Alphavirus-Based Vaccines
Abstract
:1. Introduction
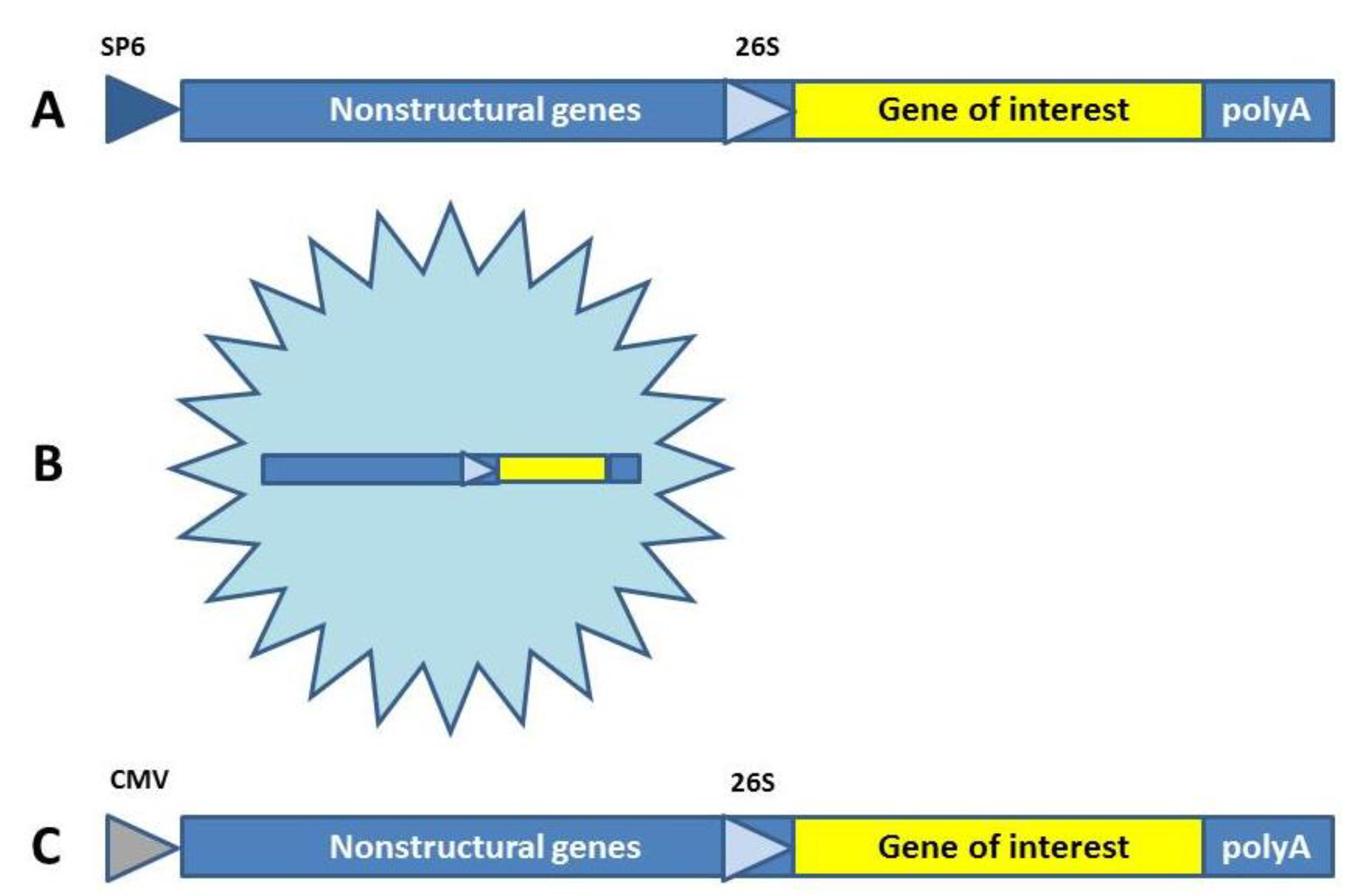
2. Viral Vaccine Approaches
| Virus | Target | Vector/Delivery | Immunization | Response | Reference |
|---|---|---|---|---|---|
| BVDV | E2 | VEE/Particles | Calf | BVDV protection | [23] |
| NS3 (p80) | SFV/DNA | Mouse | CTL, CMI | [24] | |
| CMV | gB/pp65-1E1 | VEE/Particles | Human Phase I | Neutralizing Abs | [25] |
| CSFV | E2 | SFV/DNA | Swine | CSFV protection | [26] |
| Dengue | PrME, E85 | VEE/Particles | Macaque | Dengue protection | [27] |
| Ebola | NP | VEE/Particles | Mouse | Ebola protection | [21] |
| NP, GP | VEE/Particles | Guinea pig | Ebola protection | [22] | |
| VP24, 30, 35, 30 | VEE/Particles | Mouse | Ebola protection | [28] | |
| Hepatitis B | cAg | SIN/DNA | Mouse | Specific Abs | [29] |
| sAg | SIN/DNA | Mouse | Specific Abs | [29] | |
| Hepatitis C | cAg | SFV/Particles, DNA | Mouse | CTL | [30] |
| NS3 | SFV/Particles | Mouse | Cellular | [31] | |
| nsPs | SFV/Particles | Mouse | CD8+ T-cell response | [32] | |
| HeV | Glycoprotein | VEE/Particles | Mouse | Neutralizing Abs | [33] |
| HIV-1 | Env | SFV/Particles | Mouse | Humoral | [17] |
| gp41 | SFV/Particles | Mouse | Monoclonal Abs | [18] | |
| MA/CA | VEE/Particles | Mouse | Humoral, CTL | [19] | |
| HPV | 16E7 | SFV/DNA | Mouse | CTL | [34] |
| 16E7-VP22 | SIN/Particles | Mouse | CD8+ T-cell response | [35] | |
| HSV-1 | gpB | SIN/Particles | Mouse | HSV protection | [36] |
| gpB | SIN/DNA | Mouse | CTL, protection | [37] | |
| IBDV | VP2 | SFV/Particles, DNA | Chicken | Specific Abs | [38] |
| Influenza | HA | SFV/Particles | Mouse | Systemic response | [15] |
| HA | VEE/Particles | Chicken | Influenza protection | [16] | |
| HA | VEE/Particles | Swine | Influenza protection | [20] | |
| NP | SFV/Particles, RNA | Mouse | Humoral, CTL | [39] | |
| ISAV | HE | SAV/Particles | Salmon | ISAV protection | [40] |
| JEV | prM-E, NS1-2A | SIN/Particles | Mouse | JEV Abs | [41] |
| Lassa | N | VEE/Particles | Mouse | Immune response | [42] |
| LIV | prME | SFV/Particles | Mouse | LIV protection | [43] |
| prME, NS1 | SFV/Particles | Sheep | LIV protection | [44] | |
| MBGV | GP, NP, VP35 | VEE/Particles | Guinea pig | MBGV protection | [45] |
| GP, NP | VEE/Particles | Macaque | MBGV protection | [46] | |
| Measles | HA, FUd | SIN/DNA | Mouse | Measles protection | [47] |
| HA, FUd | SIN-VEE/Particles | Macaque | Measles protection | [48] | |
| MVE | prME, E | SFV/Particles | Mouse | Neutralizing Abs | [49] |
| NiV | Glycoproteins | VEE/Particles | Mouse | Neutralizing Abs | [33] |
| NLV | VLP | VEE/Particles | Mouse | Immune response | [50] |
| Rabies | G | SIN/DNA | Mouse | Rabies protection | [51] |
| RSV | F, G | SFV/DNA, RNA | Mouse | RSV protection | [52] |
| F, G | SFV/Particles | Mouse | RSV protection | [53] | |
| RVFV | Gn | VEE/Particles | Mouse | RVFV protection | [54] |
| SARS-CoV | Glycoprotein | VEE/Particles | Mouse | SARS-CoV protection | [55] |
| SEOV | M, S | SIN/Particles, DNA | Hamster | SEOV protection | [56] |
| SHIV | Env | SFV/Particles | Macaque | T-cell proliferative response | [57] |
| SUDV | GP | VEE/Particles | Primate | SUDV protection | [58] |
| Vaccinia | A33R, B5R | VEE/Particles | Mouse | Vaccinia protection | [59] |
3. Non-Viral Targets
| Agent | Target | Vector/Delivery | Immunization | Response | Reference |
|---|---|---|---|---|---|
| B. anthracis | PA | SIN/Particles | Mouse | B. anthracis protection | [70] |
| B. abortus | IF3 | SFV/Particles | Mouse | Brucella protection | [69] |
| C. botulinum | BoNTA-Hc | SFV/DNA | Mouse | Abs, lymphoproliferative response | [68] |
| Malaria | CS | SIN/Particles | Mouse | Malaria protection | [71] |
| M. tuberculosis | Ag85A | SIN/DNA | Mouse | Protection | [67] |
| P. falciparum | Ag Pf332 | SFV/Particles-RNA | Mouse | Immunological memory | [66] |
| Prion | NP | SFV/Particles | Mouse | Monoclonal Abs | [72] |
| Staphylococcus | enterotoxin B | VEE/Particles | Mouse | Protection | [73] |
4. Tumor Vaccine Approaches
| Target | Gene | Vector/Delivery | Immunization | Response | Reference |
|---|---|---|---|---|---|
| Brain tumor | IL-12 | SFV/Particles | Mouse | Immunogenicity | [77] |
| Endostatin | SFV/Particles | Mouse | Inhibited tumor growth | [78] | |
| LacZ | SFV/Particles | Mouse | Tumor protection | [79] | |
| gp100, IL-18 | SIN/DNA | Mouse | Tumor protection | [80] | |
| HER2/neu | SIN/DNA | Mouse | Tumor protection | [81] | |
| HER2/neu | SIN/DNA | Mouse | Tumor protection | [82] | |
| HER2/neu | SIN/DNA, paclitaxel | Mouse | Tumor regression | [83] | |
| Neu | VEE/Particles | Rat | Anti-tumor immunity | [84] | |
| Neu | VEE /Particles, DCs | Mouse | Tumor regression | [85] | |
| Cervical cancer | HPVE6-E7 | SFV/Particles | Mouse | Tumor protection | [86] |
| HPVE6-E7 | SFV/Particles | Mouse | Tumor regression | [87] | |
| HPV-CRT | SIN/Particles | Mouse | Tumor protection | [88] | |
| HPVE7 | VEE/Particles | Mouse | Tumor protection | [89] | |
| HPVE6E7+IL12 | SFV/Particles | Mouse | Anti-tumor activity | [90] | |
| HPVE7-VP22 | SIN/Particles | Mouse | CD8+ T-cell response | [91] | |
| Colon cancer | SFV vector | SFV/Particles | Mouse | Anti-tumor effect | [92] |
| Endothelial | VEGFR-2 | SFV/Particles | Mouse | Antibody response | [93] |
