West Nile Virus in the United States — A Historical Perspective
Abstract
:1. Introduction
2. Status of Vector-Borne Encephalitides in 1999 in the United States
4. Outbreak Response to Zoonotic Diseases in the U.S.
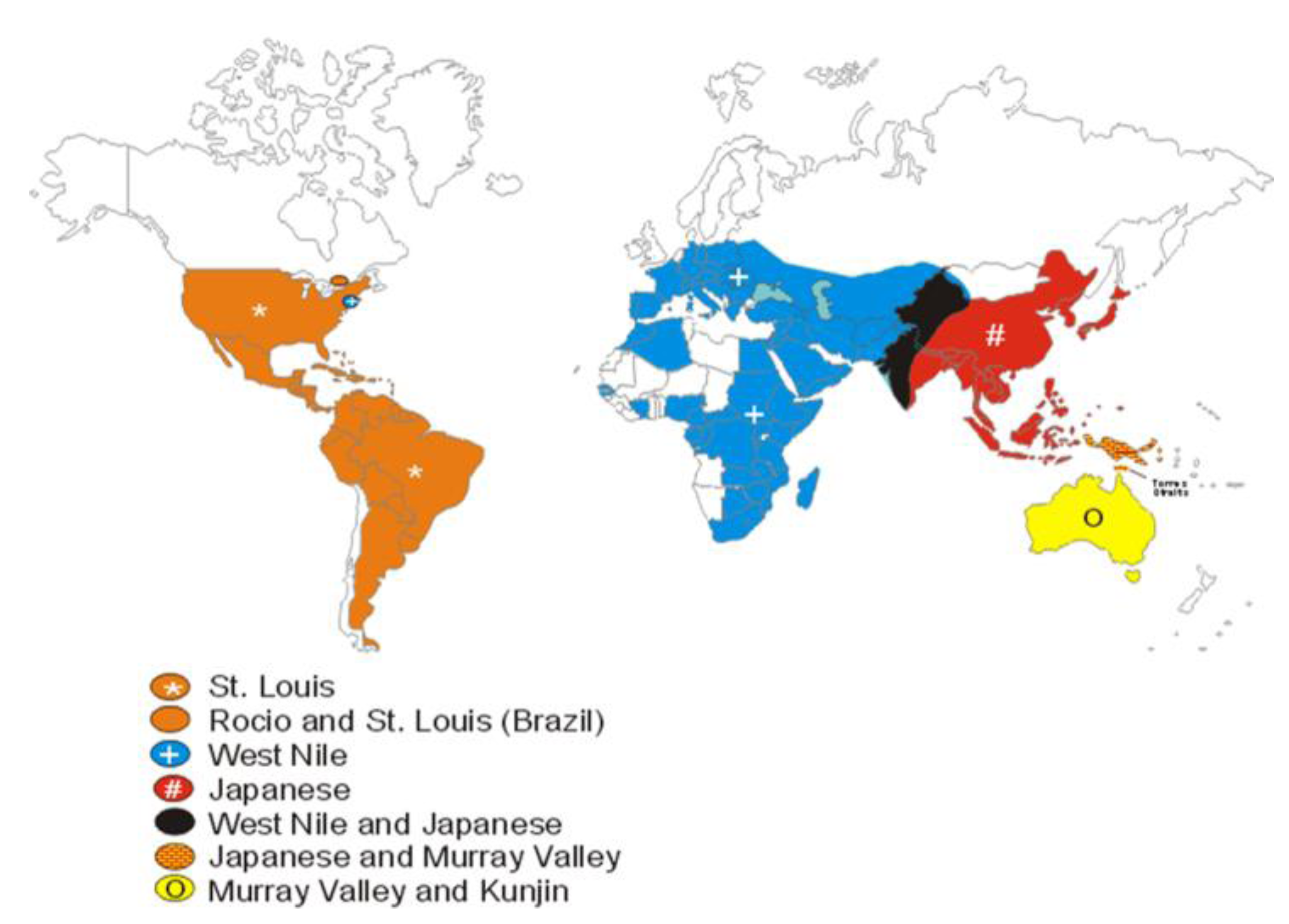
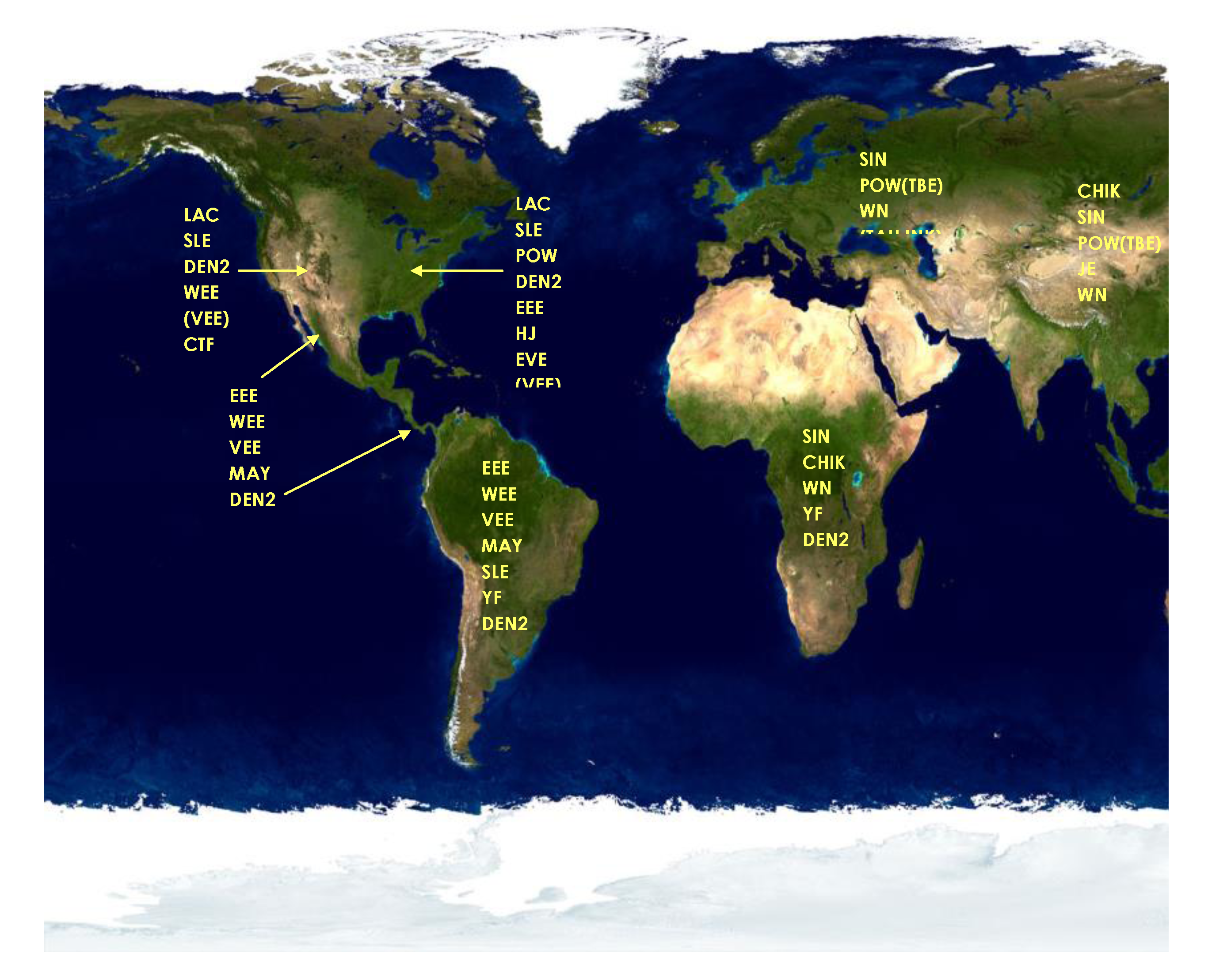
5. Discovery of Human Mosquito-borne Encephalitis in New York City
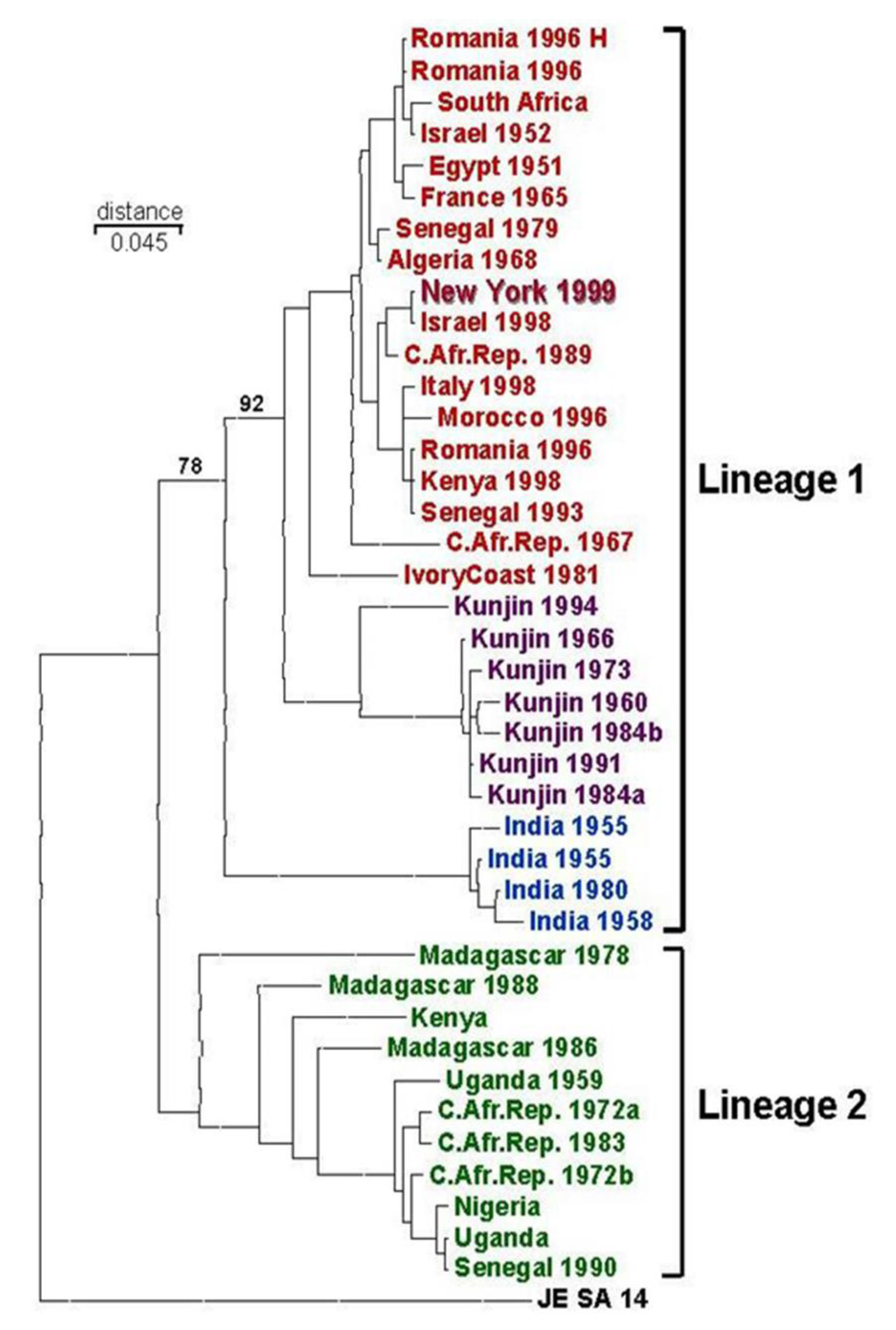
6. Possible Origins of the 1999 WNV Outbreak

7. Spread of WNV in the Western Hemisphere
