KSHV-Mediated Angiogenesis in Tumor Progression
Abstract
:1. Introduction
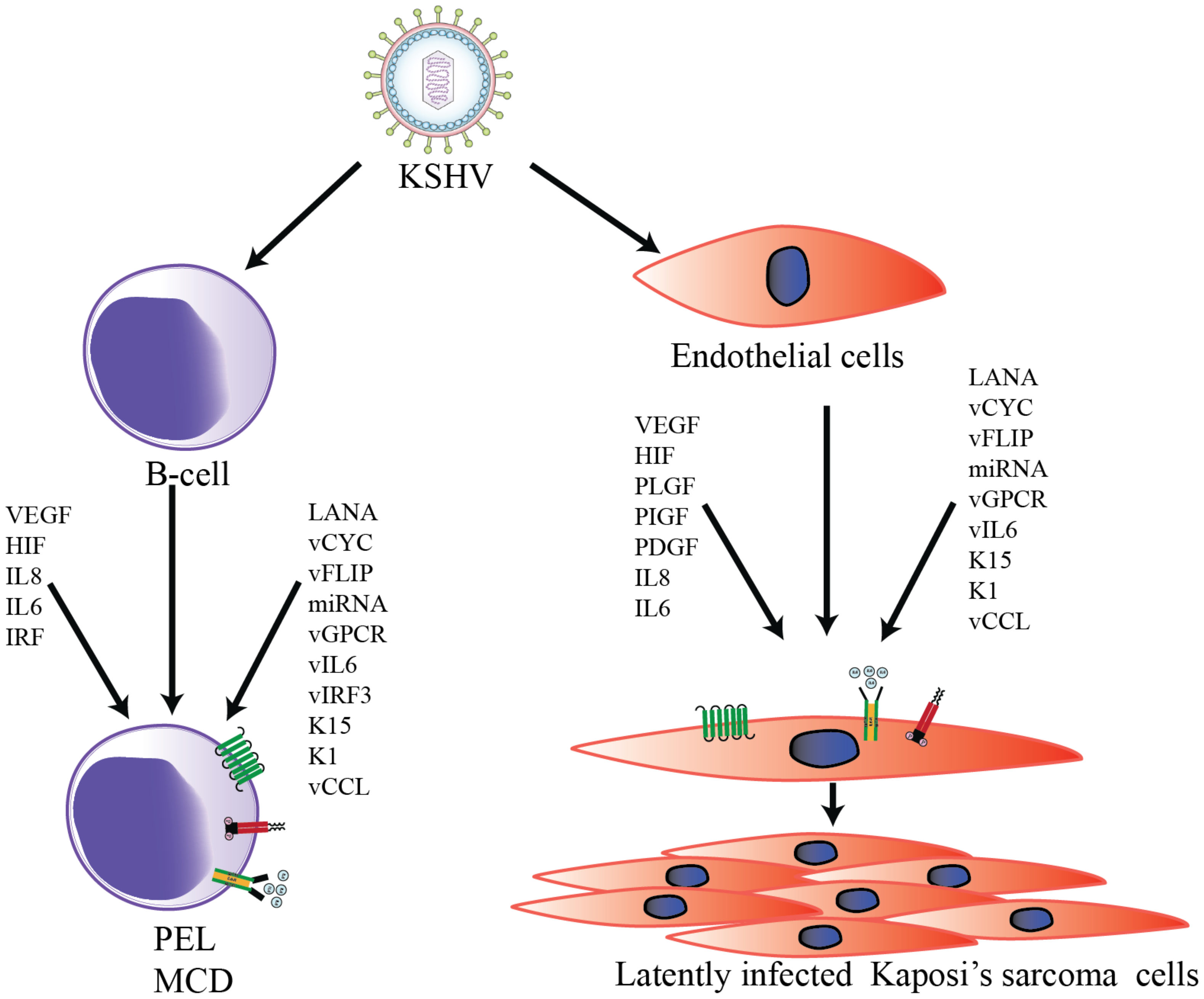
2. KSHV-Associated Human Malignancies
2.1. Kaposi’s Sarcoma (KS)
2.2. Primary Effusion Lymphoma (PEL)
2.3. Multicentric Castleman’s Disease (MCD)
3. KSHV-Mediated Angiogenesis
3.1. Cellular Factors
3.2. Viral Factors
4. Mouse Models for Studying KS-Angiogenesis
5. Current Treatment Strategies for KS Tumors
6. Summary
Acknowledgments
Conflicts of Interest
References
- Sturzl, M.; Zietz, C.; Monini, P.; Ensoli, B. Human herpesvirus-8 and kaposi’s sarcoma: Relationship with the multistep concept of tumorigenesis. Adv. Cancer Res. 2001, 81, 125–159. [Google Scholar] [PubMed]
- Verma, S.C.; Robertson, E.S. Molecular biology and pathogenesis of kaposi sarcoma-associated herpesvirus. FEMS Microbiol. Lett. 2003, 222, 155–163. [Google Scholar] [CrossRef]
- Chang, Y.; Cesarman, E.; Pessin, M.S.; Lee, F.; Culpepper, J.; Knowles, D.M.; Moore, P.S. Identification of herpesvirus-like DNA sequences in aids-associated kaposi’s sarcoma. Science 1994, 266, 1865–1869. [Google Scholar] [CrossRef] [PubMed]
- Cesarman, E.; Chang, Y.; Moore, P.S.; Said, J.W.; Knowles, D.M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in aids-related body-cavity-based lymphomas. N. Engl. J. Med. 1995, 332, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Soulier, J.; Grollet, L.; Oksenhendler, E.; Cacoub, P.; Cazals-Hatem, D.; Babinet, P.; d’Agay, M.F.; Clauvel, J.P.; Raphael, M.; Degos, L.; et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric castleman’s disease. Blood 1995, 86, 1276–1280. [Google Scholar] [PubMed]
- Deloose, S.T.; Smit, L.A.; Pals, F.T.; Kersten, M.J.; van Noesel, C.J.; Pals, S.T. High incidence of kaposi sarcoma-associated herpesvirus infection in HIV-related solid immunoblastic/plasmablastic diffuse large B-cell lymphoma. Leukemia 2005, 19, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Marshall, V.; Uldrick, T.; Leighty, R.; Labo, N.; Wyvill, K.; Aleman, K.; Polizzotto, M.N.; Little, R.F.; Yarchoan, R.; et al. Sequence analysis of kaposi sarcoma-associated herpesvirus (KSHV) microRNAs in patients with multicentric castleman disease and KSHV-associated inflammatory cytokine syndrome. J. Infect. Dis. 2012, 205, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Spear, P.G.; Longnecker, R. Herpesvirus entry: An update. J. Virol. 2003, 77, 10179–10185. [Google Scholar] [CrossRef] [PubMed]
- Lagunoff, M.; Bechtel, J.; Venetsanakos, E.; Roy, A.M.; Abbey, N.; Herndier, B.; McMahon, M.; Ganem, D. De novo infection and serial transmission of kaposi’s sarcoma-associated herpesvirus in cultured endothelial cells. J. Virol. 2002, 76, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Bechtel, J.T.; Liang, Y.; Hvidding, J.; Ganem, D. Host range of kaposi’s sarcoma-associated herpesvirus in cultured cells. J. Virol. 2003, 77, 6474–6481. [Google Scholar] [CrossRef] [PubMed]
- Uppal, T.; Banerjee, S.; Sun, Z.; Verma, S.C.; Robertson, E.S. KSHV LANA—The master regulator of KSHV latency. Viruses 2014, 6, 4961–4998. [Google Scholar] [CrossRef] [PubMed]
- DiMaio, T.A.; Gutierrez, K.D.; Lagunoff, M. Latent KSHV infection of endothelial cells induces integrin beta3 to activate angiogenic phenotypes. PLoS Pathog. 2011, 7, e1002424. [Google Scholar] [CrossRef] [PubMed]
- Bais, C.; Santomasso, B.; Coso, O.; Arvanitakis, L.; Raaka, E.G.; Gutkind, J.S.; Asch, A.S.; Cesarman, E.; Gershengorn, M.C.; Mesri, E.A. G-protein-coupled receptor of kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 1998, 391, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.; Philpott, N.J.; Cesarman, E. The kaposi’s sarcoma-associated herpesvirus g protein-coupled receptor has broad signaling effects in primary effusion lymphoma cells. J. Virol. 2003, 77, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Cavallin, L.E.; Goldschmidt-Clermont, P.; Mesri, E.A. Molecular and cellular mechanisms of KSHV oncogenesis of kaposi’s sarcoma associated with HIV/aids. PLoS Pathog. 2014, 10, e1004154. [Google Scholar] [CrossRef] [PubMed]
- DiMaio, T.A.; Gutierrez, K.D.; Lagunoff, M. Kaposi’s sarcoma-associated herpesvirus downregulates transforming growth factor beta2 to promote enhanced stability of capillary-like tube formation. J. Virol. 2014, 88, 14301–14309. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Bratoeva, M.; Toole, B.P.; Qin, Z.; Parsons, C. KSHV activation of VEGF secretion and invasion for endothelial cells is mediated through viral upregulation of emmprin-induced signal transduction. Int. J. Cancer 2012, 131, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.W.; Greene, W.; Ye, F.; Gao, S.J. Kaposi’s sarcoma-associated herpesvirus disrupts adherens junctions and increases endothelial permeability by inducing degradation of ve-cadherin. J. Virol. 2008, 82, 11902–11912. [Google Scholar] [CrossRef] [PubMed]
- Gbabe, O.F.; Okwundu, C.I.; Dedicoat, M.; Freeman, E.E. Treatment of severe or progressive kaposi’s sarcoma in HIV-infected adults. Cochrane Database Syst. Rev. 2014, 8, Cd003256. [Google Scholar] [PubMed]
- Cornali, E.; Zietz, C.; Benelli, R.; Weninger, W.; Masiello, L.; Breier, G.; Tschachler, E.; Albini, A.; Sturzl, M. Vascular endothelial growth factor regulates angiogenesis and vascular permeability in kaposi’s sarcoma. Am. J. Pathol. 1996, 149, 1851–1869. [Google Scholar] [PubMed]
- Gessain, A.; Duprez, R. Spindle cells and their role in kaposi’s sarcoma. Int. J. Biochem. Cell Biol. 2005, 37, 2457–2465. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.A.; Sturzl, M.A.; Blasig, C.; Schreier, A.; Guo, H.G.; Reitz, M.; Opalenik, S.R.; Browning, P.J. Expression of human herpesvirus 8-encoded cyclin d in kaposi’s sarcoma spindle cells. J. Natl. Cancer Inst. 1997, 89, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, P.; Espigol-Frigole, G.; McCormick, P.J.; Salvucci, O.; Maric, D.; Uldrick, T.S.; Polizzotto, M.N.; Yarchoan, R.; Tosato, G. Kaposi sarcoma herpesvirus promotes endothelial-to-mesenchymal transition through notch-dependent signaling. Cancer Res. 2012, 72, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Ganem, D. KSHV infection and the pathogenesis of kaposi’s sarcoma. Annu Rev. Pathol. 2006, 1, 273–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Trotter, M.W.; Lagos, D.; Bourboulia, D.; Henderson, S.; Makinen, T.; Elliman, S.; Flanagan, A.M.; Alitalo, K.; Boshoff, C. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in kaposi sarcoma. Nat. Genet. 2004, 36, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Foreman, K.; Shin, J.W.; Hirakawa, S.; Curry, C.L.; Sage, D.R.; Libermann, T.; Dezube, B.J.; Fingeroth, J.D.; Detmar, M. Lymphatic reprogramming of blood vascular endothelium by kaposi sarcoma-associated herpesvirus. Nat. Genet. 2004, 36, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Ciufo, D.M.; Cannon, J.S.; Poole, L.J.; Wu, F.Y.; Murray, P.; Ambinder, R.F.; Hayward, G.S. Spindle cell conversion by kaposi’s sarcoma-associated herpesvirus: Formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J. Virol. 2001, 75, 5614–5626. [Google Scholar] [CrossRef] [PubMed]
- Moses, A.V.; Jarvis, M.A.; Raggo, C.; Bell, Y.C.; Ruhl, R.; Luukkonen, B.G.; Griffith, D.J.; Wait, C.L.; Druker, B.J.; Heinrich, M.C.; et al. Kaposi’s sarcoma-associated herpesvirus-induced upregulation of the c-kit proto-oncogene, as identified by gene expression profiling, is essential for the transformation of endothelial cells. J. Virol. 2002, 76, 8383–8399. [Google Scholar] [CrossRef] [PubMed]
- Grundhoff, A.; Ganem, D. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in kaposi sarcoma pathogenesis. J. Clin. Investig. 2004, 113, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.L.; Gustin, J.K.; Moses, A.V.; Dezube, B.J.; Pantanowitz, L. Kaposi sarcoma pathogenesis: A triad of viral infection, oncogenesis and chronic inflammation. Transl. Biomed. 2010, 1, 172. [Google Scholar] [PubMed]
- Dourmishev, L.A.; Dourmishev, A.L.; Palmeri, D.; Schwartz, R.A.; Lukac, D.M. Molecular genetics of kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8) epidemiology and pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 175–212. [Google Scholar] [CrossRef] [PubMed]
- Pantanowitz, L.; Dezube, B.J. Kaposi sarcoma in unusual locations. BMC Cancer 2008, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Mohanna, S.; Maco, V.; Bravo, F.; Gotuzzo, E. Epidemiology and clinical characteristics of classic kaposi’s sarcoma, seroprevalence, and variants of human herpesvirus 8 in south america: A critical review of an old disease. Int. J. Infect. Dis. 2005, 9, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.; Polizzotto, M.N.; Uldrick, T.S.; Yarchoan, R. Kaposi sarcoma-associated herpesvirus-associated malignancies: Epidemiology, pathogenesis, and advances in treatment. Semin. Oncol. 2015, 42, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Horenstein, M.G.; Moontasri, N.J.; Cesarman, E. The pathobiology of kaposi’s sarcoma: Advances since the onset of the aids epidemic. J. Cutan Pathol. 2008, 35 (Suppl. 2), 40–44. [Google Scholar] [CrossRef] [PubMed]
- Ablashi, D.V.; Chatlynne, L.G.; Whitman, J.E., Jr.; Cesarman, E. Spectrum of kaposi’s sarcoma-associated herpesvirus, or human herpesvirus 8, diseases. Clin. Microbiol. Rev. 2002, 15, 439–464. [Google Scholar] [CrossRef] [PubMed]
- Nador, R.G.; Cesarman, E.; Chadburn, A.; Dawson, D.B.; Ansari, M.Q.; Sald, J.; Knowles, D.M. Primary effusion lymphoma: A distinct clinicopathologic entity associated with the kaposi’s sarcoma-associated herpes virus. Blood 1996, 88, 645–656. [Google Scholar] [PubMed]