| Glioma | B16, 203 | SFV/Particles | Mouse | Tumor protection | [94] |
| Kidney cancer | IL-12 | SFV/Encapsulated particles | Human Phase I | 5-fold IL-12 expression | [95] |
| Melanoma | MDA/trp-2 | VEE/Particles | Mouse | Therapeutic effect | [76] |
| IL-12 | SFV/Particles | Mouse | Tumor eradication | [96] | |
| IL-12 | SFV/Encapsulated particles | Human Phase I | 5-fold IL-12 expression | [95] | |
| MUC18/MCAM | SIN/DNA | Mouse | Tumor protection | [97] | |
| Metastatic | CEA | VEE/Particles | Human Phase I | CEA Abs, extended survival | [98] |
| PSMA | VEE/Particles | Human Phase I | PSMA Abs | [99] | |
| Prostate cancer | PSMA | VEE/Particles | Mouse | Tumor response | [100] |
| STEAP | VEE/DNA | Mouse | Anti-tumor response | [101] | |
| PSCA | VEE/Particles | Mouse | Tumor protection | [102] | |
| Tumor | β-galactosidase | SFV/RNA | Mouse | Tumor protection | [74] |
| IL-12 | SFV/Particles | Mouse | Tumor protection | [103] | |
| Tumor antigen | MHC class II | SFV/Particles-DNA | Mouse | Immunogenicity | [104] |
| P815 | SFV/Particles | Mouse | CTL, tumor protection | [105] | |
| trp-1 | SIN/DNA | Mouse | Antitumor activity | [91] |
5. Vaccines against Alphaviruses
| Virus | Gene | Vector Delivery | Immunization | Response | Reference |
|---|---|---|---|---|---|
| CHIK | TSI-GSD-218 | CHIK infection | Human Phase II | Neutralizing Abs | [115] |
| CHIK | Glycoprotein | CHIK infection | Macaques | Neutralizing Abs | [116] |
| CHIK | IRES | CHIK infection | Vero cells | Mosquito resistance | [117] |
| CHIK | C, E1 VLPs | CHIK infection | Primates | CHIK protection | [118] |
| CHIK | nsP3, E1 siRNA | CHIK infection | Vero cells | Reduced CHIK titer | [119] |
| CHIK | miRNAs | CHIK infection | Mouse | Reduced CHIK replication | [120] |
| EEE | EEE/WEE | EEE infection | Mouse | EEE protection | [114] |
| VEE | VEE att | VEE infection | Mouse | VEE protection | [112] |
| VEE | VEE V3526 | VEE infection | Mouse | VEE protection | [113] |
| VEE | VEE TC-83 | VEE infection | Mouse | VEE protection | [121] |
| VEE | 26S | VEE infection | Macaques | VEE protection | [122] |
| VEE | CHIK genes | Chimeric VEE-CHIK | Mosquito | Reduced infectivity | [123] |
| VEE | RdRp miRNA | VEE infection | BHK cells | Inhibition of VEE replication | [124] |
| WNV | WNV att | WNV Nanopatch | Mouse | Abs | [125] |
6. Clinical Trials for Alphavirus Vaccines
7. Conclusions
Conflicts of Interest
References and Notes
- Strauss, J.H.; Strauss, E.G. The Alphaviruses: Gene Expression, Replication and Evolution. Micobiol. Rev. 1994, 58, 491–562. [Google Scholar]
- Kelvin, A.A. Outbreak of Chikungunya in the Republic of Congo and the global picture. J. Infect. Dev. Countries 2011, 5, 441–444. [Google Scholar]
- Mathiot, C.C.; Grimaud, G.; Garry, P. An outbreak of Semliki Forest virus infections in Central African Republic. Am. J. Trop. Med. Hyg. 1990, 42, 386–393. [Google Scholar]
- Weaver, S.C.; Salas, R.; Rico-Hesse, R. Remergence of epidemic Venezuelan equine encephalomyelitis in South America. VEE Study Group. Lancet 1996, 348, 436–440. [Google Scholar] [CrossRef]
- Liljeström, P.; Garoff, H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology 1991, 9, 1356–1361. [Google Scholar] [CrossRef]
- Xiong, C.; Levis, R.; Shen, P.; Schlesinger, S.; Rice, C.M.; Huang, H.V. Sindbis virus: An efficient, broad host range vector for gene expression in animal cells. Science 1989, 243, 1188–1191. [Google Scholar]
- Davis, N.L.; Brown, K.W.; Johnston, R.E. In vitro synthesis of infectious Venezuelan equine encephalitis virus RNA from a cDNA clone: Analysis of a viable deletion mutant. Virology 1989, 171, 189–204. [Google Scholar] [CrossRef]
- Smerdou, C.; Liljeström, P. Two-helper RNA system for production of recombinant Semliki Forest virus particles. J. Virol. 1999, 73, 1092–1098. [Google Scholar]
- Lundstrom, K.; Schweizer, C.; Rotmann, D.; Hermann, D.; Schneider, E.M. Semliki Forest virus vectors: efficient vehicles for in vitro and in vivo gene delivery. FEBS Lett. 2001, 504, 99–103. [Google Scholar] [CrossRef]
- Lundstrom, K. Semliki Forest virus vectors for rapid and high-level expression of integral membrane proteins. Biochim. Biophys. Acta 2003, 1610, 90–96. [Google Scholar] [CrossRef]
- Ehrengruber, M.U.; Lundstrom, K.; Schweitzer, C.; Heuss, C.; Schlesinger, S.; Gähwiler, B.H. Recombinant Semliki Forest virus and Sindbis virus infect efficiently neurons in hippocampal slice cultures. Proc. Natl. Acad. Sci. USA 1999, 96, 7041–7046. [Google Scholar]
- Lundstrom, K.; Richards, J.G.; Pink, J.R.; Jenck, F. Efficient in vivo expression of a reporter gene in rat brain after injection of recombinant replication-deficient Semliki Forest virus. Gene Ther. Mol. Biol. 1999, 3, 15–23. [Google Scholar]
- Lundstrom, K. Alphavirus vectors for vaccine production and gene therapy. Exp. Rev. Vacc. 2003, 2, 447–459. [Google Scholar] [CrossRef]
- Tonkin, D.R.; Whitmore, R.; Johnston, R.E.; Barro, M. Infected dendritic cells are sufficient to mediate the adjuvant activity generated by Venezuelan equine encephalitis virus replication particles. Vaccine 2012, 30, 4532–4542. [Google Scholar] [CrossRef]
