The Antiviral Effect of Baicalin on Enterovirus 71 In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus and Chemicals
2.2. Antibodies
2.3. Cytotoxicity Assay of Baicalin
2.4. Plaque Reduction Assay
2.5. Antiviral Activity of Baicalin
2.6. Virus Absorption Assay and Time Course Analysis
2.7. Reverse Transcription PCR (RT-PCR)
2.8. Western Blot Analysis
| Genes | Primer sequence | Length of product (bp) |
|---|---|---|
| VP1 | FP: 5'-TGGCAGATGTGATTGAGAG-3' | 137 |
| RP: 5'-GGCTTGAAGTGCTGGTA-3' | ||
| 2A | FP: 5'-ACCTTAGGGTAGTAAACAGACAC-3' | 262 |
| RP: 5'-GCAAGCATAAGATGGGACT-3' | ||
| 3C | FP: 5'-GACCATCTGGGTAGAGCAT-3' | 143 |
| RP: 5'-CTTCTGGGATAAACTTGGTG-3' | ||
| 3D | FP: 5'-GGAAGTCTCGCCTGATTG-3' | 478 |
| RP: 5'-CAGCAGGAGTCATAGTCAGC-3' | ||
| GAPDH | FP: 5'-GGATTTGGTCGTATTGGG-3' | 205 |
| RP: 5'-GGAAGATGGTGATGGGATT-3' |
2.9. RD Cell Apoptosis by Annexin V-FITC/PI Dual Staining and Flow Cytometry
2.10. Statistical Analyses
3. Results
3.1. Cytotoxicity of Baicalin on RD Cells
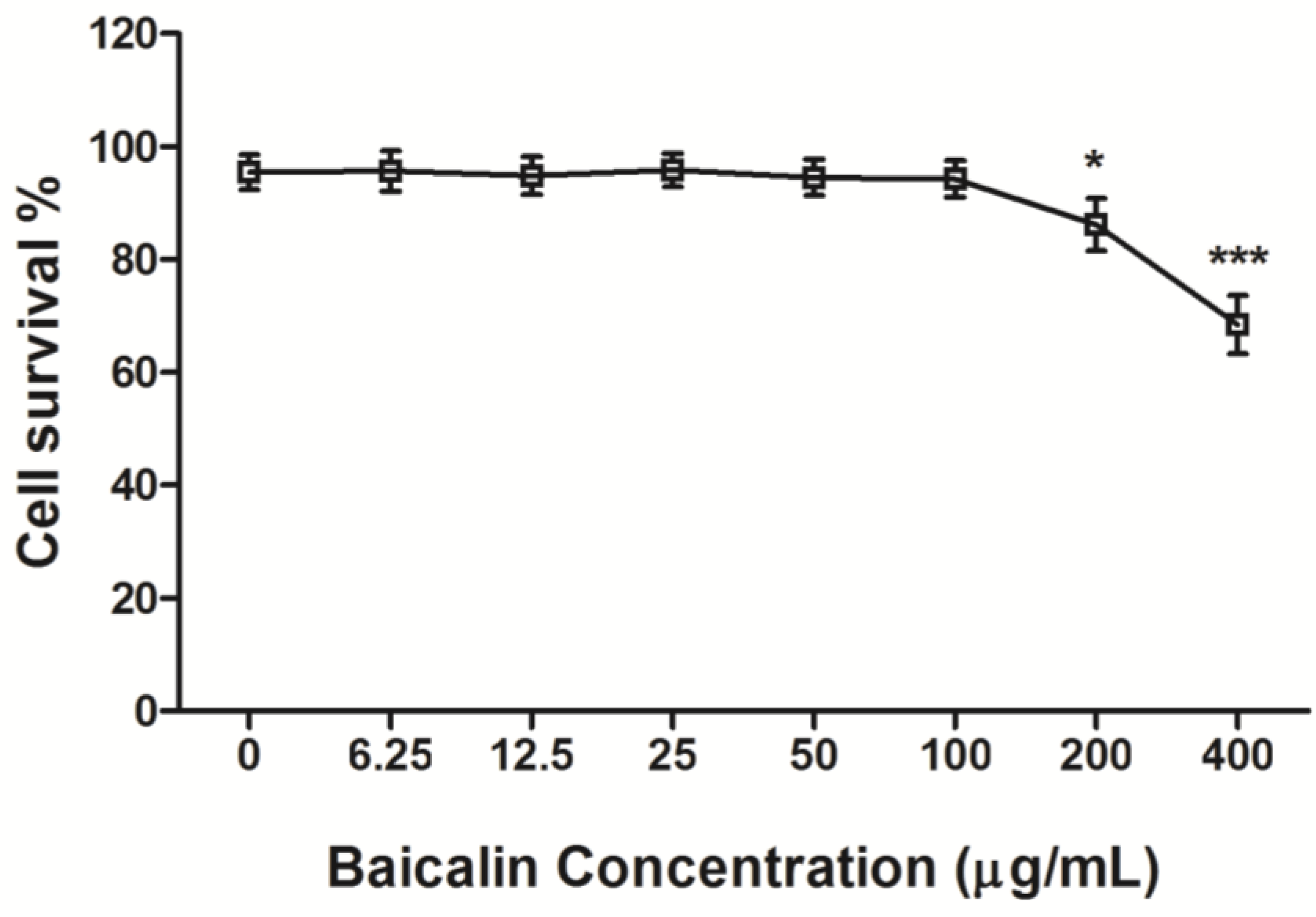
3.2. The Antiviral Efficiency of Baicalin on EV71 Replication and Absorption
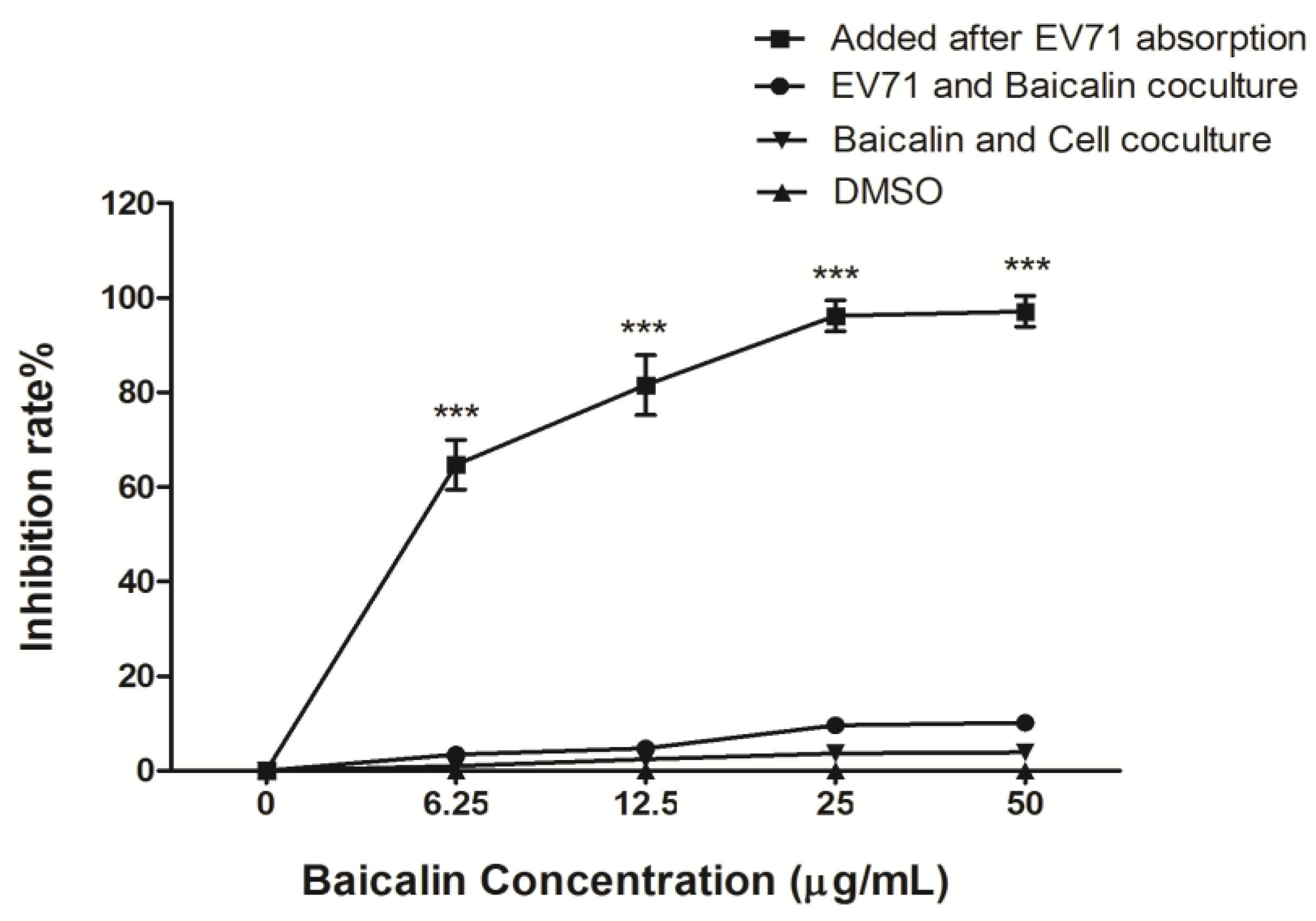
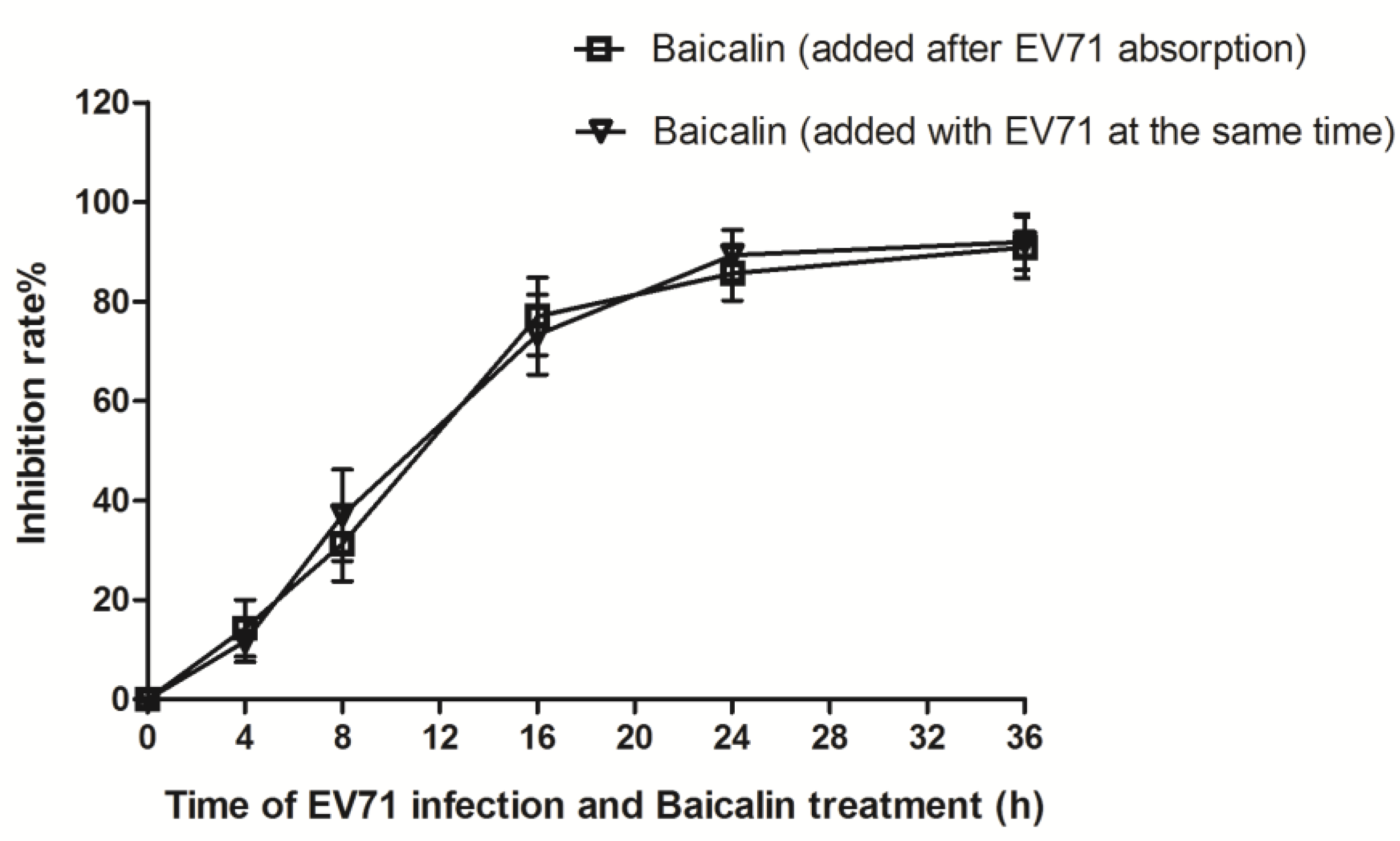
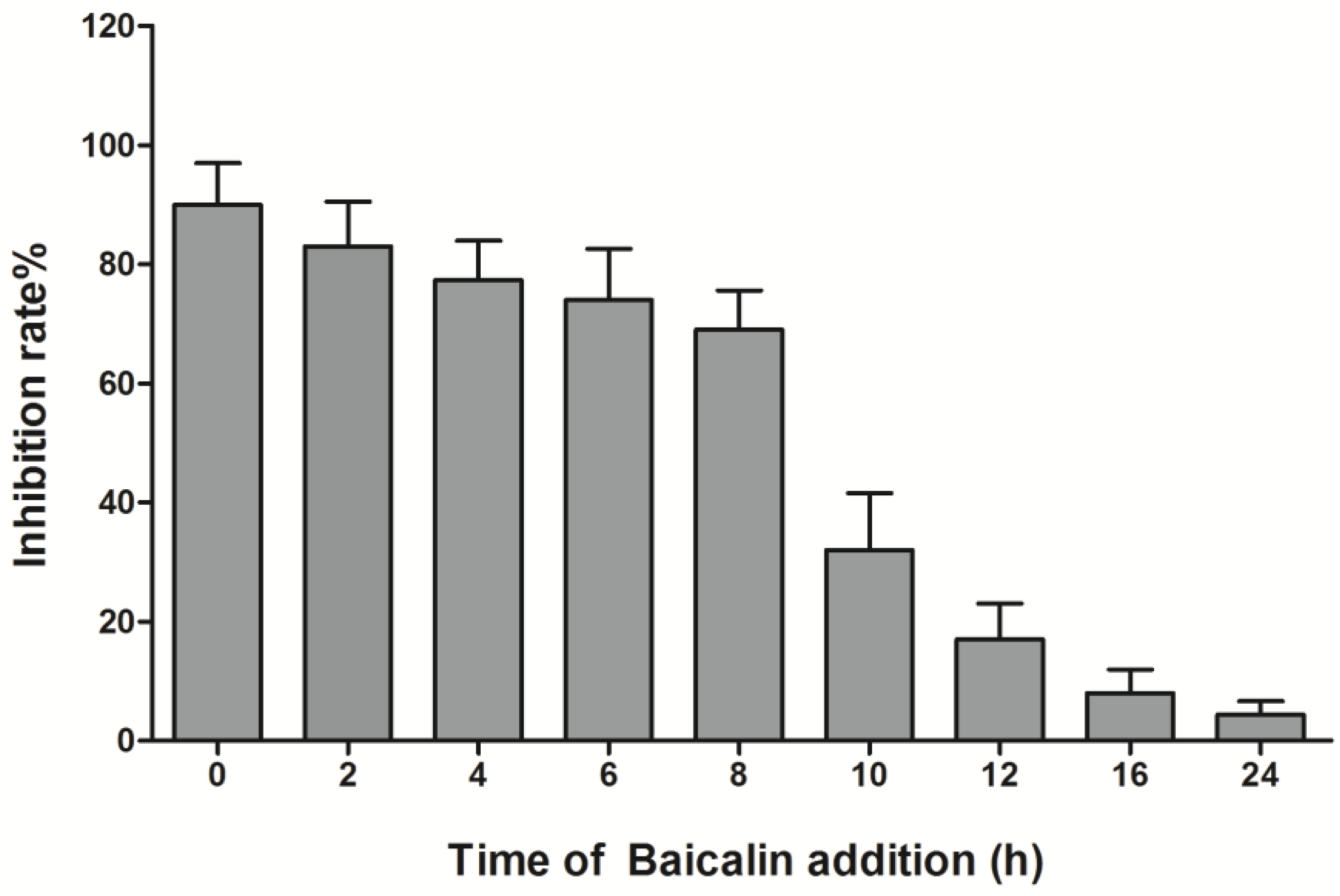
3.3. Time-Course Analysis of Baicalin on EV71 Replication
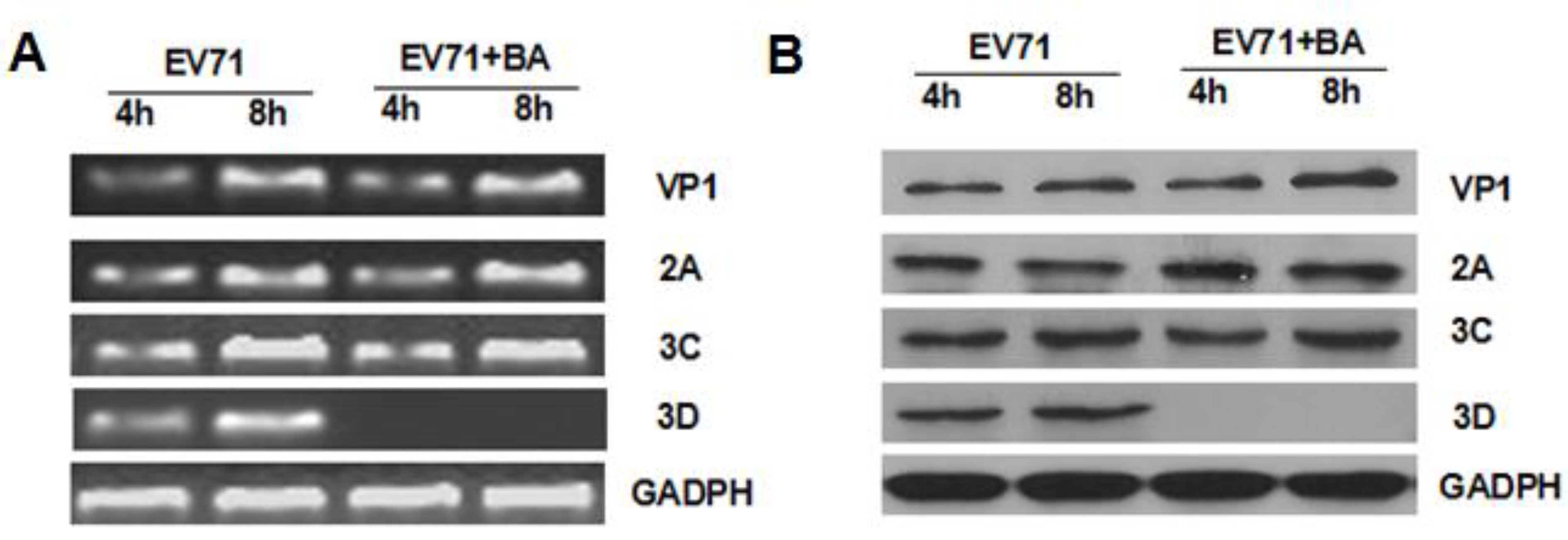
3.4. Effects of Baicalin on the Expression of EV71/VP1, 2A, 3C, and 3D
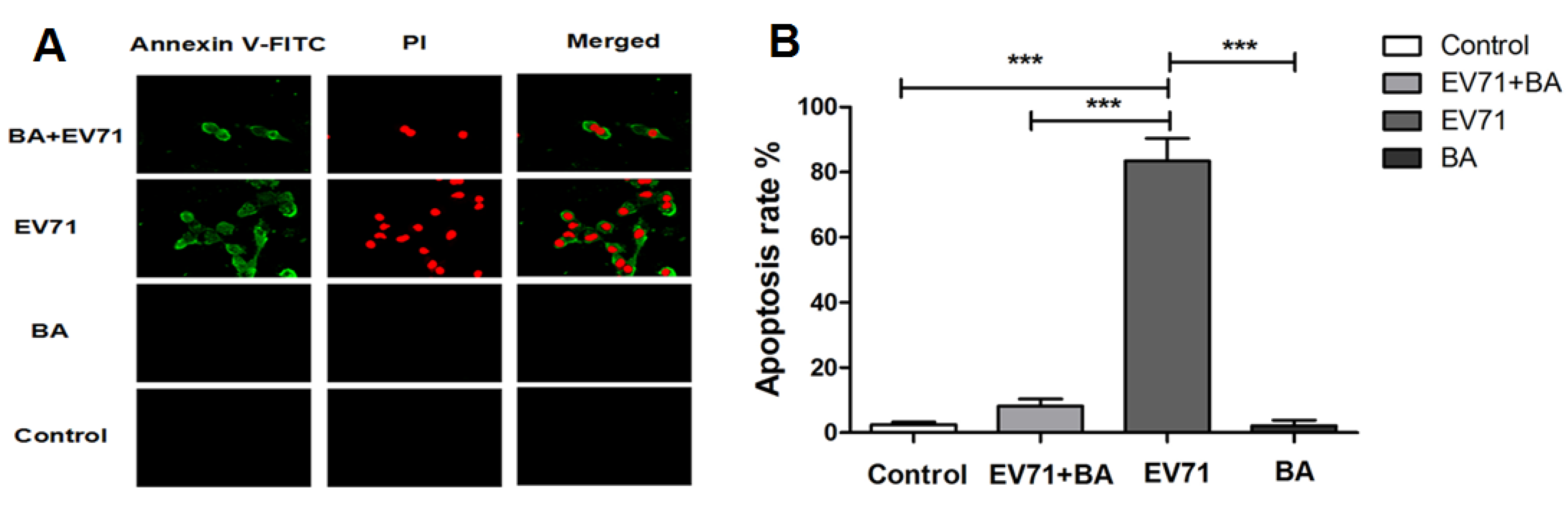
3.5. The Effect of Baicalin on EV71-Induced Apoptosis in RD Cells
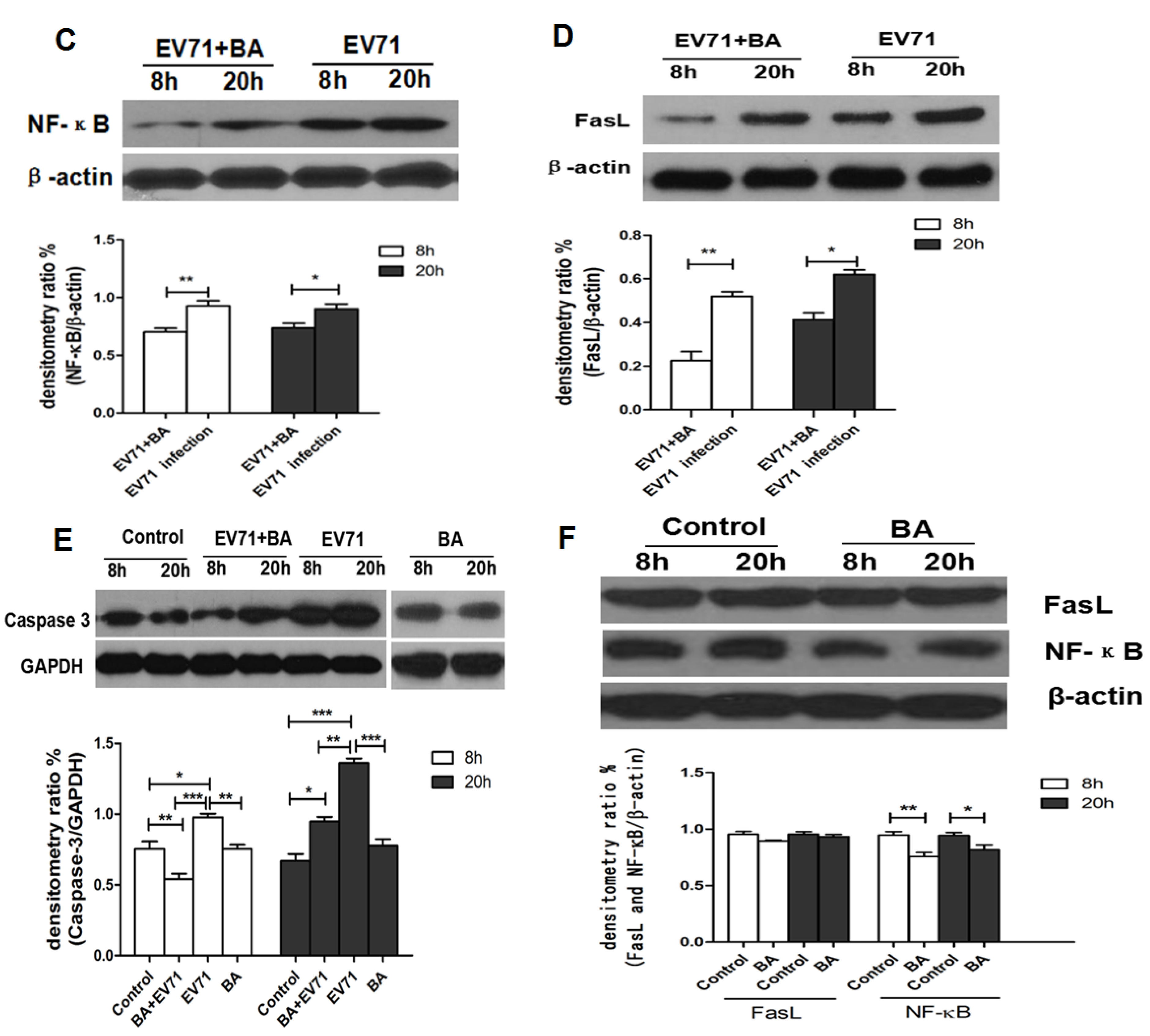
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, T.Y.; Liu, Y.C.; Jheng, J.R.; Tsai, H.P.; Jan, J.T.; Wong, W.R.; Horng, J.T. Anti-enterovirus 71 activity screening of Chinese herbs with anti-infection and inflammation activities. Am. J. Chin. Med. 2009, 37, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Han, J.F.; Cao, R.Y.; Tian, X.; Yu, M.; Qin, E.D.; Qin, C.F. Producing infectious enterovirus type 71 in a rapid strategy. Virol. J. 2010, 7, e116. [Google Scholar] [CrossRef] [PubMed]