- Ueda, K.; Ito, E.; Karayama, M.; Ohsaki, E.; Nakano, K.; Watanabe, S. KSHV-infected pel cell lines exhibit a distinct gene expression profile. Biochem. Biophys. Res. Commun. 2010, 394, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Ohsaki, E.; Nakano, K.; Zheng, X. Characterization of kaposi’s sarcoma-associated herpesvirus-related lymphomas by DNA microarray analysis. Leuk. Res. Treat. 2011, 2011, 726964. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, D.P.; Damania, B. Kaposi sarcoma associated herpesvirus pathogenesis (KSHV)—An update. Curr. Opin. Virol. 2013, 3, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Webster-Cyriaque, J.; Duus, K.; Cooper, C.; Duncan, M. Oral ebv and KSHV infection in HIV. Adv. Dent. Res. 2006, 19, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Ito, J.; Takeda, N.; Gasyu, I.; Okazaki, T.; Sakaguchi, M.; Osawa, N.; Tanaka, Y.; Oka, K.; Uzu, T.; et al. A case of castleman’s disease complicated with nephrotic syndrome due to glomerulopathy mimicking membranoproliferative glomerulonephritis. Am. J. Med. Sci. 2008, 335, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Cesarman, E.; Spina, M.; Gloghini, A.; Schulz, T.F. HIV-associated lymphomas and gamma-herpesviruses. Blood 2009, 113, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Bower, M.; Newsom-Davis, T.; Naresh, K.; Merchant, S.; Lee, B.; Gazzard, B.; Stebbing, J.; Nelson, M. Clinical features and outcome in HIV-associated multicentric castleman’s disease. J. Clin. Oncol. 2011, 29, 2481–2486. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; De Paoli, P.; Gloghini, A.; Vaccher, E. KSHV-associated multicentric castleman disease: A tangle of different entities requiring multitarget treatment strategies. Int. J. Cancer 2015, 137, 251–261. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, K.J.; Dalqleish, A.G.; Browning, M.J.; Steward, W.P.; Harris, A.L. The relationship between angiogenesis and the immune response in carcinogenesis and the progression of malignant disease. Eur. J. Cancer 2000, 36, 51–69. [Google Scholar] [CrossRef]
- Sakakibara, S.; Tosato, G. Regulation of angiogenesis in malignancies associated with epstein-barr virus and kaposi’s sarcoma-associated herpes virus. Future Microbiol. 2009, 4, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Tandle, A.; Blazer, D.G., 3rd; Libutti, S.K. Antiangiogenic gene therapy of cancer: Recent developments. J. Transl. Med. 2004, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Schulz, T.F.; Cesarman, E. Kaposi sarcoma-associated herpesvirus: Mechanisms of oncogenesis. Curr. Opin. Virol. 2015, 14, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, J.M. Ultrastructure of kaposi sarcoma. Ultrastruct. Pathol. 2008, 32, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Gramolelli, S.; Schulz, T.F. The role of kaposi sarcoma-associated herpesvirus in the pathogenesis of kaposi sarcoma. J. Pathol. 2015, 235, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.W.; Xie, J.; Ye, F.; Gao, S.J. Kaposi’s sarcoma-associated herpesvirus infection promotes invasion of primary human umbilical vein endothelial cells by inducing matrix metalloproteinases. J. Virol. 2007, 81, 7001–7010. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhang, W.; Bakken, T.; Schutten, M.; Toth, Z.; Jung, J.U.; Gill, P.; Cannon, M.; Gao, S.J. Cancer angiogenesis induced by kaposi sarcoma-associated herpesvirus is mediated by EZH2. Cancer Res. 2012, 72, 3582–3592. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.P.; Damania, B. Aktivation of PI3K/AKT/mTOR signaling pathway by KSHV. Front. Immunol. 2012, 3, 401. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Jaffe, E.S.; Chang, Y.; Jones, K.; Teruya-Feldstein, J.; Moore, P.S.; Tosato, G. Angiogenesis and hematopoiesis induced by kaposi’s sarcoma-associated herpesvirus-encoded interleukin-6. Blood 1999, 93, 4034–4043. [Google Scholar] [PubMed]
- Boshoff, C.; Endo, Y.; Collins, P.D.; Takeuchi, Y.; Reeves, J.D.; Schweickart, V.L.; Siani, M.A.; Sasaki, T.; Williams, T.J.; Gray, P.W.; et al. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science 1997, 278, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Stine, J.T.; Wood, C.; Hill, M.; Epp, A.; Raport, C.J.; Schweickart, V.L.; Endo, Y.; Sasaki, T.; Simmons, G.; Boshoff, C.; et al. KSHV-encoded CC chemokine VMIP-III is a CCR4 agonist, stimulates angiogenesis, and selectively chemoattracts TH2 cells. Blood 2000, 95, 1151–1157. [Google Scholar] [PubMed]
- Liu, C.; Okruzhnov, Y.; Li, H.; Nicholas, J. Human herpesvirus 8 (HHV-8)-encoded cytokines induce expression of and autocrine signaling by vascular endothelial growth factor (VEGF) in HHV-8-infected primary-effusion lymphoma cell lines and mediate VEGF-independent antiapoptotic effects. J. Virol. 2001, 75, 10933–10940. [Google Scholar] [CrossRef] [PubMed]
- Bais, C.; Van Geelen, A.; Eroles, P.; Mutlu, A.; Chiozzini, C.; Dias, S.; Silverstein, R.L.; Rafii, S.; Mesri, E.A. Kaposi’s sarcoma associated herpesvirus g protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/ KDR. Cancer Cell 2003, 3, 131–143. [Google Scholar] [CrossRef]
- Wang, L.; Wakisaka, N.; Tomlinson, C.C.; DeWire, S.M.; Krall, S.; Pagano, J.S.; Damania, B. The kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) K1 protein induces expression of angiogenic and invasion factors. Cancer Res. 2004, 64, 2774–2781. [Google Scholar] [CrossRef] [PubMed]
- Breen, E.C. VEGF in biological control. J. Cell. Biochem. 2007, 102, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, M.L.; De Palma, M. Macrophage regulation of tumor angiogenesis: Implications for cancer therapy. Mol. Aspects Med. 2011, 32, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, F.; Markham, P.D.; Gendelman, R.; Watanabe, Y.; Kao, V.; Kowalski, K.; Sonnabend, J.A.; Pintus, A.; Gallo, R.C.; Ensoli, B. Vascular endothelial growth factor and basic fibroblast growth factor present in kaposi’s sarcoma (KS) are induced by inflammatory cytokines and synergize to promote vascular permeability and KS lesion development. Am. J. Pathol. 1998, 152, 1433–1443. [Google Scholar] [PubMed]
- Akula, S.M.; Ford, P.W.; Whitman, A.G.; Hamden, K.E.; Bryan, B.A.; Cook, P.P.; McCubrey, J.A. B-raf-dependent expression of vascular endothelial growth factor-a in kaposi sarcoma-associated herpesvirus-infected human B cells. Blood 2005, 105, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, R.; Sehgal, I.; D’Auvergne, O.; Kousoulas, K.G. Kaposi’s sarcoma-associated herpesvirus glycoproteins B and k8.1 regulate virion egress and synthesis of vascular endothelial growth factor and viral interleukin-6 in BCBL-1 cells. J. Virol. 2010, 84, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Masood, R.; Cesarman, E.; Smith, D.L.; Gill, P.S.; Flore, O. Human herpesvirus-8-transformed endothelial cells have functionally activated vascular endothelial growth factor/vascular endothelial growth factor receptor. Am. J. Pathol. 2002, 160, 23–29. [Google Scholar] [CrossRef]
- Sivakumar, R.; Sharma-Walia, N.; Raghu, H.; Veettil, M.V.; Sadagopan, S.; Bottero, V.; Varga, L.; Levine, R.; Chandran, B. Kaposi’s sarcoma-associated herpesvirus induces sustained levels of vascular endothelial growth factors a and c early during in vitro infection of human microvascular dermal endothelial cells: Biological implications. J. Virol. 2008, 82, 1759–1776. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Damania, B. Kaposi’s sarcoma-associated herpesvirus confers a survival advantage to endothelial cells. Cancer Res. 2008, 68, 4640–4648. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; McGough, R.; Aswad, B.; Block, J.A.; Terek, R. Hypoxia induces HIF-1alpha and VEGF expression in chondrosarcoma cells and chondrocytes. J. Orthop. Res. 2004, 22, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Brocato, J.; Chervona, Y.; Costa, M. Molecular responses to hypoxia-inducible factor 1alpha and beyond. Mol. Pharmacol. 2014, 85, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.J.; Kappel, A.; Goodall, G.J. Hypoxia-inducible factor-1alpha mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia. Mol. Biol. Cell 2002, 13, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Carroll, P.A.; Kenerson, H.L.; Yeung, R.S.; Lagunoff, M. Latent kaposi’s sarcoma-associated herpesvirus infection of endothelial cells activates hypoxia-induced factors. J. Virol. 2006, 80, 10802–10812. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.C.; Joo, C.H.; Gack, M.U.; Lee, H.R.; Jung, J.U. Kaposi’s sarcoma-associated herpesvirus viral ifn regulatory factor 3 stabilizes hypoxia-inducible factor-1 alpha to induce vascular endothelial growth factor expression. Cancer Res. 2008, 68, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Dai, L.; Slomiany, M.G.; Toole, B.P.; Parsons, C. Direct activation of emmprin and associated pathogenesis by an oncogenic herpesvirus. Cancer Res. 2010, 70, 3884–3889. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.H.; Liu, L.Z. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv. Cancer Res. 2009, 102, 19–65. [Google Scholar] [CrossRef] [PubMed]