- Malone, J.G.; Berglund, P.J.; Liljestrom, P.; Rhodes, G.H.; Malone, R.W. Mucosal immune responses associated with polynucleotide vaccination. Behring Inst. Mitt. 1997, 98, 63–72. [Google Scholar]
- Schultz-Cherry, S.; Dybing, J.K.; Davis, N.L.; Williamson, C.; Suarez, D.L.; Johnston, R.; Perdue, M.L. Influenza virus (A/HK/156/97) hemagglutinin expressed by an alphavirus replicon system protects against lethal infection with Hong Kong-origin H5N1 viruses. Virology 2000, 278, 55–59. [Google Scholar] [CrossRef]
- Brand, D.; Lemiale, F.; Turbica, I.; Buzelay, L.; Brunet, S.; Branet, F. Comparative analysis of humoral immune responses to HIV type 1 envelope glycoproteins in mice immunized with a DNA vaccine, recombinant Semliki Forest virus RNA, or recombinant Semliki Forest virus particles. AIDS Res. Hum. Retroviruses 1998, 14, 1369–1377. [Google Scholar] [CrossRef]
- Giraud, A.; Ataman-Onal, Y.; Battail, N.; Piga, N.; Brand, D.; Mandrand, B.; Verrier, B. Generation of monoclonal antibodies to native human immunodeficiency virus type 1 envelope glycoprotein by immunization of mice with naked RNA. J. Virol. Methods 1999, 79, 75–84. [Google Scholar] [CrossRef]
- Caley, I.J.; Betts, M.R.; Irlbeck, D.M.; Davis, N.L.; Swanstrom, R.; Frelinger, J.A.; Johnston, R.E. Humoral, mucosal, and cellular immunity in response to a human immunodeficiency virus type 1 immunogen expressed by a Venezuelan equine encephalitis virus vaccine vector. J. Virol. 1997, 71, 3031–3038. [Google Scholar]
- Bosworth, B.; Erdman, M.M.; Stine, D.L.; Harris, I.; Irwin, C.; Jens, M.; Loynachan, A.; Kamrud, K.; Harris, D.L. Replicon particle vaccine protects swine against influenza. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, e99–e103. [Google Scholar]
- Wilson, J.A.; Hart, M.K. Protection from Ebola virus mediated by cytotoxic T-lymphocytes specific for the viral nucleoprotein. J. Virol. 2001, 75, 2660–2664. [Google Scholar] [CrossRef]
- Pushko, P.; Bray, M.; Ludwig, G.V.; Parker, M.; Schmaljohn, A.; Sanchez, A.; Jahrling, P.B.; Smith, J.F. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine 2000, 19, 142–153. [Google Scholar] [CrossRef]
- Loy, J.D.; Grander, J.; Mogler, M.; Vander Veen, R.; Ridpath, J; Harris, D.H.; Kamrud, K. Development and evaluation of a replicon particle vaccine expressing the E2 glycoprotein of bovine viral diarrhea virus (BVDV) in cattle. Virol. J. 2013, 10, e35. [Google Scholar] [CrossRef]
- Reddy, J.R.; Kwang, J.; Varthakavi, V.; Lechtenberg, K.F.; Minocha, H.C. Semiliki forest virus vector carrying the bovine viral diarrhea virus NS3 (p80) cDNA induced immune responses in mice and expressed BVDV protein in mammalian cells. Comp. Immunol. Microbiol. Infect. Dis. 1999, 22, 231–246. [Google Scholar]
- Bernstein, D.I.; Reap, E.A.; Katen, K.; Watson, A.; Smith, K.; Norberg, P.; Olmsted, R.A.; Hoeper, A.; Morris, J.; Negri, S.; et al. Randomized, double-blind, Phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine 2009, 28, 484–493. [Google Scholar] [CrossRef]
- Sun, Y.; Li, N.; Li, H.Y.; Li, M.; Qiu, H.J. Enhanced immunity against classical swine fever in pigs induced by prime-boost immunization using an alphavirus replicon-vectored DNA vaccine and a recombinant adenovirus. Vet. Immunol. Immunopathol. 2010, 137, 20–27. [Google Scholar] [CrossRef]
- White, L.J.; Sariol, C.A.; Mattocks, M.D.; Wahala, M.P.B.W.; Yingsiwaphat, V.; Collier, M.L.; Whitley, J.; Mikkelsen, R.; Rodriguez, I.V.; Martinez, M.I.; et al. An alphavirus vector-based tetravalent dengue vaccine induces a rapid and protective immune response in macaques that differs qualitatively from immunity induced by live virus infection. J. Virol. 2013, 87, 3409–3424. [Google Scholar] [CrossRef]
- Wilson, J.A.; Bray, M.; Bakken, R.; Hart, M.K. Vaccine potential of Ebola virus VP24, VP30, VP35, and VP40 proteins. Virology 2001, 286, 384–390. [Google Scholar] [CrossRef]
- Driver, D.A.; Latham, E.M.; Polo, J.M.; Belli, B.A.; Banks, T.A.; Chada, S.; Brumm, D.; Chang, S.M.; Mento, S.J.; Dolly, D.J.; et al. Layered amplification of gene expression with a DNA gene delivery system. Ann. N. Y. Acad. Sci. 1995, 772, 261–264. [Google Scholar] [CrossRef]
- Vidalin, O.; Fournillier, A.; Renard, N.; Chen, M.; Depla, E.; Boucreux, D.; Brinster, C.; Baumert, T.; Nakano, I.; Fukuda, Y.; et al. Use of conventional or replicating nucleic acid-based vaccines and recombinant Semliki forest virus-derived particles for the induction of immune responses against hepatitis C virus core and E2 antigens. Virology 2000, 276, 259–270. [Google Scholar] [CrossRef]
- Brinster, C.; Chen, M.; Boucreux, D.; Paranhos-Baccala, G.; Liljeström, P.; Lemmonier, F.; Inchasupé, G. Hepatitis C virus non-structural protein 3-specific cellular immune responses following single or combined immunization with DNA or recombinant Semliki Forest virus particles. J. Gen. Virol. 2002, 83, 369–381. [Google Scholar]
- Ip, P.P.; Boerma, A.; Regts, J.; Meijerhof, T.; Wilschut, J.; Nijman, H.V.; Daemen, T. Alphavirus-based vaccines encoding non-structural proteins of Hepatitis C virus induce robust and protective T cell responses. Mol. Ther. 2013. [Google Scholar] [CrossRef]
- Defang, G.N.; Khetawat, D.; Broder, C.C.; Quinnan, G.V., Jr. Induction of neutralizing antibodies to Hendra and Nipah glycoproteins using a Venezuelan equine encephalitis virus in vivo expression system. Vaccine 2010, 29, 212–220. [Google Scholar] [CrossRef]