- Kiener, T.K.; Jia, Q.; Lim, X.F.; He, F.; Meng, T.; Chow, V.T.; Kwang, J. Characterization and specificity of the linear epitope of the enterovirus 71 VP2 protein. Virol. J. 2012, 9, e55. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Qi, J.; Chen, Z.; Xu, X.; Gao, F.; Lin, D.; Qian, W.; Liu, H.; Jiang, H.; Yan, J.; et al. Enterovirus 71 and coxsackievirus A16 3C proteases: Binding to rupintrivir and their substrates and anti-hand, foot, and mouth disease virus drug design. J. Virol. 2011, 85, 10319–10331. [Google Scholar] [PubMed]
- Wei, Y.; Shi, L.; Wang, K.; Liu, M.; Yang, Q.; Yang, Z.; Ke, S. Discovery of Gramine Derivatives That Inhibit the Early Stage of EV71 Replication in Vitro. Molecules 2014, 19, 8949–8964. [Google Scholar] [CrossRef] [PubMed]
- Zu, M.; Yang, F.; Zhou, W.; Liu, A.; Du, G.; Zheng, L. In vitro anti-influenza virus and anti-inflammatory activities of theaflavin derivatives. Antivir. Res. 2012, 94, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lu, Z.; Lv, Z.; Yu, T.; Yang, P.; Shen, Y.; Ding, Y.; Fu, D.; Zhang, X.; Fu, Q.; et al. The Fas/Fas ligand death receptor pathway contributes to phenylalanine-induced apoptosis in cortical neurons. PLoS ONE 2013, 8, e71553. [Google Scholar] [PubMed]
- Shi, W.; Li, X.; Hou, X.; Peng, H.; Jiang, Q.; Shi, M.; Ji, Y.; Liu, X.; Liu, J.; et al. Differential apoptosis gene expressions of rhabdomyosarcoma cells in response to enterovirus 71 infection. BMC Infect. Dis. 2012, 12, e327. [Google Scholar]
