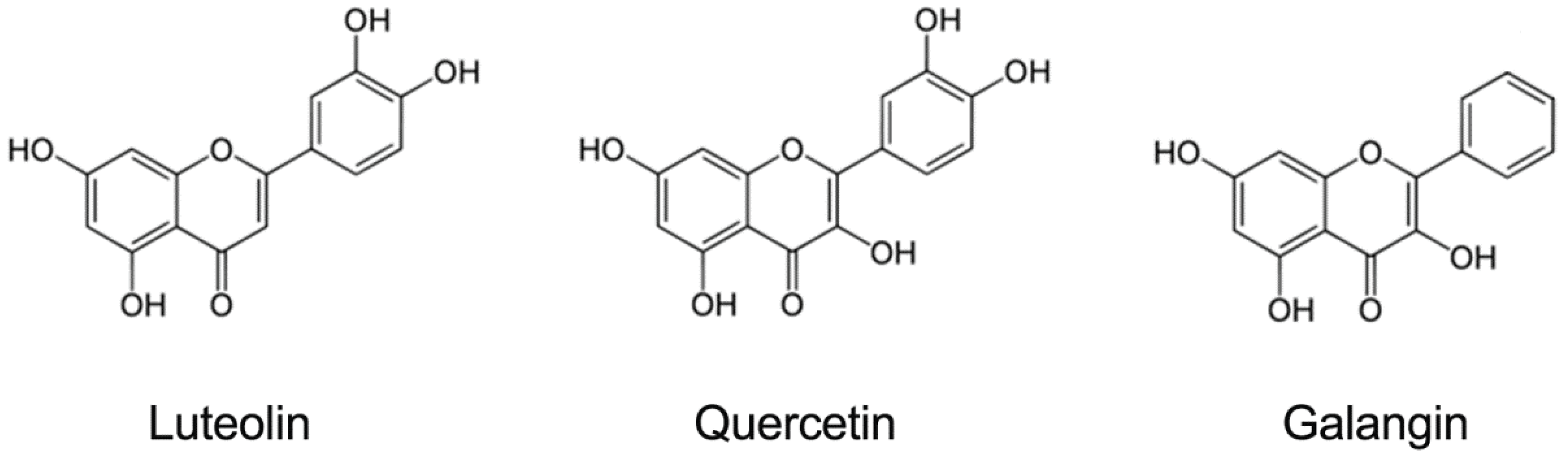
Identification of Luteolin as Enterovirus 71 and Coxsackievirus A16 Inhibitors through Reporter Viruses and Cell Viability-Based Screening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Drug Library
2.2. Viruses
2.2.1. Wild-Type Viruses
2.2.2. EV71/CA16-Luciferase Pseudoviruses
2.2.3. CA16-EGFP and EV71-EGFP Viruses
2.2.3.1. Construction of Plasmids
2.2.3.2. Virus Production
2.2.3.3. Virus Titration
2.2.3.4. Reverse Transcription PCR (RT-PCR)
2.3. Reporter Virus-Based Primary Screening
2.4. Cell Viability-Based Secondary Screening
2.6. Plaque Reduction Assay