| Location | State | Year | Clinical Cases | Serosurvey Sample Size | Identified Infection Rate | Estimated Infections |
|---|---|---|---|---|---|---|
| NYC (Queens) | NY | 1999 | 62 | 677 | 2.6% | 8200 |
| NYC (Staten Island) | NY | 2000 | 10 | 871 | 0.46% | 1574 |
| Suffolk County | NY | 2000 | 0 | 834 | 0.12% | 121 |
| Fairfield County | CT | 2000 | 1 | 731 | 0% | 0 |
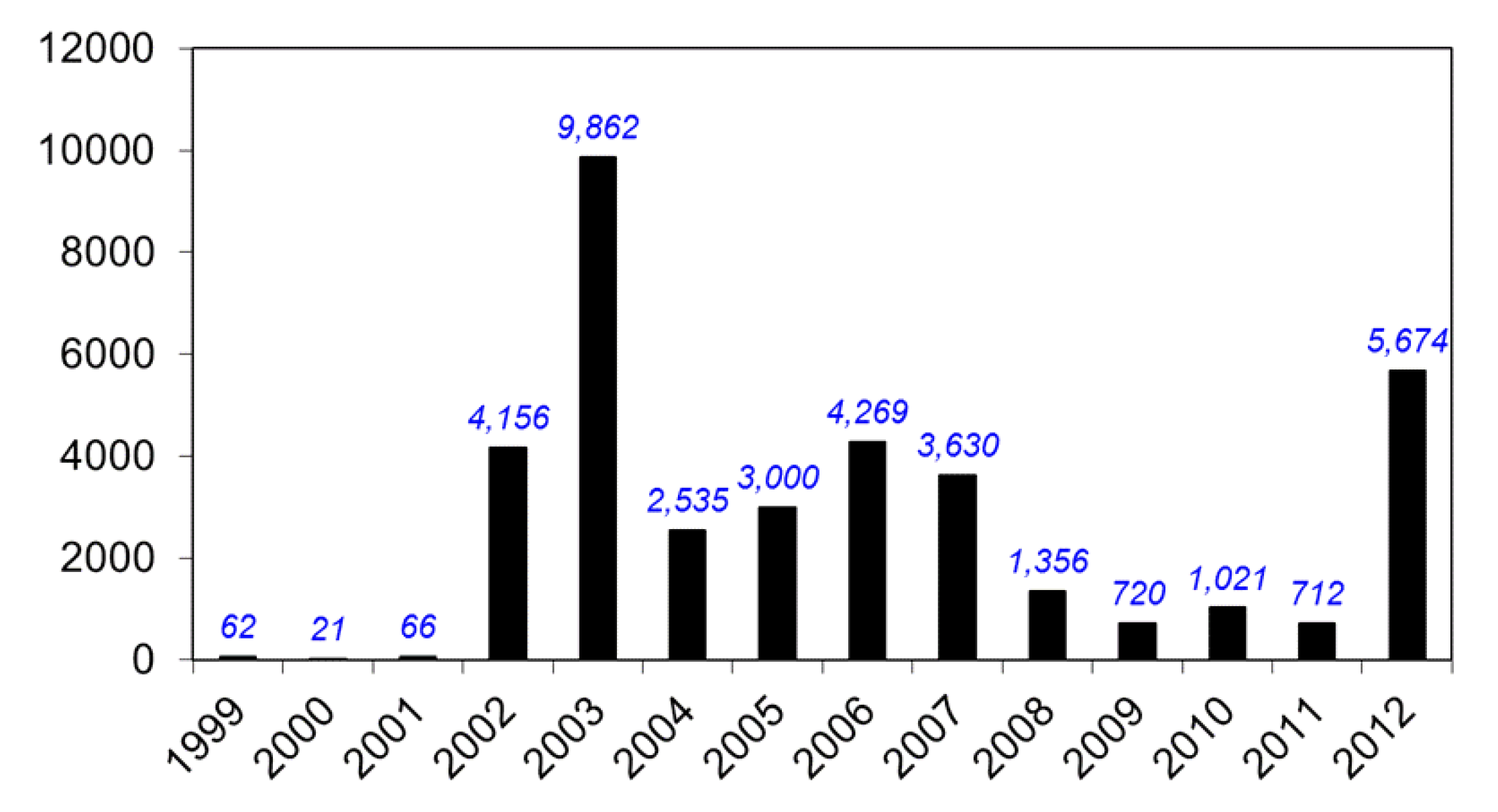
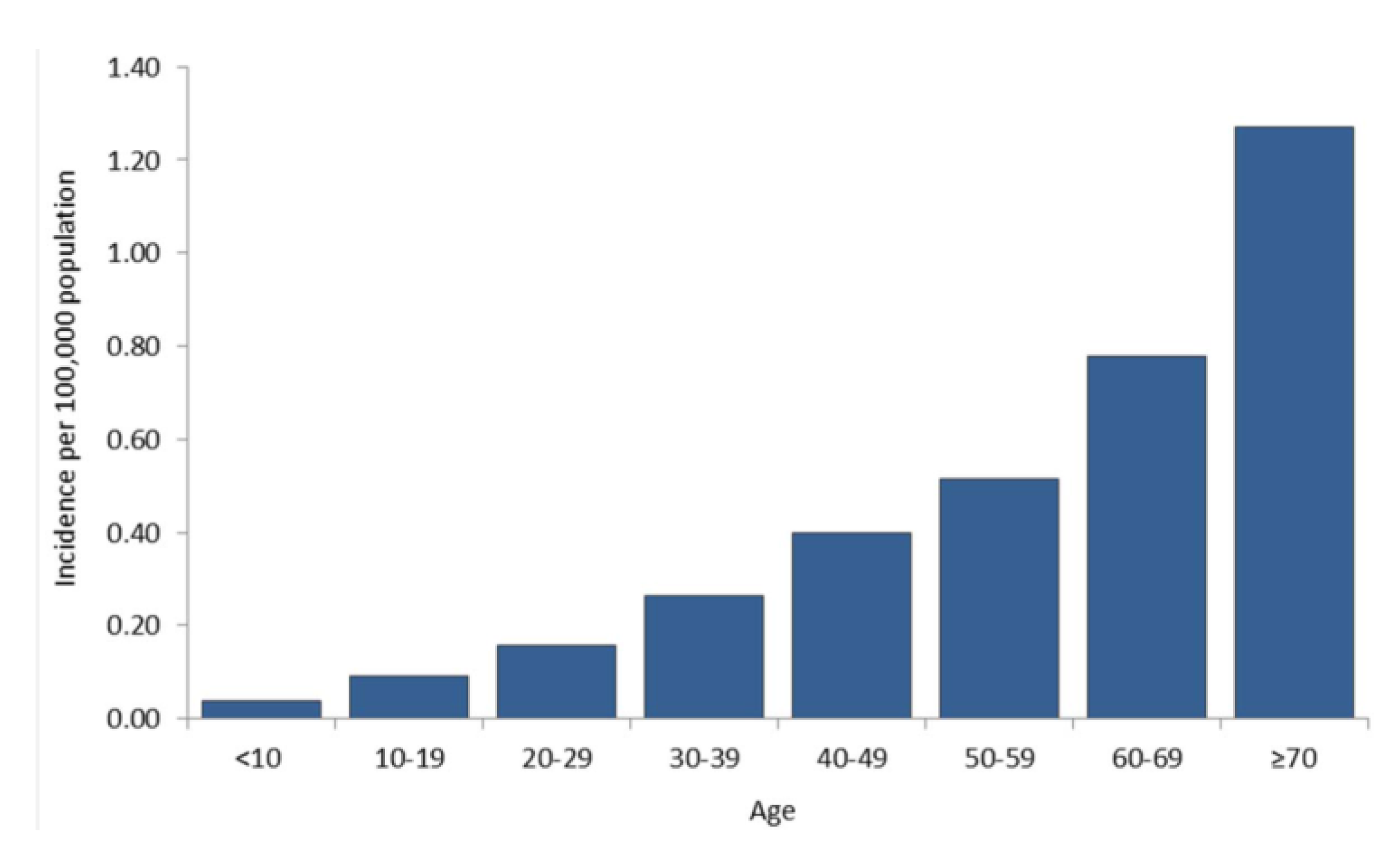
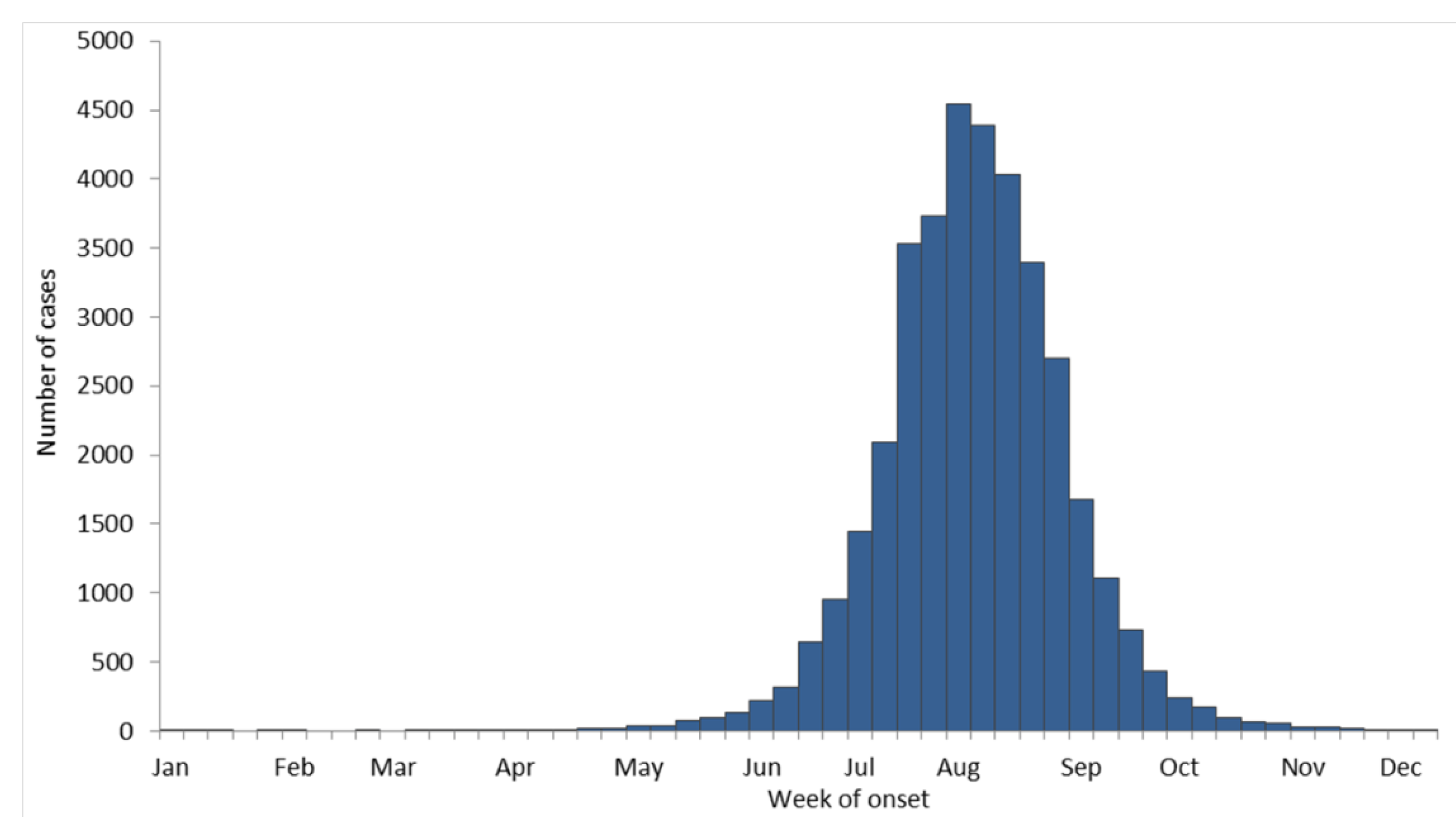
8. Federal Government Response to WNV