- Zhu, G.; Zheng, Y.; Zhang, L.; Shi, Y.; Li, W.; Liu, Z.; Peng, B.; Yin, J.; Liu, W.; He, X. Coxsackievirus A16 infection triggers apoptosis in RD cells by inducing ER stress. Biochem. Biophys. Res. Commun. 2013, 441, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, Q.; Xu, L. Herpes simplex virus 2 infects human endothelial ECV304 cells and induces cell apoptosis synergistically with ox-LDL. J. Toxicol. Sci. 2014, 39, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Boonnak, K.; Vogel, L.; Feldmann, F.; Feldmann, H.; Legge, K.L.; Subbarao, K. Lymphopenia Associated with Highly Virulent H5N1 Virus Infection Due to Plasmacytoid Dendritic Cell—Mediated Apoptosis of T Cells. J. Immunol. 2014, 192, 5906–5912. [Google Scholar] [CrossRef] [PubMed]
- Vachirayonstien, T.; Promkhatkaew, D.; Bunjob, M.; Chueyprom, A.; Chavalittumrong, P.; Sawanpanyalert, P. Molecular evaluation of extracellular activity of medicinal herb Clinacanthus nutans against herpes simplex virus type-2. Natl. Prod. Res. 2010, 24, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, X.Y. Research progress of Chinese herbal medicine Radix isatidis (banlangen). Am. J. Chin. Med. 2013, 41, 743–764. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.F.; Cai, B.L.; Guan, S.M.; Li, Y.; Wu, J.Z.; Wang, Y.; Liu, B. Baicalin induces human mucoepidermoid carcinoma Mc3 cells apoptosis in vitro and in vivo. Investig. New Drugs 2011, 29, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Hour, M.J.; Huang, S.H.; Chang, C.Y.; Lin, Y.K.; Wang, C.Y.; Chang, Y.S.; Lin, C.W. Baicalein, ethyl acetate, and chloroform extracts of Scutellaria baicalensis inhibit the neuraminidase activity of pandemic 2009 H1N1 and seasonal influenza A viruses. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Q.; Fu, T.; Dongyan, Y.; Mikovits, J.A.; Ruscetti, F.W.; Wang, J.M. Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry. Biochem. Biophys. Res. Commun. 2000, 276, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ping, J.; Xu, H.D.; Fu, H.J.; Zhou, Z.H. Synergistic effect of a novel oxymatrine-baicalin combination against hepatitis B virus replication, alpha smooth muscle actin expression and type I collagen synthesis in vitro. World J. Gastroenterol. 2006, 12, 5153–5159. [Google Scholar] [PubMed]