- Badescu, A.; Couvelard, A.; Handra-Luca, A. AKT pathway protein expression in gastrointestinal kaposi sarcomas: Relevance for tumor biology. APMIS 2014, 122, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E.; Lipp, P.; Bootman, M.; Ridley, S.H.; Coadwell, J.; Ronnstrand, L.; Lennartsson, J.; Holmes, A.B.; Painter, G.F.; Thuring, J.; et al. Dapp1 undergoes a PI 3-kinase-dependent cycle of plasma-membrane recruitment and endocytosis upon cell stimulation. Curr. Biol. 2000, 10, 1403–1412. [Google Scholar] [CrossRef]
- Toker, A. Protein kinases as mediators of phosphoinositide 3-kinase signaling. Mol. Pharmacol. 2000, 57, 652–658. [Google Scholar] [PubMed]
- Gingras, A.C.; Kennedy, S.G.; O’Leary, M.A.; Sonenberg, N.; Hay, N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the AKT(PKB) signaling pathway. Genes Dev. 1998, 12, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Beevers, C.S.; Li, F.; Liu, L.; Huang, S. Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells. Int. J. Cancer 2006, 119, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Hay, N.; Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Windgassen, A.; Nogueira, V.; Chen, C.C.; Skeen, J.E.; Sonenberg, N.; Hay, N. AKT activates the mammalian target of rapamycin by regulating cellular ATP level and ampk activity. J. Biol. Chem. 2005, 280, 32081–32089. [Google Scholar] [CrossRef] [PubMed]
- Montaner, S.; Sodhi, A.; Pece, S.; Mesri, E.A.; Gutkind, J.S. The kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of AKT/protein kinase B. Cancer Res. 2001, 61, 2641–2648. [Google Scholar] [PubMed]
- Uddin, S.; Hussain, A.R.; Al-Hussein, K.A.; Manogaran, P.S.; Wickrema, A.; Gutierrez, M.I.; Bhatia, K.G. Inhibition of phosphatidylinositol 3’-kinase/AKT signaling promotes apoptosis of primary effusion lymphoma cells. Clin. Cancer Res. 2005, 11, 3102–3108. [Google Scholar] [CrossRef] [PubMed]
- Sin, S.H.; Roy, D.; Wang, L.; Staudt, M.R.; Fakhari, F.D.; Patel, D.D.; Henry, D.; Harrington, W.J., Jr.; Damania, B.A.; Dittmer, D.P. Rapamycin is efficacious against primary effusion lymphoma (PEL) cell lines in vivo by inhibiting autocrine signaling. Blood 2007, 109, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Curry, C.L.; Reed, L.L.; Golde, T.E.; Miele, L.; Nickoloff, B.J.; Foreman, K.E. Gamma secretase inhibitor blocks notch activation and induces apoptosis in kaposi’s sarcoma tumor cells. Oncogene 2005, 24, 6333–6344. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Pekkonen, P.; Laurinavicius, S.; Sugiyama, N.; Henderson, S.; Gunther, T.; Rantanen, V.; Kaivanto, E.; Aavikko, M.; Sarek, G.; et al. KSHV-initiated notch activation leads to membrane-type-1 matrix metalloproteinase-dependent lymphatic endothelial-to-mesenchymal transition. Cell Host Microbe 2011, 10, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Schumacher, N.; Maier, M.; Sendtner, M.; Gessler, M. The notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004, 18, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Steidl, C.; Wagner, T.U.; Lang, E.; Jakob, P.M.; Friedl, P.; Knobeloch, K.P.; Gessler, M. Combined loss of Hey1 and Heyl causes congenital heart defects because of impaired epithelial to mesenchymal transition. Circ. Res. 2007, 100, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Sharff, K.A.; Song, W.X.; Luo, X.; Tang, N.; Luo, J.; Chen, J.; Bi, Y.; He, B.C.; Huang, J.; Li, X.; et al. Hey1 basic helix-loop-helix protein plays an important role in mediating BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. J. Biol. Chem. 2009, 284, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.L.; Hankenson, K.D. Integration of bmp, wnt, and notch signaling pathways in osteoblast differentiation. J. Cell. Biochem. 2011, 112, 3491–3501. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, Z.; Xia, T.; Li, X.; Liang, D.; Lin, X.; Wen, H.; Lan, K. Latency-associated nuclear antigen of kaposi sarcoma-associated herpesvirus promotes angiogenesis through targeting notch signaling effector Hey1. Cancer Res. 2014, 74, 2026–2037. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yu, F.X.; Kim, Y.C.; Meng, Z.; Naipauer, J.; Looney, D.J.; Liu, X.; Gutkind, J.S.; Mesri, E.A.; Guan, K.L. Kaposi sarcoma-associated herpesvirus promotes tumorigenesis by modulating the hippo pathway. Oncogene 2015, 34, 3536–3546. [Google Scholar] [CrossRef] [PubMed]
- Badouel, C.; McNeill, H. Snapshot: The hippo signaling pathway. Cell 2011, 145, 484–484. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Quiqley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Impola, U.; Cuccuru, M.A.; Masala, M.V.; Jeskanen, L.; Cottoni, F. Preliminary communication: Matrix metalloproteinases in kaposi’s sarcoma. Br. J. Dermatol. 2003, I49, 905–907. [Google Scholar] [CrossRef]
- Meade-Tollin, L.C.; Way, D.; Witte, M.H. Expression of multiple matrix metalloproteinases and urokinase type plasminogen activator in cultured kaposi sarcoma cells. Acta Histochem. 1999, 101, 305–316. [Google Scholar] [CrossRef]
- Cianfrocca, M.; Cooley, T.P.; Lee, J.Y.; Rudek, M.A.; Scadden, D.T.; Ratner, L.; Pluda, J.M.; Figg, W.D.; Krown, S.E.; Dezube, B.J.; et al. Matrix metalloproteinase inhibitor COL-3 in the treatment of AIDS-related kaposi’s sarcoma: A phase I aids malignancy consortium study. J. Clin. Oncol. 2002, 20, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Brekken, R.; McMahon, G.; Vu, T.H.; Itoh, T.; Tamaki, K.; Tanzawa, K.; Thorpe, P.; Itohara, S.; Werb, Z.; et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000, 2, 737–744. [Google Scholar] [PubMed]
- Ye, F.C.; Blackbourn, D.J.; Mengel, M.; Xie, J.P.; Qian, L.W.; Greene, W.; Yeh, I.T.; Graham, D.; Gao, S.J. Kaposi’s sarcoma-associated herpesvirus promotes angiogenesis by inducing angiopoietin-2 expression via AP-1 and ETS1. J. Virol. 2007, 81, 3980–3991. [Google Scholar] [CrossRef] [PubMed]
- Metheny-Barlow, L.J.; Li, L.Y. The enigmatic role of angiopoietin-1 in tumor angiogenesis. Cell Res. 2003, 13, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Sako, K.; Noda, K.; Zhang, J.; Minami, M.; Mochizuki, N. Angiopoietin-1/Tie2 receptor signaling in vascular quiescence and angiogenesis. Histol. Histopathol. 2010, 25, 387–396. [Google Scholar] [PubMed]
- Brown, L.F.; Dezube, B.J.; Tognazzi, K.; Dvorak, H.F.; Yancopoulos, G.D. Expression of Tie1, Tie2, and angiopoietins 1, 2, and 4 in kaposi’s sarcoma and cutaneous angiosarcoma. Am. J. Pathol. 2000, 156, 2179–2183. [Google Scholar] [CrossRef]
- Zheng, X.; Ohsaki, E.; Ueda, K. Mechanism of angiopoietin-1 upregulation in kaposi’s sarcoma-associated herpesvirus-infected PEL cell lines. J. Virol. 2015, 89, 4786–4797. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Maemura, K.; Imai, Y.; Harada, T.; Kawanami, D. Endothelial PAS domain protein 1 gene promotes angiogenesis through the transactivation of both vascular endothelial growth factor and its receptor. Circ. Res. 2004, 95, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Jham, B.C.; Ma, T.; Hu, J.; Chaisuparat, R.; Friedman, E.R.; Pandolfi, P.P.; Schneider, A.; Sodhi, A.; Montaner, S. Amplification of the angiogenic signal through the activation of the TSC/mTOR/HIF axis by the KSHV VGPCR in kaposi’s sarcoma. PLoS ONE 2011, 6, e19103. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, A.; Chaisuparat, R.; Hu, J.; Ramsdell, A.K.; Manning, B.D.; Sausville, E.A.; Sawai, E.T.; Molinolo, A.; Gutkind, J.S.; Montaner, S. The TSC2/mTOR pathway drives endothelial cell transformation induced by the kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor. Cancer Cell 2006, 10, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Jham, B.C.; Hu, J.; Friedman, E.R.; Basile, J.R.; Molinolo, A.; Sodhi, A.; Montaner, S. Viral g protein-coupled receptor up-regulates angiopoietin-like 4 promoting angiogenesis and vascular permeability in kaposi’s sarcoma. Proc. Natl. Acad. Sci. USA 2010, 107, 14363–14368. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Patel, H.; Babapoor-Farrokhran, S.; Franklin, R.; Semenza, G.L.; Sodhi, A.; Montaner, S. KSHV induces aerobic glycolysis and angiogenesis through HIF-1-dependent upregulation of pyruvate kinase 2 in kaposi’s sarcoma. Angiogenesis 2015, 18, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.A.; Bala, K.; Busche, G.; Weidner-Glunde, M.; Santag, S.; Kati, S.; Gramolelli, S.; Damas, M.; Dittrich-Breiholz, O.; Kracht, M.; et al. The inflammatory kinase MAP4K4 promotes reactivation of kaposi’s sarcoma herpesvirus and enhances the invasiveness of infected endothelial cells. PLoS Pathog. 2013, 9, e1003737. [Google Scholar] [CrossRef] [PubMed]
- Sharma-Walia, N.; Paul, A.G.; Bottero, V.; Sadagopan, S.; Veettil, M.V.; Kerur, N.; Chandran, B. Kaposi’s sarcoma associated herpes virus (KSHV) induced cox-2: A key factor in latency, inflammation, angiogenesis, cell survival and invasion. PLoS Pathog. 2010, 6, e1000777. [Google Scholar] [CrossRef] [PubMed]
- Montaner, S.; Sodhi, A.; Molinolo, A.; Bugge, T.H.; Sawai, E.T.; He, Y.; Li, Y.; Ray, P.E.; Gutkind, J.S. Endothelial infection with KSHV genes in vivo reveals that VGPCR initiates kaposi’s sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 2003, 3, 23–36. [Google Scholar] [CrossRef]