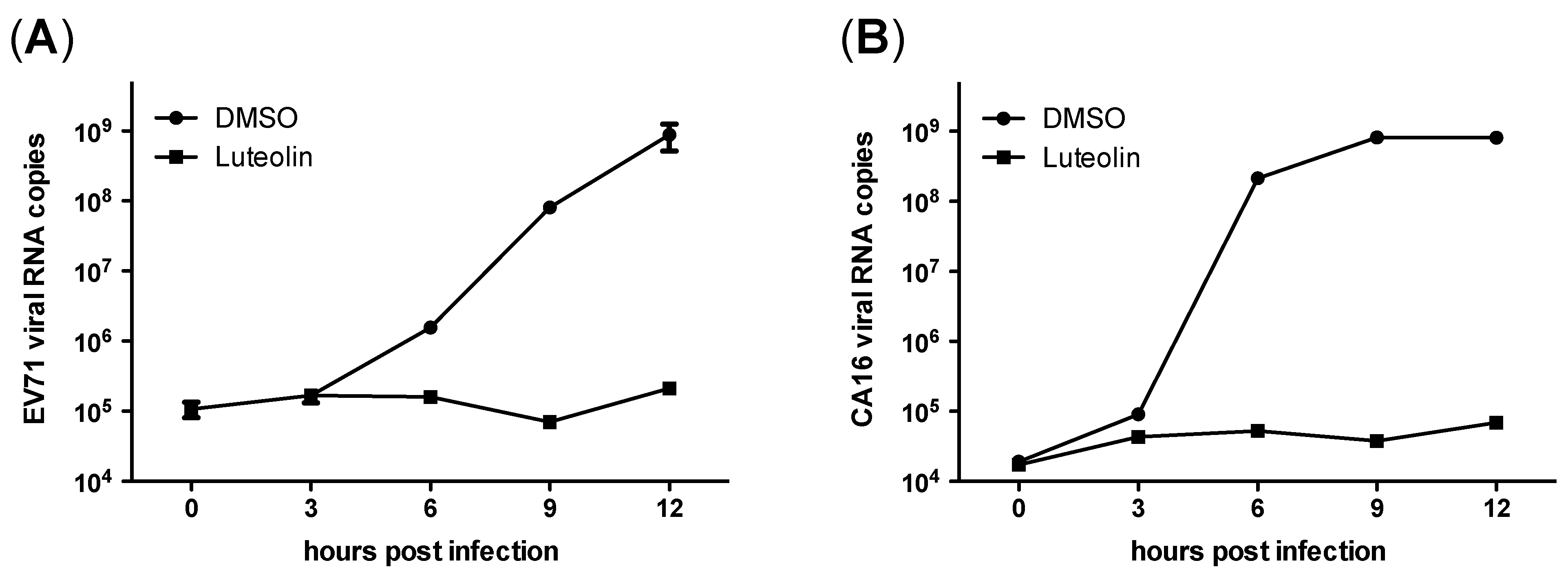
2.7. Viral Replication Assay
2.8. Real-Time RT-PCR of Viral RNA
3. Results
3.1. Construction and Characterization of EGFP Reporter Viruses
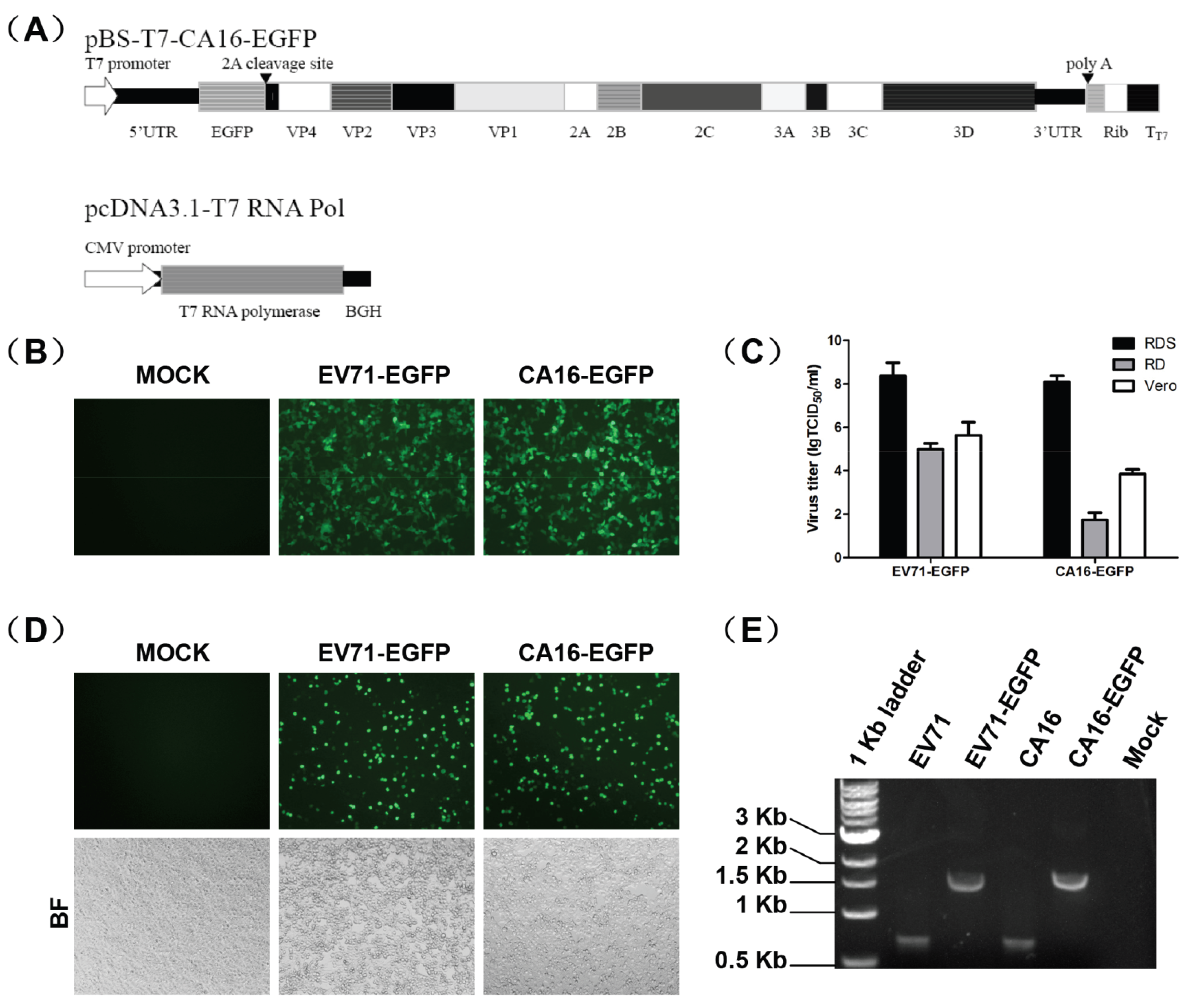
3.2. HTS Screening System to Identify Anti-EV71 and CA16 Drugs
3.2.1. Primary Screening Using Reporter Virus-Based Methods

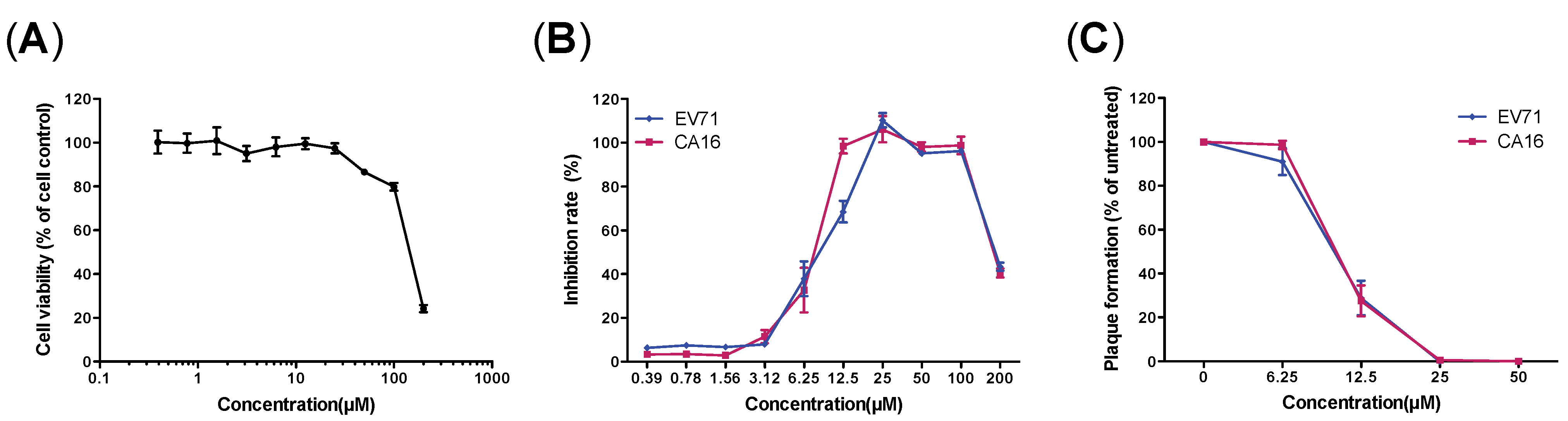
3.2.2. Secondary Screening Using Cell Viability-Based Methods
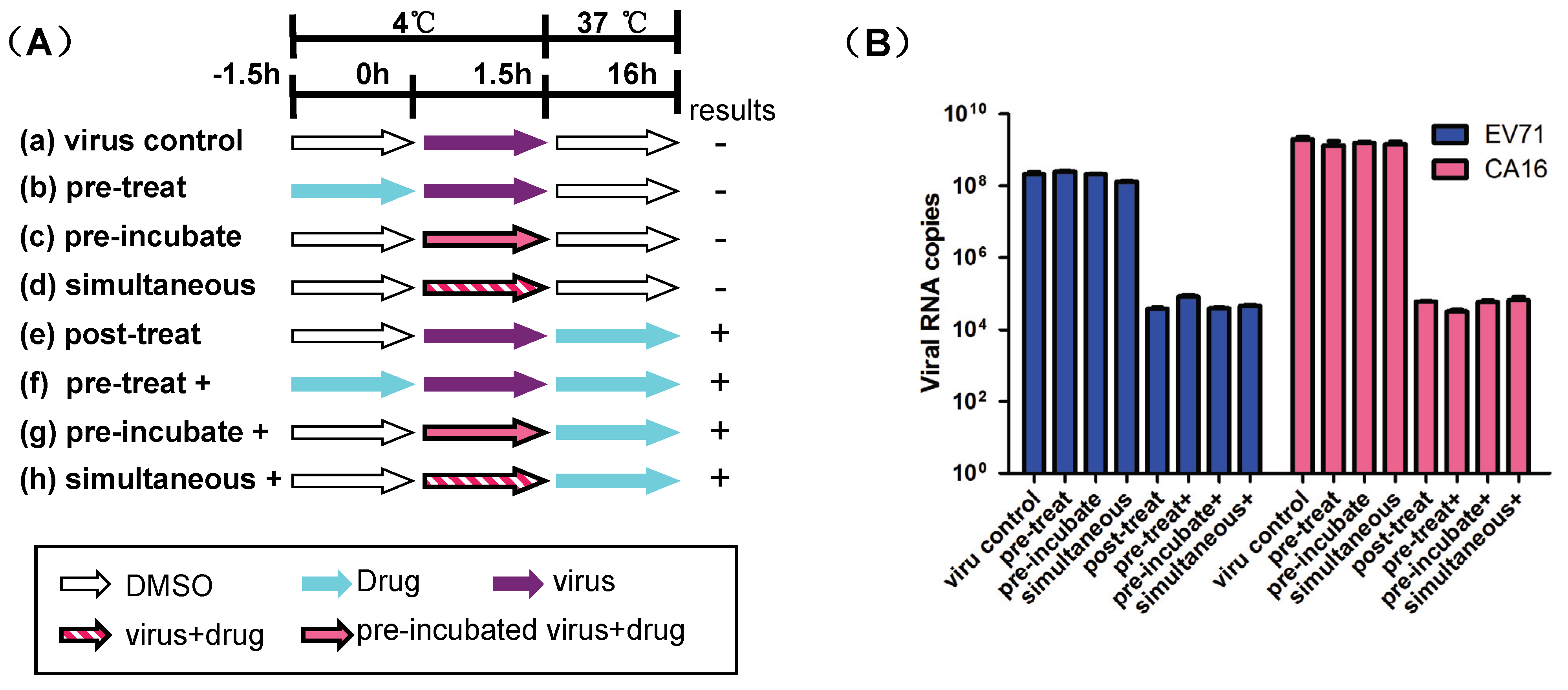
3.4. Luteolin Targets the Post-Attachment Stage of EV71 and CA16 Infection

4. Discussion
Supplementary Files
