Clomiphene and Its Isomers Block Ebola Virus Particle Entry and Infection with Similar Potency: Potential Therapeutic Implications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Cells
2.3. Preparation of EBOV trVLPs and Entry Reporter VLPs
2.4. trVLP Infection and Parallel Cell Viability Assays
2.5. VLP Entry and Parallel Cell Viability Assays
2.6. Analysis of Eight-Point Dose Response Data
3. Results
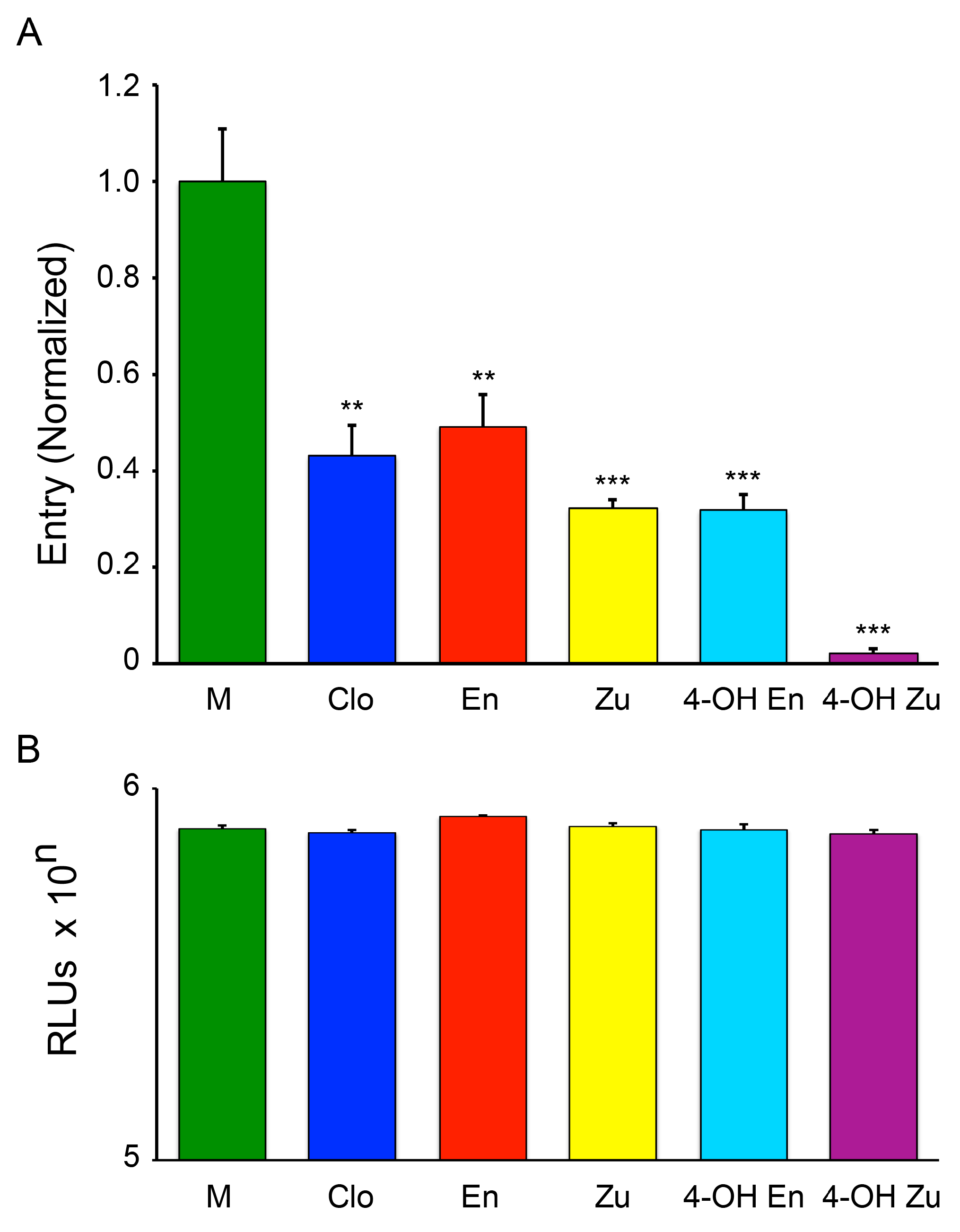
3.1. The Two Clomiphene Isomers and Their Primary Metabolites Block EBOV Entry and Replication
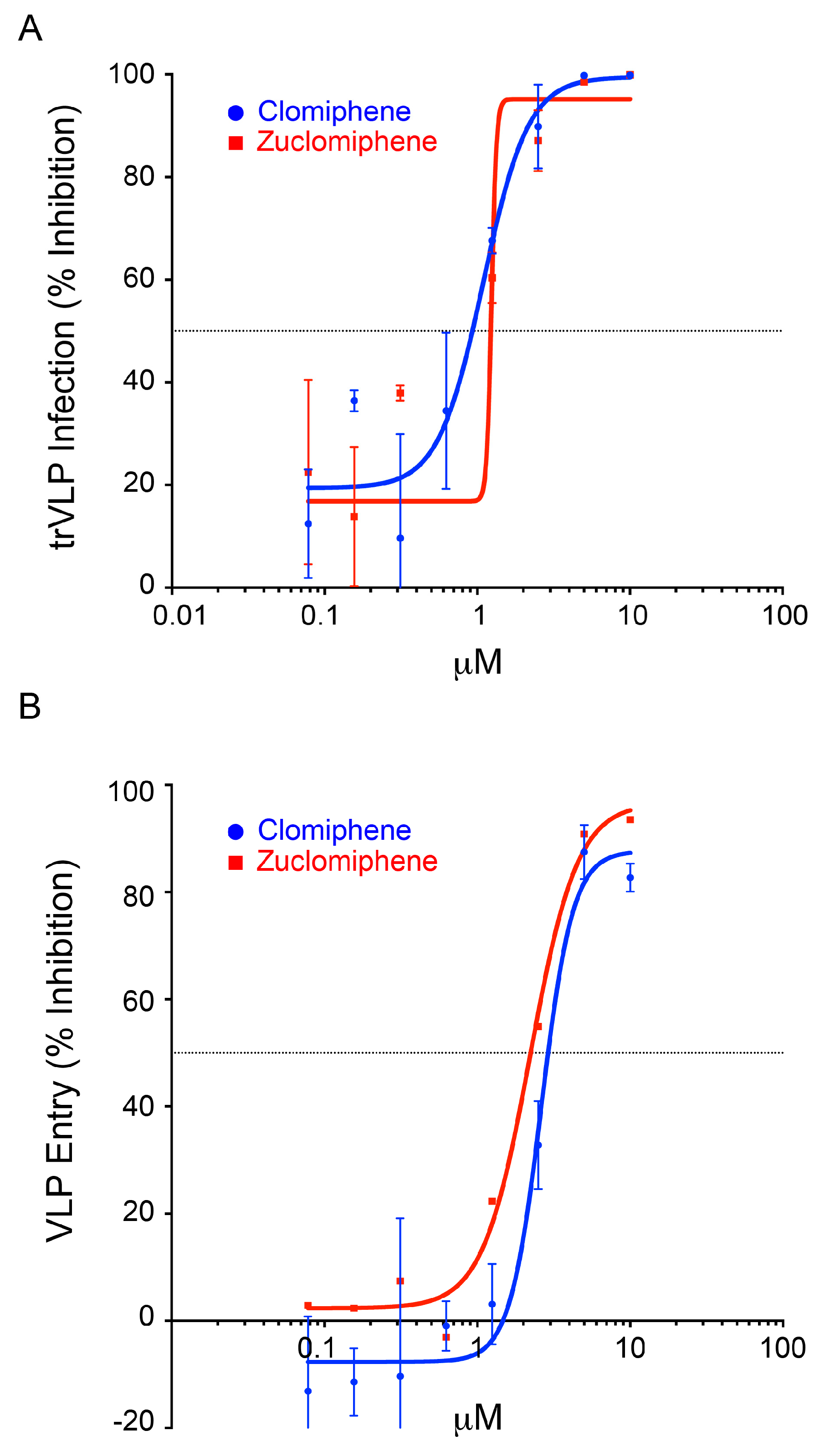
3.2. Clomiphene, Enclomiphene, and Zuclomiphene Inhibit EBOV trVLP Infection and VLP Entry with Equal Potency
3.3. Enclomiphene Blocks trVLP Infection in Vero E6 Cells as well as Entry Mediated by the Makona EBOV and Angola MARV GPs
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- de La Vega, M.-A.; Stein, D.; Kobinger, G.P. Ebolavirus Evolution: Past and Present. PLoS Pathog. 2015, 11, e1005221. [Google Scholar]
- World Health Organization. Ebola Situation Reports. Available online: http://apps.who.int/ebola/ebola-situation-reports (accessed on 15 April 2016).