- Tomlinson, C.C.; Damania, B. The K1 protein of kaposi’s sarcoma-associated herpesvirus activates the AKT signaling pathway. J. Virol. 2004, 78, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.A.; Punjabi, A.S.; Lagunoff, M. Activation of AKT through gp130 receptor signaling is required for kaposi’s sarcoma-associated herpesvirus-induced lymphatic reprogramming of endothelial cells. J. Virol. 2008, 82, 8771–8779. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, C.; Wei, F.; Zhang, L.; Mo, X.; Feng, Y.; Xu, J.; Yuan, Z.; Robertson, E.; Cai, Q. Constitutive activation of interleukin-13/stat6 contributes to kaposi’s sarcoma-associated herpesvirus-related primary effusion lymphoma cell proliferation and survival. J. Virol. 2015, 89, 10416–10426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, S.; Wang, Y.; Brulois, K.; Lan, K.; Jung, J.U.; Feng, P. Herpesviral g protein-coupled receptors activate nfat to induce tumor formation via inhibiting the serca calcium atpase. PLoS Pathog. 2015, 11, e1004768. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolo, D.L.; Cannon, M.; Liu, Y.F.; Renne, R.; Chadburn, A.; Boshoff, C.; Cesarman, E. KSHV LANA inhibits TGF-beta signaling through epigenetic silencing of the TGF-beta type II receptor. Blood 2008, 111, 4731–4740. [Google Scholar] [CrossRef] [PubMed]
- Botto, S.; Totonchy, J.E.; Gustin, J.K.; Moses, A.V. Kaposi sarcoma herpesvirus induces HO-1 during de novo infection of endothelial cells via viral miRNA-dependent and -independent mechanisms. MBio 2015, 6, e00668. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, A.; Montaner, S.; Patel, V.; Zohar, M.; Bais, C.; Mesri, E.A.; Gutkind, J.S. The kaposi’s sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha. Cancer Res. 2000, 60, 4873–4880. [Google Scholar] [PubMed]
- Angius, F.; Uda, S.; Piras, E.; Spolitu, S.; Ingianni, A.; Batetta, B.; Pompei, R. Neutral lipid alterations in human herpesvirus 8-infected huvec cells and their possible involvement in neo-angiogenesis. BMC Microbiol. 2015, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Xiao, Y.; Qu, Z. Oncovirus kaposi sarcoma herpesvirus (KSHV) represses tumor suppressor PDLIM2 to persistently activate nuclear factor kappab (NF-kappaB) and stat3 transcription factors for tumorigenesis and tumor maintenance. J. Biol. Chem. 2015, 290, 7362–7368. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.; Azzi, S.; Leclair, H.M.; Georges, S.; Carlotti, A.; Treps, L.; Galan-Moya, E.M.; Alexia, C.; Dupin, N.; Bidere, N.; et al. The guanine exchange factor SWAP70 mediates vGPCR-induced endothelial plasticity. Cell Commun. Signal. 2015, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Lei, X.; Gao, S.J. Mechanisms of kaposi’s sarcoma-associated herpesvirus latency and reactivation. Adv. Virol. 2011, 2011, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gantt, S.; Casper, C. Human herpesvirus 8-associated neoplasms: The roles of viral replication and antiviral treatment. Curr. Opin. Infect. Dis. 2011, 24, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Santag, S.; Jager, W.; Karsten, C.B.; Kati, S.; Pietrek, M.; Steinemann, D.; Sarek, G.; Ojala, P.M.; Schulz, T.F. Recruitment of the tumour suppressor protein p73 by kaposi’s sarcoma herpesvirus latent nuclear antigen contributes to the survival of primary effusion lymphoma cells. Oncogene 2013, 32, 3676–3685. [Google Scholar] [CrossRef] [PubMed]
- Friborg, J., Jr.; Kong, W.; Hottiger, M.O.; Nabel, G.J. P53 inhibition by the LANA protein of KSHV protects against cell death. Nature 1999, 402, 889–894. [Google Scholar] [PubMed]
- Watanabe, T.; Sugaya, M.; Atkins, A.M.; Aquilino, E.A.; Yang, A.; Borris, D.L.; Brady, J.; Blauvelt, A. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen prolongs the life span of primary human umbilical vein endothelial cells. J. Virol. 2003, 77, 6188–6196. [Google Scholar] [CrossRef] [PubMed]
- Bubman, D.; Guasparri, I.; Cesarman, E. Deregulation of c-Myc in primary effusion lymphoma by kaposi’s sarcoma herpesvirus latency-associated nuclear antigen. Oncogene 2007, 26, 4979–4986. [Google Scholar] [CrossRef] [PubMed]
- Fujimuro, M.; Wu, F.Y.; ApRhys, C.; Kajumbula, H.; Young, D.B.; Hayward, G.S.; Hayward, S.D. A novel viral mechanism for dysregulation of beta-catenin in kaposi’s sarcoma-associated herpesvirus latency. Nat. Med. 2003, 9, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Moore, P.S.; Talbot, S.J.; Boshoff, C.H.; Zarkowska, T.; Godden, K.; Paterson, H.; Weiss, R.A.; Mittnacht, S. Cyclin encoded by KS herpesvirus. Nature 1996, 382, 410. [Google Scholar] [CrossRef] [PubMed]
- Godden-Kent, D.; Talbot, S.J.; Boshoff, C.; Chang, Y.; Moore, P.; Weiss, R.A.; Mittnacht, S. The cyclin encoded by kaposi’s sarcoma-associated herpesvirus stimulates CDK6 to phosphorylate the retinoblastoma protein and histone H1. J. Virol. 1997, 71, 4193–4198. [Google Scholar] [PubMed]
- Swanton, C.; Mann, D.J.; Fleckenstein, B.; Neipel, F.; Peters, G.; Jones, N. Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature 1997, 390, 184–187. [Google Scholar] [PubMed]
- Verschuren, E.W.; Hodgson, J.G.; Gray, J.W.; Kogan, S.; Jones, N.; Evan, G.I. The role of p53 in suppression of KSHV cyclin-induced lymphomagenesis. Cancer Res. 2004, 64, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Sarek, G.; Jarviluoma, A.; Moore, H.M.; Tojkander, S.; Vartia, S.; Biberfeld, P.; Laiho, M.; Ojala, P.M. Nucleophosmin phosphorylation by v-cyclin-CDK6 controls KSHV latency. PLoS Pathog. 2010, 6, e1000818. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, C.; Podgrabinska, S.; Skobe, M.; Ganem, D. Activation of NF-kappaB by the latent vFLIP gene of kaposi’s sarcoma-associated herpesvirus is required for the spindle shape of virus-infected endothelial cells and contributes to their proinflammatory phenotype. J. Virol. 2006, 80, 7179–7185. [Google Scholar] [CrossRef] [PubMed]
- Ballon, G.; Akar, G.; Cesarman, E. Systemic expression of kaposi sarcoma herpesvirus (KSHV) vFLIP in endothelial cells leads to a profound proinflammatory phenotype and myeloid lineage remodeling in vivo. PLoS Pathog. 2015, 11, e1004581. [Google Scholar] [CrossRef] [PubMed]
- Guasparri, I.; Keller, S.A.; Cesarman, E. KSHV vFLIP is essential for the survival of infected lymphoma cells. J. Exp. Med. 2004, 199, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Sun, Y.; Sun, R.; Rettig, M.B. Kaposi’s sarcoma-associated herpesvirus encoded vFLIP induces cellular IL-6 expression: The role of the NF-kappaB and JNK/AP1 pathways. Oncogene 2003, 22, 3371–3385. [Google Scholar] [CrossRef] [PubMed]
- Umbach, J.L.; Cullen, B.R. In-depth analysis of kaposi’s sarcoma-associated herpesvirus microRNA expression provides insights into the mammalian microRNA-processing machinery. J. Virol. 2010, 84, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Wang, C.; Li, W.; Lu, W.; Bai, Z.; Qin, D.; Yan, Q.; Zhu, J.; Krueger, B.J.; Renne, R.; et al. A KSHV microRNA directly targets g protein-coupled receptor kinase 2 to promote the migration and invasion of endothelial cells by inducing CXCR2 and activating AKT signaling. PLoS Pathog. 2015, 11, e1005171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, R.; Lin, X.; Liang, D.; Deng, Q.; Lan, K. Kaposi’s sarcoma-associated herpesvirus-encoded microRNA miR-K12–11 attenuates transforming growth factor beta signaling through suppression of SMAD5. J. Virol. 2012, 86, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Samols, M.A.; Skalsky, R.L.; Maldonado, A.M.; Riva, A.; Lopez, M.C.; Baker, H.V.; Renne, R. Identification of cellular genes targeted by KSHV-encoded microRNAs. PLoS Pathog. 2007, 3, e65. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.; Henderson, S.; Lagos, D.; Nikitenko, L.; Coulter, E.; Roberts, S.; Gratrix, F.; Plaisance, K.; Renne, R.; Bower, M.; et al. KSHV-encoded miRNAs target MAF to induce endothelial cell reprogramming. Genes Dev. 2010, 24, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Bala, K.; Bosco, R.; Gramolelli, S.; Haas, D.A.; Kati, S.; Pietrek, M.; Havemeier, A.; Yakushko, Y.; Singh, V.V.; Dittrich-Breiholz, O.; et al. Kaposi’s sarcoma herpesvirus K15 protein contributes to virus-induced angiogenesis by recruiting PLCgamma1 and activating NFAT1-dependent RCAN1 expression. PLoS Pathog. 2012, 8, e1002927. [Google Scholar] [CrossRef] [PubMed]
- Gramolelli, S.; Weidner-Glunde, M.; Abere, B.; Viejo-Borbolla, A.; Bala, K.; Ruckert, J.; Kremmer, E.; Schulz, T.F. Inhibiting the recruitment of PLCgamma1 to kaposi’s sarcoma herpesvirus K15 protein reduces the invasiveness and angiogenesis of infected endothelial cells. PLoS Pathog. 2015, 11, e1005105. [Google Scholar] [CrossRef] [PubMed]
- Steinbruck, L.; Gustems, M.; Medele, S.; Schulz, T.F.; Lutter, D.; Hammerschmidt, W. K1 and K15 of kaposi’s sarcoma-associated herpesvirus are partial functional homologues of latent membrane protein 2A of epstein-barr virus. J. Virol. 2015, 89, 7248–7261. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, M.M.; Pietrek, M.; Dittrich-Breiholz, O.; Kracht, M.; Schulz, T.F. Modulation of host gene expression by the K15 protein of kaposi’s sarcoma-associated herpesvirus. J. Virol. 2007, 81, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Havemeier, A.; Gramolelli, S.; Pietrek, M.; Jochmann, R.; Sturzl, M.; Schulz, T.F. Activation of NF-kappaB by the kaposi’s sarcoma-associated herpesvirus K15 protein involves recruitment of the NF-kappaB-inducing kinase, IkappaB kinases, and phosphorylation of p65. J. Virol. 2014, 88, 13161–13172. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, J.A.; Johnston, B.P.; McCormick, C. Viral activation of MK2-hsp27-p115RhoGEF-RhoA signaling axis causes cytoskeletal rearrangements, p-body disruption and are-mRNA stabilization. PLoS Pathog. 2015, 11, e1004597. [Google Scholar] [CrossRef] [PubMed]