9. Current Status of WNV and other Flaviviruses in the U.S.
10. Concluding Remarks
- Determine current and future geographic distribution of WNV
- Study bird migration as a mechanism of WNV dispersal
- Study vector and vertebrate host relationships and range
- Identify and investigate virus persistence mechanisms
- Characterize mosquito biology, behavior, vector competence, surveillance, and control
- Develop and evaluate prevention strategies
- Improve laboratory diagnosis
- Determine the full clinical spectrum of disease and the long-term prognosis in humans
- Identify genetic relationships and the molecular basis of virulence
- Develop vaccines for animals and humans
- Develop antiviral therapy for WNV and other flaviviruses
- Determine the economic cost of the WNV epidemic/epizootic
- Investigate alternate modes of WNV transmission to humans
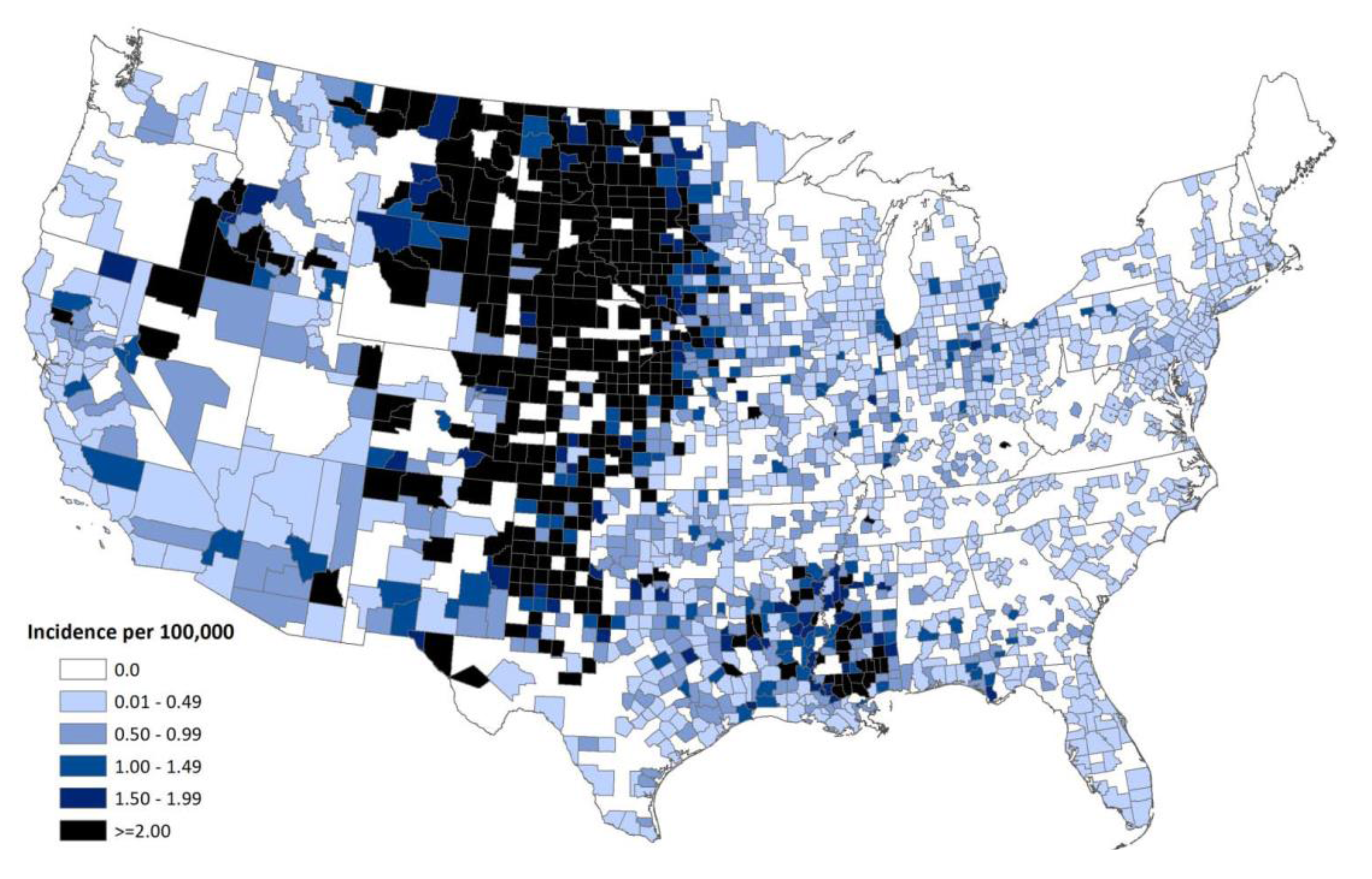

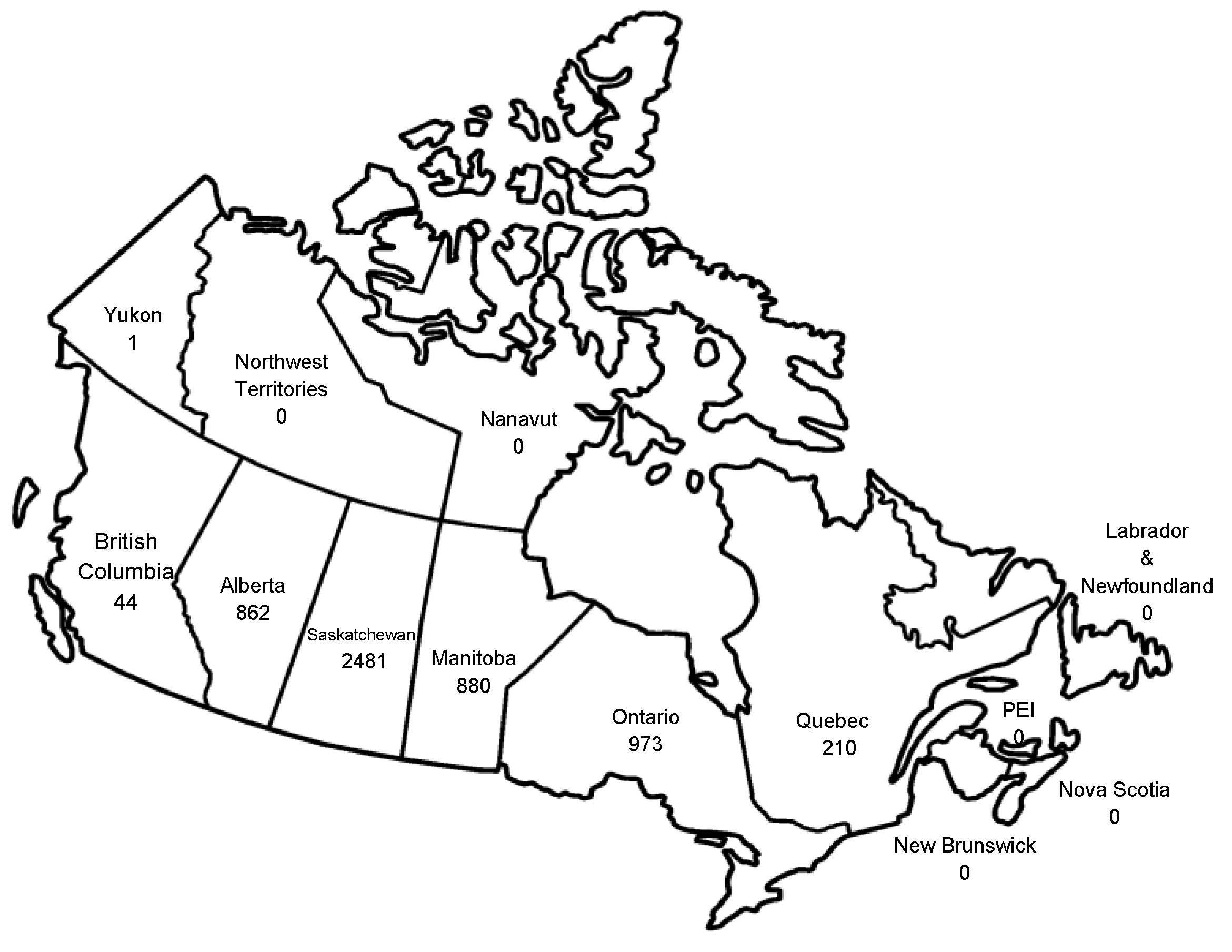
Acknowledgements
Conflicts of Interest
References and Notes
- Roehrig, J.T.; Layton, M.; Smith, P.; Campbell, G.L.; Nasci, R.; Lanciotti, R.S. The emergence of West Nile virus in North America: Ecology, epidemiology, and surveillance. Curr. Top. Microbiol. Immunol. 2002, 267, 223–240. [Google Scholar]
- Jones, S.C.; Morris, J.; Hill, G.; Alderman, M.; Ratard, R.C. St. Louis encephalitis outbreak in Louisiana in 2001. J. La State Med. Soc. 2002, 154, 303–306. [Google Scholar]
- St. Louis encephalitis outbreak—Arkansas, 1991. MMWR Morb. Mortal. Wkly. Rep. 1991, 40, 605–607.