- Hsu, K.F.; Hung, C.F.; Cheng, W.F.; He, L.; Slater, L.A.; Ling, M.; Wu, T.C. Enhancement of suicidal DNA vaccine potency by linking Mycobacterium tuberculosis heat shock protein 70 to an antigen. Gene Ther. 2001, 8, 376–383. [Google Scholar]
- Cheng, W.F.; Hung, C.H.; Chai, C.Y.; Hsu, K.F.; He, L.; Ling, M.; Slater, L.A.; Roden, R.B.; Wu, T.C. Enhancement of sindbis virus self-replicating RNA vaccine potency by linkage of herpes simplex virus type 1 VP22 protein to antigen. J. Virol. 2001, 75, 2368–2376. [Google Scholar] [CrossRef]
- Schlesinger, S.; Dubensky, T.W. Alphavirus vectors for gene expression and vaccines. Curr. Opin. Biotechnol. 1999, 10, 434–439. [Google Scholar] [CrossRef]
- Hariharan, M.J.; Driver, D.A.; Townsend, K.; Brumm, D.; Polo, J.M.; Belli, B.A.; Catton, D.J.; Hsu, D.; Mittelstaedt, D.; McCormack, J.E.; et al. DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector. J. Virol. 1998, 72, 950–958. [Google Scholar]
- Phenix, K.V.; Wark, K.; Luke, C.J.; Skinner, M.A.; Smyth, J.A.; Mawhinney, K.A.; Todd, D. Recombinant Semliki Forest virus vector exhibits potential for avian virus vaccine development. Vaccine 2001, 19, 3116–3123. [Google Scholar] [CrossRef]
- Zhou, X.; Berglund, P.; Rhodes, G.; Parker, S.E.; Jondal, M.; Liljestrom, P. Self-replicating Semliki Forest virus RNA as recombinant vaccine. Vaccine 1994, 12, 1510–1514. [Google Scholar] [CrossRef]
- Wolf, A.; Hodneland, K.; Frost, P.; Braaen, S.; Rimstad, E. A hemagglutinin-esterase-expressing salmonid alphavirus replicon protects Atlantic salmon (Salmo salar) against infectious salmon anemia (ISA). Vaccine 2013, 31, 661–669. [Google Scholar] [CrossRef]
- Pugachev, K.V.; Mason, P.W.; Shope, R.E.; Frey, T.K. Double-subgenomic Sindbis virus recombinants expressing immunogenic proteins of Japanese encephalitis virus induce significant protection in mice against lethal JEV infection. Virology 1995, 212, 587–594. [Google Scholar]
- Pushko, P.; Parker, M.; Ludwig, G.V.; Davis, N.L.; Johnston, R.E.; Smith, J.F. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: Expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology 1997, 239, 389–401. [Google Scholar]
- Fleeton, M.N.; Liljeström, P.; Sheahan, B.J.; Atkins, G.J. Recombinant Semliki Forest virus particles expressing louping ill virus antigens induce a better protective response than plasmid-based DNA vaccines or an inactivated whole particle vaccine. J. Gen. Virol. 2000, 81, 749–758. [Google Scholar]
- Morris-Downes, M.M.; Phenix, K.V.; Smyth, J.; Sheahan, B.J.; Lileqvist, S.; Mooney, D.A.; Liljestrom, P.; Todd, D.; Atkins, G.J. Semliki Forest virus-based vaccines: Persistence, distribution and pathological analysis in two animal systems. Vaccine 2001, 19, 1978–1988. [Google Scholar] [CrossRef]
- Hevey, M.; Negley, D.; VanderZanden, L.; Tammariello, R.F.; Geisbert, J.; Smalljohn, C.; Smith, J.F.; Jahrling, P.B.; Smalljohn, A.L. Marburg virus vaccines: Comparing classical and new approaches. Vaccine 2001, 20, 586–593. [Google Scholar] [CrossRef]
- Hevey, M.; Negley, D.; Pushko, P.; Smith, J.; Schmaljohn, A. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology 1998, 251, 28–37. [Google Scholar] [CrossRef]
- Pasetti, M.F.; Ramirez, K.; Resendiz-Albor, A.; Ulmer, J.; Barry, E.M.; Levine, M.M. Sindbis virus-based measles DNA vaccines protect cotton rats against respiratory measles: Relevance of antibodies, mucosal and systemic antibody-secreting cells, memory B cells, and Th1-type cytokines as correlates of immunity. J. Virol. 2009, 83, 2789–2794. [Google Scholar] [CrossRef]
- Pan, C.H.; Greer, C.E.; Hauer, D.; Legg, H.S.; Lee, E.Y.; Bergen, M.J.; Lau, B.; Adams, R.J.; Polo, J.M.; Griffin, D.E. A chimeric alphavirus replicon particle vaccine expressing the hemagglutinin and fusion proteins protects juvenile and infant rhesus macaques from measles. J. Virol. 2010, 84, 3798–3807. [Google Scholar] [CrossRef]
- Colombage, G.; Hall, R.; Pavy, M.; Lobigs, M. DNA-based and alphavirus-vectored immunisation with prM and E proteins elicits long-lived and protective immunity against the flavivirus, Murray Valley encephalitis virus. Virology 1998, 250, 151–163. [Google Scholar] [CrossRef]
- Harrington, P.R.; Yount, B.; Johnston, R.E.; Davis, N.; Moe, C.; Baris, R.S. Systemic, mucosal, and heterotypic immune induction in mice inoculated with Venezuelan equine encephalitis replicons expressing Norwalk virus-like particles. J. Virol. 2002, 76, 730–742. [Google Scholar] [CrossRef]
- Saxena, S.; Dahiya, S.S.; Sonwane, A.A.; Patel, C.L.; Saini, M.; Rai, A.; Gupta, P.K. A sindbis virus replicon-based DNA vaccine encoding the rabies virus glycoprotein elicits immune responses and complete protection in mice from lethal challenge. Vaccine 2008, 26, 6592–6601. [Google Scholar] [CrossRef]
- Fleeton, M.N.; Chen, M.; Berglund, P.; Rhodes, G.; Parker, S.E.; Murphy, M.; Atkins, G.J.; Liljestrom, P. Self-replicative RNA vaccines elicit protection against influenza A virus, respiratory syncytial virus, and a tickborne encephalitis virus. J. Infect. Dis. 2001, 183, 1395–1398. [Google Scholar] [CrossRef]
