Antiretroviral Adherence and Use of Antihypertensives, Statins, and Antidiabetics Among Elderly People with HIV: A 5-Year Real-World Study in Southern Italy
Abstract
1. Introduction
2. Methods
2.1. Data Source and Study Population
2.2. Study Measures
2.3. Statistical Analyses
3. Results
3.1. Subject Characteristics and ART Regimens, 2018–2023
3.2. Treatment-Naive Individuals
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3TC | Lamivudine |
| ABC | Abacavir |
| ART | Antiretroviral therapy |
| ASCVD | Atherosclerotic cardiovascular disease |
| ATC | Anatomical Therapeutic Chemical |
| CAGR | Compound annual growth rate |
| CVD | Cardiovascular disease |
| DDD | Defined daily dose |
| EMA | European Medicines Agency |
| FDCs | Fixed-dose combinations |
| FTC | Emtricitabine |
| LHUs | Local Health Units |
| MTR | Multiple-Tablet Regimen |
| NNRTI | Non-nucleoside reverse transcriptase inhibitor |
| NRTI | Nucleoside/nucleotide reverse transcriptase inhibitor |
| INSTI | Integrase strand transfer inhibitor |
| OD | Once-daily |
| PDC | Proportion of days covered |
| PI/bs | Boosted protease inhibitors |
| PWH | People with HIV |
| STRs | Single-tablet regimens |
| TAF | Tenofovir alafenamide fumarate |
| TDF | Tenofovir disoproxil fumarate |
References
- Teeraananchai, S.; Kerr, S.; Amin, J.; Ruxrungtham, K.; Law, M. Life Expectancy of HIV-positive People after Starting Combination Antiretroviral Therapy: A Meta-analysis. HIV Med. 2017, 18, 256–266. [Google Scholar] [CrossRef]
- Wandeler, G.; Johnson, L.F.; Egger, M. Trends in Life Expectancy of HIV-Positive Adults on Antiretroviral Therapy across the Globe: Comparisons with General Population. Curr. Opin. HIV AIDS 2016, 11, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, G.; Milic, J. The Interplay Between Frailty and Intrinsic Capacity in Aging and HIV Infection. AIDS Res. Hum. Retroviruses 2019, 35, 1013–1022. [Google Scholar] [CrossRef]
- Tseng, A.; Seet, J.; Phillips, E.J. The Evolution of Three Decades of Antiretroviral Therapy: Challenges, Triumphs and the Promise of the Future. Br. J. Clin. Pharmacol. 2015, 79, 182–194. [Google Scholar] [CrossRef]
- Kelly, S.G.; Masters, M.C.; Taiwo, B.O. Initial Antiretroviral Therapy in an Integrase Inhibitor Era. Infect. Dis. Clin. N. Am. 2019, 33, 681–692. [Google Scholar] [CrossRef]
- EACS. European AIDS Clinical Society (EACS) EACS Guidelines, Version n.11.0. 2021. Available online: https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf (accessed on 20 April 2025).
- Maggiolo, F.; Taramasso, L.; Valenti, D.; Blanchi, S.; Centorrino, F.; Comi, L.; Di Biagio, A. B/F/TAF Forgiveness to Non-Adherence. Sex. Transm. Infect. 2024, 100, 418–422. [Google Scholar] [CrossRef]
- Paterson, D.L.; Swindells, S.; Mohr, J.; Brester, M.; Vergis, E.N.; Squier, C.; Wagener, M.M.; Singh, N. Adherence to Protease Inhibitor Therapy and Outcomes in Patients with HIV Infection. Ann. Intern. Med. 2000, 133, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Komandt, M.; Canfield, S.; Lengel, M.; Gilmore, V.; Kilcrease, C. Correlation between Medication Adherence Using Proportion of Days Covered and Achieving Viral Suppression in Patients Living with HIV. J. Manag. Care Spec. Pharm. 2023, 29, 1129–1137. [Google Scholar] [CrossRef]
- Feinstein, M.J.; Hsue, P.Y.; Benjamin, L.A.; Bloomfield, G.S.; Currier, J.S.; Freiberg, M.S.; Grinspoon, S.K.; Levin, J.; Longenecker, C.T.; Post, W.S.; et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation 2019, 140, e98–e124. [Google Scholar] [CrossRef] [PubMed]