- Wan, Q.; Wang, H.; Han, X.; Lin, Y.; Yang, Y.; Gu, L.; Zhao, J.; Wang, L.; Huang, L.; Li, Y.; et al. Baicalin inhibits TLR7/MYD88 signaling pathway activation to suppress lung inflammation in mice infected with influenza A virus. Biomed. Rep. 2014, 2, 437–441. [Google Scholar] [PubMed]
- Liu, J.; Yang, Y.; Xu, Y.; Ma, C.; Qin, C.; Zhang, L. Lycorine reduces mortality of human enterovirus 71-infected mice by inhibiting virus replication. Virol. J. 2011, 8, e483. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.C.; Chen, T.C.; Fang, M.Y.; Yen, K.J.; Shih, S.R.; Hsu, J.T.A.; Tseng, C.P. Inhibition of enterovirus 71 replication and the viral 3D polymerase by aurintricarboxylic acid. J. Antimicrob. Chemother. 2010, 65, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Wu, C.F.; Hsiao, N.W.; Chang, C.Y.; Li, S.W.; Wan, L.; Lin, Y.J.; Lin, W.Y. Aloe-emodin is an interferon-inducing agent with antiviral activity against Japanese encephalitis virus and enterovirus 71. Int. J. Antimicrob. Agents 2008, 32, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Schnellrath, L.C.; Damaso, C.R. Potent antiviral activity of brequinar against the emerging Cantagalo virus in cell culture. Int. J. Antimicrob. Agents 2011, 38, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Chiu, L.; Ooi, V.; Chan, P.; Ang, P. Antiviral property and mode of action of a sulphated polysaccharide from Sargassum patens against herpes simplex virus type 2. Int. J. Antimicrob. Agents 2004, 24, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yin, Z.; Li, L.; Cheng, A.; Jia, R.; Xu, J.; Wang, Y.; Yao, X.; Lv, C.; Zhao, X.; et al. Antiviral activity of sulfated Chuanminshen violaceum polysaccharide against duck enteritis virus in vitro. Antivir. Res. 2013, 98, 344–351. [Google Scholar] [PubMed]