- Chen, M.; Hu, K.F.; Rozell, B.; Orvell, C.; Morein, B.; Liljestrom, P. Vaccination with recombinant alphavirus or immune-stimulating complex antigen against respiratory syncytial virus. J. Immunol. 2002, 169, 3208–3216. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Heise, M.T.; Ross, T.M. Vaccination with DNA plasmids expressing Gn coupled to C3d or alphavirus replicons expressing gn protects mice against Rift Valley fever virus. PLoS Negl. Trop. Dis. 2010, 4, e725. [Google Scholar] [CrossRef]
- Sheahan, T.; Whitmore, A.; Long, K.; Ferris, M.; Rockx, B.; Funkhouser, W.; Donaldson, E.; Gralinski, L.; Collier, M.; Heise, M.; et al. Successful vaccination strategies that protect aged mice from lethal challenge from influenza virus and heterologous severe acute respiratory syndrome coronavirus. J. Virol. 2011, 85, 217–230. [Google Scholar] [CrossRef]
- Kamrud, K.I.; Hooper, J.W.; Elgh, F.; Schmaljohn, C.S. Comparison of the protective efficacy of naked DNA, DNA-based Sindbis replicon, and packaged Sindbis replicon vectors expressing Hantavirus structural genes in hamsters. Virology 1999, 263, 209–219. [Google Scholar] [CrossRef]
- Berglund, P.; Quesada-Rolander, M.; Putkonen, P.; Biberfeld, G.; Thorstensson, R.; Liljestrom, P. Outcome of immunization of cynomolgus monkeys with recombinant Semliki Forest virus encoding human immunodeficiency virus type 1 envelope protein and challenge with a high dose of SHIV-4 virus. AIDS Res. Hum. Retroviruses 1997, 13, 1487–1495. [Google Scholar] [CrossRef]
- Herbert, A.S.; Kuehne, A.I.; Barth, J.F.; Ortiz, R.A.; Nichols, D.K.; Zak, S.E.; Stonier, S.W.; Muhammad, M.A.; Bakken, R.R.; Prugar, L.I.; et al. Venezuelan equine encephalitis virus replicon particle vaccine protects nonhuman primates from intramuscular and aerosol challenge with ebolavirus. J. Virol. 2013, 87, 4952–4964. [Google Scholar] [CrossRef]
- Hooper, J.W.; Ferro, A.M.; Golden, J.W.; Silvera, P.; Dudek, J.; Alterson, K.; Custer, M.; Rivers, B.; Morris, J.; Owens, G.; et al. Molecular smallpox vaccine delivered by alphavirus replicons elicits protective immunity in mice and non-human primates. Vaccine 2009, 28, 494–511. [Google Scholar] [CrossRef]
- Yang, S.G.; Wo, J.E.; Li, M.W.; Mi, F.F.; Yu, C.B.; Lv, G.L.; Cao, H.C.; Lu, H.F.; Wang, B.H.; Zhu, H.; et al. Construction and cellular immune response induction of HA-based alphavirus replicon vaccines against human-avian influenza (H5N1). Vaccine 2009, 27, 7451–7458. [Google Scholar] [CrossRef]
- Xu, R.; Srivastava, I.K.; Greer, C.E.; Zarkikh, I.; Kraft, Z.; Kuller, L.; Polo, J.M.; Barnett, S.W.; Stamatatos, L. Characterization of immune responses elicited in macaques immunized sequentially with chimeric VEE/SIN alphavirus replicon particles expressing SIVGag and/or HIVEnv and with recombinant HIVgp140Env protein. AIDS Res. Hum. Retroviruses 2006, 22, 1022–1030. [Google Scholar]
- Barnett, S.W.; Burke, B.; Sun, Y.; Kan, E.; Legg, H.; Lian, Y.; Bost, K.; Zhou, F.; Goodsell, A.; Zur Megede, J.; et al. Antibody-mediated protection against mucosal simian-human immunodeficiency virus challenge of macaques immunized with alphavirus replicon particles and boosted with trimeric envelope glycoprotein in MF59 adjuvant. J. Virol. 2010, 84, 5975–5985. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, F.; Fang, L.; Xu, J.; Li, B.; Jiang, Y.; Chen, H.; Xiao, S. Enhanced immunogenicity induced by an alphavirus replicon-based pseudotyped baculovirus vaccine against porcine reproductive and respiratory syndrome virus. J. Virol. Methods 2013, 187, 251–258. [Google Scholar] [CrossRef]
- Wolf, A.; Hodneland, K.; Frost, P.; Hoeijmakers, M.; Rimstad, E. Salmonid alphavirus-based replicon vaccine against infectious salmon anemia (ISA): Impact on infectious route and interactions of the replicon vector. Fish Shellfish Immunol. 2014, 36, 383–392. [Google Scholar] [CrossRef]
- Xu, C.; Mutoloki, S.; Evensen, O. Superior protection against conferred by inactivated whole virus vaccine over subunit and DNA vaccines against salmonid alphavirus infection in Atlantic salmon (Salmon salar L). Vaccine 2012, 30, 3918–3928. [Google Scholar] [CrossRef]
- Andersson, C.; Vasconcelos, N.M.; Sievertzon, M.; Haddad, D.; Liljeqvist, S. Comparative immunization study using RNA and DNA constructs encoding a part of the Plasmodium falciparum antigen Pf332. Scand. J. Immunol. 2001, 54, 117–124. [Google Scholar] [CrossRef]
- Kirman, J.R.; Turon, T.; Su, H.; Li, A.; Kraus, C.; Polo, J.M.; Belisle, J.; Morris, S.; Seder, R.A. Enhanced immunogenicity to Mycobacterium tuberculosis by vaccination with an alphavirus plasmid replicon expressing antigen 85A. Infect. Immun. 2003, 71, 575–579. [Google Scholar] [CrossRef]
- Li, N.; Yu, Y.Z.; Yu, W.Y.; Sun, Z.W. Enhancement of the immunogenicity of DNA replicon vaccine of Clostridium botulinum neurotoxin serotype A by GM-CSF gene adjuvant. Immunopharmacol. Immunotoxicol. 2011, 33, 211–219. [Google Scholar] [CrossRef]
- Cabrera, A.; Sáez, D.; Céspedes, S.; Andrews, E.; Oñate, A. Vaccination with recombinant Semliki Forest virus particles expressing translation initiation factor 3 of Brucella abortus induces protective immunity in BALB/c mice. Immunobiology 2009, 214, 467–474. [Google Scholar] [CrossRef]