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Qiu, J. Enterovirus 71 infection: A new threat to global public health? Lancet Neurol. 2008, 7, 868–869. [Google Scholar] [CrossRef]
- Fujimoto, T.; Chikahira, M.; Yoshida, S.; Ebira, H.; Hasegawa, A.; Totsuka, A.; Nishio, O. Outbreak of central nervous system disease associated with hand, foot, and mouth disease in japan during the summer of 2000: Detection and molecular epidemiology of enterovirus 71. Microbiol. Immunol. 2002, 46, 621–627. [Google Scholar] [CrossRef]
- Chan, L.G.; Parashar, U.D.; Lye, M.S.; Ong, F.G.; Zaki, S.R.; Alexander, J.P.; Ho, K.K.; Han, L.L.; Pallansch, M.A.; Suleiman, A.B.; et al. Deaths of children during an outbreak of hand, foot, and mouth disease in sarawak, malaysia: Clinical and pathological characteristics of the disea. For the outbreak study group. Clin. Infect. Dis. 2000, 31, 678–683. [Google Scholar] [CrossRef]
- Ahmad, K. Hand, foot, and mouth disease outbreak reported in singapore. Lancet 2000, 356, 1338. [Google Scholar] [CrossRef]
- WHO. Wpro hand, foot and mouth disease situation update, 15 January 2014. Available online: http://www.wpro.who.int/emerging_diseases/HFMD.Biweekly.15Jan2014.pdf?ua=1 (accessed on 17 May 2014).