- Shin, H.B.; Choi, M.S.; Ryu, B.; Lee, N.R.; Kim, H.I.; Choi, H.E.; Chang, J.; Lee, K.T.; Jang, D.S.; Inn, K.S.; et al. Antiviral activity of carnosic acid against respiratory syncytial virus. Virol. J. 2013, 10, e303. [Google Scholar]
- Choi, Y.H.; Kim, G.Y.; Lee, H.H. Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated raW 264.7 macrophages through Toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and NF-κB signaling pathways. Drug Des. Dev. Ther. 2014, 8, 1941–1953. [Google Scholar]
- Jung, Y.C.; Kim, M.E.; Yoon, J.H.; Park, P.R.; Youn, H.Y.; Lee, H.W.; Lee, J.S. Anti-inflammatory effects of galangin on lipopolysaccharide-activated macrophages via ERK and NF-κB pathway regulation. Immunopharmacol. Immunotoxicol. 2014, 36, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qiao, H.; Lv, Y.; Wang, J.; Chen, X.; Hou, Y.; Tan, R.; Li, E. Apigenin inhibits enterovirus-71 infection by disrupting viral RNA association with trans-acting factors. PLoS ONE 2014, 9, e110429. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Su, W.; Jin, J.; Chen, J.; Li, X.; Zhang, X.; Sun, M.; Sun, S.; Fan, P.; An, D.; et al. Identification of luteolin as enterovirus 71 and coxsackievirus A16 inhibitors through reporter viruses and cell viability-based screening. Viruses 2014, 6, 2778–2795. [Google Scholar] [PubMed]
- Burnett, B.; Jia, Q.; Zhao, Y.; Levy, R. A medicinal extract of Scutellaria baicalensis and Acacia catechu acts as a dual inhibitor of cyclooxygenase and 5-lipoxygenase to reduce inflammation. J. Med. Food 2007, 10, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.; Wu, Y.; Leu, Y.; Yeh, S.; Chou, C. Scutellariae radix suppresses hepatitis B virus production in human hepatoma cells. Front. Biosci. (Elite Ed.) 2009, 2, 1538–1547. [Google Scholar] [CrossRef]