- King, C.A. Kaposi’s sarcoma-associated herpesvirus kaposin B induces unique monophosphorylation of stat3 at serine 727 and MK2-mediated inactivation of the stat3 transcriptional repressor trim28. J. Virol. 2013, 87, 8779–8791. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.; Ganem, D. The kaposin b protein of KSHV activates the p38/MK2 pathway and stabilizes cytokine mRNAs. Science 2005, 307, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Kang, J.; Lee, H.N.; Aguilar, B.; Kafka, D.; Lee, S.; Choi, I.; Lee, J.; Ramu, S.; Haas, J.; et al. Kaposin-b enhances the prox1 mRNA stability during lymphatic reprogramming of vascular endothelial cells by kaposi’s sarcoma herpes virus. PLoS Pathog. 2010, 6, e1001046. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dittmer, D.P.; Tomlinson, C.C.; Fakhari, F.D.; Damania, B. Immortalization of primary endothelial cells by the K1 protein of kaposi’s sarcoma-associated herpesvirus. Cancer Res. 2006, 66, 3658–3666. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, S.; Maeng, H.; Young, D.P.; Prakash, O.; Fayad, L.E.; Younes, A.; Samaniego, F. K1 protein of human herpesvirus 8 suppresses lymphoma cell Fas-mediated apoptosis. Blood 2007, 109, 2174–2182. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Veazey, R.; Williams, K.; Li, M.; Guo, J.; Neipel, F.; Fleckenstein, B.; Lackner, A.; Desrosiers, R.C.; Jung, J.U. Deregulation of cell growth by the K1 gene of kaposi’s sarcoma-associated herpesvirus. Nat. Med. 1998, 4, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Ishido, S.; Wang, C.; Lee, B.S.; Cohen, G.B.; Jung, J.U. Downregulation of major histocompatibility complex class i molecules by kaposi’s sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 2000, 74, 5300–5309. [Google Scholar] [CrossRef] [PubMed]
- Coscoy, L.; Ganem, D. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J. Clin. Investig. 2001, 107, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Ishido, S.; Choi, J.K.; Lee, B.S.; Wang, C.; DeMaria, M.; Johnson, R.P.; Cohen, G.B.; Jung, J.U. Inhibition of natural killer cell-mediated cytotoxicity by kaposi’s sarcoma-associated herpesvirus K5 protein. Immunity 2000, 13, 365–374. [Google Scholar] [CrossRef]
- Mansouri, M.; Viswanathan, K.; Douglas, J.L.; Hines, J.; Gustin, J.; Moses, A.V.; Fruh, K. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of kaposi’s sarcoma-associated herpesvirus. J. Virol. 2009, 83, 9672–9681. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.S.; Boshoff, C.; Weiss, R.A.; Chang, Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 1996, 274, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Molden, J.; Chang, Y.; You, Y.; Moore, P.S.; Goldsmith, M.A. A kaposi’s sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J. Biol. Chem. 1997, 272, 19625–19631. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.; Neipel, F.; Fleckenstein, B.; Savino, R.; Ciliberto, G.; Kalden, J.R.; Gramatzki, M. Human herpesvirus type 8 interleukin-6 homologue is functionally active on human myeloma cells. Blood 1998, 91, 1858–1863. [Google Scholar] [PubMed]
- Cousins, E.; Gao, Y.; Sandford, G.; Nicholas, J. Human herpesvirus 8 viral interleukin-6 signaling through gp130 promotes virus replication in primary effusion lymphoma and endothelial cells. J. Virol. 2014, 88, 12167–12172. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, A.D.; Cavallin, L.E.; Vincent, L.; Chiozzini, C.; Eroles, P.; Duran, E.M.; Asgari, Z.; Hooper, A.T.; La Perle, K.M.; Hilsher, C.; et al. In vivo-restricted and reversible malignancy induced by human herpesvirus-8 KSHV: A cell and animal model of virally induced kaposi’s sarcoma. Cancer Cell 2007, 11, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Emuss, V.; Lagos, D.; Pizzey, A.; Gratrix, F.; Henderson, S.R.; Boshoff, C. KSHV manipulates notch signaling by DLL4 and JAG1 to alter cell cycle genes in lymphatic endothelia. PLoS Pathog. 2009, 5, e1000616. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, A.; Montaner, S.; Patel, V.; Gomez-Roman, J.J.; Li, Y.; Sausville, E.A.; Sawai, E.T.; Gutkind, J.S. AKT plays a central role in sarcomagenesis induced by kaposi’s sarcoma herpesvirus-encoded g protein-coupled receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 4821–4826. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Galisteo, R.; Molinolo, A.A.; Wetzker, R.; Hirsch, E.; Gutkind, J.S. PI3Kgamma mediates kaposi’s sarcoma-associated herpesvirus vGPCR-induced sarcomagenesis. Cancer Cell 2011, 19, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Rivas, C.; Thlick, A.E.; Parravicini, C.; Moore, P.S.; Chang, Y. Kaposi’s sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 2001, 75, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Wies, E.; Mori, Y.; Hahn, A.; Kremmer, E.; Sturzl, M.; Fleckenstein, B.; Neipel, F. The viral interferon-regulatory factor-3 is required for the survival of KSHV-infected primary effusion lymphoma cells. Blood 2008, 111, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.; Garcia, M.A.; Domingo-Gil, E.; Arroyo, J.; Nombela, C.; Rivas, C. The latency protein LANA2 from kaposi’s sarcoma-associated herpesvirus inhibits apoptosis induced by dsRNA-activated protein kinase but not RNAse l activation. J. Gen. Virol. 2003, 84, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Wies, E.; Hahn, A.S.; Schmidt, K.; Viebahn, C.; Rohland, N.; Lux, A.; Schellhorn, T.; Holzer, A.; Jung, J.U.; Neipel, F. The kaposi’s sarcoma-associated herpesvirus-encoded vIRF-3 inhibits cellular IRF-5. J. Biol. Chem. 2009, 284, 8525–8538. [Google Scholar] [CrossRef] [PubMed]
- Endres, M.J.; Garlisi, C.G.; Xiao, H.; Shan, L.; Hedrick, J.A. The kaposi’s sarcoma-related herpesvirus (KSHV)-encoded chemokine vMIP-I is a specific agonist for the cc chemokine receptor (CCR)8. J. Exp. Med. 1999, 189, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.S.; Grone, H.J.; Rocken, M.; Klier, C.; Gu, S.; Wank, R.; Proudfoot, A.E.; Nelson, P.J.; Weber, C. Selective recruitment of TH2-type cells and evasion from a cytotoxic immune response mediated by viral macrophage inhibitory protein-II. Eur. J. Immunol. 2001, 31, 2458–2466. [Google Scholar] [CrossRef]
- Chen, W.; Hilton, I.B.; Staudt, M.R.; Burd, C.E.; Dittmer, D.P. Distinct p53, p53:LANA, and LANA complexes in kaposi’s sarcoma—Associated herpesvirus lymphomas. J. Virol. 2010, 84, 3898–3908. [Google Scholar] [CrossRef] [PubMed]
- Radkov, S.A.; Kellam, P.; Boshoff, C. The latent nuclear antigen of kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene hras transforms primary rat cells. Nat. Med. 2000, 6, 1121–1127. [Google Scholar] [PubMed]
- Liu, J.; Martin, H.J.; Liao, G.; Hayward, S.D. The kaposi’s sarcoma-associated herpesvirus LANA protein stabilizes and activates c-Myc. J. Virol. 2007, 81, 10451–10459. [Google Scholar] [CrossRef] [PubMed]
- Paudel, N.; Sadagopan, S.; Balasubramanian, S.; Chandran, B. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen and angiogenin interact with common host proteins, including annexin A2, which is essential for survival of latently infected cells. J. Virol. 2012, 86, 1589–1607. [Google Scholar] [CrossRef] [PubMed]
- Sadagopan, S.; Valiya Veettil, M.; Paudel, N.; Bottero, V.; Chandran, B. Kaposi’s sarcoma-associated herpesvirus-induced angiogenin plays roles in latency via the phospholipase C gamma pathway: Blocking angiogenin inhibits latent gene expression and induces the lytic cycle. J. Virol. 2011, 85, 2666–2685. [Google Scholar] [CrossRef] [PubMed]
- Sharma-Walia, N.; Patel, K.; Chandran, K.; Marginean, A.; Bottero, V.; Kerur, N.; Paul, A.G. Cox-2/pge2: Molecular ambassadors of kaposi’s sarcoma-associated herpes virus oncoprotein-v-FLIP. Oncogenesis 2012, 1, e5. [Google Scholar] [CrossRef] [PubMed]
- Sturzl, M.; Hohenadl, C.; Zietz, C.; Castanos-Velez, E.; Wunderlich, A.; Ascherl, G.; Biberfeld, P.; Monini, P.; Browning, P.J.; Ensoli, B. Expression of K13/v-FLIP gene of human herpesvirus 8 and apoptosis in kaposi’s sarcoma spindle cells. J. Natl. Cancer Inst. 1999, 91, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Kwun, H.J.; da Silva, S.R.; Shah, I.M.; Blake, N.; Moore, P.S.; Chang, Y. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mimics epstein-barr virus EBNA1 immune evasion through central repeat domain effects on protein processing. J. Virol. 2007, 81, 8225–8235. [Google Scholar] [CrossRef] [PubMed]
- Zaldumbide, A.; Ossevoort, M.; Wiertz, E.J.; Hoeben, R.C. In cis inhibition of antigen processing by the latency-associated nuclear antigen I of kaposi sarcoma herpes virus. Mol. Immunol. 2007, 44, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, D.; Lin, X.; Robertson, E.S.; Lan, K. Kaposi’s sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen reduces interleukin-8 expression in endothelial cells and impairs neutrophil chemotaxis by degrading nuclear p65. J. Virol. 2011, 85, 8606–8615. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, N.; Flamand, L. Kaposi sarcoma-associated herpesvirus latency-associated nuclear antigen inhibits interferon (IFN) beta expression by competing with IFN regulatory factor-3 for binding to IFNB promoter. J. Biol. Chem. 2010, 285, 7208–7221. [Google Scholar] [CrossRef] [PubMed]