- Varkey, J.B.; Shantha, J.G.; Crozier, I.; Kraft, C.S.; Lyon, G.M.; Mehta, A.K.; Kumar, G.; Smith, J.R.; Kainulainen, M.H.; Whitmer, S.; et al. Persistence of Ebola Virus in Ocular Fluid during Convalescence. N. Engl. J. Med. 2015, 372, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.V.; Kibuuka, H.; Millard, M.; Wakabi, S.; Lukwago, L.; Taylor, A.; Eller, M.A.; Eller, L.A.; Michael, N.L.; Honko, A.N.; et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: A retrospective cohort study. Lancet Infect. Dis. 2015, 15, 905–912. [Google Scholar] [CrossRef]
- Deen, G.F.; Knust, B.; Broutet, N.; Sesay, F.R.; Formenty, P.; Ross, C.; Thorson, A.E.; Massaquoi, T.A.; Marrinan, J.E.; Ervin, E.; et al. Ebola RNA Persistence in Semen of Ebola Virus Disease Survivors - Preliminary Report. N. Engl. J. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Mate, S.E.; Kugelman, J.R.; Nyenswah, T.G.; Ladner, J.T.; Wiley, M.R.; Cordier-Lassalle, T.; Christie, A.; Schroth, G.P.; Gross, S.M.; Davies-Wayne, G.J.; et al. Molecular Evidence of Sexual Transmission of Ebola Virus. N. Engl. J. Med. 2015, 373, 2448–2454. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Wong, G.; Audet, J.; Bello, A.; Fernando, L.; Alimonti, J.B.; Fausther-Bovendo, H.; Wei, H.; Aviles, J.; Hiatt, E.; et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014, 514, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.T. PREVAIL II: A Randomized Controlled Trial of ZMapp™ in Acute Ebola Virus Infection. In Proceddings of Retroviruses and Opportunistic Infections, Boston, MA, USA, 22–25 February 2016.
- Almansa, R.; Eiros, J.M.; Fedson, D.; Bermejo-Martin, J.F. Hyperimmune serum from healthy vaccinated individuals for Ebola virus disease? Lancet Glob. Health 2014, 2, e686. [Google Scholar] [CrossRef]
- Dye, J.M.; Herbert, A.S.; Kuehne, A.I.; Barth, J.F.; Muhammad, M.A.; Zak, S.E.; Ortiz, R.A.; Prugar, L.I.; Pratt, W.D. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc. Natl. Acad. Sci. USA 2012, 109, 5034–5039. [Google Scholar] [CrossRef] [PubMed]
- Enserink, M. Ebola drugs still stuck in lab. Science 2014, 345, 364–365. [Google Scholar] [CrossRef] [PubMed]
- Oestereich, L.; Lüdtke, A.; Wurr, S.; Rieger, T.; Muñoz-Fontela, C.; Günther, S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antivir. Res. 2014, 105, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Thi, E.P.; Mire, C.E.; Lee, A.C.H.; Geisbert, J.B.; Zhou, J.Z.; Agans, K.N.; Snead, N.M.; Deer, D.J.; Barnard, T.R.; Fenton, K.A.; et al. Lipid nanoparticle siRNA treatment of Ebola-virus-Makona-infected nonhuman primates. Nature 2015, 521, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Misasi, J.; Gilman, M.S.A.; Kanekiyo, M.; Gui, M.; Cagigi, A.; Mulangu, S.; Corti, D.; Ledgerwood, J.E.; Lanzavecchia, A.; Cunningham, J.; et al. Structural and molecular basis for Ebola virus neutralization by protective human antibodies. Science 2016, 351, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Corti, D.; Misasi, J.; Mulangu, S.; Stanley, D.A.; Kanekiyo, M.; Wollen, S.; Ploquin, A.; Doria-Rose, N.A.; Staupe, R.P.; Bailey, M.; et al. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science 2016, 351, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Bornholdt, Z.A.; Turner, H.L.; Murin, C.D.; Li, W.; Sok, D.; Souders, C.A.; Piper, A.E.; Goff, A.; Shamblin, J.D.; Wollen, S.E.; et al. Isolation of potent neutralizing antibodies from a survivor of the 2014 Ebola virus outbreak. Science 2016, 351, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Furuyama, W.; Marzi, A.; Nanbo, A.; Haddock, E.; Maruyama, J.; Miyamoto, H.; Igarashi, M.; Yoshida, R.; Noyori, O.; Feldmann, H.; et al. Discovery of an antibody for pan-ebolavirus therapy. Sci. Rep. 2016, 6, 20514. [Google Scholar] [CrossRef] [PubMed]
