1542 | 24.1 |
| Italy | 7.5 | 13,099 | 59.8 | 0.22 | 2451 | 41.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartl, J.; Otto, B.; Madden, R.G.; Webb, G.; Woolson, K.L.; Kriston, L.; Vettorazzi, E.; Lohse, A.W.; Dalton, H.R.; Pischke, S. Hepatitis E Seroprevalence in Europe: A Meta-Analysis. Viruses 2016, 8, 211. https://doi.org/10.3390/v8080211
Hartl J, Otto B, Madden RG, Webb G, Woolson KL, Kriston L, Vettorazzi E, Lohse AW, Dalton HR, Pischke S. Hepatitis E Seroprevalence in Europe: A Meta-Analysis. Viruses. 2016; 8(8):211. https://doi.org/10.3390/v8080211
Chicago/Turabian StyleHartl, Johannes, Benjamin Otto, Richie Guy Madden, Glynn Webb, Kathy Louise Woolson, Levente Kriston, Eik Vettorazzi, Ansgar W. Lohse, Harry Richard Dalton, and Sven Pischke. 2016. "Hepatitis E Seroprevalence in Europe: A Meta-Analysis" Viruses 8, no. 8: 211. https://doi.org/10.3390/v8080211
APA StyleHartl, J., Otto, B., Madden, R. G., Webb, G., Woolson, K. L., Kriston, L., Vettorazzi, E., Lohse, A. W., Dalton, H. R., & Pischke, S. (2016). Hepatitis E Seroprevalence in Europe: A Meta-Analysis. Viruses, 8(8), 211. https://doi.org/10.3390/v8080211