- Nayak, M.K.; Agrawal, A.S.; Bose, S.; Naskar, S.; Bhowmick, R.; Chakrabarti, S.; Sarkar, S.; Chawla-Sarkar, M. Antiviral activity of baicalin against influenza virus H1N1-pdm09 is due to modulation of NS1-mediated cellular innate immune responses. J. Antimicrob. Chemother. 2014, 69, 1298–1310. [Google Scholar]
- Garcia, C.C.; Acosta, E.G.; Carro, A.C.; Fernandez Belmonte, M.C.; Bomben, R.; Duschatzky, C.B.; Perotti, M.; Schuff, C.; Damonte, E.B. Virucidal activity and chemical composition of essential oils from aromatic plants of central west Argentina. Nat. Prod. Commun. 2010, 5, 1307–1310. [Google Scholar] [PubMed]
- Schnitzler, P.; Reichling, J. Efficacy of plant products against herpetic infections. HNO 2011, 59, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Faral-Tello, P.; Mirazo, S.; Dutra, C.; Pérez, A.; Geis-Asteggiante, L.; Frabasile, S.; Koncke, E.; Davyt, D.; Cavallaro, L.; Heinzen, H.; et al. Cytotoxic, virucidal, and antiviral activity of South American plant and algae extracts. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; He, Y.Q.; Yi, L.N.; Zan, H.; Kung, H.F.; He, M.L. Viral kinetics of enterovirus 71 in human abdomyosarcoma cells. World J. Gastroenterol. 2011, 17, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Chen, T.C.; Weng, K.F.; Chang, S.C.; Chen, L.L.; Shih, S.R. Viral and host proteins involved in picornavirus life cycle. J. Biomed. Sci. 2009, 16, e103. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Xu, M.; Yin, Z. Antiviral drug discovery for the treatment of enterovirus 71 infections. Antivir. Res. 2013, 97, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xi, X.; Lei, X.; Zhang, X.; Cui, S.; Wang, J.; Jin, Q.; Zhao, Z. Enterovirus 71 protease 2A pro targets MAVS to inhibit anti-viral type I interferon responses. PLoS Pathog. 