Pandemic Influenza A (H1N1) Virus Infection Increases Apoptosis and HIV-1 Replication in HIV-1 Infected Jurkat Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture and HIV-1 Infection
2.3. Pandemic Influenza A (H1N1) Virus Infection
2.4. Real-Time PCR
2.5. Caspase-3 Assay
2.6. Cell Viability Assay
2.7. Subcellular Protein Fractionation
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
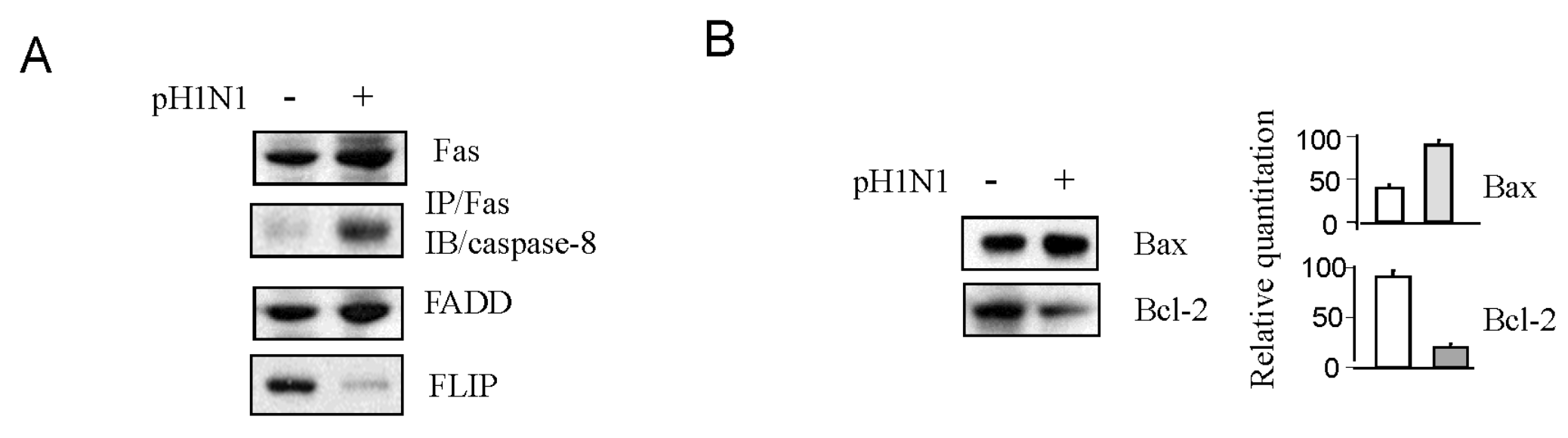
3.1. Pandemic 2009 Influenza A (H1N1) Virus (pH1N1) Infection Induction of Apoptotic Death in HIV-1 Infected Cells
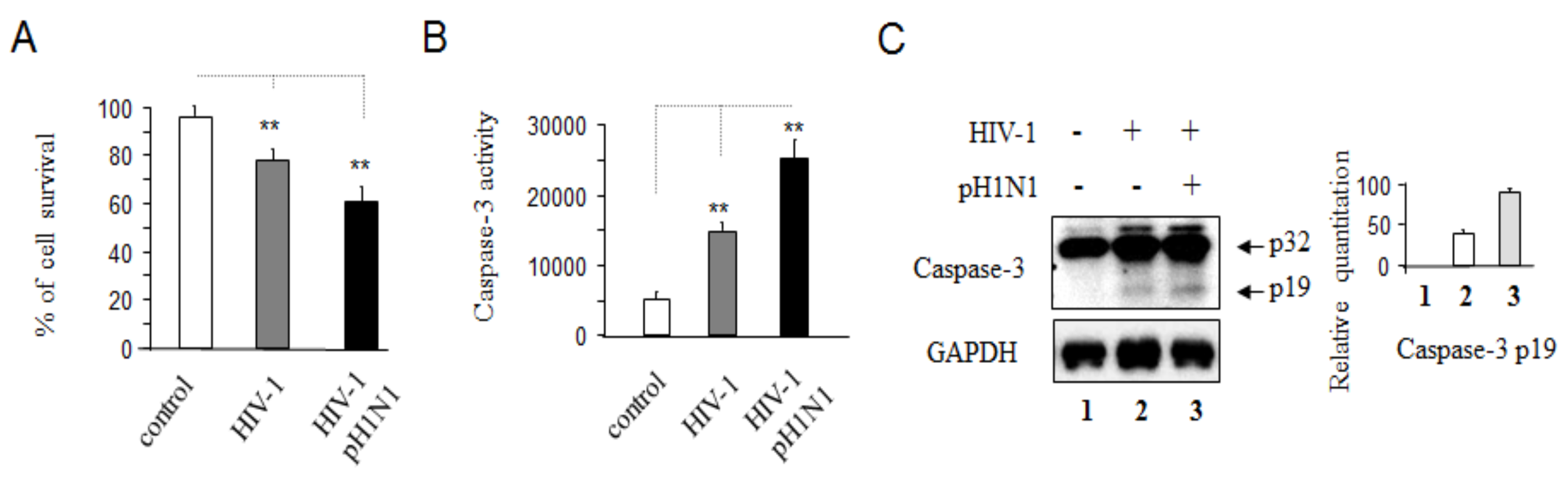
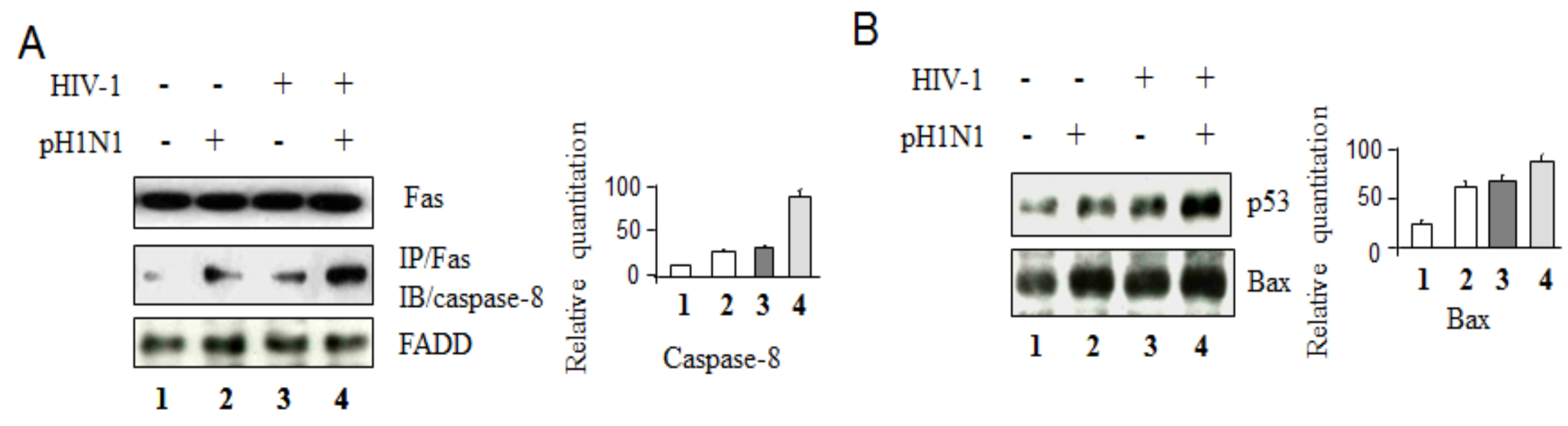
3.2. Pandemic Influenza A (H1N1) Virus (pH1N1) Infection Could Activate Both Apoptotic Pathways
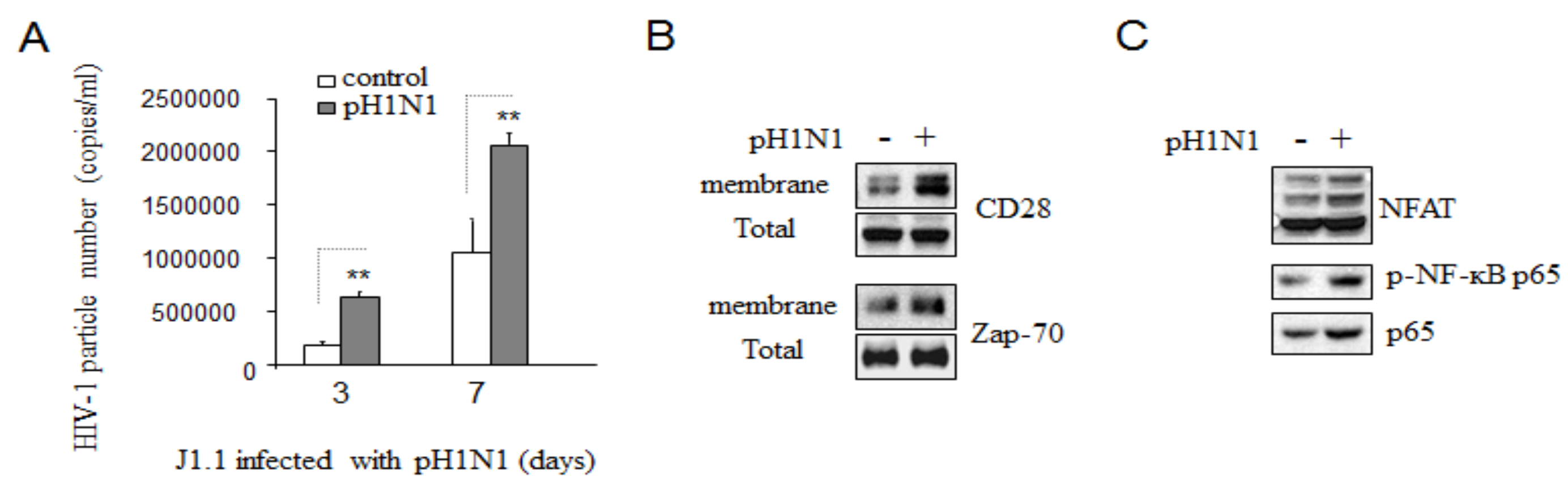