- Ang, L.W.; Koh, B.K.; Chan, K.P.; Chua, L.T.; James, L.; Goh, K.T. Epidemiology and control of hand, foot and mouth disease in singapore, 2001–2007. Ann. Acad. Med. Singapore 2009, 38, 106–112. [Google Scholar]
- Solomon, T.; Lewthwaite, P.; Perera, D.; Cardosa, M.J.; McMinn, P.; Ooi, M.H. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 2010, 10, 778–790. [Google Scholar] [CrossRef]
- Rabenau, H.F.; Richter, M.; Doerr, H.W. Hand, foot and mouth disease: Seroprevalence of coxsackie a16 and enterovirus 71 in germany. Med. Microbiol. Immunol. 2010, 199, 45–51. [Google Scholar] [CrossRef]
- Li, W.; Yi, L.; Su, J.; Lu, J.; Zeng, H.; Guan, D.; Ma, C.; Zhang, W.; Xiao, H.; Li, H.; et al. Seroepidemiology of human enterovirus71 and coxsackievirusa16 among children in guangdong province, china. BMC Infect. Dis. 2013, 13, 322. [Google Scholar] [CrossRef]
- Yip, C.C.; Lau, S.K.; Zhou, B.; Zhang, M.X.; Tsoi, H.W.; Chan, K.H.; Chen, X.C.; Woo, P.C.; Yuen, K.Y. Emergence of enterovirus 71 "double-recombinant" strains belonging to a novel genotype d originating from southern china: First evidence for combination of intratypic and intertypic recombination events in ev71. Arch. Virol. 2010, 155, 1413–1424. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Z.; Yang, W.; Ren, J.; Tan, X.; Wang, Y.; Mao, N.; Xu, S.; Zhu, S.; Cui, A.; et al. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in fuyang city of china. Virol. J. 2010, 7, 94. [Google Scholar] [CrossRef]
- Shang, L.; Xu, M.; Yin, Z. Antiviral drug discovery for the treatment of enterovirus 71 infections. Antivir. Res. 2013, 97, 183–194. [Google Scholar] [CrossRef]
- Liang, Z.-L.; Mao, Q.-Y.; Wang, Y.-P.; Zhu, F.-C.; Li, J.-X.; Yao, X.; Gao, F.; Wu, X.; Xu, M.; Wang, J. Progress on the research and development of inactivated ev71 whole-virus vaccines. Hum. Vaccin. Immunother. 2013, 9, 1701–1705. [Google Scholar] [CrossRef]
- Zhu, Q.C.; Wang, Y.; Liu, Y.P.; Zhang, R.Q.; Li, X.; Su, W.H.; Long, F.; Luo, X.D.; Peng, T. Inhibition of enterovirus 71 replication by chrysosplenetin and penduletin. Eur. J. Pharm. Sci. 2011, 44, 392–398. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Wang, W.; Zhang, Y.; Yang, Z.; Jin, Y.; Ge, H.M.; Li, E.; Yang, G. Glycyrrhizic acid as the antiviral component of glycyrrhiza uralensis fisch. Against coxsackievirus a16 and enterovirus 71 of hand foot and mouth disease. J. Ethnopharmacol. 2013, 147, 114–121. [Google Scholar] [CrossRef]
- Yang, Y.; Xiu, J.; Zhang, X.; Zhang, L.; Yan, K.; Qin, C.; Liu, J. Antiviral effect of matrine against human enterovirus 71. Molecules 2012, 17, 10370–10376. [Google Scholar] [CrossRef]
- Chen, T.C.; Chang, H.Y.; Lin, P.F.; Chern, J.H.; Hsu, J.T.; Chang, C.Y.; Shih, S.R. Novel antiviral agent dtrip-22 targets rna-dependent rna polymerase of enterovirus 71. Antimicrob. Agents Chemother. 2009, 53, 2740–2747. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Z.; Qin, B.; Zhang, X.; Chen, L.; Hu, Y.; Yuan, Z. Rupintrivir is a promising candidate for treating severe cases of enterovirus-71 infection: Evaluation of antiviral efficacy in a murine infection model. Antivir. Res. 2013, 97, 264–269. [Google Scholar] [CrossRef]
