Regulation of the Wnt/β-Catenin Signaling Pathway by Human Papillomavirus E6 and E7 Oncoproteins
Abstract
:1. Introduction
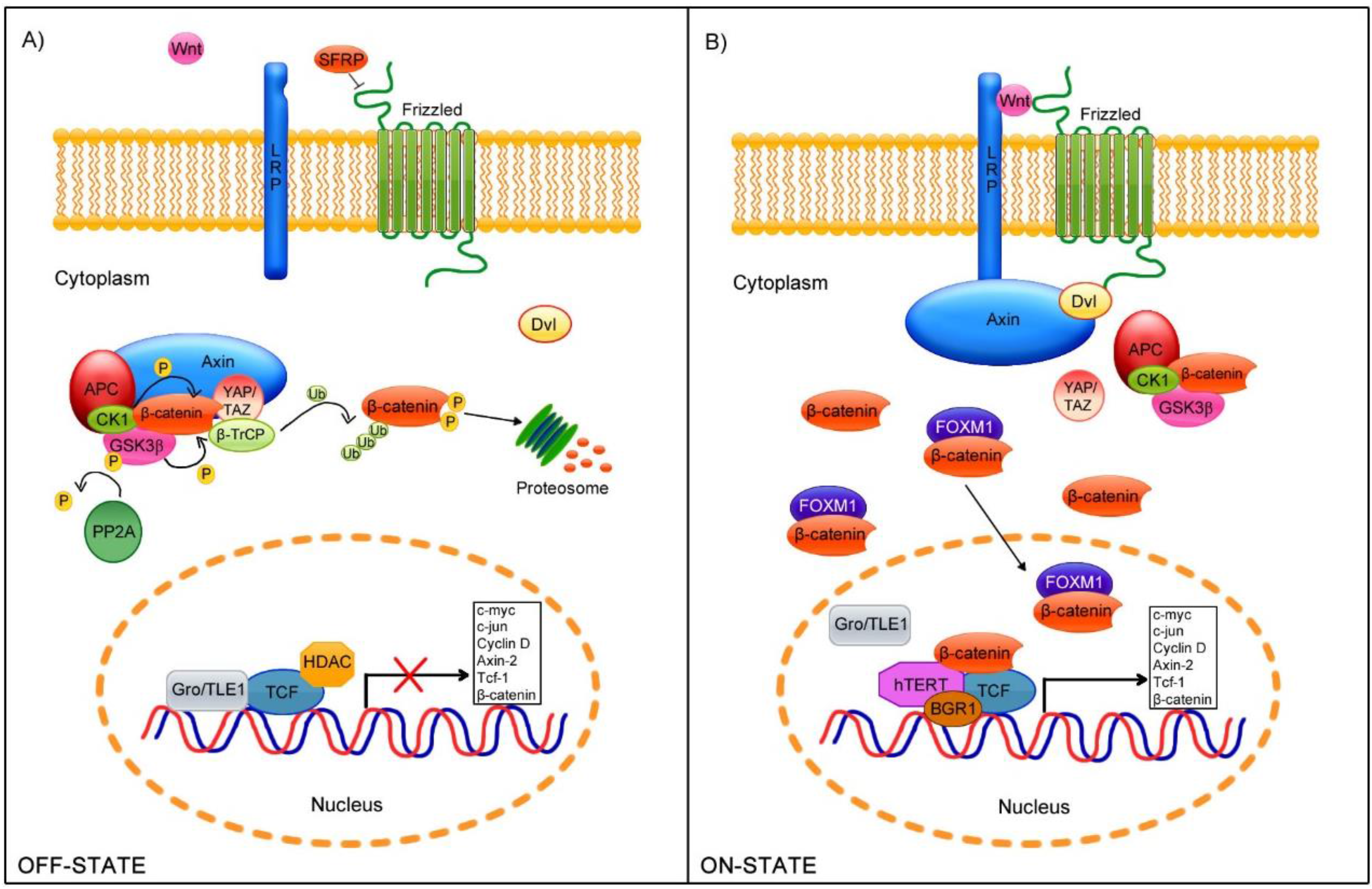
2. Wnt/β-Catenin Cell Signaling Pathway
3. Human Papillomavirus
HPV Genome
4. E6 and E7 in Cellular Transformation
| E6-Interactions | Biological Effects |
|---|---|
| Protein PDZ-domain | Degradation of proteins harboring PDZ domains, with a loss of cell architecture and polarity [93]. |
| E6AP | Degradation of targets such as p53 [83]. Activation of hTERT transcription, inducing immortalization [94]. |
| Bak, FADD Procaspase 8 | Induction of respective protein degradation, suppressing apoptosis [95,96]. |
| BRCA1 | Activation of estrogen receptor ER signaling pathway [97]. |
| Tyk2 | Impairment of Tyk2 activation thereby inhibiting IFN-induced signaling [98]. |
| CBP/p300 | Down-regulation of p53 activity by targeting the transcriptional coactivator CBP [99]. |
| NFX1-91 | Degradation of NFX1-91 and activation of hTERT [100]. |
| c-Myc | Increased hTERT gene expression [101]. |
| Dvl2 | β-catenin stabilization and Wnt signaling activation [102]. |
| E7-Interactions | Biological effects |
| pRb family proteins | Disruption of pRb-E2F complexes thereby initiating the E2F-mediated transcription [103]. |
| AP1 | Transactivation of members of AP1 family [104]. |
| Cyclin A/CDK2 | Regulation of cell cycle [105]. |
| Cyclin E/CDK2 | Regulation of cell cycle (binding through p107) [106]. |
| p21 | Inactivation of p21, modulating CDK and PCNA inhibitory functions [107]. |
| MPP2 | Enhancement of MPP2-specific transcriptional activity [108]. |
| p600 | Contribution to anchorage-independent growth and transformation [109]. |
| Mi2 | Complexes with HDAC to promote the E2F2-mediated transcription [110]. |