- Warren, T.K.; Wells, J.; Panchal, R.G.; Stuthman, K.S.; Garza, N.L.; van Tongeren, S.A.; Dong, L.; Retterer, C.J.; Eaton, B.P.; Pegoraro, G.; et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature 2014. [Google Scholar] [CrossRef] [PubMed]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016, 531, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Johansen, L.M.; Brannan, J.M.; Delos, S.E.; Shoemaker, C.J.; Stossel, A.; Lear, C.; Hoffstrom, B.G.; Dewald, L.E.; Schornberg, K.L.; Scully, C.; et al. FDA-Approved Selective Estrogen Receptor Modulators Inhibit Ebola Virus Infection. Sci. Transl. Med. 2013, 5, 190ra79. [Google Scholar] [CrossRef] [PubMed]
- Johansen, L.M.; Brannan, J.M.; Delos, S.E.; Shoemaker, C.J.; Stossel, A.; Lear, C.; Hoffstrom, B.G.; Dewald, L.E.; Schornberg, K.L.; Scully, C.; et al. A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity. Sci. Transl. Med. 2015, 7, 290ra89. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsova, J.; Sun, W.; Martínez-Romero, C.; Tawa, G.; Shinn, P.; Chen, C.Z.; Schimmer, A.; Sanderson, P.; McKew, J.C.; Zheng, W.; et al. Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg. Microbes Infect. 2014, 3, e84. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Lear-Rooney, C.M.; Johansen, L.; Varhegyi, E.; Chen, Z.W.; Olinger, G.G.; Rong, L. Inhibition of Ebola and Marburg Virus Entry by G Protein-Coupled Receptor Antagonists. J. Virol. 2015, 89, 9932–9938. [Google Scholar] [CrossRef] [PubMed]
- Madrid, P.B.; Chopra, S.; Manger, I.D.; Gilfillan, L.; Keepers, T.R.; Shurtleff, A.C.; Green, C.E.; Iyer, L.V.; Dilks, H.H.; Davey, R.A.; et al. A Systematic Screen of FDA-Approved Drugs for Inhibitors of Biological Threat Agents. PLoS ONE 2013, 8, e60579. [Google Scholar] [CrossRef] [PubMed]
- Dickey, R.P.; Holtkamp, D.E. Development, pharmacology and clinical experience with clomiphene citrate. Hum. Reprod. Update 1996, 2, 483–506. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.; Collins, J.; Vandekerckhove, P. Clomiphene citrate for ovulation induction in women with oligo-amenorrhoea. Cochrane Database Syst. Rev. 2000, CD000056. [Google Scholar] [CrossRef]
- Jungheim, E.S.; Odibo, A.O. Fertility treatment in women with polycystic ovary syndrome: a decision analysis of different oral ovulation induction agents. Fertil. Steril. 2010, 94, 2659–2664. [Google Scholar] [CrossRef] [PubMed]
- Guay, A.T.; Bansal, S.; Hodge, M.B. Possible hypothalamic impotencemale counterpart to hypothalamic amenorrhea? Urology 1991, 38, 317–322. [Google Scholar] [CrossRef]
- Shoemaker, C.J.; Schornberg, K.L.; Delos, S.E.; Scully, C.; Pajouhesh, H.; Olinger, G.G.; Johansen, L.M.; White, J.M. Multiple cationic amphiphiles induce a niemann-pick C phenotype and inhibit ebola virus entry and infection. PLoS ONE 2013, 8, e56265. [Google Scholar] [CrossRef]