- Kwun, H.J.; da Silva, S.R.; Qin, H.; Ferris, R.L.; Tan, R.; Chang, Y.; Moore, P.S. The central repeat domain 1 of kaposi’s sarcoma-associated herpesvirus (KSHV) latency associated-nuclear antigen 1 (LANA1) prevents cis MHC class i peptide presentation. Virology 2011, 412, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Thakker, S.; Purushothaman, P.; Gupta, N.; Challa, S.; Cai, Q.; Verma, S.C. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen inhibits major histocompatibility complex class II expression by disrupting enhanceosome assembly through binding with the regulatory factor x complex. J. Virol. 2015, 89, 5536–5556. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.M.; Wang, L.; West, J.A.; Dittmer, D.P.; Damania, B. Latent kaposi’s sarcoma-associated herpesvirus infection of monocytes downregulates expression of adaptive immune response costimulatory receptors and proinflammatory cytokines. J. Virol. 2012, 86, 3916–3923. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Murakami, M.; Si, H.; Robertson, E.S. A potential alpha-helix motif in the amino terminus of LANA encoded by kaposi’s sarcoma-associated herpesvirus is critical for nuclear accumulation of HIF-1alpha in normoxia. J. Virol. 2007, 81, 10413–10423. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Lan, K.; Verma, S.C.; Si, H.; Lin, D.; Robertson, E.S. Kaposi’s sarcoma-associated herpesvirus latent protein LANA interacts with HIF-1 alpha to upregulate rta expression during hypoxia: Latency control under low oxygen conditions. J. Virol. 2006, 80, 7965–7975. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lee, H.; Yoon, D.W.; Albrecht, J.C.; Fleckenstein, B.; Neipel, F.; Jung, J.U. Kaposi’s sarcoma-associated herpesvirus encodes a functional cyclin. J. Virol. 1997, 71, 1984–1991. [Google Scholar] [PubMed]
- Godfrey, A.; Anderson, J.; Papanastasiou, A.; Takeuchi, Y.; Boshoff, C. Inhibiting primary effusion lymphoma by lentiviral vectors encoding short hairpin rna. Blood 2005, 105, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- Grundhoff, A.; Ganem, D. Mechanisms governing expression of the v-FLIP gene of kaposi’s sarcoma-associated herpesvirus. J. Virol. 2001, 75, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Bieleski, L.; Talbot, S.J. Kaposi’s sarcoma-associated herpesvirus vcyclin open reading frame contains an internal ribosome entry site. J. Virol. 2001, 75, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.; Chew, Y.P.; Fallis, L.; Freddersdorf, S.; Boshoff, C.; Weiss, R.A.; Lu, X.; Mittnacht, S. Degradation of p27(Kip) cdk inhibitor triggered by kaposi’s sarcoma virus cyclin-cdk6 complex. EMBO J. 1999, 18, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Jarviluoma, A.; Ojala, P.M. Cell signaling pathways engaged by KSHV. Biochim. Biophys. Acta 2006, 1766, 140–158. [Google Scholar] [CrossRef] [PubMed]
- Verschuren, E.W.; Jones, N.; Evan, G.I. The cell cycle and how it is steered by kaposi’s sarcoma-associated herpesvirus cyclin. J. Gen. Virol. 2004, 85, 1347–1361. [Google Scholar] [CrossRef] [PubMed]
- Bertin, J.; Armstrong, R.C.; Ottilie, S.; Martin, D.A.; Wang, Y.; Banks, S.; Wang, G.H.; Senkevich, T.G.; Alnemri, E.S.; Moss, B.; et al. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc. Natl. Acad. Sci. USA 1997, 94, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Vincenz, C.; Buller, M.; Dixit, V.M. A novel family of viral death effector domain-containing molecules that inhibit both CD-95- and tumor necrosis factor receptor-1-induced apoptosis. J. Biol. Chem. 1997, 272, 9621–9624. [Google Scholar] [CrossRef] [PubMed]
- Thome, M.; Schneider, P.; Hofmann, K.; Fickenscher, H.; Meinl, E.; Neipel, F.; Mattmann, C.; Burns, K.; Bodmer, J.L.; Schroter, M.; et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 1997, 386, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.M.; Jasmin, A.; Eby, M.T.; Hood, L. Modulation of the NF-kappaB pathway by virally encoded death effector domains-containing proteins. Oncogene 1999, 18, 5738–5746. [Google Scholar] [CrossRef] [PubMed]
- Chugh, P.; Matta, H.; Schamus, S.; Zachariah, S.; Kumar, A.; Richardson, J.A.; Smith, A.L.; Chaudhary, P.M. Constitutive NF-kappaB activation, normal Fas-induced apoptosis, and increased incidence of lymphoma in human herpes virus 8 K13 transgenic mice. Proc. Natl. Acad. Sci. USA 2005, 102, 12885–12890. [Google Scholar] [CrossRef] [PubMed]
- Matta, H.; Chaudhary, P.M. Activation of alternative NF-kappaB pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (vFLIP). Proc. Natl. Acad. Sci. USA 2004, 101, 9399–9404. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.; Dutia, B.; Rhind, S.; Stewart, J.P.; Talbot, S.J. Expression in a recombinant murid herpesvirus 4 reveals the in vivo transforming potential of the K1 open reading frame of kaposi’s sarcoma-associated herpesvirus. J. Virol. 2004, 78, 8878–8884. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, S.; Sewer, A.; Lagos-Quintana, M.; Sheridan, R.; Sander, C.; Grasser, F.A.; van Dyk, L.F.; Ho, C.K.; Shuman, S.; Chien, M.; et al. Identification of microRNAs of the herpesvirus family. Nat. Methods 2005, 2, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Samols, M.A.; Hu, J.; Skalsky, R.L.; Renne, R. Cloning and identification of a microRNA cluster within the latency-associated region of kaposi’s sarcoma-associated herpesvirus. J. Virol. 2005, 79, 9301–9305. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; Pu, X.M.; Zhao, Z.F.; Zhao, Y.N.; Kang, X.J.; Wu, W.D.; Zou, Y.M.; Wu, C.Y.; Qu, Y.Y.; Zhang, D.Z.; et al. The expression profiles of microRNAs in kaposi’s sarcoma. Tumour Biol. 2015, 36, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Lu, S.; Zhang, Z.; Gonzalez, C.M.; Damania, B.; Cullen, B.R. Kaposi’s sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc. Natl. Acad. Sci. USA 2005, 102, 5570–5575. [Google Scholar] [CrossRef] [PubMed]
- Catrina Ene, A.M.; Borze, I.; Guled, M.; Costache, M.; Leen, G.; Sajin, M.; Ionica, E.; Chitu, A.; Knuutila, S. MicroRNA expression profiles in kaposi’s sarcoma. Pathol. Oncol. Res. 2014, 20, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Viollet, C.; Davis, D.A.; Reczko, M.; Ziegelbauer, J.M.; Pezzella, F.; Ragoussis, J.; Yarchoan, R. Next-generation sequencing analysis reveals differential expression profiles of miRNA-mRNA target pairs in KSHV-infected cells. PLoS ONE 2015, 10, e0126439. [Google Scholar] [CrossRef] [PubMed]
- Chugh, P.E.; Sin, S.H.; Ozgur, S.; Henry, D.H.; Menezes, P.; Griffith, J.; Eron, J.J.; Damania, B.; Dittmer, D.P. Systemically circulating viral and tumor-derived microRNAs in KSHV-associated malignancies. PLoS Pathog. 2013, 9, e1003484. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.R.; Kara, M.; Coleman, C.B.; Grau, K.R.; Oko, L.M.; Krueger, B.J.; Renne, R.; van Dyk, L.F.; Tibbetts, S.A. Virus-encoded microRNAs facilitate gammaherpesvirus latency and pathogenesis in vivo. MBio 2014, 5, e00981–14. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, A.M.; Das, S.; Xiao, Z.; Andresson, T.; Kieffer-Kwon, P.; Happel, C.; Ziegelbauer, J. Proteomic screening of human targets of viral microRNAs reveals functions associated with immune evasion and angiogenesis. PLoS Pathog. 2013, 9, e1003584. [Google Scholar] [CrossRef] [PubMed]
- Forte, E.; Raja, A.N.; Shamulailatpam, P.; Manzano, M.; Schipma, M.J.; Casey, J.L.; Gottwein, E. MicroRNA-mediated transformation by the kaposi’s sarcoma-associated herpesvirus kaposin locus. J. Virol. 2015, 89, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, D.; Happel, C.; Ziegelbauer, J.M. Kaposi’s sarcoma-associated herpesvirus microRNAs repress breakpoint cluster region protein expression, enhance RAC1 activity, and increase in vitro angiogenesis. J. Virol. 2015, 89, 4249–4261. [Google Scholar] [CrossRef] [PubMed]
- Quinn, S.R.; O’Neill, L.A. The role of microRNAs in the control and mechanism of action of IL-10. Curr. Top. Microbiol. Immunol. 2014, 380, 145–155. [Google Scholar] [PubMed]
- Yoo, J.; Lee, H.N.; Choi, I.; Choi, D.; Chung, H.K.; Kim, K.E.; Lee, S.; Aguilar, B.; Kang, J.; Park, E.; et al. Opposing regulation of PROX1 by interleukin-3 receptor and notch directs differential host cell fate reprogramming by kaposi sarcoma herpes virus. PLoS Pathog. 2012, 8, e1002770. [Google Scholar] [CrossRef] [PubMed]
- Pyakurel, P.; Pak, F.; Mwakigonja, A.R.; Kaaya, E.; Heiden, T.; Biberfeld, P. Lymphatic and vascular origin of kaposi’s sarcoma spindle cells during tumor development. Int. J. Cancer 2006, 119, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Lee, B.S.; Shim, S.N.; Li, M.; Jung, J.U. Identification of the novel K15 gene at the rightmost end of the kaposi’s sarcoma-associated herpesvirus genome. J. Virol. 2000, 74, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.V.; Wang, H.W.; Koumi, A.; Hollyman, D.; Endo, Y.; Ye, H.; Du, M.Q.; Boshoff, C. K15 protein of kaposi’s sarcoma-associated herpesvirus is latently expressed and binds to HAX-1, a protein with antiapoptotic function. J. Virol. 2002, 76, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.H.; Wu, M.F.; Wu, Y.H.; Chang, S.J.; Lin, S.F.; Sharp, T.V.; Wang, H.W. The M type K15 protein of kaposi’s sarcoma-associated herpesvirus regulates microRNA expression via its SH2-binding motif to induce cell migration and invasion. J. Virol. 2009, 83, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, M.M.; Glenn, M.; Rainbow, L.; Kieser, A.; Henke-Gendo, C.; Schulz, T.F. Activation of mitogen-activated protein kinase and nf-kappab pathways by a kaposi’s sarcoma-associated herpesvirus K15 membrane protein. J. Virol. 2003, 77, 9346–9358. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Choi, Y.K.; Choi, J.K. Multi-transmembrane protein K15 of kaposi’s sarcoma-associated herpesvirus targets Lyn kinase in the membrane raft and induces NFAT/AP1 activities. Exp. Mol. Med. 2008, 40, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.B.; Nicholas, J. Autocrine and paracrine promotion of cell survival and virus replication by human herpesvirus 8 chemokines. J. Virol. 