3.3. Pandemic Influenza A (H1N1) Infection Enhanced HIV-1 Replication
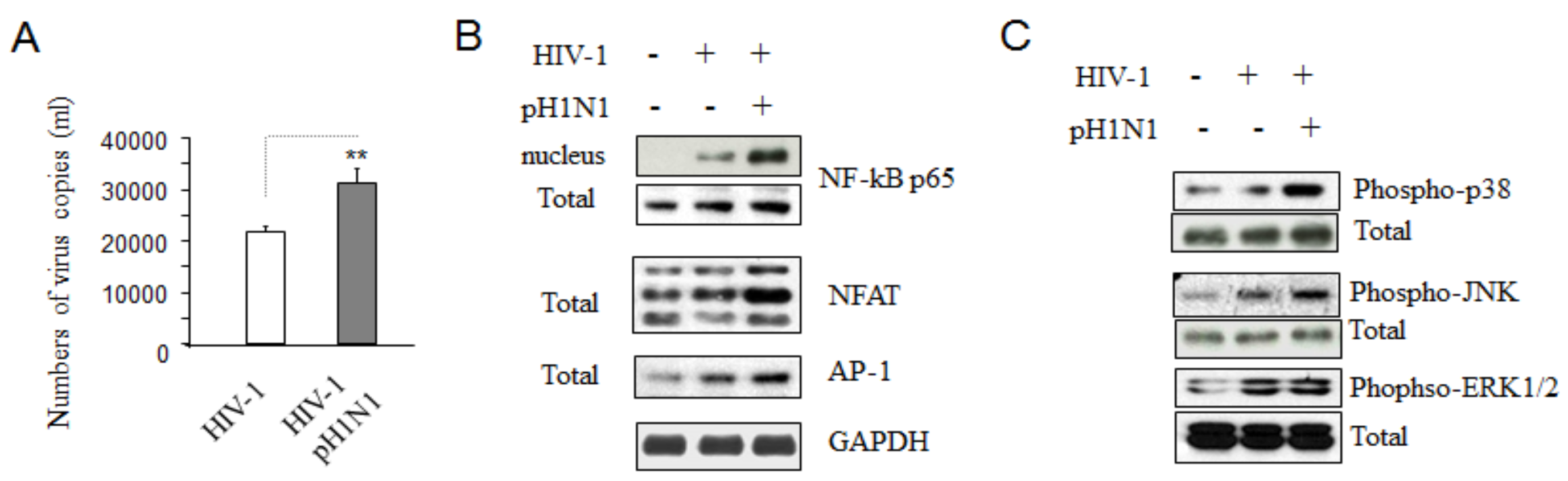
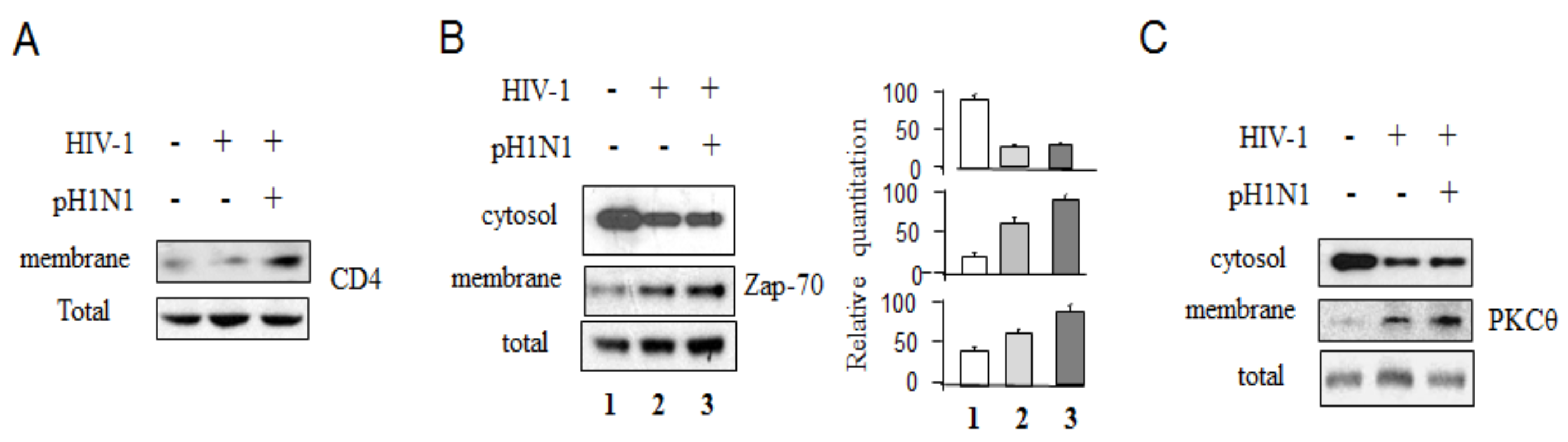
3.4. Pandemic Influenza A (H1N1) Infection Activated TCR Signaling in HIV-1 Infected Cells
3.5. Pandemic Influenza A (H1N1) Infection Reactivated HIV-1 Replication in Latently Infected Cells
4. Discussion
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interests
References
- HIV/AIDS. WHO. Available online: http://www.who.int/mediacentre/factsheets/fs360/en/ (accessed on 19 July 2014).
- Stimating Seasonal Influenza-Associated Deaths in the United States: CDC Study Confirms Variability of Flu. CDC. Available online: http://www.cdc.gov/flu/about/disease/us_flu-related_deaths.htm (accessed on 18 March 2015).