- Mingo, R.M.; Simmons, J.A.; Shoemaker, C.J.; Nelson, E.A.; Schornberg, K.L.; D'Souza, R.S.; Casanova, J.E.; White, J.M. Ebola Virus and Severe Acute Respiratory Syndrome Coronavirus Display Late Cell Entry Kinetics: Evidence that Transport to NPC1+ Endolysosomes Is a Rate-Defining Step. J. Virol. 2015, 89, 2931–2943. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.A.; D’Souza, R.S.; Ruas, M.; Galione, A.; Casanova, J.E.; White, J.M. Ebolavirus Glycoprotein Directs Fusion through NPC1+ Endolysosomes. J. Virol. 2015, 90, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.S.; Krause, T.B.; Mittler, E.; Jangra, R.K.; Chandran, K. Direct Visualization of Ebola Virus Fusion Triggering in the Endocytic Pathway. MBio 2016, 7, e01857–15. [Google Scholar] [CrossRef] [PubMed]
- Herbert, A.S.; Davidson, C.; Kuehne, A.I.; Bakken, R.; Braigen, S.Z.; Gunn, K.E.; Whelan, S.P.; Brummelkamp, T.R.; Twenhafel, N.A.; Chandran, K.; et al. Niemann-pick C1 is essential for ebolavirus replication and pathogenesis in vivo. MBio 2015, 6, e00565–15. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.H.; Guthrie, S.C. Agonistic and antagonistic effects of clomiphene citrate and its isomers. Biol. Reprod. 1981, 25, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, S.L.; Berrodin, T.J.; Jenkins, S.F.; Sindoni, D.M.; Deecher, D.C.; Frail, D.E. Effect of estrogen agonists and antagonists on induction of progesterone receptor in a rat hypothalamic cell line. Endocrinology 1999, 140, 3928–3937. [Google Scholar] [CrossRef] [PubMed]
- Kaminetsky, J.; Hemani, M.L. Clomiphene citrate and enclomiphene for the treatment of hypogonadal androgen deficiency. Expert Opin. Investig. Drugs 2009, 18, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Mikkelson, T.J.; Kroboth, P.D.; Cameron, W.J.; Dittert, L.W.; Chungi, V.; Manberg, P.J. Single-dose pharmacokinetics of clomiphene citrate in normal volunteers. Fertil. Steril. 1986, 46, 392–396. [Google Scholar] [CrossRef]
- Mürdter, T.E.; Kerb, R.; Turpeinen, M.; Schroth, W.; Ganchev, B.; Böhmer, G.M.; Igel, S.; Schaeffeler, E.; Zanger, U.; Brauch, H.; et al. Genetic polymorphism of cytochrome P450 2D6 determines oestrogen receptor activity of the major infertility drug clomiphene via its active metabolites. Hum. Mol. Genet. 2012, 21, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Wiehle, R.D.; Fontenot, G.K.; Hsu, K.; Podolski, J. The Isomers of Clomiphene Citrate Have Dissimilar Dispositions Once Ingested: Results of a Mouse ADE Study. Manuscript preparation. 2016. [Google Scholar]
- Fontenot, G.K.; Wiehle, R.D.; Podolski, J.S. Differential effects of isomers of clomiphene citrate on reproductive tissues in male mice. BJU Int. 2016, 117, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Wiehle, R.; Cunningham, G.R.; Pitteloud, N.; Wike, J.; Hsu, K.; Fontenot, G.K.; Rosner, M.; Dwyer, A.; Podolski, J. Testosterone restoration using enclomiphene citrate in men with secondary hypogonadism: a pharmacodynamic and pharmacokinetic study. BJU Int. 2013, 112, 1188–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watt, A.; Moukambi, F.; Banadyga, L.; Groseth, A.; Callison, J.; Herwig, A.; Ebihara, H.; Feldmann, H.; Hoenen, T. A Novel Life Cycle Modeling System for Ebola Virus Shows a Genome Length-Dependent Role of VP24 in Virus Infectivity. J. Virol. 2014, 88, 10511–10524. [Google Scholar] [CrossRef] [PubMed]
- Hoenen, T.; Watt, A.; Mora, A.; Feldmann, H. Modeling The Lifecycle Of Ebola Virus Under Biosafety Level 2 Conditions With Virus-like Particles Containing Tetracistronic Minigenomes. J. Vis. Exp. 2014. [Google Scholar] [CrossRef] [PubMed]
- GraphPad Software QuickCalcs. Available online: http://www.graphpad.com/quickcalcs/Ecanything1/ (accessed on 5 July 2016).