2008, 82, 6501–6513. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Brinkmann, M.M.; Pietrek, M.; Ottinger, M.; Dittrich-Breiholz, O.; Kracht, M.; Schulz, T.F. Functional characterization of the M-type K15-encoded membrane protein of kaposi’s sarcoma-associated herpesvirus. J. Gen. Virol. 2007, 88, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Lee, S.H.; Feng, P.; Chang, H.; Cho, N.H.; Jung, J.U. Characterization of the kaposi’s sarcoma-associated herpesvirus K1 signalosome. J. Virol. 2005, 79, 12173–12184. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Swamy, O.R.; Peng, X.; Tang, Z.Y.; Li, L.; Larson, J.E.; Cohen, J.C.; Gill, J.; Farr, G.; Wang, S.; et al. Activation of src kinase Lyn by the kaposi sarcoma-associated herpesvirus K1 protein: Implications for lymphomagenesis. Blood 2005, 105, 3987–3994. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Liu, Z.Y.; Anand, A.R.; Zhang, X.; Brown, L.F.; Dezube, B.J.; Gill, P.; Ganju, R.K. Alpha-chemokine-mediated signal transduction in human kaposi’s sarcoma spindle cells. Biochim. Biophys. Acta 2004, 1691, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Tang, Z.Y.; Peng, X.; Coleman, R.; Gill, J.; Farr, G.; Samaniego, F. Tumorigenesis and aberrant signaling in transgenic mice expressing the human herpesvirus-8 K1 gene. J. Natl. Cancer Inst. 2002, 94, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Alvarez, X.; Ishido, S.; Lackner, A.A.; Jung, J.U. Inhibition of intracellular transport of B cell antigen receptor complexes by kaposi’s sarcoma-associated herpesvirus K1. J. Exp. Med. 2000, 192, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Hu, M.; Hao, T.; Li, W.; Xue, X.; Xue, M.; Zhu, X.; Zhou, F.; Qin, D.; Yan, Q.; et al. MiRNA-891a-5p mediates HIV-1 tat and KSHV ORF-K1 synergistic induction of angiogenesis by activating NF-kappaB signaling. Nucleic Acids Res. 2015, 43, 9362–9378. [Google Scholar] [CrossRef] [PubMed]
- Lagunoff, M.; Lukac, D.M.; Ganem, D. Immunoreceptor tyrosine-based activation motif-dependent signaling by kaposi’s sarcoma-associated herpesvirus K1 protein: Effects on lytic viral replication. J. Virol. 2001, 75, 5891–5898. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Guo, J.; Li, M.; Choi, J.K.; DeMaria, M.; Rosenzweig, M.; Jung, J.U. Identification of an immunoreceptor tyrosine-based activation motif of K1 transforming protein of kaposi’s sarcoma-associated herpesvirus. Mol. Cell Biol. 1998, 18, 5219–5228. [Google Scholar] [CrossRef] [PubMed]
- Rezza, G.; Andreoni, M.; Dorrucci, M.; Pezzotti, P.; Monini, P.; Zerboni, R.; Salassa, B.; Colangeli, V.; Sarmati, L.; Nicastri, E.; et al. Human herpesvirus 8 seropositivity and risk of kaposi’s sarcoma and other acquired immunodeficiency syndrome-related diseases. J. Natl. Cancer Inst. 1999, 91, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Tosato, G. Pathogenesis and manifestations of human herpesvirus-8-associated disorders. Semin. Hematol. 2003, 40, 143–153. [Google Scholar] [CrossRef]
- Xue, M.; Yao, S.; Hu, M.; Li, W.; Hao, T.; Zhou, F.; Zhu, X.; Lu, H.; Qin, D.; Yan, Q.; et al. HIV-1 NEF and KSHV oncogene K1 synergistically promote angiogenesis by inducing cellular miR-718 to regulate the PTEN/AKT/mTOR signaling pathway. Nucleic Acids Res. 2014, 42, 9862–9879. [Google Scholar] [CrossRef] [PubMed]
- Coscoy, L.; Sanchez, D.J.; Ganem, D. A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J. Cell Biol. 2001, 155, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Coscoy, L.; Ganem, D. Kaposi’s sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl. Acad. Sci. USA 2000, 97, 8051–8056. [Google Scholar] [CrossRef] [PubMed]
- Brulois, K.; Jung, J.U. Interplay between kaposi’s sarcoma-associated herpesvirus and the innate immune system. Cytokine Growth Factor Rev. 2014, 25, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Manes, T.D.; Hoer, S.; Muller, W.A.; Lehner, P.J.; Pober, J.S. Kaposi’s sarcoma-associated herpesvirus K3 and K5 proteins block distinct steps in transendothelial migration of effector memory CD4+ T cells by targeting different endothelial proteins. J. Immunol. 2010, 184, 5186–5192. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.; Douglas, J.; Rose, P.P.; Gouveia, K.; Thomas, G.; Means, R.E.; Moses, A.V.; Fruh, K. Kaposi sarcoma herpesvirus K5 removes CD31/pecam from endothelial cells. Blood 2006, 108, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.; Rose, P.P.; Moses, A.V.; Fruh, K. Remodeling of endothelial adherens junctions by kaposi’s sarcoma-associated herpesvirus. J. Virol. 2008, 82, 9615–9628. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Means, R.; Lang, S.; Jung, J.U. Downregulation of gamma interferon receptor 1 by kaposi’s sarcoma-associated herpesvirus K3 and K5. J. Virol. 2007, 81, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Lang, S.M.; Means, R.E. The march family E3 ubiquitin ligase K5 alters monocyte metabolism and proliferation through receptor tyrosine kinase modulation. PLoS Pathog. 2011, 7, e1001331. [Google Scholar] [CrossRef] [PubMed]
- Neipel, F.; Albrecht, J.C.; Ensser, A.; Huang, Y.Q.; Li, J.J.; Friedman-Kien, A.E.; Fleckenstein, B. Human herpesvirus 8 encodes a homolog of interleukin-6. J. Virol. 1997, 71, 839–842. [Google Scholar] [PubMed]
- Nicholas, J.; Ruvolo, V.R.; Burns, W.H.; Sandford, G.; Wan, X.; Ciufo, D.; Hendrickson, S.B.; Guo, H.G.; Hayward, G.S.; Reitz, M.S. Kaposi’s sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat. Med. 1997, 3, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Guo, Y.; Yao, S.; Yan, Q.; Xue, M.; Hao, T.; Zhou, F.; Zhu, J.; Qin, D.; Lu, C. Synergy between kaposi’s sarcoma-associated herpesvirus (KSHV) VIL-6 and HIV-1 nef protein in promotion of angiogenesis and oncogenesis: Role of the AKT signaling pathway. Oncogene 2014, 33, 1986–1996. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Chauhan, D.; Teoh, G.; Raje, N.; Treon, S.P.; Tai, Y.T.; Shima, Y.; Anderson, K.C. Characterization of signaling cascades triggered by human interleukin-6 versus kaposi’s sarcoma-associated herpes virus-encoded viral interleukin 6. Clin. Cancer Res. 2000, 6, 1180–1189. [Google Scholar] [PubMed]
- Osborne, J.; Moore, P.S.; Chang, Y. KSHV-encoded viral IL-6 activates multiple human IL-6 signaling pathways. Hum. Immunol. 1999, 60, 921–927. [Google Scholar] [CrossRef]
- Sun, R.; Lin, S.F.; Staskus, K.; Gradoville, L.; Grogan, E.; Haase, A.; Miller, G. Kinetics of kaposi’s sarcoma-associated herpesvirus gene expression. J. Virol. 1999, 73, 2232–2242. [Google Scholar] [PubMed]
- Mesri, E.A.; Cesarman, E.; Boshoff, C. Kaposi’s sarcoma and its associated herpesvirus. Nat. Rev. Cancer 2010, 10, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Tosato, G. Role of vascular endothelial growth factor/vascular permeability factor in the pathogenesis of kaposi’s sarcoma-associated herpesvirus-infected primary effusion lymphomas. Blood 1999, 94, 4247–4254. [Google Scholar] [PubMed]
- Ensoli, B.; Sturzl, M. Kaposi’s sarcoma: A result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev. 1998, 9, 63–83. [Google Scholar] [CrossRef]
- Guo, H.G.; Browning, P.; Nicholas, J.; Hayward, G.S.; Tschachler, E.; Jiang, Y.W.; Sadowska, M.; Raffeld, M.; Colombini, S.; Gallo, R.C.; et al. Characterization of a chemokine receptor-related gene in human herpesvirus 8 and its expression in kaposi’s sarcoma. Virology 1997, 228, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, L.; Geras-Raaka, E.; Varma, A.; Gershengorn, M.C.; Cesarman, E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 1997, 385, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, A.; Montaner, S.; Gutkind, J.S. Does dysregulated expression of a deregulated viral GPCR trigger kaposi’s sarcomagenesis? FASEB J. 2004, 18, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Cesarman, E.; Nador, R.G.; Bai, F.; Bohenzky, R.A.; Russo, J.J.; Moore, P.S.; Chang, Y.; Knowles, D.M. Kaposi’s sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in kaposi’s sarcoma and malignant lymphoma. J. Virol. 1996, 70, 8218–8223. [Google Scholar] [PubMed]
- Cannon, M. The KSHV and other human herpesviral G protein-coupled receptors. Curr. Top. Microbiol. Immunol. 2007, 312, 137–156. [Google Scholar] [PubMed]
- De Munnik, S.M.; Smit, M.J.; Leurs, R.; Vischer, H.F. Modulation of cellular signaling by herpesvirus-encoded G protein-coupled receptors. Front. Pharmacol. 2015, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Morris, C.A.; Zhuo, Y.; Shelby, B.D.; Levy, D.R.; Lasky, J.A. Activation of prommp-2 and SRC by HHV8 vGPCR in human pulmonary arterial endothelial cells. J. Mol. Cell. Cardiol. 2007, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; Barnard, S.; Blackbourn, D.J.; Davison, A.J. Transcription mapping of human herpesvirus 8 genes encoding viral interferon regulatory factors. J. Gen. Virol. 2003, 84, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Jenner, R.G.; Alba, M.M.; Boshoff, C.; Kellam, P. Kaposi’s sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J. Virol. 2001, 75, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Fontela, C.; Collado, M.; Rodriguez, E.; Garcia, M.A.; Alvarez-Barrientos, A.; Arroyo, J.; Nombela, C.; Rivas, C. Identification of a nuclear export signal in the KSHV latent protein LANA2 mediating its export from the nucleus. Exp. Cell Res. 2005, 311, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Lubyova, B.; Kellum, M.J.; Frisancho, J.A.; Pitha, P.M. Stimulation of c-Myc transcriptional activity by vIRF-3 of kaposi sarcoma-associated herpesvirus. J. Biol. Chem. 2007, 282, 31944–31953. [Google Scholar] [CrossRef] [PubMed]