- Kumar, A.; Zarychanski, R.; Pinto, R.; Cook, D.J.; Marshall, J.; Lacroix, J.; Stelfox, T.; Bagshaw, S.; Choong, K.; Lamontagne, F.; et al. Canadian Critical Care Trials Group H1N1 Collaborative. Critically ill patients with 2009 influenza A (H1N1) infection in Canada. JAMA 2009, 302, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Louie, J.K.; Acosta, M.; Winter, K.; Jean, C.; Gavali, S.; Schechter, R.; Vugia, D.; Harriman, K.; Matyas, B.; Glaser, C.A.; et al. California Pandemic (H1N1) Working Group. Factors associated with death or hospitalization due to pandemic 2009 influenza A (H1N1) infection in California. JAMA 2009, 302, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Ormsby, C.E.; de la Rosa-Zamboni, D.; Vázquez-Pérez, J.; Ablanedo-Terrazas, Y.; Vega-Barrientos, R.; Gómez-Palacio, M.; Murakami-Ogasawara, A.; Ibarra-Ávalos, J.A.; Romero-Rodríguez, D.; Avila-Ríos, S.; et al. Severe 2009 pandemic influenza A (H1N1) infection and increased mortality in patients with late and advanced HIV disease. AIDS 2011, 25, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.; Simonsen, L.; Sample, J.; Kang, J.W.; Miller, M.; Madhi, S.A.; Campsmith, M.; Viboud, C. Influenza-related mortality among adults aged 25–54 years with AIDS in South Africa and the United States of America. Clin. Infect. Dis. 2012, 55, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Tse, H.; To, K.K.W.; Wen, X.; Chen, H.; Chan, K.H.; Tsoi, H.W.; Li, I.W.; Yuen, K.Y. Clinical and virological factors associated with viremia in pandemic influenza A/H1N1/2009 virus infection. PLoS ONE 2011, 6, e22534. [Google Scholar] [CrossRef] [PubMed]
- Oughton, M.; Dascal, A.; Laporta, D.; Charest, H.; Afilalo, M.; Miller, M. Evidence of viremia in 2 cases of severe pandemic influenza A H1N1/09. Diagn. Microbiol. Infect. Dis. 2011, 70, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Xie, H.; Campbell, A.P.; Kuypers, J.; Leisenring, W.; Boudreault, A.A.; Englund, J.A.; Corey, L.; Boeckh, M. Influenza viral RNA detection in blood as a marker to predict disease severity in hematopoietic cell transplant recipients. J Infect. Dis. 2012, 206, 1872–1877. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zoueva, O.; Zhao, J.; Ye, Z.; Hewlett, I. Stability and infectivity of novel pandemic influenza A (H1N1) virus in blood-derived matrices under different storage conditions. BMC Infect. Dis. 2011, 11, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tan, J.; Zhao, J.; Ye, Z.; Hewlett, I. Highly pathogenic avian influenza A virus (H5N1) can be transmitted in ferrets by transfusion. BMC Infect. Dis. 2014, 14, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Thitithanyanont, A.; Engering, A.; Ekchariyawat, P.; Wiboon-ut, S.; Limsalakpetch, A.; Yongvanitchit, K.; Kum-Arb, U.; Kanchongkittiphon, W.; Utaisincharoen, P.; Sirisinha, S.; et al. High susceptibility of human dendritic cells to avian influenza H5N1 virus infection and protection by IFN-alpha and TLR ligands. J. Immunol. 2007, 179, 5220–5227. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Kobelt, D.; Steinkasserer, A.; Menke, A.; Hobom, G.; Behrends, U.; Bornkamm, G.W.; Mautner, J. Efficient generation and expansion of antigen-specific CD4+ T cells by recombinant influenza viruses. Eur. J. Immunol. 2003, 33, 3331–3341. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Dudziak, D.; Dirmeier, U.; Hobom, G.; Riedel, A.; Schlee, M.; Staudt, L.M.; Rosenwald, A.; Behrends, U.; Bornkamm, G.W.; et al. Active NF-kappaB signalling is a prerequisite for influenza virus infection. J. Gen. Virol. 2004, 85, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Mohl, J.; Joshi, A. HIV-1 induced bystander apoptosis. Viruses 2012, 4, 3020–3043. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Barbeau, B.; Sato, S.; Boivin, G.; Goyette, N.; Tremblay, M.J. Syncytium formation and HIV-1 replication are both accentuated by purified influenza and virus-associated neuraminidase. J. Biol. Chem. 2002, 277, 9825–9833. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Barbeau, B.; Sato, S.; Tremblay, M.J. Neuraminidase from a bacterial source enhances both HIV-1-mediated syncytium formation and the virus binding/entry process. Virology 2001, 284, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tan, J.; Zoueva, O.; Zhao, J.; Ye, Z.; Hewlett, I. Novel pandemic influenza A (H1N1) virus infection modulates apoptotic pathways that impact its replication in A549 cells. Microbes Infect. 2014, 16, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Montecalvo, A.; Kane, L.P. Regulation of NF-κB induction by TCR/CD28. Immuno. Res. 2011, 50, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Farrow, M.A.; Kim, E.Y.; Wolinsky, S.M.; Sheehy, A.M. NFAT and IRF proteins regulate transcription of the anti-HIV gene, APOBEC3G. J. Biol. Chem. 2011, 286, 2567–2577. [Google Scholar] [CrossRef] [PubMed]
- Copeland, K.F. Modulation of HIV-1 transcription by cytokines and chemokines. Mini. Rev. Med. Chem. 2005, 5, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.S.; Sang, W.W.; Ruan, Z.; Wang, Y.O. Akt/Nox2/NF-κB signaling pathway is involved in Tat-induced HIV-1 long terminal repeat (LTR) transactivation. Chin. Med. J. 2010, 123, 2440–2445. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ragupathy, V.; Zhao, J.; Hewlett, I. Molecules from apoptotic pathways modulate HIV-1 replication in Jurkat cells. Biochem. Biophys. Res. Commun. 2011, 414, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wang, X.; Devadas, K.; Zhao, J.; Zhang, P.; Hewlett, I. Some mechanisms of FLIP expression in inhibition of HIV-1 replication in Jurkat cells, CD4+ T cells and PBMCs. J. Cell. Physiol. 2013, 228, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Ganser-Pornillos, B.K.; Yeager, M.; Pornillos, O. Assembly and architecture of HIV. Adv. Exp. Med. Biol. 2012, 726, 441–465. [Google Scholar] [PubMed]
- Moss, J.A. HIV/AIDS Review. Radiol. Technol. 2013, 84, 247–267. [Google Scholar] [PubMed]
- Luo, C.; Wang, K.; Liu, D.; Li, Y.; Zhao, Q. The Functional Roles of Lipid Rafts in T Cell Activation, Immune Diseases and HIV Infection and Prevention. Cell. Mol. Immun. 2008, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pietiäinen, V.M.; Marjomäki, V.; Heino, J.; Hyypiä, T. Viral entry, lipid rafts and caveosomes. Ann. Med. 2005, 37, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Hiscott, J.; Kwon, H.; Genin, P. Hostile takeovers: Viral appropriation of the NF-kB pathway. J. Clin. Investig. 2001, 107, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Chun, T.W.; Fauci, A.S. HIV reservoirs: Pathogenesis and obstacles to viral eradication and cure. AIDS 2012, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Perez-Padilla, R.; de la Rosa-Zamboni, D.; Ponce de Leon, S.; Hernandez, M.; Quinones-Falconi, F.; Bautista, E.; Ramirez-Venegas, A.; Rojas-Serrano, J.; Ormsby, C.E.; Corrales, A.; et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N. Engl. J. Med. 2009, 361, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Cherit, G.; Lapinsky, S.; Macias, A.; Pinto, R.; Espinosa-Perez, L.; de la Torre, A.; Poblano-Morales, M.; Baltazar-Torres, J.; Bautista, E.; Martinez, A.; et al. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA 2009, 302, 1880–1887. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, V.S.; Olsen, C.W.; Dybdahl-Sissoko, N.; Evans, D. Apoptosis: A mechanism of cell killing by influenza A and B viruses. J. Virol. 1994, 68, 3667–3673. [Google Scholar]