- Ghobadi, C.; Mirhosseini, N.; Shiran, M.R.; Moghadamnia, A.; Lennard, M.S.; Ledger, W.L.; Rostami-Hodjegan, A. Single-dose pharmacokinetic study of clomiphene citrate isomers in anovular patients with polycystic ovary disease. J. Clin. Pharmacol. 2009, 49, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Rostami-Hodjegan, A.; Lennard, M.S.; Tucker, G.T.; Ledger, W.L. Monitoring plasma concentrations to individualize treatment with clomiphene citrate. Fertil. Steril. 2004, 81, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Young, S.L.; Opsahl, M.S.; Fritz, M.A. Serum concentrations of enclomiphene and zuclomiphene across consecutive cycles of clomiphene citrate therapy in anovulatory infertile women. Fertil. Steril. 1999, 71, 639–644. [Google Scholar] [CrossRef]
- Szutu, M.; Morgan, D.J.; McLeish, M.; Phillipou, G.; Blackman, G.L.; Cox, L.W.; Dollman, W. Pharmacokinetics of intravenous clomiphene isomers. Br. J. Clin. Pharmacol. 1989, 27, 639–640. [Google Scholar] [CrossRef] [PubMed]

| Drug | Assay | Set 1 | Set 2 | ||
|---|---|---|---|---|---|
| IC50 (μM) | IC90 (μM) | IC50 (μM) | IC90 (μM) | ||
| Clomiphene | trVLP | 1.2 | 1.4 | 0.9 | 2.2 |
| Entry | 1.4 | 4.1 | 2.9 | 4.4 * | |
| Enclomiphene | trVLP | 1.0 | 2.8 | ||
| Entry | 1.2 | 1.6 | |||
| Zuclomiphene | trVLP | 1.2 | 1.4 | ||
| Entry | 2.2 | 5.6 | |||
| Compound Administered | Compound Analyzed | Dose (mg) a | Route | Sex | Status | Ave. of n | C (max) (μM) | AUC (ng/mL/h) (last time) | T (max) | T 1/2 (h) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clomiphene | 50 | Oral | F | Healthy | 24 | [37] | |||||
| Enclomiphene b | 0.02 | 42 (24 h) | 3.7 | --- | |||||||
| Zuclomiphene b | 0.02 | 662 (288 h) | 6.8 | --- | |||||||
| Clomiphene | 50 | Oral | F | Anov. | 9 | [45] | |||||
| Enclomiphene | 0.01 | 65 (72 h) | 3 | --- | |||||||
| Zuclomiphene | 0.04 | 1289 (456 h) | 7 | --- | |||||||
| Clomiphene | 50 | IV | F | Anov. | [49] | ||||||
| Enclomiphene | N/A, P1 | 0.66 | 373 (24 h) | --- | 60 | ||||||
| N/A, P2 | 1.21 | 862 (24 h) | --- | 283 | |||||||
| Zuclomiphene | N/A, P1 | 0.15 | 134 (24 h) | --- | 341 | ||||||
| N/A, P2 | 0.26 | 255 (24 h) | 802 | ||||||||
| Enclomiphene | Enclomiphene c | 25 | oral | M | ITT | 16 | 0.04 | --- | 2.5 | --- | [41] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, E.A.; Barnes, A.B.; Wiehle, R.D.; Fontenot, G.K.; Hoenen, T.; White, J.M. Clomiphene and Its Isomers Block Ebola Virus Particle Entry and Infection with Similar Potency: Potential Therapeutic Implications. Viruses 2016, 8, 206. https://doi.org/10.3390/v8080206
Nelson EA, Barnes AB, Wiehle RD, Fontenot GK, Hoenen T, White JM. Clomiphene and Its Isomers Block Ebola Virus Particle Entry and Infection with Similar Potency: Potential Therapeutic Implications. Viruses. 2016; 8(8):206. https://doi.org/10.3390/v8080206
Chicago/Turabian StyleNelson, Elizabeth A., Alyson B. Barnes, Ronald D. Wiehle, Gregory K. Fontenot, Thomas Hoenen, and Judith M. White. 2016. "Clomiphene and Its Isomers Block Ebola Virus Particle Entry and Infection with Similar Potency: Potential Therapeutic Implications" Viruses 8, no. 8: 206. https://doi.org/10.3390/v8080206
APA StyleNelson, E. A., Barnes, A. B., Wiehle, R. D., Fontenot, G. K., Hoenen, T., & White, J. M. (2016). Clomiphene and Its Isomers Block Ebola Virus Particle Entry and Infection with Similar Potency: Potential Therapeutic Implications. Viruses, 8(8), 206. https://doi.org/10.3390/v8080206