- Baresova, P.; Musilova, J.; Pitha, P.M.; Lubyova, B. P53 tumor suppressor protein stability and transcriptional activity are targeted by kaposi’s sarcoma-associated herpesvirus-encoded viral interferon regulatory factor 3. Mol. Cell Biol. 2014, 34, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Villar, L.; Lopitz-Otsoa, F.; Gallego, P.; Munoz-Fontela, C.; Gonzalez-Santamaria, J.; Campagna, M.; Shou-Jiang, G.; Rodriguez, M.S.; Rivas, C. Kaposi’s sarcoma-associated herpesvirus protein LANA2 disrupts pml oncogenic domains and inhibits PML-mediated transcriptional repression of the survivin gene. J. Virol. 2009, 83, 8849–8858. [Google Scholar] [CrossRef] [PubMed]
- Haque, N.S.; Fallon, J.T.; Taubman, M.B.; Harpel, P.C. The chemokine receptor CCR8 mediates human endothelial cell chemotaxis induced by I-309 and kaposi sarcoma herpesvirus-encoded vMIP-I and by lipoprotein(a)-stimulated endothelial cell conditioned medium. Blood 2001, 97, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Pekkonen, P.; Jarviluoma, A.; Zinovkina, N.; Cvrljevic, A.; Prakash, S.; Westermarck, J.; Evan, G.I.; Cesarman, E.; Verschuren, E.W.; Ojala, P.M. KSHV viral cyclin interferes with T-cell development and induces lymphoma through CDK6 and notch activation in vivo. Cell Cycle 2014, 13, 3670–3684. [Google Scholar] [CrossRef] [PubMed]
- Zhi, H.; Zahoor, M.A.; Shudofsky, A.M.; Giam, C.Z. KSHV vcyclin counters the senescence/G1 arrest response triggered by NF-kappaB hyperactivation. Oncogene 2015, 34, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Eklund, L.; Bry, M.; Alitalo, K. Mouse models for studying angiogenesis and lymphangiogenesis in cancer. Mol. Oncol. 2013, 7, 259–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Kang, G.; Kumar, P.; Lu, W.; Li, Y.; Zhou, Y.; Li, Q.; Wood, C. Humanized-blt mouse model of kaposi’s sarcoma-associated herpesvirus infection. Proc. Natl. Acad. Sci. USA 2014, 111, 3146–3151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, L.; Lu, X.; Feldman, E.R.; Keyes, L.R.; Wang, Y.; Fan, H.; Feng, H.; Xia, Z.; Sun, J.; et al. Recombinant murine gamma herpesvirus 68 carrying KSHV G protein-coupled receptor induces angiogenic lesions in mice. PLoS Pathog. 2015, 11, e1005001. [Google Scholar] [CrossRef] [PubMed]
- Berkova, Z.; Wang, S.; Sehgal, L.; Patel, K.P.; Prakash, O.; Samaniego, F. Lymphoid hyperplasia and lymphoma in KSHV K1 transgenic mice. Histol. Histopathol. 2015, 30, 559–568. [Google Scholar] [PubMed]
- Ojala, P.M.; Schulz, T.F. Manipulation of endothelial cells by KSHV: Implications for angiogenesis and aberrant vascular differentiation. Semin. Cancer Biol. 2014, 26, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ashlock, B.M.; Ma, Q.; Issac, B.; Mesri, E.A. Productively infected murine kaposi’s sarcoma-like tumors define new animal models for studying and targeting KSHV oncogenesis and replication. PLoS ONE 2014, 9, e87324. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- De Falco, S. Antiangiogenesis therapy: An update after the first decade. Korean J. Intern. Med. 2014, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Uehara, T.; Osawa, M.; Fukumoto, H.; Mine, S.; Ueda, K.; Hasegawa, H.; Katano, H. Fumagillin, a potent angiogenesis inhibitor, induces kaposi sarcoma-associated herpesvirus replication in primary effusion lymphoma cells. Biochem. Biophys. Res. Commun. 2015, 463, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.R.; Mohan, R.; Crews, C.M. The antiangiogenic agent TNP-470 requires p53 and p21CIP/WAF for endothelial cell growth arrest. Proc. Natl. Acad. Sci. USA 2000, 97, 12782–12787. [Google Scholar] [CrossRef] [PubMed]
- Ardavanis, A.; Doufexis, D.; Kountourakis, P.; Rigatos, G. A kaposi’s sarcoma complete clinical response after sorafenib administration. Ann. Oncol. 2008, 19, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Uldrick, T.S.; Wyvill, K.M.; Kumar, P.; O’Mahony, D.; Bernstein, W.; Aleman, K.; Polizzotto, M.N.; Steinberg, S.M.; Pittaluga, S.; Marshall, V.; et al. Phase ii study of bevacizumab in patients with hiv-associated kaposi’s sarcoma receiving antiretroviral therapt. J. Clin Oncol. 2012, 30, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Krown, S.E.; Roy, D.; Lee, J.Y.; Dezube, B.J.; Reid, E.G.; Venkataramanan, R.; Han, K.; Cesarman, E.; Dittmer, D.P. Rapamycin with antiretroviral therapy in aids-associated kaposi sarcoma: An aids malignancy consortium study. J. Acquir. Immune Defic. Syndr. 2012, 59, 447–454. [Google Scholar] [CrossRef] [PubMed]
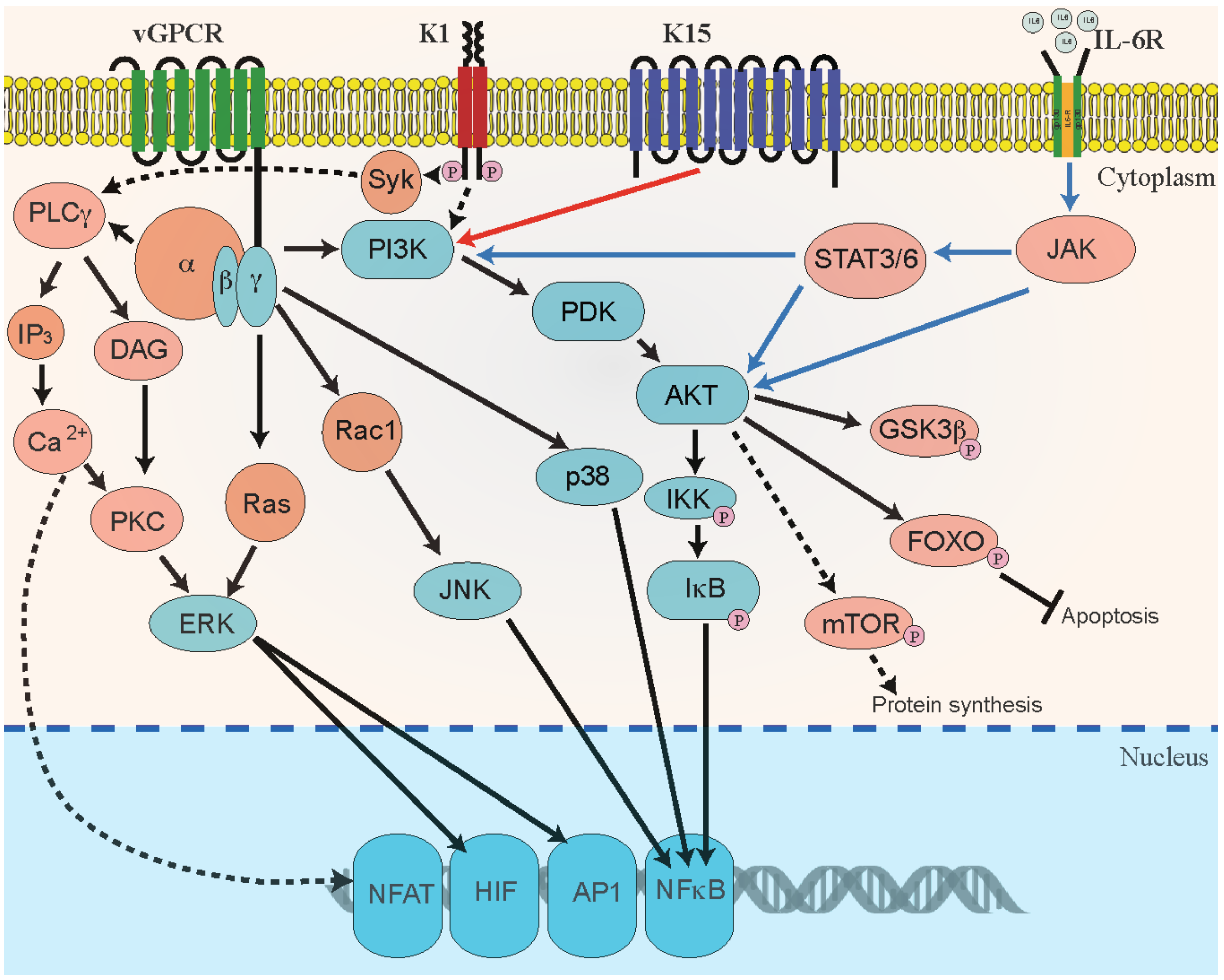
| KSHV Gene | KSHV Protein | Function | Reference |
|---|---|---|---|
| LANA | Latency Associated Nuclear Antigen | Apart from KSHV genome persistence, it inhibits p53, pRB and extends the lifespan of latently infected cells | [125,126,127,128,129] |
| vCYC | Homologue of cellular cyclin D | Primarily regulates cell cycle and promotes oncogene-induced senescence | [130,131,132,133,134] |
| vFLIP | Homologue of FLICE inhibitory protein | Regulates activation of NF-κB and apoptosis. Additionally may contribute to PEL survival and spindle cell formation | [53,135,136,137,138] |
| miRNA | Micro RNAs | Contributes to B cell expansion and transformation of rat mesenchymal precursor cells. Downregulates TGFβ signaling and MAF transcription factor. Contributes to cell proliferation and angiogenesis | [139,140,141,142,143] |
| K15 | Viral membrane protein | Induce cell proliferation and angiogenesis. Activates cellular signaling pathways to induces various pro-survival and paracrine-mediated pro-angiogenic cellular cytokines and chemokines, including IL6, IL8, CXCL3, and Cox2 | [144,145,146,147,148] |
| Kaposin B | Kaposin | Regulates cell signaling and reprogramming of vascular endothelial cells | [149,150,151,152] |
| K1 | Variable ITAM-Containing Protein (VIP) | Activates cellular signaling pathways and induces angiogenesis | [60,113,146,153,154,155] |
| K5 | Modulator of immune recognition (MIR2) | Viral E3 ligases capable of ubiquitinating MHC-I, ICAM-1, B7-2, Tetherin (CD317/BST2) | [156,157,158,159] |
| vIL6 | Viral Interleukin-6 | Homologues of cellular IL-6. Activate JAK/STAT, MAPK, and PI3K/Akt signaling to induce VEGF pathways to regulate B-cell proliferation | [55,160,161,162,163] |
| vGPCR | Viral G-protein-coupled receptor (vGPCR) | Homologue of cellular IL-8 receptor. vGPCR activates cellular signaling and induce secretion of proinflammatory cytokines and angiogenic growth factors contributing to angioproliferative tumors | [107,112,164,165,166,167] |
| vIRF3 | Viral interferon regulatory factor-3 | Homologues of cellular interferon: Inhibitor of IFN1, p53, NFκB RelA, and p300. Activates HIF-1α and VEGF | [73,168,169,170,171] |
| vCCL | Viral CC-Chemokine Ligands (vCCLs) | Homologues of cellular chemokines: viral CC-chemokine ligand 1 (vCCL1, (vMIP1), vCCL2 (vMIP2), and vCCL3 (vMIP3), respectively. Modulates signaling through chemokine receptors to promote cell proliferation and angiogenesis | [56,57,172,173] |
| Glycoprotein B | Glycoprotein | Activates VEGF secretion | [17,74] |
| K8.1 | Glycoprotein | Activates VEGF secretion | [65,74] |
| Name of the Drug | Manufacturer | Target | Efficacy | Reference |
|---|---|---|---|---|
| Imatinib | (Gleevec, Novartis, Basel, Switzerland) | An inhibitor of tyrosine kinases such as Abl, PDGFR and c-kit. | Treatment with imatinib resulted in partial regression of KS tumors in about one-third AIDS-KS patients on combination retroviral therapy | [278] |
| Sorafenib | (Nexavar, Bayer Healthcare Pharmaceuticals, West Haven, CT, USA) | A small molecule inhibitor of tyrosine-kinases that inhibits VEGFR, PDGFR, FGFR, c-kit, Raf and stem cell factor receptor. | The use of Sorafenib prevented brain metastasis progression and led to unexpected complete remission in a patient with cardiovascular risk and classical KS | [279] |
| Bevacizumab | (Avastin, Genentech, San Francisco, CA, USA) | Anti-VEGF-A monoclonal antibody that binds to VEGF and neutralizes its action. | The drug induced complete and partial remission of HIV-KS lesions in 3/16 and 2/16 patients respectively, while receiving highly active antiretroviral therapy (HAART) | [280] |
| Sirolimus | (Rapamune, Pfizer Inc., New York, NY, USA) | Mammalian target of rapamycin (mTOR) inhibitor. | Sirolimus inhibited the progression of dermal KS lesions in kidney-transplant patients being treated with calcineurin inhibitors | [281] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purushothaman, P.; Uppal, T.; Sarkar, R.; Verma, S.C. KSHV-Mediated Angiogenesis in Tumor Progression. Viruses 2016, 8, 198. https://doi.org/10.3390/v8070198
Purushothaman P, Uppal T, Sarkar R, Verma SC. KSHV-Mediated Angiogenesis in Tumor Progression. Viruses. 2016; 8(7):198. https://doi.org/10.3390/v8070198
Chicago/Turabian StylePurushothaman, Pravinkumar, Timsy Uppal, Roni Sarkar, and Subhash C. Verma. 2016. "KSHV-Mediated Angiogenesis in Tumor Progression" Viruses 8, no. 7: 198. https://doi.org/10.3390/v8070198
APA StylePurushothaman, P., Uppal, T., Sarkar, R., & Verma, S. C. (2016). KSHV-Mediated Angiogenesis in Tumor Progression. Viruses, 8(7), 198. https://doi.org/10.3390/v8070198