2013, 9, e1003231. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Weng, L.; Zhang, N.; Arita, M.; Li, R.; Chen, L.; Toyoda, T. Biochemical characterization of enterovirus 71 3D RNA polymerase. Biochim. Biophys. Acta Gene Regul. Mech. 2011, 1809, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Weng, K.F.; Chang, S.C.; Lin, J.Y.; Huang, P.N.; Shih, S.R. Development of antiviral agents for enteroviruses. J. Antimicrob. Chemother. 2008, 62, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wimmer, E.; Paul, A.V. Cis-acting RNA elements in human and animal plus-strand RNA viruses. Biochim. Biophys. Acta Gene Regul. Mech. 2009, 1789, 495–517. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Haenni, A.L. Expression strategies of ambisense viruses. Virus Res. 2003, 93, 141–150. [Google Scholar] [CrossRef]
- Kaiser, W.J.; Upton, J.W.; Mocarski, E.S. Viral modulation of programmed necrosis. Curr. Opin. Virol. 2013, 3, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Iwai, A.; Shiozaki, T.; Miyazaki, T. Relevance of signaling molecules for apoptosis induction on influenza A virus replication. Biochem. Biophys. Res. Commun. 2013, 441, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Pruijssers, A.J.; Hengel, H.; Abel, T.W.; Dermody, T.S. Apoptosis induction influences reovirus replication and virulence in newborn mice. J. Virol. 2013, 87, 12980–12989. [Google Scholar] [CrossRef] [PubMed]
- Holloway, G.; Truong, T.T.; Coulson, B.S. Rotavirus antagonizes cellular antiviral responses by inhibiting the nuclear accumulation of STAT1, STAT2, and NF-κB. J. Virol. 2009, 83, 4942–4951. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Zou, J.; Zhang, B.; Yuan, Z. Dengue virus subgenomic RNA induces apoptosis through the Bcl-2-mediated PI3k/Akt signaling pathway. Virology 2014, 448, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Mankan, A.K.; Lawless, M.W.; Gray, S.G.; Kelleher, D.; McManus, R. NF-κB regulation: The nuclear response. J. Cell. Mol. Med. 2009, 13, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Tung, W.H.; Hsieh, H.L.; Lee, I.T.; Yang, C.M. Enterovirus 71 modulates a COX-2/PGE2/cAMP-dependent viral replication in human neuroblastoma cells: Role of the c-Src/EGFR/p42/p44 MAPK/CREB signaling pathway. J. Cell. Biochem. 2011, 112, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Xin, Z.Y.; Liang, Y.; Ly, H. NF-κB signaling differentially regulates influenza virus RNA synthesis. J. Virol. 2008, 82, 9880–9889. [Google Scholar] [CrossRef] [PubMed]
- La Frazia, S.; Amici, C.; Santoro, M.G. Antiviral activity of proteasome inhibitors in herpes simplex virus-1 infection: Role of nuclear factor-κB. Antivir. Ther. 2006, 11, 995–1004. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, Y.; Wu, T.; Jin, Y.; Cheng, J.; Wan, C.; Qian, W.; Xing, F.; Shi, W. The Antiviral Effect of Baicalin on Enterovirus 71 In Vitro. Viruses 2015, 7, 4756-4771. https://doi.org/10.3390/v7082841
Li X, Liu Y, Wu T, Jin Y, Cheng J, Wan C, Qian W, Xing F, Shi W. The Antiviral Effect of Baicalin on Enterovirus 71 In Vitro. Viruses. 2015; 7(8):4756-4771. https://doi.org/10.3390/v7082841
Chicago/Turabian StyleLi, Xiang, Yuanyuan Liu, Tingting Wu, Yue Jin, Jianpin Cheng, Changbiao Wan, Weihe Qian, Fei Xing, and Weifeng Shi. 2015. "The Antiviral Effect of Baicalin on Enterovirus 71 In Vitro" Viruses 7, no. 8: 4756-4771. https://doi.org/10.3390/v7082841
APA StyleLi, X., Liu, Y., Wu, T., Jin, Y., Cheng, J., Wan, C., Qian, W., Xing, F., & Shi, W. (2015). The Antiviral Effect of Baicalin on Enterovirus 71 In Vitro. Viruses, 7(8), 4756-4771. https://doi.org/10.3390/v7082841