- Tsai, T.F.; Canfield, M.A.; Reed, C.M.; Flannery, V.L.; Sullivan, K.H.; Reeve, G.R.; Bailey, R.E.; Poland, J.D. Epidemiological aspects of a St. Louis encephalitis outbreak in Harris County, Texas, 1986. J. Infect. Dis. 1988, 157, 351–356. [Google Scholar] [CrossRef]
- Tasi, T.F.; Smith, G.C.; Ndukwu, M.; Jakob, W.L.; Happ, C.M.; Kirk, L.J.; Francy, D.B.; Lampert, K.J. Entomologic studies after a St. Louis encephalitis epidemic in Grand Junction, Colorado. Am. J. Epidemiol. 1988, 128, 285–297. [Google Scholar]
- Day, J.F.; Stark, L.M. Frequency of Saint Louis encephalitis virus in humans from Florida, USA: 1990–1999. J. Med. Entomol 2000, 37, 626–633. [Google Scholar] [CrossRef]
- Calisher, C.H.; Karabatsos, N.; Dalrymple, J.M.; Shope, R.E.; Porterfield, J.S.; Westaway, E.G.; Brandt, W.E. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 1989, 70, 37–43. [Google Scholar] [CrossRef]
- Kuno, G.; Chang, G.J.; Tsuchiya, K.R.; Karabatsos, N.; Cropp, C.B. Phylogeny of the genus flavivirus. J. Virol. 1998, 72, 73–83. [Google Scholar]
- Kuno, G. Universal diagnostic RT-PCR protocol for arboviruses. J. Virol. Methods 1998, 72, 27–41. [Google Scholar]
- Day, J.F.; Stark, L.M. Eastern equine encephalitis transmission to emus (Dromaius novaehollandiae) in Volusia County, Florida: 1992 through 1994. J. Am. Mosq. Control. Assoc. 1996, 12, 429–436. [Google Scholar]
- Calisher, C.H. Medically important arboviruses of the United States and Canada. Clin. Microbiol. Rev. 1994, 7, 89–116. [Google Scholar]
- Johnson, A.J.; Martin, D.A.; Karabatsos, N.; Roehrig, J.T. Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. J. Clin. Microbiol. 2000, 38, 1827–1831. [Google Scholar]
- Martin, D.A.; Muth, D.A.; Brown, T.; Johnson, A.J.; Karabatsos, N.; Roehrig, J.T. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J. Clin. Microbiol. 2000, 38, 1823–1826. [Google Scholar]
- Lanciotti, R.S.; Roehrig, J.T. Arboviruses. In Manual of clinical laboratory immunology, 6th ed.; Rose, N.R., Hamilton, R.G., Detrick, B., Eds.; American Society for Microbiology: Washington, DC, USA, 2006; pp. 757–765. [Google Scholar]
- Nash, D.; Mostashari, F.; Fine, A.; Miller, J.; O’Leary, D.; Murray, K.; Huang, A.; Rosenberg, A.; Greenberg, A.; Sherman, M.; et al. The outbreak of West Nile virus infection in the New York City area in 1999. N. Engl. J. Med. 2001, 344, 1807–1814. [Google Scholar]
- Asnis, D.S.; Conetta, R.; Teixeira, A.A.; Waldman, G.; Sampson, B.A. The west nile virus outbreak of 1999 in new york: The Flushing hospital experience. Clin. Infect. Dis. 2000, 30, 413–418. [Google Scholar] [CrossRef]
- Asnis, D.S.; Conetta, R.; Waldman, G.; Teixeira, A.A. The West Nile virus encephalitis outbreak in the United States (1999–2000): From Flushing, New York, to beyond its borders. Ann. NY Acad. Sci. 2001, 951, 161–171. [Google Scholar]
- Briese, T.; Glass, W.G.; Lipkin, W.I. Detection of West Nile virus sequences in cerebrospinal fluid. Lancet 2000, 355, 1614–1615. [Google Scholar] [CrossRef]
- Briese, T.; Jia, X.Y.; Huang, C.; Grady, L.J.; Lipkin, W.I. Identification of a Kunjin/West Nile-like flavivirus in brains of patients with New York encephalitis. Lancet 1999, 354, 1261–1262. [Google Scholar]
- Hall, R.A.; Broom, A.K.; Smith, D.W.; Mackenzie, J.S. The ecology and epidemiology of Kunjin virus. Curr. Top. Microbiol. Immunol. 2002, 267, 253–269. [Google Scholar]
- Lanciotti, R.S.; Roehrig, J.T.; Deubel, V.; Smith, J.; Parker, M.; Steele, K.; Crise, B.; Volpe, K.E.; Crabtree, M.B.; Scherret, J.H.; et al. Origin of thWest Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 1999, 286, 2333–2337. [Google Scholar] [CrossRef]
- Shieh, W.J.; Guarner, J.; Layton, M.; Fine, A.; Miller, J.; Nash, D.; Campbell, G.L.; Roehrig, J.T.; Gubler, D.J.; Zaki, S.R. The role of pathology in an investigation of an outbreak of West Nile encephalitis in New York, 1999. Emerg. Infect. Dis. 2000, 6, 370–372. [Google Scholar]
- Anderson, J.F.; Andreadis, T.G.; Vossbrinck, C.R.; Tirrell, S.; Wakem, E.M.; French, R.A.; Garmendia, A.E.; Van Kruiningen, H.J. Isolation of West Nile virus from mosquitoes, crows, and a Cooper’s hawk in Connecticut. Science 1999, 286, 2331–2333. [Google Scholar]
- Tsai, T.F.; Popovici, F.; Cernescu, C.; Campbell, G.L.; Nedelcu, N.I. West Nile encephalitis epidemic in Southeastern Romania. Lancet 1998, 352, 767–771. [Google Scholar]
- Murgue, B.; Murri, S.; Triki, H.; Deubel, V.; Zeller, H.G. West Nile in the Mediterranean basin: 1950–2000. Ann. NY Acad. Sci. 2001, 951, 117–126. [Google Scholar]
- Bin, H.; Grossman, Z.; Pokamunski, S.; Malkinson, M.; Weiss, L.; Duvdevani, P.; Banet, C.; Weisman, Y.; Annis, E.; Gandaku, D.; et al. West Nile fever in Israel 1999–2000: From geese to humans. Ann. NY Acad. Sci. 2001, 951, 127–142. [Google Scholar]
- L’Vov, D.K.; Butenko, A.M.; Gaidamovich, S.; Larichev, V.F.; Leshchinskaia, E.V.; Zhukov, A.N.; Lazorenko, V.V.; Aliushin, A.M.; Petrov, V.R.; Trikhanov, S.T.; et al. Epidemic outbreak of meningitis and meningoencephalitis, caused by West Nile virus, in the Krasnodar territory and Volgograd region (preliminary report). Vopr. Virusol. 2000, 45, 37–38. [Google Scholar]