- Thomas, J.M.; Moen, S.T.; Gnade, B.T.; Vargas-Inchaustegui, D.A.; Foltz, S.M.; Suarez, G.; Heidner, H.W.; König, R.; Chopra, A.K.; Peterson, J.W. Recombinant Sindbis virus vectors designed to express protective antigen of Bacillus anthracis protect animals from anthrax and display synergy with ciprofloxacin. Clin. Vaccine Immunol. 2009, 16, 1696–1699. [Google Scholar] [CrossRef]
- Tsuji, M.; Bergmann, C.C.; Takita-Sonoda, Y.; Murata, K.; Rodrigues, E.G.; Nussenzweig, R.S.; Zavala, F. Recombinant Sindbis viruses expressing a cytotoxic T-lymphocyte epitope of a malaria parasite or of influenza virus elicit protection against the corresponding pathogen in mice. J. Virol. 1998, 72, 6907–6910. [Google Scholar]
- Krasemann, S.; Jürgens, T.; Bodemer, W. Generation of monoclonal antibodies against prion proteins with an unconventional nucleic acid-based immunization strategy. J. Biotechnol. 1999, 73, 119–129. [Google Scholar] [CrossRef]
- Lee, J.S.; Dyas, B.K.; Nystrom, S.S.; Lind, C.M.; Smith, J.F.; Ullrich, R.G. Immune protection against staphylococcal enterotoxin-induced toxic shock by vaccination with a Venezuelan equine encephalitis virus replicon. J. Infect. Dis. 2002, 185, 1192–1196. [Google Scholar] [CrossRef]
- Ying, H.; Zaks, T.Z.; Wang, R.F.; Irvine, K.R.; Kammula, U.S.; Marincola, F.M.; Leitner, W.W.; Restifo, N.P. Cancer therapy using a self-replicating RNA vaccine. Nat. Med. 1999, 5, 823–827. [Google Scholar] [CrossRef]
- Leitner, W.W.; Hwang, L.N.; deVeer, M.J.; Zhou, A.; Silverman, R.H.; Williams, B.R.; Dubensky, T.B.; Ying, H.; Restifo, N.P. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat. Med. 2003, 9, 33–39. [Google Scholar]
- Avogadri, F.; Merghoub, T.; Maughan, M.F.; Hirschhorn-Cymerman, D.; Morris, J.; Ritter, E.; Olmsted, R.; Houghton, A.N.; Wolchok, J.D. Alphavirus replicon particles expressing TRP-2 provide potent therapeutic effect on melanoma through activation of humoral and cellular immunity. PLoS One 2010, 5, e12670. [Google Scholar]
- Yamanaka, R.; Zullo, S.A.; Ramsey, J.; Yajima, N.; Tsuchiya, N.; Tanaka, R.; Blaese, M.; Xanthopoulos, K.G. Marked enhancement of antitumor immune responses in mouse brain tumor models by genetically modified dendritic cells producing Semliki Forest virus-mediated interleukin-12. J. Neurosurg. 2002, 97, 611–618. [Google Scholar] [CrossRef]
- Yamanaka, R.; Zullo, S.A.; Ramsey, J.; Onodera, M.; Tanaka, R.; Blaese, M.; Xanthopoulos, K.G. Induction of therapeutic antitumor antiangiogenesis by intratumoral injection of genetically engineered endostatin-producing Semliki Forest virus. Cancer Gene Ther. 2001, 8, 796–802. [Google Scholar]
- Yamanaka, R.; Zullo, S.A.; Tanaka, R.; Blaese, M.; Xanthopoulos, K.G. Enhancement of antitumor immune response in glioma models in mice by genetically modified dendritic cells pulsed with Semliki forest virus-mediated complementary DNA. J. Neurosurg. 2001, 94, 474–481. [Google Scholar] [CrossRef]
- Yamanaka, R.; Xanthopoulos, K.G. Induction of antigen-specific immune responses against malignant brain tumors by intramuscular injection of sindbis DNA encoding gp100 and IL-18. DNA Cell Biol. 2005, 24, 317–324. [Google Scholar] [CrossRef]
- Lachman, L.B.; Rao, X.M.; Kremer, R.H.; Ozpolat, B.; Kiriakova, G.; Price, J.E. DNA vaccination against neu reduces breast cancer incidence and metastasis in mice. Cancer Gene Ther. 2001, 8, 259–268. [Google Scholar]
- Wang, X.; Wang, J.P.; Rao, X.M.; Price, J.E.; Zhou, H.S.; Lachman, L.B. Prime-boost vaccination with plasmid and adenovirus gene vaccines control HER2/neu+ metastatic breast cancer in mice. Breast Cancer Res. 2005, 7, R580–R588. [Google Scholar] [CrossRef]
- Eralp, Y.; Wang, X.; Wang, J.P.; Maughan, M.F.; Polo, J.M.; Lachman, L.B. Doxorubicin and paclitaxel enhance the antitumor efficacy of vaccines directed against HER 2/neu in a murine mammary carcinoma model. Breast Cancer Res. 2004, 6, R275–R283. [Google Scholar] [CrossRef]
- Laust, A.K.; Sur, B.W.; Wang, K.; Hubby, B.; Smith, J.F.; Nelson, E.L. VRP immunotherapy targeting neu: Treatment efficacy and evidence for immunoediting in a stringent rat mammary tumor model. Breast Cancer Res. Treat. 2007, 106, 371–382. [Google Scholar] [CrossRef]
- Moran, T.P.; Burgents, J.E.; Long, B.; Ferrer, I.; Jaffee, E.M.; Tisch, R.M.; Johnston, R.E.; Serody, J.S. Alphaviral vector-transduced dendritic cells are successful therapeutic vaccines against neu-overexpressing tumors in wild-type mice. Vaccine 2007, 25, 6604–6612. [Google Scholar] [CrossRef]
- Daemen, T.; Regts, J.; Holtrop, M.; Wilschut, J. Immunization strategy against cervical cancer involving an alphavirus vector expressing high levels of a stable fusion protein of human papillomavirus 16 E6 and E7. Gene Ther. 2002, 9, 85–94. [Google Scholar]
- Daemen, T.; Riezebos-Brilman, A.; Regts, J.; Dontje, B.; van der Zee, A.; Wilschut, J. Superior therapeutic efficacy of alphavirus-mediated immunization against human papilloma virus type 16 antigens in a murine tumour model: Effects of the route of immunization. Antivir. Ther. 2004, 9, 733–742. [Google Scholar]
- Cheng, W.F.; Lee, C.N.; Su, Y.N.; Chai, C.Y.; Chang, M.C.; Polo, J.M.; Hung, C.F.; Wu, T.C.; Hsieh, C.Y.; Chen, C.A. Sindbis virus replicon particles encoding calreticulin linked to a tumor antigen generate long-term tumor-specific immunity. Cancer Gene Ther. 2006, 13, 873–885. [Google Scholar]