| IRF1 | Abrogation of transactivation function of IRF1 [111]. |
| p48 | Down-regulation of IFN α-mediated signal transduction [112]. |
| p27 | Abolishment of p27’s cell cycle inhibitory function, which endows the cell with invasive properties [113]. |
| PP2A | Inhibition of PP2A catalytic activity [114]. |
5. Wnt/β-Catenin Signaling in HPV-Related Cancers
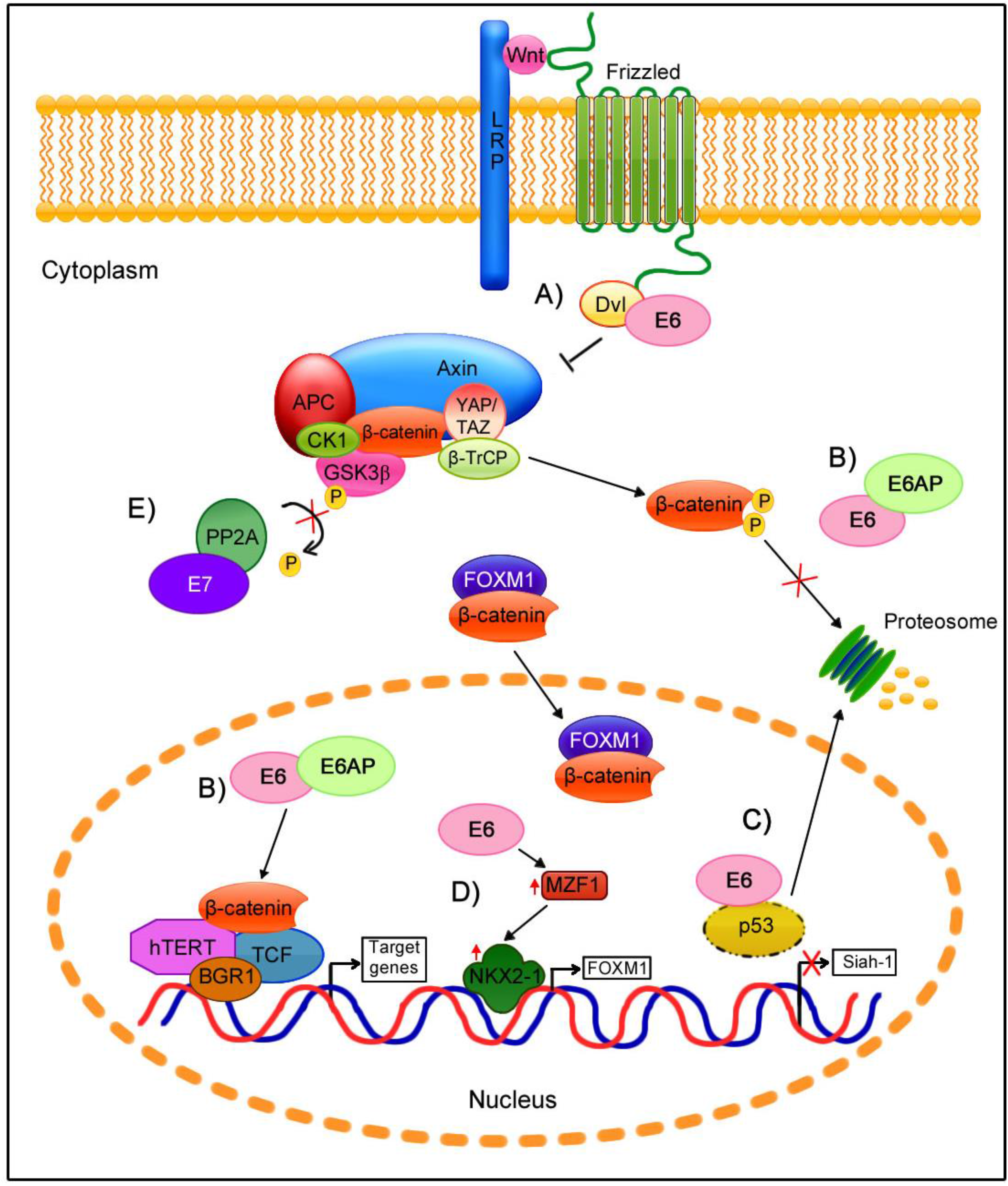
6. Wnt/β-Catenin Cell Signaling Regulation by E6 and E7 Oncoproteins
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, C.H.; Ji, T.; Chen, C.F.; Hoang, B.H. Wnt signaling in osteosarcoma. Adv. Exp. Med. Biol. 2014, 804, 33–45. [Google Scholar] [PubMed]
- Takigawa, Y.; Brown, A.M. Wnt signaling in liver cancer. Curr. Drug Targets 2008, 9, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Krausova, M.; Korinek, V. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal. 2014, 26, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Khramtsov, A.I.; Khramtsova, G.F.; Tretiakova, M.; Huo, D.; Olopade, O.I.; Goss, K.H. Wnt/β-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am. J. Pathol. 2010, 176, 2911–2920. [Google Scholar] [CrossRef] [PubMed]
- Rampias, T.; Boutati, E.; Pectasides, E.; Sasaki, C.; Kountourakis, P.; Weinberger, P.; Psyrri, A. Activation of Wnt signaling pathway by human papillomavirus E6 and E7 oncogenes in HPV16-positive oropharyngeal squamous carcinoma cells. Mol. Cancer Res. 2010, 8, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sastre, M.A.; Gonzalez-Maya, L.; Delgado, R.; Lizano, M.; Tsubaki, G.; Mohar, A.; Garcia-Carranca, A. Abnormal distribution of E-cadherin and β-catenin in different histologic types of cancer of the uterine cervix. Gynecol. Oncol. 2005, 97, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available online: http://globocan.iarc.fr (accessed on 6 May 2015).
- Zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Kreimer, A.R.; Viscidi, R.; Pawlita, M.; Fakhry, C.; Koch, W.M.; Westra, W.H.; Gillison, M.L. Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 2007, 356, 1944–1956. [Google Scholar] [CrossRef] [PubMed]
- Daling, J.R.; Madeleine, M.M.; Johnson, L.G.; Schwartz, S.M.; Shera, K.A.; Wurscher, M.A.; Carter, J.J.; Porter, P.L.; Galloway, D.A.; McDougall, J.K. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer 2004, 101, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Bilir, B.; Kucuk, O.; Moreno, C.S. Wnt signaling blockage inhibits cell proliferation and migration, and induces apoptosis in triple-negative breast cancer cells. J. Transl. Med. 2013, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yang, L.; Chang, H.; Dai, G.; Wang, F.; Duan, X.; Guo, L.; Zhang, Y.; Chen, G. Wnt/β-catenin signaling regulates the proliferation and differentiation of mesenchymal progenitor cells through the p53 pathway. PLoS ONE 2014, 9, e97283. [Google Scholar] [CrossRef] [PubMed]
- Gradl, D.; Kuhl, M.; Wedlich, D. The Wnt/Wg signal transducer β-catenin controls fibronectin expression. Mol. Cell. Biol. 1999, 19, 5576–5587. [Google Scholar] [PubMed]
- Dollar, G.L.; Weber, U.; Mlodzik, M.; Sokol, S.Y. Regulation of Lethal giant larvae by Dishevelled. Nature 2005, 437, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Brown, A.; Papkoff, J.; Scambler, P.; Shackleford, G.; McMahon, A.; Moon, R.; Varmus, H. A new nomenclature for int-1 and related genes: The Wnt gene family. Cell 1991, 64, 231. [Google Scholar] [CrossRef]
- Nusse, R.; Varmus, H.E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 1982, 31, 99–109. [Google Scholar] [CrossRef]
- Van Ooyen, A.; Nusse, R. Structure and nucleotide sequence of the putative mammary oncogene int-1; proviral insertions leave the protein-encoding domain intact. Cell 1984, 39, 233–240. [Google Scholar] [CrossRef]
- Rijsewijk, F.; Schuermann, M.; Wagenaar, E.; Parren, P.; Weigel, D.; Nusse, R. The drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell 1987, 50, 649–657. [Google Scholar] [CrossRef]
- The Wnt Homepage. Available online: http://web.stanford.edu/group/nusselab/cgi-bin/wnt/ (accessed on 6 May 2015).
- Bafico, A.; Gazit, A.; Wu-Morgan, S.S.; Yaniv, A.; Aaronson, S.A. Characterization of Wnt-1 and Wnt-2 induced growth alterations and signaling pathways in NIH3T3 fibroblasts. Oncogene 1998, 16, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Julius, M.A.; Giarre, M.; Zheng, Z.; Brown, A.M.; Kitajewski, J. Transformation by Wnt family proteins correlates with regulation of β-catenin. Cell Growth Differ. 1997, 8, 1349–1358. [Google Scholar] [PubMed]
- Ohira, T.; Gemmill, R.M.; Ferguson, K.; Kusy, S.; Roche, J.; Brambilla, E.; Zeng, C.; Baron, A.; Bemis, L.; Erickson, P.; et al. WNT7a induces E-cadherin in lung cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 10429–10434. [Google Scholar] [CrossRef] [PubMed]
- Fagotto, F.; Guger, K.; Gumbiner, B.M. Induction of the primary dorsalizing center in Xenopus by the Wnt/GSK/β-catenin signaling pathway, but not by Vg1, Activin or Noggin. Development 1997, 124, 453–460. [Google Scholar] [PubMed]
- Ye, J.; Yang, T.; Guo, H.; Tang, Y.; Deng, F.; Li, Y.; Xing, Y.; Yang, L.; Yang, K. Wnt10b promotes differentiation of mouse hair follicle melanocytes. Int. J. Med. Sci. 2013, 10, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Zhang, K.; Ye, J.X.; Lian, X.H.; Yang, T. Wnt10b promotes growth of hair follicles via a canonical Wnt signalling pathway. Clin. Exp. Dermatol. 2011, 36, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Louis, I.; Heinonen, K.M.; Chagraoui, J.; Vainio, S.; Sauvageau, G.; Perreault, C. The signaling protein Wnt4 enhances thymopoiesis and expands multipotent hematopoietic progenitors through β-catenin-independent signaling. Immunity 2008, 29, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Chen, S.; Cai, S.; Dong, L.; Liu, L.; Yang, Y.; Guo, F.; Lu, X.; He, H.; Chen, Q.; et al. Wnt5a through noncanonical Wnt/JNK or Wnt/PKC signaling contributes to the differentiation of mesenchymal stem cells into type II alveolar epithelial cells in vitro. PLoS ONE 2014, 9, e90229. [Google Scholar] [CrossRef] [PubMed]
- Slusarski, D.C.; Corces, V.G.; Moon, R.T. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 1997, 390, 410–413. [Google Scholar] [PubMed]
- Flaherty, M.P.; Dawn, B. Noncanonical Wnt11 signaling and cardiomyogenic differentiation. Trends Cardiovasc. Med. 2008, 18, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Kilander, M.B.; Dahlstrom, J.; Schulte, G. Assessment of Frizzled 6 membrane mobility by FRAP supports G protein coupling and reveals WNT-Frizzled selectivity. Cell Signal. 2014, 26, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Caddy, J.; Wilanowski, T.; Darido, C.; Dworkin, S.; Ting, S.B.; Zhao, Q.; Rank, G.; Auden, A.; Srivastava, S.; Papenfuss, T.A.; et al. Epidermal wound repair is regulated by the planar cell polarity signaling pathway. Dev. Cell 2010, 19, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, J.N.; Damrau, C.; Paudyal, A.; Bogani, D.; Wells, S.; Greene, N.D.; Stanier, P.; Copp, A.J. Genetic interactions between planar cell polarity genes cause diverse neural tube defects in mice. Dis. Models Mech. 2014, 7, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Babayeva, S.; Zilber, Y.; Torban, E. Planar cell polarity pathway regulates actin rearrangement, cell shape, motility, and nephrin distribution in podocytes. Am. J. Physiol. Ren. Physiol. 2011, 300, F549–F560. [Google Scholar] [CrossRef] [PubMed]