- L’Vov, D.K.; Butenko, A.M.; Vyshemirskii, O.I.; Gaidamovich, S.; Gromashevskii, V.L.; Larichev, V.F.; Morozova, T.N.; Skvortsova, T.M.; Khutoretskaia, N.V.; Shishkina, E.O.; et al. Isolation of the West Nile fever virus from human patients during an epidemic outbreak in the Volgograd and Astrakhan regions. Vopr. Virusol. 2000, 45, 9–12. [Google Scholar]
- Lvov, D.K.; Butenko, A.M.; Gromashevsky, V.L.; Larichev, V.P.; Gaidamovich, S.Y.; Vyshemirsky, O.I.; Zhukov, A.N.; Lazorenko, V.V.; Salko, V.N.; Kovtunov, A.I.; et al. Isolation of two strains of West Nile virus during an outbreak in southern Russia, 1999. Emerg Infect. Dis 2000, 6, 373–376. [Google Scholar]
- Murgue, B.; Zeller, H.; Deubel, V. The ecology and epidemiology of West Nile virus in Africa, Europe and Asia. Curr. Top. Microbiol. Immunol. 2002, 267, 195–221. [Google Scholar]
- Deubel, V.; Fiette, L.; Gounon, P.; Drouet, M.T.; Khun, H.; Huerre, M.; Banet, C.; Malkinson, M.; Despres, P. Variations in biological features of West Nile viruses. Ann. NY Acad. Sci. 2001, 951, 195–206. [Google Scholar]
- Malkinson, M.; Weisman, Y.; Pokamonski, S.; King, R.; Deubel, V. Intercontinental transmission of West Nile virus by migrating white storks. Emerg. Infect. Dis. 2001, 7, 540. [Google Scholar] [CrossRef]
- Malkinson, M.; Banet, C.; Weisman, Y.; Pokamunski, S.; King, R.; Drouet, M.T.; Deubel, V. Introduction of West Nile virus in the Middle East by migrating white storks. Emerg. Infect. Dis. 2002, 8, 392–397. [Google Scholar] [CrossRef]
- Malkinson, M.; Banet, C. The role of birds in the ecology of West Nile virus in Europe and Africa. Curr. Top. Microbiol. Immunol. 2002, 267, 309–322. [Google Scholar]
- Berthet, F.X.; Zeller, H.G.; Drouet, M.T.; Rauzier, J.; Digoutte, J.P.; Deubel, V. Extensive nucleotide changes and deletions within the envelope glycoprotein gene of Euro-African West Nile viruses. J. Gen. Virol. 1997, 78, 2293–2297. [Google Scholar]
- Hall, R.A.; Burgess, G.W.; Kay, B.H.; Clancy, P. Monoclonal antibodies to Kunjin and Kokobera viruses. Immunol. Cell Biol. 1991, 69, 47–49. [Google Scholar]
- Adams, S.C.; Broom, A.K.; Sammels, L.M.; Hartnett, A.C.; Howard, M.J.; Coelen, R.J.; Mackenzie, J.S.; Hall, R.A. Glycosylation and antigenic variation among Kunjin virus isolates. Virology 1995, 206, 49–56. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Ebel, G.D.; Deubel, V.; Kerst, A.J.; Murri, S.; Meyer, R.; Bowen, M.; McKinney, N.; Morrill, W.E.; Crabtree, M.B.; et al. Complete genome sequences and phylogenetic analysis of West Nile virus strains isolated from the United States, Europe, and the Middle East. Virology 2002, 298, 96–105. [Google Scholar] [CrossRef]
- Monath, T.P. Aedes albopictus, an exotic mosquito vector in the United States. Ann. Intern. Med. 1986, 105, 449–451. [Google Scholar]
- Moore, C.G.; Francy, D.B.; Eliason, D.A.; Monath, T.P. Aedes albopictus in the United States: Rapid spread of a potential disease vector. J. Am. Mosq. Control Assoc. 1988, 4, 356–361. [Google Scholar]
- Hofhuis, A.; Reimerink, J.; Reusken, C.; Scholte, E.J.; Boer, A.; Takken, W.; Koopmans, M. The hidden passenger of lucky bamboo: Do imported Aedes albopictus mosquitoes cause dengue virus transmission in the Netherlands? Vector Borne Zoonotic Dis. 2009, 9, 217–220. [Google Scholar]
- Scholte, E.J.; Dijkstra, E.; Blok, H.; de Vries, A.; Takken, W.; Hofhuis, A.; Koopmans, M.; de Boer, A.; Reusken, C.B. Accidental importation of the mosquito Aedes albopictus into the Netherlands: A survey of mosquito distribution and the presence of dengue virus. Med. Vet. Entomol. 2008, 22, 352–358. [Google Scholar]
- Zhong, D.; Lo, E.; Hu, R.; Metzger, M.E.; Cummings, R.; Bonizzoni, M.; Fujioka, K.K.; Sorvillo, T.E.; Kluh, S.; Healy, S.P.; et al. Genetic analysis of invasive Aedes albopictus populations in Los Angeles County, California and its potential public health impact. PLoS One 2013, 8, e68586. [Google Scholar]
- Miller, B.R.; Nasci, R.S.; Godsey, M.S.; Savage, H.M.; Lutwama, J.J.; Lanciotti, R.S.; Peters, C.J. First field evidence for natural vertical transmission of West Nile virus in Culex univittatus complex mosquitoes from Rift Valley province, Kenya. Am. J. Trop. Med. Hyg. 2000, 62, 240–246. [Google Scholar]
- Baqar, S.; Hayes, C.G.; Murphy, J.R.; Watts, D.M. Vertical transmission of West Nile virus by Culex and Aedes species mosquitoes. Am. J. Trop. Med. Hyg. 1993, 48, 757–762. [Google Scholar]
- Dohm, D.J.; Sardelis, M.R.; Turell, M.J. Experimental vertical transmission of West Nile virus by Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 2002, 39, 640–644. [Google Scholar]
- Mostashari, F.; Bunning, M.L.; Kitsutani, P.T.; Singer, D.A.; Nash, D.; Cooper, M.J.; Katz, N.; Liljebjelke, K.A.; Biggerstaff, B.J.; Fine, A.D.; et al. Epidemic West Nile encephalitis, New York, 1999: Results of a household-based seroepidemiological survey. Lancet 2001, 358, 261–264. [Google Scholar]
- Serosurveys for West Nile virus infection—New York and Connecticut counties, 2000. MMWR Morb. Mortal. Wkly. Rep. 2001, 50, 37–39.