- Tumpey, T.M.; Lu, X.; Morken, T.; Zaki, S.R.; Katz, J.M. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J. Virol. 2000, 74, 6105–6116. [Google Scholar] [CrossRef] [PubMed]
- Riera, M.; Payeras, A.; Marcos, M.A.; Viasus, D.; Farinas, M.C.; Segura, F.; Torre-Cisneros, J.; Martín-Quirós, A.; Rodríguez-Baño, J.; Vila, J.; et al. Clinical presentation and prognosis of the 2009 H1N1 influenza A infection in HIV-1-infected patients: A Spanish multicenter study. AIDS 2010, 24, 2461–2467. [Google Scholar] [CrossRef] [PubMed]
- Feiterna-Sperling, C.; Edelmann, A.; Nickel, R.; Magdorf, K.; Bergmann, F.; Rautenberg, P.; Schweiger, B.; Wahn, V.; Krüger, D.H.; Hofmann, J. Pandemic influenza A (H1N1) outbreak among 15 school-aged HIV-1-infected children. Clin. Infect. Dis. 2010, 51, e90–e94. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, G.; Rezza, G.; Fois, A.G.; Naitana, A.G.; Piredda, G.; Pirina, P.; Mura, M.S. Acute respiratory distress syndrome due to influenza virus A/H1N1v in a patient with HIV/HCV co-infection. Int. J. STD AIDS 2011, 22, 234–235. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.; Marcos, M.A.; Hoyo-Ulloa, I.; Antón, A.; Sánchez, M.; Vilella, A.; Larrousse, M.; Pérez, I.; Moreno, A.; Trilla, A.; et al. Influenza A H1N1 in HIV-infected adults. HIV Med. 2011, 12, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Neff-LaFord, H.; Teske, S.; Bushnell, T.P.; Lawrence, B.P. Aryl hydrocarbon receptor activation during influenza virus infection unveils a novel pathway of IFN-gamma production by phagocytic cells. J. Immunol. 2007, 179, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Rockx, B.; Baas, T.; Zornetzer, G.A.; Haagmans, B.; Sheahan, T.; Frieman, M.; Dyer, M.D.; Teal, T.H.; Proll, S.; van den Brand, J.; et al. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J. Virol. 2009, 83, 7062–7074. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.W.; Yuen, K.M.; Yu, W.C.; Ho, C.C.; Nicholls, J.M.; Peiris, J.S.; Chan, M.C. Influenza H5N1 and H1N1 virus replication and innate immune responses in bronchial epithelial cells are influenced by the state of differentiation. PLoS ONE 2010, 5, e8713. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Jia, Y.; Wang, S.; Li, H.; Wu, D.; Wang, G.; Chen, J.L. Role of Itk signalling in the interaction between influenza A virus and T-cells. J. Gen. Virol. 2012, 93, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, M.; Hatta, Y.; Kawaoka, Y.; Malherbe, L.P. Antigen signal strength during priming determines effector CD4 T cell function and antigen sensitivity during influenza virus challenge. J. Immunol. 2014, 193, 2812–2820. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Tan, J.; Biswas, S.; Zhao, J.; Devadas, K.; Ye, Z.; Hewlett, I. Pandemic Influenza A (H1N1) Virus Infection Increases Apoptosis and HIV-1 Replication in HIV-1 Infected Jurkat Cells. Viruses 2016, 8, 33. https://doi.org/10.3390/v8020033
Wang X, Tan J, Biswas S, Zhao J, Devadas K, Ye Z, Hewlett I. Pandemic Influenza A (H1N1) Virus Infection Increases Apoptosis and HIV-1 Replication in HIV-1 Infected Jurkat Cells. Viruses. 2016; 8(2):33. https://doi.org/10.3390/v8020033
Chicago/Turabian StyleWang, Xue, Jiying Tan, Santanu Biswas, Jiangqin Zhao, Krishnakumar Devadas, Zhiping Ye, and Indira Hewlett. 2016. "Pandemic Influenza A (H1N1) Virus Infection Increases Apoptosis and HIV-1 Replication in HIV-1 Infected Jurkat Cells" Viruses 8, no. 2: 33. https://doi.org/10.3390/v8020033
APA StyleWang, X., Tan, J., Biswas, S., Zhao, J., Devadas, K., Ye, Z., & Hewlett, I. (2016). Pandemic Influenza A (H1N1) Virus Infection Increases Apoptosis and HIV-1 Replication in HIV-1 Infected Jurkat Cells. Viruses, 8(2), 33. https://doi.org/10.3390/v8020033