- Boutin, C.; Labedan, P.; Dimidschstein, J.; Richard, F.; Cremer, H.; Andre, P.; Yang, Y.; Montcouquiol, M.; Goffinet, A.M.; Tissir, F. A dual role for planar cell polarity genes in ciliated cells. Proc. Natl. Acad. Sci. USA 2014, 111, E3129–E3138. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, N.; Iioka, H.; Miyakoshi, A.; Ueno, N. PKCδ is essential for Dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev. 2003, 17, 1663–1676. [Google Scholar] [CrossRef] [PubMed]
- Fanto, M.; Weber, U.; Strutt, D.I.; Mlodzik, M. Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr. Biol. 2000, 10, 979–988. [Google Scholar] [CrossRef]
- Kuhl, M.; Sheldahl, L.C.; Malbon, C.C.; Moon, R.T. Ca2+/Calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J. Biol. Chem. 2000, 275, 12701–12711. [Google Scholar] [CrossRef] [PubMed]
- Sheldahl, L.C.; Park, M.; Malbon, C.C.; Moon, R.T. Protein Kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr. Biol. 1999, 9, 695–698. [Google Scholar] [CrossRef]
- Kuhl, M.; Geis, K.; Sheldahl, L.C.; Pukrop, T.; Moon, R.T.; Wedlich, D. Antagonistic regulation of convergent extension movements in Xenopus by Wnt/β-catenin and Wnt/Ca2+ signaling. Mech. Dev. 2001, 106, 61–76. [Google Scholar] [CrossRef]
- Okamoto, K.; Narayanan, R.; Lee, S.H.; Murata, K.; Hayashi, Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc. Natl. Acad. Sci. USA 2007, 104, 6418–6423. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Symes, A.J.; Kane, C.A.; Freeman, A.; Nariculam, J.; Munson, P.; Thrasivoulou, C.; Masters, J.R.; Ahmed, A. A novel role for Wnt/Ca2+ signaling in actin cytoskeleton remodeling and cell motility in prostate cancer. PLoS ONE 2010, 5, e10456. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Schaer, D.J.; Bachli, E.B.; Kurrer, M.O.; Schoedon, G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Kishida, S.; Yamamoto, H.; Murai, H.; Koyama, S.; Kikuchi, A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998, 17, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Sobrado, P.; Jedlicki, A.; Bustos, V.H.; Allende, C.C.; Allende, J.E. Basic region of residues 228–231 of protein kinase CK1α is involved in its interaction with axin: Binding to axin does not affect the kinase activity. J. Cell. Biochem. 2005, 94, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Papkoff, J.; Rubinfeld, B.; Schryver, B.; Polakis, P. Wnt-1 regulates free pools of catenins and stabilizes APC-catenin complexes. Mol. Cell. Biol. 1996, 16, 2128–2134. [Google Scholar] [PubMed]
- Azzolin, L.; Panciera, T.; Soligo, S.; Enzo, E.; Bicciato, S.; Dupont, S.; Bresolin, S.; Frasson, C.; Basso, G.; Guzzardo, V.; et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 2014, 158, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Semenov, M.; Han, C.; Baeg, G.H.; Tan, Y.; Zhang, Z.; Lin, X.; He, X. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002, 108, 837–847. [Google Scholar] [CrossRef]
- Sakanaka, C. Phosphorylation and regulation of β-catenin by casein kinase Iε. J. Biochem. 2002, 132, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Fu, C.; Ishikawa, S.; Stella, A.; Kojima, M.; Shitoh, K.; Schreiber, E.M.; Day, B.W.; Liu, B. APC is essential for targeting phosphorylated β-catenin to the SCFβ-TrCP ubiquitin ligase. Mol. Cell 2008, 32, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.T.; Strack, P.; Beer-Romero, P.; Chu, C.Y.; Elledge, S.J.; Harper, J.W. The SCFβ-TrCP—Ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 1999, 13, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Cong, F.; Schweizer, L.; Varmus, H. Wnt signals across the plasma membrane to activate the β-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development 2004, 131, 5103–5115. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Galceran, J.; Grosschedl, R. Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 signaling and association with β-catenin. Mol. Cell. Biol. 1998, 18, 4807–4818. [Google Scholar] [PubMed]
- Schneider, S.; Steinbeisser, H.; Warga, R.M.; Hausen, P. β-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech. Dev. 1996, 57, 191–198. [Google Scholar] [CrossRef]
- Tauriello, D.V.; Jordens, I.; Kirchner, K.; Slootstra, J.W.; Kruitwagen, T.; Bouwman, B.A.; Noutsou, M.; Rudiger, S.G.; Schwamborn, K.; Schambony, A.; et al. Wnt/β-catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled. Proc. Natl. Acad. Sci. USA 2012, 109, E812–E820. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Wang, J.; Liu, B.; Pan, W.; Farr, G.H., 3rd; Flynn, C.; Yuan, H.; Takada, S.; Kimelman, D.; Li, L.; et al. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell 2001, 7, 801–809. [Google Scholar] [CrossRef]