- Chen, T.C.; Liu, S.C.; Huang, P.N.; Chang, H.Y.; Chern, J.H.; Shih, S.R. Antiviral activity of pyridyl imidazolidinones against enterovirus 71 variants. J. Biomed. Sci. 2008, 15, 291–300. [Google Scholar] [CrossRef]
- Arita, M.; Wakita, T.; Shimizu, H. Characterization of pharmacologically active compounds that inhibit poliovirus and enterovirus 71 infectivity. J. Gen. Virol. 2008, 89, 2518–2530. [Google Scholar] [CrossRef]
- Noble, C.G.; Chen, Y.L.; Dong, H.; Gu, F.; Lim, S.P.; Schul, W.; Wang, Q.Y.; Shi, P.Y. Strategies for development of dengue virus inhibitors. Antivir. Res. 2010, 85, 450–462. [Google Scholar] [CrossRef]
- Gudmundsdottir, H.S.; Olafsdottir, K.; Franzdottir, S.R.; Andresdottir, V. Construction and characterization of an infectious molecular clone of maedi-visna virus that expresses green fluorescent protein. J. Virol. Meth. 2010, 168, 98–102. [Google Scholar] [CrossRef]
- Marriott, A.C.; Hornsey, C.A. Reverse genetics system for chandipura virus: Tagging the viral matrix protein with green fluorescent protein. Virus Res. 2011, 160, 166–172. [Google Scholar] [CrossRef]
- Kim, C.S.; Keum, S.J.; Jang, S.K. Generation of a cell culture-adapted hepatitis c virus with longer half life at physiological temperature. PLoS One 2011, 6, e22808. [Google Scholar]
- Tsueng, G.; Tabor-Godwin, J.M.; Gopal, A.; Ruller, C.M.; Deline, S.; An, N.; Frausto, R.F.; Milner, R.; Crocker, S.J.; Whitton, J.L.; et al. Coxsackievirus preferentially replicates and induces cytopathic effects in undifferentiated neural progenitor cells. J. Virol. 2011, 85, 5718–5732. [Google Scholar] [CrossRef]
- Shang, B.; Deng, C.; Ye, H.; Xu, W.; Yuan, Z.; Shi, P.Y.; Zhang, B. Development and characterization of a stable egfp enterovirus 71 for antiviral screening. Antivir. Res. 2013, 97, 198–205. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Yamashita, Y.; Li, J.; Hanagata, N.; Minowa, T.; Takemura, T.; Koike, S. Scavenger receptor b2 is a cellular receptor for enterovirus 71. Nat. Med. 2009, 15, 798–801. [Google Scholar] [CrossRef]
- Li, X.; Fan, P.; Jin, J.; Su, W.; An, D.; Xu, L.; Sun, S.; Zhang, Y.; Meng, X.; Gao, F.; et al. Establishment of cell lines with increased susceptibility to ev71/ca16 by stable overexpression of scarb2. Virol. J. 2013, 10, 250. [Google Scholar] [CrossRef]
- Jin, J.; Ma, H.; Xu, L.; An, D.; Sun, S.; Huang, X.; Kong, W.; Jiang, C. Development of a coxsackievirus a16 neutralization assay based on pseudoviruses for measurement of neutralizing antibody titer in human serum. J. Virol. Meth. 2013, 187, 362–367. [Google Scholar] [CrossRef]
- Jin, J.; Xu, L.; Guo, S.; Sun, S.; Zhang, S.; Zhu, C.; Kong, W.; Jiang, C. Safe and objective assay of enterovirus 71 neutralizing antibodies via pseudovirus. Chem. Res. Chin. Univ. 2012, 28, 91–95. [Google Scholar]
- Troupin, C.; Dehee, A.; Schnuriger, A.; Vende, P.; Poncet, D.; Garbarg-Chenon, A. Rearranged genomic rna segments offer a new approach to the reverse genetics of rotaviruses. J. Virol. 2010, 84, 6711–6719. [Google Scholar] [CrossRef]