- Velders, M.P.; McElhiney, S.; Cassetti, M.C.; Eiben, G.L.; Higgins, T.; Kovacs, G.R.; Elmishad, A.G.; Kast, W.M.; Smith, L.R. Eradication of established tumors by vaccination with Venezuelan equine encephalitis virus replicon particles delivering human papillomavirus 16 E7 RNA. Cancer Res. 2001, 61, 7861–7867. [Google Scholar]
- Riezebos-Brilman, A.; Regts, J.; Chen, M.; Wilschut, J.; Daemen, T. Augmentation of alphavirus vector-induced human papilloma virus-specific immune and anti-tumour responses by co-expression of interleukin-12. Vaccine 2009, 27, 701–707. [Google Scholar] [CrossRef]
- Cheng, W.F.; Hung, C.F.; Hsu, K.F.; Chai, C.Y.; He, L.; Polo, J.M.; Slater, L.A.; Ling, M.; Wu, T.C. Cancer immunotherapy using Sindbis virus replicon particles encoding a VP22-antigen fusion. Hum. Gene Ther. 2002, 13, 553–568. [Google Scholar]
- Smyth, J.W.; Fleeton, M.N.; Sheahan, B.J.; Atkins, G.J. Treatment of rapidly growing K-BALB and CT26 mouse tumours using Semliki Forest virus and its derived vector. Gene Ther. 2005, 12, 147–159. [Google Scholar]
- Lyons, J.A.; Sheahan, B.J.; Galbraith, S.E.; Mehra, R.; Atkins, G.J.; Fleeton, M.N. Inhibition of angiogenesis by a Semliki Forest virus vector expressing VEGFR-2 reduces tumour growth and metastasis in mice. Gene Ther. 2007, 14, 503–513. [Google Scholar]
- Yamanaka, R.; Zullo, S.A.; Tanaka, R.; Ramsey, J.; Blaese, M.; Xanthopoulos, K.G. Induction of a therapeutic antitumor immunological response by intratumoral injection of genetically engineered Semliki Forest virus to produce interleukin-12. Neurosurg. Focus 2000, 9, e7. [Google Scholar]
- Lundstrom, K. Biology and application of alphaviruses in gene therapy. Gene Ther. 2005, 12, S92–S97. [Google Scholar] [CrossRef]
- Quetglas, J.I.; Dubrot, J.; Bezunartea, J.; Sanmamed, M.F.; Hervas-Stubbs, S.; Smerdou, C.; Melero, I. Immunotherapeutic synergy between anti-CD137 mAb and intratumoral administration of a cytopathic Semliki Forest virus encoding IL-12. Mol. Ther. 2012, 20, 1664–1675. [Google Scholar] [CrossRef]
- Leslie, M.C.; Zhao, Y.J.; Lachman, L.B.; Hwu, P.; Wu, G.J.; Bar-Eli, M. Immunization against MUC18/MCAM, a novel antigen that drives melanoma invasion and metastasis. Gene Ther. 2007, 14, 316–323. [Google Scholar]
- Morse, M.A.; Hobeika, A.C.; Osada, T.; Berglund, P.; Hubby, B.; Negri, S.; Niedzwiecki, G.; Devi, G.R.; Burnett, G.K.; Clay, T.M. An alphavirus vector overcomes the presence of neutralizing antibodies and elevated numbers of Tregs to induce immune responses in humans with advanced cancer. J. Clin. Invest. 2010, 120, 3234–3241. [Google Scholar] [CrossRef]
- Slovin, S.F.; Kehoe, M.; Durso, R.; Fernandez, C.; Olson, W.; Gao, J.P.; Israel, R.; Sher, H.I.; Morris, S. A phase I dose escalation trial of vaccine replicon particles (VRP) expressing prostate-specific membrane antigen (PSMA) in subjects with prostate cancer. Vaccine 2013, 31, 943–949. [Google Scholar] [CrossRef]
- Durso, R.J.; Andjelic, S.; Gardner, J.P.; Margitich, D.J.; Donovan, G.P.; Arrigale, R.R.; Wang, X.; Maughan, M.F.; Talarico, T.L.; Olmsted, R.A. A novel alphavirus vaccine encoding prostate-specific membrane antigen elicits potent cellular and humoral immune responses. Clin. Cancer Res. 2007, 13, 3999–4008. [Google Scholar] [CrossRef]
- Garcia-Hernandez, M.L.; Gray, A.; Hubby, B.; Kast, W.M. In vivo effects of vaccination with six-transmembrane epithelial antigen of the prostate: A candidate antigen for treating prostate cancer. Cancer Res. 2007, 67, 1344–1351. [Google Scholar] [CrossRef]
- Garcia-Hernandez, M.L.; Gray, A.; Hubby, B.; Klinger, O.J.; Kast, WM. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer Res. 2008, 68, 861–869. [Google Scholar] [CrossRef]
- Colmenero, P.; Chen, M.; Castaños-Velez, E.; Liljeström, P.; Jondal, M. Immunotherapy with recombinant SFV-replicons expressing the P815A tumor antigen or IL-12 induces tumor regression. Int. J. Cancer 2002, 98, 554–560. [Google Scholar] [CrossRef]
- Ying, H.; Zeng, G.; Black, K.L. Innovative cancer vaccine strategies based on the identification of tumour-associated antigens. BioDrugs 2001, 15, 819–831. [Google Scholar] [CrossRef]
- Colmenero, P.; Liljeström, P.; Jondal, M. Induction of P815 tumor immunity by recombinant Semliki Forest virus expressing the P1A gene. Gene Ther. 1999, 6, 1728–1733. [Google Scholar] [CrossRef]
- Leitner, W.W.; Ying, H.; Driver, D.A.; Dubensky, T.W.; Restifo, N.P. Enhancement of tumor-specific immune response with plasmid DNA replicon vectors. Cancer Res. 2000, 60, 51–55. [Google Scholar]
- Daemen, T.; Pries, F.; Bungener, L.; Kraak, M.; Regts, J.; Wilschut, J. Genetic immunization against cervical carcinoma: induction of cytotoxic T lymphocyte activity with a recombinant alphavirus vector expressing human papillomavirus type 16 E6 and E7. Gene Ther. 2000, 7, 1859–1866. [Google Scholar]
- Cheng, W.F.; Hung, C.F.; Hsu, K.F.; Chai, C.Y.; He, L.; Ling, M.; Slater, L.A.; Roden, R.B.; Wu, T.C. Enhancement of sindbis virus self-replicating RNA vaccine potency by targeting antigen to endosomal/lysosomal compartments. Hum. Gene Ther. 2001, 12, 235–252. [Google Scholar]