- Davidson, G.; Wu, W.; Shen, J.; Bilic, J.; Fenger, U.; Stannek, P.; Glinka, A.; Niehrs, C. Casein kinase 1 γ couples Wnt receptor activation to cytoplasmic signal transduction. Nature 2005, 438, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.; Huang, S. FoxM1 and Wnt/β-catenin signaling in glioma stem cells. Cancer Res. 2012, 72, 5658–5662. [Google Scholar] [CrossRef] [PubMed]
- Korinek, V.; Barker, N.; Willert, K.; Molenaar, M.; Roose, J.; Wagenaar, G.; Markman, M.; Lamers, W.; Destree, O.; Clevers, H. Two members of the Tcf family implicated in Wnt/β-catenin signaling during embryogenesis in the mouse. Mol. Cell. Biol. 1998, 18, 1248–1256. [Google Scholar] [PubMed]
- Daniels, D.L.; Weis, W.I. β-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat. Struct. Mol. Biol. 2005, 12, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, C.; Duan, X.; Ren, F.; Li, S.; Jin, Z.; Wang, Y.; Feng, Y.; Liu, Z.; Chang, Z. CREPT/RPRD1B, a recently identified novel protein highly expressed in tumors, enhances the β-catenin·TCF4 transcriptional activity in response to Wnt signaling. J. Biol. Chem. 2014, 289, 22589–22599. [Google Scholar] [CrossRef] [PubMed]
- Labalette, C.; Renard, C.A.; Neuveut, C.; Buendia, M.A.; Wei, Y. Interaction and functional cooperation between the LIM protein FHL2, CBP/p300, and β-catenin. Mol. Cell. Biol. 2004, 24, 10689–10702. [Google Scholar] [CrossRef] [PubMed]
- Takemaru, K.I.; Moon, R.T. The transcriptional coactivator CBP interacts with β-catenin to activate gene expression. J. Cell Biol. 2000, 149, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Hurlstone, A.; Musisi, H.; Miles, A.; Bienz, M.; Clevers, H. The chromatin remodelling factor Brg-1 interacts with β-catenin to promote target gene activation. EMBO J. 2001, 20, 4935–4943. [Google Scholar] [CrossRef] [PubMed]
- Bandapalli, O.R.; Dihlmann, S.; Helwa, R.; Macher-Goeppinger, S.; Weitz, J.; Schirmacher, P.; Brand, K. Transcriptional activation of the β-catenin gene at the invasion front of colorectal liver metastases. J. Pathol. 2009, 218, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Lindsay, L.; Hoots, B.; Keys, J.; Franceschi, S.; Winer, R.; Clifford, G.M. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int. J. Cancer 2007, 121, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.R.; Duensing, S.; Brake, T.; Munger, K.; Lambert, P.F.; Arbeit, J.M. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003, 63, 4862–4871. [Google Scholar] [PubMed]
- Song, S.; Pitot, H.C.; Lambert, P.F. The human papillomavirus type 16 E6 gene alone is sufficient to induce carcinomas in transgenic animals. J. Virol. 1999, 73, 5887–5893. [Google Scholar] [PubMed]
- De Villiers, E.M. Cross-roads in the classification of papillomaviruses. Virology 2013, 445, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Bzhalava, D.; Guan, P.; Franceschi, S.; Dillner, J.; Clifford, G. A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology 2013, 445, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Munoz, N.; Bosch, F.X.; de Sanjose, S.; Herrero, R.; Castellsague, X.; Shah, K.V.; Snijders, P.J.; Meijer, C.J.; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Samarzija, I.; Beard, P. Hedgehog pathway regulators influence cervical cancer cell proliferation, survival and migration. Biochem. Biophys. Res. Commun. 2012, 425, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Branca, M.; Ciotti, M.; Santini, D.; Bonito, L.D.; Benedetto, A.; Giorgi, C.; Paba, P.; Favalli, C.; Costa, S.; Agarossi, A.; et al. Activation of the ERK/MAP kinase pathway in cervical intraepithelial neoplasia is related to grade of the lesion but not to high-risk human papillomavirus, virus clearance, or prognosis in cervical cancer. Am. J. Clin. Pathol. 2004, 122, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Weijzen, S.; Zlobin, A.; Braid, M.; Miele, L.; Kast, W.M. HPV16 E6 and E7 oncoproteins regulate Notch-1 expression and cooperate to induce transformation. J. Cell. Physiol. 2003, 194, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Uren, A.; Fallen, S.; Yuan, H.; Usubutun, A.; Kucukali, T.; Schlegel, R.; Toretsky, J.A. Activation of the canonical Wnt pathway during genital keratinocyte transformation: A model for cervical cancer progression. Cancer Res. 2005, 65, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J. Molecular biology of human papillomavirus infection and cervical cancer. Clin. Sci. Lond. 2006, 110, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.B.; Day, P.M.; Trus, B.L. The papillomavirus major capsid protein L1. Virology 2013, 445, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Roden, R.B. L2, the minor capsid protein of papillomavirus. Virology 2013, 445, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Bergvall, M.; Melendy, T.; Archambault, J. The E1 proteins. Virology 2013, 445, 35–56. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A. The papillomavirus E2 proteins. Virology 2013, 445, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J. The E4 protein; structure, function and patterns of expression. Virology 2013, 445, 80–98. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin-Drubin, M.E.; Munger, K. Oncogenic activities of human papillomaviruses. Virus Res. 2009, 143, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Scheffner, M.; Huibregtse, J.M.; Vierstra, R.D.; Howley, P.M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993, 75, 495–505. [Google Scholar] [CrossRef]