- Lu, J.; Yi, L.; Zhao, J.; Yu, J.; Chen, Y.; Lin, M.C.; Kung, H.F.; He, M.L. Enterovirus 71 disrupts interferon signaling by reducing the level of interferon receptor 1. J. Virol. 2012, 86, 3767–3776. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Marschall, M.; Freitag, M.; Weiler, S.; Sorg, G.; Stamminger, T. Recombinant green fluorescent protein-expressing human cytomegalovirus as a tool for screening antiviral agents. Antimicrob. Agents Chemother. 2000, 44, 1588–1597. [Google Scholar] [CrossRef]
- Zuo, J.; Quinn, K.K.; Kye, S.; Cooper, P.; Damoiseaux, R.; Krogstad, P. Fluoxetine is a potent inhibitor of coxsackievirus replication. Antimicrob. Agents Chemother. 2012, 56, 4838–4844. [Google Scholar] [CrossRef]
- Pirrone, V.; Passic, S.; Wigdahl, B.; Rando, R.F.; Labib, M.; Krebs, F.C. A styrene-alt-maleic acid copolymer is an effective inhibitor of r5 and x4 human immunodeficiency virus type 1 infection. J. Biomed. Biotechnol. 2010, 2010, 1–11. [Google Scholar]
- Nijhuis, M.; van Maarseveen, N.; Schuurman, R.; Verkuijlen, S.; de Vos, M.; Hendriksen, K.; van Loon, A.M. Rapid and sensitive routine detection of all members of the genus enterovirus in different clinical specimens by real-time pcr. J. Clin. Microbiol. 2002, 40, 3666–3670. [Google Scholar] [CrossRef]
- Zhang, J.H. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
- Hung, H.C.; Chen, T.C.; Fang, M.Y.; Yen, K.J.; Shih, S.R.; Hsu, J.T.; Tseng, C.P. Inhibition of enterovirus 71 replication and the viral 3d polymerase by aurintricarboxylic acid. J. Antimicrob. Chemother 2010, 65, 676–683. [Google Scholar] [CrossRef]
- Chang, J.S.; Wang, K.C.; Chiang, L.C. Sheng-ma-ge-gen-tang inhibited enterovirus 71 infection in human foreskin fibroblast cell line. J. Ethnopharmacol. 2008, 119, 104–108. [Google Scholar] [CrossRef]
- Ho, H.Y.; Cheng, M.L.; Weng, S.F.; Leu, Y.L.; Chiu, D.T. Antiviral effect of epigallocatechin gallate on enterovirus 71. J. Agr. Food Chem. 2009, 57, 6140–6147. [Google Scholar] [CrossRef]
- Wang, C.Y.; Huang, S.C.; Zhang, Y.; Lai, Z.R.; Kung, S.H.; Chang, Y.S.; Lin, C.W. Antiviral ability of kalanchoe gracilis leaf extract against enterovirus 71 and coxsackievirus a16. Evid. base. Compl. Alternative Med. 2012, 2012, 503165. [Google Scholar]
- Yang, Y.; Xiu, J.; Liu, J.; Zhang, L.; Li, X.; Xu, Y.; Qin, C.; Zhang, L. Chebulagic acid, a hydrolyzable tannin, exhibited antiviral activity in vitro and in vivo against human enterovirus 71. Int. J. Mol. Sci. 2013, 14, 9618–9627. [Google Scholar]
- Campagnola, G.; Gong, P.; Peersen, O.B. High-throughput screening identification of poliovirus rna-dependent rna polymerase inhibitors. Antivir. Res. 2011, 91, 241–251. [Google Scholar] [CrossRef]
- Falah, N.; Violot, S.; Decimo, D.; Berri, F.; Foucault-Grunenwald, M.L.; Ohlmann, T.; Schuffenecker, I.; Morfin, F.; Lina, B.; Riteau, B.; et al. Ex vivo and in vivo inhibition of human rhinovirus replication by a new pseudosubstrate of viral 2a protease. J. Virol. 2012, 86, 691–704. [Google Scholar] [CrossRef]