- Eiben, G.L.; Velders, M.P.; Schreiber, H.; Cassetti, M.C.; Pullen, J.K.; Smith, L.R.; Kast, W.M. Establishment of an HLA-A*0201 human papillomavirus type 16 tumor model to determine the efficacy of vaccination strategies in HLA-A*0201 transgenic mice. Cancer Res. 2002, 62, 5792–5799. [Google Scholar]
- Cassetti, M.C.; McElhiney, S.P.; Shahabi, V.; Pullen, J.K.; Le, Poole, I.; Eiben, G.L.; Smith, L.R.; Kast, W.M. Antitumor efficacy of Venezuelan equine encephalitis virus replicon particles encoding mutated HPV16 E6 and E7 genes. Vaccine 2004, 22, 520–527. [Google Scholar] [CrossRef]
- Yamanaka, R.; Tsuchiya, N.; Yajima, N.; Honma, J.; Hasegawa, H.; Tanaka, R.; Ramsey, J.; Blaese, R.M.; Xanthopoulos, K.G. Induction of an antitumor immunological response by an intratumoral injection of dendritic cells pulsed with genetically engineered Semliki Forest virus to produce interleukin-18 combined with the systemic administration of interleukin-12. J. Neurosurg. 2003, 99, 746–753. [Google Scholar] [CrossRef]
- Bennett, A.M.; Elvin, S.J.; Wright, A.J.; Jones, S.M.; Phillpotts, R.J. An immunological profile of Balb/c mice protected from airborne challenge following vaccination with a live attenuated Venezuelan equine encephalitis virus vaccine. Vaccine 2000, 19, 337–347. [Google Scholar] [CrossRef]
- Hart, M.K.; Caswell-Stephan, K.; Bakken, R.; Tammariello, R.; Pratt, W.; Davis, N.; Johnston, R.E.; Smith, J.; Steele, K. Improved mucosal protection against Venezuelan equine encephalitis virus is induced by the molecularly defined, live-attenuated V3526 vaccine candidate. Vaccine 2000, 18, 3067–3075. [Google Scholar] [CrossRef]
- Schoepp, R.J.; Smith, J.F.; Parker, M.D. Recombinant chimeric western and eastern equine encephalitis viruses as potential vaccine candidates. Virology 2002, 302, 299–309. [Google Scholar] [CrossRef]
- Edelman, R.; Tacket, C.O.; Wasserman, S.S.; Bodison, S.A.; Perry, J.G.; Mangiafico, J.A. Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am. J. Trop. Med. Hyg. 2000, 62, 681–685. [Google Scholar]
- Mallilankaraman, K.; Shedlock, D.J.; Bao, H.; Kawalekar, O.U.; Fagone, P.; Ramanathan, A.A.; Ferraro, B. A DNA vaccine against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl. Trop. Dis. 2011, 5, e928. [Google Scholar] [CrossRef]
- Kim, D.Y.; Atasheva, S.; Foy, N.J.; Wang, E.; Frolova, E.I.; Weaver, S.; Frolov, I. Design of chimeric alphaviruses with a programmed, attenuated, cell type-restricted phenotype. J. Virol. 2011, 85, 4363–4376. [Google Scholar]
- Kramer, R.M.; Zeng, Y.; Sahni, N.; Kueltzo, L.A.; Schwartz, R.M.; Srivastava, I.K.; Crane, L.; Joshi, S.B.; Volkin, D.B.; Middaugh, C.R. Development of a stable virus-like particle vaccine formulation against Chikungunya virus and investigation of the effects of polyanions. J. Pharm. Sci. 2013, 102, 4305–4314. [Google Scholar] [CrossRef]
- Dash, P.K.; Tiwari, M.; Santhosh, S.R.; Parida, M.; Lakshmana Rao, P.V. RNA interference mediated inhibition of Chikungunya virus replication in mammalian cells. Biochem. Biophys. Res. Commun. 2008, 376, 718–722. [Google Scholar] [CrossRef]
- Kamrud, K.I.; Coffield, V.M.; Owens, G.; Goodman, C.; Alterson, K.; Custer, M.; Murphy, M.A.; Lewis, W.; Timberlake, S.; Wansley, E.K. In vitro and in vivo characterization of microRNA-targeted alphavirus replicon and helper RNAs. J. Virol. 2010, 84, 7713–7725. [Google Scholar] [CrossRef]
- Elvin, S.J.; Bennett, A.M.; Phillpotts, R.J. Role for mucosal immune responses and cell-mediated immune functions in protection from airborne challenge with Venezuelan equine encephalitis virus. J. Med. Virol. 2002, 67, 384–393. [Google Scholar] [CrossRef]
- Dupuy, L.C.; Richards, M.J.; Reed, D.S.; Schmaljohn, C.S. Immunogenicity and protective efficacy of a DNA vaccine against Venezuelan equine encephalitis virus aerosol challenge in nonhuman primates. Vaccine 2010, 28, 7345–7350. [Google Scholar] [CrossRef]
- Darwin, J.R.; Kenney, J.L.; Weaver, S.C. Transmission potential of two chimeric Chikungunya vaccine candidates in the urban mosquito vectors, Aedes aegypti and Ae. albopictus. Am. J. Trop. Med. Hyg. 2011, 84, 1012–1015. [Google Scholar] [CrossRef]
- Bhomia, M.; Sharma, A.; Gayen, M.; Gupta, P.; Maheshwari, R.K. Artificial microRNAs can effectively inhibit replication of Venezuelan equine encephalitis virus. Antivir. Res. 2013, 100, 429–434. [Google Scholar] [CrossRef]
- Prow, T.W.; Chen, X.; Prow, N.A.; Fernando, G.J.; Tan, C.S.; Raphael, A.P.; Chang, D.; Ruutu, M.D.; Jenkins, D.W.; Pyke, A. Nanopatch-targeted skin vaccination against West Nile Virus and Chikungunya virus in mice. Small 2010, 6, 1776–1784. [Google Scholar] [CrossRef]
- Berglund, P.; Sjöberg, M.; Garoff, H.; Atkins, G.J.; Sheahan, B.J.; Liljestrom, P. Semliki Forest virus expression system: Production of conditionally infectious recombinant particles. Biotechnology 1993, 11, 916–920. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lundstrom, K. Alphavirus-Based Vaccines. Viruses 2014, 6, 2392-2415. https://doi.org/10.3390/v6062392
Lundstrom K. Alphavirus-Based Vaccines. Viruses. 2014; 6(6):2392-2415. https://doi.org/10.3390/v6062392
Chicago/Turabian StyleLundstrom, Kenneth. 2014. "Alphavirus-Based Vaccines" Viruses 6, no. 6: 2392-2415. https://doi.org/10.3390/v6062392
APA StyleLundstrom, K. (2014). Alphavirus-Based Vaccines. Viruses, 6(6), 2392-2415. https://doi.org/10.3390/v6062392