- Huh, K.; Zhou, X.; Hayakawa, H.; Cho, J.Y.; Libermann, T.A.; Jin, J.; Harper, J.W.; Munger, K. Human papillomavirus type 16 E7 oncoprotein associates with the cullin 2 ubiquitin ligase complex, which contributes to degradation of the retinoblastoma tumor suppressor. J. Virol. 2007, 81, 9737–9747. [Google Scholar] [CrossRef] [PubMed]
- Pett, M.; Coleman, N. Integration of high-risk human papillomavirus: A key event in cervical carcinogenesis? J. Pathol. 2007, 212, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Lambert, P.F. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: Implications for cervical carcinogenesis. Proc. Natl. Acad. Sci. USA 1995, 92, 1654–1658. [Google Scholar] [CrossRef] [PubMed]
- Sashiyama, H.; Shino, Y.; Kawamata, Y.; Tomita, Y.; Ogawa, N.; Shimada, H.; Kobayashi, S.; Asano, T.; Ochiai, T.; Shirasawa, H. Immortalization of human esophageal keratinocytes by E6 and E7 of human papillomavirus type 16. Int. J. Oncol. 2001, 19, 97–103. [Google Scholar] [CrossRef] [PubMed]
- McCance, D.J.; Kopan, R.; Fuchs, E.; Laimins, L.A. Human papillomavirus type 16 alters human epithelial cell differentiation in vitro. Proc. Natl. Acad. Sci. USA 1988, 85, 7169–7173. [Google Scholar] [CrossRef] [PubMed]
- Arbeit, J.M.; Howley, P.M.; Hanahan, D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc. Natl. Acad. Sci. USA 1996, 93, 2930–2935. [Google Scholar] [CrossRef] [PubMed]
- Durst, M.; Gallahan, D.; Jay, G.; Rhim, J.S. Glucocorticoid-enhanced neoplastic transformation of human keratinocytes by human papillomavirus type 16 and an activated ras oncogene. Virology 1989, 173, 767–771. [Google Scholar] [CrossRef]
- Pei, X.F.; Meck, J.M.; Greenhalgh, D.; Schlegel, R. Cotransfection of HPV-18 and v-fos DNA induces tumorigenicity of primary human keratinocytes. Virology 1993, 196, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.A.; Laimins, L.A. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.A.; Thomas, M.; Banks, L.; Roberts, S. Activity of the human papillomavirus E6 PDZ-binding motif correlates with an enhanced morphological transformation of immortalized human keratinocytes. J. Cell Sci. 2003, 116, 4925–4934. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dakic, A.; Zhang, Y.; Dai, Y.; Chen, R.; Schlegel, R. HPV E6 protein interacts physically and functionally with the cellular telomerase complex. Proc. Natl. Acad. Sci. USA 2009, 106, 18780–18785. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Banks, L. Human papillomavirus (HPV) E6 interactions with Bak are conserved amongst E6 proteins from high and low risk HPV types. J. Gen. Virol. 1999, 80, 1513–1517. [Google Scholar] [PubMed]
- Garnett, T.O.; Filippova, M.; Duerksen-Hughes, P.J. Accelerated degradation of FADD and procaspase 8 in cells expressing human papilloma virus 16 E6 impairs TRAIL-mediated apoptosis. Cell Death Differ. 2006, 13, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, S.; Meng, Q.; Ma, Y.; Katiyar, P.; Schlegel, R.; Rosen, E.M. BRCA1 interaction with human papillomavirus oncoproteins. J. Biol. Chem. 2005, 280, 33165–33177. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Labrecque, S.; Gauzzi, M.C.; Cuddihy, A.R.; Wong, A.H.; Pellegrini, S.; Matlashewski, G.J.; Koromilas, A.E. The human papilloma virus (HPV)-18 E6 oncoprotein physically associates with Tyk2 and impairs Jak-STAT activation by interferon-α. Oncogene 1999, 18, 5727–5737. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H.; Degenkolbe, R.; Bernard, H.U.; O’Connor, M.J. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J. Virol. 1999, 73, 6209–6219. [Google Scholar] [PubMed]
- Gewin, L.; Myers, H.; Kiyono, T.; Galloway, D.A. Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex. Genes Dev. 2004, 18, 2269–2282. [Google Scholar] [CrossRef] [PubMed]
- Veldman, T.; Liu, X.; Yuan, H.; Schlegel, R. Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter. Proc. Natl. Acad. Sci. USA 2003, 100, 8211–8216. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Delgado, J.; Bulut, G.; Liu, X.; Cortes-Malagon, E.M.; Schlegel, R.; Flores-Maldonado, C.; Contreras, R.G.; Chung, S.H.; Lambert, P.F.; Uren, A.; et al. The E6 oncoprotein from HPV16 enhances the canonical Wnt/β-catenin pathway in skin epidermis in vivo. Mol. Cancer Res. 2012, 10, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Dyson, N.; Howley, P.M.; Munger, K.; Harlow, E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 1989, 243, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Antinore, M.J.; Birrer, M.J.; Patel, D.; Nader, L.; McCance, D.J. The human papillomavirus type 16 E7 gene product interacts with and trans-activates the AP1 family of transcription factors. EMBO J. 1996, 15, 1950–1960. [Google Scholar] [PubMed]