- McCarthy, T.A.; Hadler, J.L.; Julian, K.; Walsh, S.J.; Biggerstaff, B.J.; Hinten, S.R.; Baisley, C.; Iton, A.; Brennan, T.; Nelson, R.S.; et al. West Nile virus serosurvey and assessment of personal prevention efforts in an area with intense epizootic activity: Connecticut, 2000. Ann. NY Acad. Sci. 2001, 951, 307–316. [Google Scholar]
- Petersen, L.R.; Carson, P.J.; Biggerstaff, B.J.; Custer, B.; Borchardt, S.M.; Busch, M.P. Estimated cumulative incidence of West Nile virus infection in us adults, 1999–2010. Epidemiol. Infect. 2012, 28, 1–5. [Google Scholar]
- Nasci, R.S.; Savage, H.M.; White, D.J.; Miller, J.R.; Cropp, B.C.; Godsey, M.S.; Kerst, A.J.; Bennett, P.; Gottfried, K.; Lanciotti, R.S. West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg. Infect. Dis. 2001, 7, 742–744. [Google Scholar]
- Knight, J. U.S. zoos keep watch for cross-species killer. Nature 2002, 417. [Google Scholar] [CrossRef]
- Hunt, A.R.; Hall, R.A.; Kerst, A.J.; Nasci, R.S.; Savage, H.M.; Panella, N.A.; Gottfried, K.L.; Burkhalter, K.L.; Roehrig, J.T. Detection of West Nile virus antigen in mosquitoes and avian tissues by a monoclonal antibody-based capture enzyme immunoassay. J. Clin. Microbiol. 2002, 40, 2023–2030. [Google Scholar] [CrossRef]
- Ryan, J.; Dave, K.; Emmerich, E.; Fernandez, B.; Turell, M.; Johnson, J.; Gottfried, K.; Burkhalter, K.; Kerst, A.; Hunt, A.; et al. Wicking assays for the rapid detection of West Nile and St. Louis encephalitis viral antigens in mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2003, 40, 95–99. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kerst, A.J.; Nasci, R.S.; Godsey, M.S.; Mitchell, C.J.; Savage, H.M.; Komar, N.; Panella, N.A.; Allen, B.C.; Volpe, K.E.; et al. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a taqman reverse transcriptase-PCR assay. J. Clin. Microbiol. 2000, 38, 4066–4071. [Google Scholar]
- Davis, B.S.; Chang, G.J.; Cropp, B.; Roehrig, J.T.; Martin, D.A.; Mitchell, C.J.; Bowen, R.; Bunning, M.L. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J. Virol. 2001, 75, 4040–4047. [Google Scholar] [CrossRef]
- Bunning, M.L.; Fox, P.E.; Bowen, R.A.; Komar, N.; Chang, G.J.; Speaker, T.J.; Stephens, M.R.; Nemeth, N.; Panella, N.A.; Langevin, S.A.; et al. DNA vaccination of the american crow (Corvus brachyrhynchos) provides partial protection against lethal challenge with West Nile virus. Avian Dis. 2007, 51, 573–577. [Google Scholar] [CrossRef]
- Chang, G.J.; Davis, B.S.; Stringfield, C.; Lutz, C. Prospective immunization of the endangered california condors (Gymnogyps californianus) protects this species from lethal West Nile virus infection. Vaccine 2007, 25, 2325–2330. [Google Scholar] [CrossRef]
- Biggerstaff, B.J.; Petersen, L.R. Estimated risk of West Nile virus transmission through blood transfusion during an epidemic in Queens, New York City. Transfusion 2002, 42, 1019–1026. [Google Scholar] [CrossRef]
- Charatan, F. Organ transplants and blood transfusions may transmit West Nile virus. Br. Med. J. 2002, 325, 566. [Google Scholar] [CrossRef]
- Stephenson, J. Investigation probes risk of contracting West Nile virus via blood transfusions. JAMA 2002, 288, 1573–1574. [Google Scholar] [CrossRef]
- Armstrong, W.S.; Bashour, C.A.; Smedira, N.G.; Heupler, F.A.; Hoeltge, G.A.; Mawhorter, S.D.; Sudheendra, V.; Gordon, S.M. A case of fatal West Nile virus meningoencephalitis associated with receipt of blood transfusions after open heart surgery. Ann. Thorac. Surg. 2003, 76, 605–607. [Google Scholar] [CrossRef]
- Becker, J.L. Vector-borne illnesses and the safety of the blood supply. Curr. Hematol. Rep. 2003, 2, 511–517. [Google Scholar]
- Biggerstaff, B.J.; Petersen, L.R. Estimated risk of transmission of the West Nile virus through blood transfusion in the US, 2002. Transfusion 2003, 43, 1007–1017. [Google Scholar] [CrossRef]
- Harrington, T.; Kuehnert, M.J.; Kamel, H.; Lanciotti, R.S.; Hand, S.; Currier, M.; Chamberland, M.E.; Petersen, L.R.; Marfin, A.A. West Nile virus infection transmitted by blood transfusion. Transfusion 2003, 43, 1018–1022. [Google Scholar] [CrossRef]
- Hiatt, B.; DesJardin, L.; Carter, T.; Gingrich, R.; Thompson, C.; de Magalhaes-Silverman, M. A fatal case of West Nile virus infection in a bone marrow transplant recipient. Clin. Infect. Dis. 2003, 37, e129–e131. [Google Scholar] [CrossRef]
- Iwamoto, M.; Jernigan, D.B.; Guasch, A.; Trepka, M.J.; Blackmore, C.G.; Hellinger, W.C.; Pham, S.M.; Zaki, S.; Lanciotti, R.S.; Lance-Parker, S.E.; et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N. Engl. J. Med. 2003, 348, 2196–2203. [Google Scholar]
- Lefrere, J.J.; Allain, J.P.; Prati, D.; Sauleda, S.; Reesink, H. West Nile virus and blood donors. Lancet 2003, 361, 2083–2084. [Google Scholar]
- Intrauterine West Nile virus infection—New York, 2002. MMWR Morb. Mortal. Wkly. Rep. 2002, 51, 1135–1136.
- Government Accounting Office, USA. Available online: www.gao.gov/assets/230/229648.pdf (accessed on 1 August 2013).
- Public Health Agency of Canada. Available online: www.phac-aspc.gc.ca/wnv-vwn/index-eng.php (accessed on 15 September 2013).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Roehrig, J.T. West Nile Virus in the United States — A Historical Perspective. Viruses 2013, 5, 3088-3108. https://doi.org/10.3390/v5123088
Roehrig JT. West Nile Virus in the United States — A Historical Perspective. Viruses. 2013; 5(12):3088-3108. https://doi.org/10.3390/v5123088
Chicago/Turabian StyleRoehrig, John T. 2013. "West Nile Virus in the United States — A Historical Perspective" Viruses 5, no. 12: 3088-3108. https://doi.org/10.3390/v5123088
APA StyleRoehrig, J. T. (2013). West Nile Virus in the United States — A Historical Perspective. Viruses, 5(12), 3088-3108. https://doi.org/10.3390/v5123088