- Lee, J.C.; Shih, S.R.; Chang, T.Y.; Tseng, H.Y.; Shih, Y.F.; Yen, K.J.; Chen, W.C.; Shie, J.J.; Fang, J.M.; Liang, P.H.; et al. A mammalian cell-based reverse two-hybrid system for functional analysis of 3c viral protease of human enterovirus 71. Anal. Biochem. 2008, 375, 115–123. [Google Scholar] [CrossRef]
- Puig-Basagoiti, F.; Deas, T.S.; Ren, P.; Tilgner, M.; Ferguson, D.M.; Shi, P.Y. High-throughput assays using a luciferase-expressing replicon, virus-like particles, and full-length virus for west nile virus drug discovery. Antimicrob. Agents Chemother. 2005, 49, 4980–4988. [Google Scholar] [CrossRef]
- Phillips, T.; Jenkinson, L.; McCrae, C.; Thong, B.; Unitt, J. Development of a high-throughput human rhinovirus infectivity cell-based assay for identifying antiviral compounds. J. Virol. Meth. 2011, 173, 182–188. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Shimizu, M.; Hiyama, Y.; Itoh, K.; Hashimoto, Y.; Nakayama, M.; Horie, T.; Morita, N. Antiviral activity of natural occurring flavonoids in vitro. Chem. Pharm. Bull. (Tokyo) 1985, 33, 3881–3886. [Google Scholar] [CrossRef]
- Vrijsen, R.; Everaert, L.; Boeye, A. Antiviral activity of flavones and potentiation by ascorbate. J. Gen. Virol. 1988, 69, 1749–1751. [Google Scholar] [CrossRef]
- Mehla, R.; Bivalkar-Mehla, S.; Chauhan, A. A flavonoid, luteolin, cripples hiv-1 by abrogation of tat function. PLoS One 2011, 6, e27915. [Google Scholar]
- Manvar, D.; Mishra, M.; Kumar, S.; Pandey, V.N. Identification and evaluation of anti hepatitis c virus phytochemicals from eclipta alba. J. Ethnopharmacol. 2012, 144, 545–554. [Google Scholar] [CrossRef]
- Seelinger, G.; Merfort, I.; Schempp, C.M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Medica 2008, 74, 1667–1677. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, L.; Su, W.; Jin, J.; Chen, J.; Li, X.; Zhang, X.; Sun, M.; Sun, S.; Fan, P.; An, D.; et al. Identification of Luteolin as Enterovirus 71 and Coxsackievirus A16 Inhibitors through Reporter Viruses and Cell Viability-Based Screening. Viruses 2014, 6, 2778-2795. https://doi.org/10.3390/v6072778
Xu L, Su W, Jin J, Chen J, Li X, Zhang X, Sun M, Sun S, Fan P, An D, et al. Identification of Luteolin as Enterovirus 71 and Coxsackievirus A16 Inhibitors through Reporter Viruses and Cell Viability-Based Screening. Viruses. 2014; 6(7):2778-2795. https://doi.org/10.3390/v6072778
Chicago/Turabian StyleXu, Lin, Weiheng Su, Jun Jin, Jiawen Chen, Xiaojun Li, Xuyuan Zhang, Meiyan Sun, Shiyang Sun, Peihu Fan, Dong An, and et al. 2014. "Identification of Luteolin as Enterovirus 71 and Coxsackievirus A16 Inhibitors through Reporter Viruses and Cell Viability-Based Screening" Viruses 6, no. 7: 2778-2795. https://doi.org/10.3390/v6072778
APA StyleXu, L., Su, W., Jin, J., Chen, J., Li, X., Zhang, X., Sun, M., Sun, S., Fan, P., An, D., Zhang, H., Zhang, X., Kong, W., Ma, T., & Jiang, C. (2014). Identification of Luteolin as Enterovirus 71 and Coxsackievirus A16 Inhibitors through Reporter Viruses and Cell Viability-Based Screening. Viruses, 6(7), 2778-2795. https://doi.org/10.3390/v6072778