- Nguyen, C.L.; Munger, K. Direct association of the HPV16 E7 oncoprotein with cyclin A/CDK2 and cyclin E/CDK2 complexes. Virology 2008, 380, 21–25. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, M.C.; Ruesch, M.N.; Laimins, L.A. Human papillomavirus E7 oncoproteins bind a single form of cyclin E in a complex with cdk2 and p107. Virology 1996, 215, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.O.; Waga, S.; Harry, J.B.; Espling, E.; Stillman, B.; Galloway, D.A. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 1997, 11, 2090–2100. [Google Scholar] [CrossRef] [PubMed]
- Luscher-Firzlaff, J.M.; Westendorf, J.M.; Zwicker, J.; Burkhardt, H.; Henriksson, M.; Muller, R.; Pirollet, F.; Luscher, B. Interaction of the fork head domain transcription factor MPP2 with the human papilloma virus 16 E7 protein: Enhancement of transformation and transactivation. Oncogene 1999, 18, 5620–5630. [Google Scholar] [CrossRef] [PubMed]
- Huh, K.W.; DeMasi, J.; Ogawa, H.; Nakatani, Y.; Howley, P.M.; Munger, K. Association of the human papillomavirus type 16 E7 oncoprotein with the 600-kDa retinoblastoma protein-associated factor, p600. Proc. Natl. Acad. Sci. USA 2005, 102, 11492–11497. [Google Scholar] [CrossRef] [PubMed]
- Brehm, A.; Nielsen, S.J.; Miska, E.A.; McCance, D.J.; Reid, J.L.; Bannister, A.J.; Kouzarides, T. The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. EMBO J. 1999, 18, 2449–2458. [Google Scholar] [CrossRef] [PubMed]
- Um, S.J.; Rhyu, J.W.; Kim, E.J.; Jeon, K.C.; Hwang, E.S.; Park, J.S. Abrogation of IRF-1 response by high-risk HPV E7 protein in vivo. Cancer Lett. 2002, 179, 205–212. [Google Scholar] [CrossRef]
- Barnard, P.; McMillan, N.A. The human papillomavirus E7 oncoprotein abrogates signaling mediated by interferon-α. Virology 1999, 259, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Shah, W.; Jing, L.; Chen, H.; Wang, Y. High-risk human papillomavirus type 18 E7 caused p27 elevation and cytoplasmic localization. Cancer Biol. Ther. 2010, 9, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Pim, D.; Massimi, P.; Dilworth, S.M.; Banks, L. Activation of the protein kinase B pathway by the HPV-16 E7 oncoprotein occurs through a mechanism involving interaction with PP2A. Oncogene 2005, 24, 7830–7838. [Google Scholar] [CrossRef] [PubMed]
- Howie, H.L.; Katzenellenbogen, R.A.; Galloway, D.A. Papillomavirus E6 proteins. Virology 2009, 384, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dasgupta, J.; Ma, R.Z.; Banks, L.; Thomas, M.; Chen, X.S. Structures of a human papillomavirus (HPV) E6 polypeptide bound to MAGUK proteins: Mechanisms of targeting tumor suppressors by a high-risk HPV oncoprotein. J. Virol. 2007, 81, 3618–3626. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Laimins, L.A. Role of the PDZ domain-binding motif of the oncoprotein E6 in the pathogenesis of human papillomavirus type 31. J. Virol. 2004, 78, 12366–12377. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.; Werness, B.A.; Dyson, N.; Phelps, W.C.; Harlow, E.; Howley, P.M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989, 8, 4099–4105. [Google Scholar] [PubMed]
- Liu, X.; Clements, A.; Zhao, K.; Marmorstein, R. Structure of the human papillomavirus E7 oncoprotein and its mechanism for inactivation of the retinoblastoma tumor suppressor. J. Biol. Chem. 2006, 281, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Zerfass-Thome, K.; Zwerschke, W.; Mannhardt, B.; Tindle, R.; Botz, J.W.; Jansen-Durr, P. Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Oncogene 1996, 13, 2323–2330. [Google Scholar] [PubMed]
- Massimi, P.; Pim, D.; Banks, L. Human papillomavirus type 16 E7 binds to the conserved carboxy-terminal region of the TATA box binding protein and this contributes to E7 transforming activity. J. Gen. Virol. 1997, 78, 2607–2613. [Google Scholar] [PubMed]
- Hwang, S.G.; Lee, D.; Kim, J.; Seo, T.; Choe, J. Human papillomavirus type 16 E7 binds to E2F1 and activates E2F1-driven transcription in a retinoblastoma protein-independent manner. J. Biol. Chem. 2002, 277, 2923–2930. [Google Scholar] [CrossRef] [PubMed]
- Manzo-Merino, J.; Contreras-Paredes, A.; Vazquez-Ulloa, E.; Rocha-Zavaleta, L.; Fuentes-Gonzalez, A.M.; Lizano, M. The role of signaling pathways in cervical cancer and molecular therapeutic targets. Arch. Med. Res. 2014, 45, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.T.; Rozycka, M.; Sara, E.; Davis, E.; Smalley, M.; Young, N.; Dale, T.C.; Wooster, R. Sequence variants of the axin gene in breast, colon, and other cancers: An analysis of mutations that interfere with GSK3 binding. Genes Chromosomes Cancer 2000, 28, 443–453. [Google Scholar] [CrossRef]
- Su, T.H.; Chang, J.G.; Yeh, K.T.; Lin, T.H.; Lee, T.P.; Chen, J.C.; Lin, C.C. Mutation analysis of CTNNB1 (β-catenin) and AXIN1, the components of Wnt pathway, in cervical carcinomas. Oncol. Rep. 2003, 10, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cao, H.Z.; Zheng, P.S. LGR5 promotes the proliferation and tumor formation of cervical cancer cells through the Wnt/β-catenin signaling pathway. Oncotarget 2014, 5, 9092–9105. [Google Scholar] [PubMed]
- Rath, G.; Jawanjal, P.; Salhan, S.; Nalliah, M.; Dhawan, I. Clinical significance of inactivated glycogen synthase kinase 3β in HPV-associated cervical cancer: Relationship with Wnt/β-catenin pathway activation. Am. J. Reprod. Immunol. 2015, 73, 460–478. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.M.; Cheng, Y.W.; Wang, Y.C.; Wu, T.C.; Chen, C.Y.; Lee, H. Up-regulation of FOXM1 by E6 oncoprotein through the MZF1/NKX2-1 axis is required for human papillomavirus-associated tumorigenesis. Neoplasia 2014, 16, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Al-Shabanah, O.A.; Hafez, M.M.; Hassan, Z.K.; Sayed-Ahmed, M.M.; Abozeed, W.N.; Alsheikh, A.; Al-Rejaie, S.S. Methylation of SFRPS and APC genes in ovarian cancer infected with high risk human papillomavirus. Asian Pac. J. Cancer Prev. 2014, 15, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Van der Meide, W.F.; Snellenberg, S.; Meijer, C.J.; Baalbergen, A.; Helmerhorst, T.J.; van der Sluis, W.B.; Snijders, P.J.; Steenbergen, R.D. Promoter methylation analysis of Wnt/β-catenin signaling pathway regulators to detect adenocarcinoma or its precursor lesion of the cervix. Gynecol. Oncol. 2011, 123, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Perez-Plasencia, C.; Vazquez-Ortiz, G.; Lopez-Romero, R.; Pina-Sanchez, P.; Moreno, J.; Salcedo, M. Genome wide expression analysis in HPV16 cervical cancer: Identification of altered metabolic pathways. Infect. Agent Cancer 2007, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Fragoso-Ontiveros, V.; Maria Alvarez-Garcia, R.; Contreras-Paredes, A.; Vaca-Paniagua, F.; Alonso Herrera, L.; Lopez-Camarillo, C.; Jacobo-Herrera, N.; Lizano-Soberon, M.; Perez-Plasencia, C. Gene expression profiles induced by E6 from non-european HPV18 variants reveals a differential activation on cellular processes driving to carcinogenesis. Virology 2012, 432, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Solano, M.; Meza-Canales, I.D.; Torres-Reyes, L.A.; Alvarez-Zavala, M.; Alvarado-Ruiz, L.; Rincon-Orozco, B.; Garcia-Chagollan, M.; Ochoa-Hernandez, A.B.; Ortiz-Lazareno, P.C.; Rosl, F.; et al. Expression of WNT genes in cervical cancer-derived cells: Implication of WNT7A in cell proliferation and migration. Exp. Cell Res. 2015, 335, 39–50. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lin, J.; Ding, Y.; Liu, G.; Luo, Y.; Huang, M.; Xu, C.; Kim, T.K.; Etheridge, A.; Lin, M.; et al. A systematic study on dysregulated microRNAs in cervical cancer development. Int. J. Cancer 2015. [Google Scholar] [CrossRef] [PubMed]
- Bulut, G.; Fallen, S.; Beauchamp, E.M.; Drebing, L.E.; Sun, J.; Berry, D.L.; Kallakury, B.; Crum, C.P.; Toretsky, J.A.; Schlegel, R.; et al. β-catenin accelerates human papilloma virus type-16 mediated cervical carcinogenesis in transgenic mice. PLoS ONE 2011, 6, e27243. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, S.I.; Reed, J.C. Siah-1, SIP, and Ebi collaborate in a novel pathway for β-catenin degradation linked to p53 responses. Mol. Cell 2001, 7, 915–926. [Google Scholar] [CrossRef]
- Lichtig, H.; Gilboa, D.A.; Jackman, A.; Gonen, P.; Levav-Cohen, Y.; Haupt, Y.; Sherman, L. HPV16 E6 augments Wnt signaling in an E6AP-dependent manner. Virology 2010, 396, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Sominsky, S.; Kuslansky, Y.; Shapiro, B.; Jackman, A.; Haupt, Y.; Rosin-Arbesfeld, R.; Sherman, L. HPV16 E6 and E6AP differentially cooperate to stimulate or augment Wnt signaling. Virology 2014, 468–470, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yost, H.J.; Virshup, D.M.; Seeling, J.M. Protein phosphatase 2A and its B56 regulatory subunit inhibit Wnt signaling in Xenopus. EMBO J. 2001, 20, 4122–4131. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bello, J.O.M.; Nieva, L.O.; Paredes, A.C.; Gonzalez, A.M.F.; Zavaleta, L.R.; Lizano, M. Regulation of the Wnt/β-Catenin Signaling Pathway by Human Papillomavirus E6 and E7 Oncoproteins. Viruses 2015, 7, 4734-4755. https://doi.org/10.3390/v7082842
Bello JOM, Nieva LO, Paredes AC, Gonzalez AMF, Zavaleta LR, Lizano M. Regulation of the Wnt/β-Catenin Signaling Pathway by Human Papillomavirus E6 and E7 Oncoproteins. Viruses. 2015; 7(8):4734-4755. https://doi.org/10.3390/v7082842
Chicago/Turabian StyleBello, Jesus Omar Muñoz, Leslie Olmedo Nieva, Adriana Contreras Paredes, Alma Mariana Fuentes Gonzalez, Leticia Rocha Zavaleta, and Marcela Lizano. 2015. "Regulation of the Wnt/β-Catenin Signaling Pathway by Human Papillomavirus E6 and E7 Oncoproteins" Viruses 7, no. 8: 4734-4755. https://doi.org/10.3390/v7082842
APA StyleBello, J. O. M., Nieva, L. O., Paredes, A. C., Gonzalez, A. M. F., Zavaleta, L. R., & Lizano, M. (2015). Regulation of the Wnt/β-Catenin Signaling Pathway by Human Papillomavirus E6 and E7 Oncoproteins. Viruses, 7(8), 4734-4755. https://doi.org/10.3390/v7082842