- Kobe, E.A.; Thakkar, A.; Matai, S.; Akkaya, E.; Pagidipati, N.J.; McGarrah, R.W.; Bloomfield, G.S.; Shah, N.P. Optimizing Cardiometabolic Risk in People Living with Human Immunodeficiency Virus: A Deep Dive into an Important Risk Enhancer. Am. J. Prev. Cardiol. 2024, 20, 100888. [Google Scholar] [CrossRef]
- Islam, F.; Wu, J.; Jansson, J.; Wilson, D. Relative Risk of Cardiovascular Disease among People Living with HIV: A Systematic Review and Meta-analysis. HIV Med. 2012, 13, 453–468. [Google Scholar] [CrossRef]
- Kaplan-Lewis, E.; Aberg, J.A.; Lee, M. Aging with HIV in the ART Era. Semin. Diagn. Pathol. 2017, 34, 384–397. [Google Scholar] [CrossRef]
- Kentoffio, K.; Temu, T.M.; Shakil, S.S.; Zanni, M.V.; Longenecker, C.T. Cardiovascular Disease Risk in Women Living with HIV. Curr. Opin. HIV AIDS 2022, 17, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Grinspoon, S.K.; Fitch, K.V.; Overton, E.T.; Fichtenbaum, C.J.; Zanni, M.V.; Aberg, J.A.; Malvestutto, C.; Lu, M.T.; Currier, J.S.; Sponseller, C.A.; et al. Rationale and Design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). Am. Heart J. 2019, 212, 23–35. [Google Scholar] [CrossRef]
- Grinspoon, S.K.; Fitch, K.V.; Zanni, M.V.; Fichtenbaum, C.J.; Umbleja, T.; Aberg, J.A.; Overton, E.T.; Malvestutto, C.D.; Bloomfield, G.S.; Currier, J.S.; et al. Pitavastatin to Prevent Cardiovascular Disease in HIV Infection. N. Engl. J. Med. 2023, 389, 687–699. [Google Scholar] [CrossRef]
- Alagaratnam, J.; Van Bremen, K.; Behrens, G.M.N.; Boccara, F.; Cinque, P.; Gisslén, M.; Guaraldi, G.; Konopnicki, D.; Kowalska, J.D.; Mallon, P.W.G.; et al. Statin Use in HIV: European AIDS Clinical Society Guidance for the Primary Prevention of Cardiovascular Disease. Lancet HIV 2025, 12, e382–e392. [Google Scholar] [CrossRef]
- Cattaneo, D.; Oreni, L.; Meraviglia, P.; Minisci, D.; Astuti, N.; Antinori, S.; Gori, A.; Gervasoni, C. Polypharmacy and Aging in People Living with HIV: 6 Years of Experience in a Multidisciplinary Outpatient Clinic. Drugs Aging 2023, 40, 665–674. [Google Scholar] [CrossRef]
- Mazzitelli, M.; Pontillo, D.; Clemente, T.; Di Biagio, A.; Cenderello, G.; Rusconi, S.; Menzaghi, B.; Fornabaio, C.; Garlassi, E.; Zazzi, M.; et al. Polypharmacy, Anticholinergic Burden and Drug–Drug Interaction Assessment in People with Four-Class-Resistant HIV: Data from the PRESTIGIO Registry. J. Antimicrob. Chemother. 2024, 79, 2163–2169. [Google Scholar] [CrossRef] [PubMed]
- Althoff, K.N.; Stewart, C.N.; Humes, E.; Zhang, J.; Gerace, L.; Boyd, C.M.; Wong, C.; Justice, A.C.; Gebo, K.A.; Thorne, J.E.; et al. The Shifting Age Distribution of People with HIV Using Antiretroviral Therapy in the United States. AIDS 2022, 36, 459–471. [Google Scholar] [CrossRef]
- Govender, R.D.; Hashim, M.J.; Khan, M.A.; Mustafa, H.; Khan, G. Global Epidemiology of HIV/AIDS: A Resurgence in North America and Europe. J. Epidemiol. Glob. Health 2021, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data—United States and 6 Territories and Freely Associated States, 2022. HIV Surveillance Supplemental Report 2024;29(No. 2). 2024. Available online: https://www.cdc.gov/Hiv-Data/Nhss/National-Hiv-Prevention-and-Care-Outcomes.Html (accessed on 10 May 2025).
- Smit, M.; Brinkman, K.; Geerlings, S.; Smit, C.; Thyagarajan, K.; Sighem, A.V.; De Wolf, F.; Hallett, T.B. Future Challenges for Clinical Care of an Ageing Population Infected with HIV: A Modelling Study. Lancet Infect. Dis. 2015, 15, 810–818. [Google Scholar] [CrossRef]
- Perrone, V.; Dovizio, M.; Sangiorgi, D.; Andretta, M.; Bartolini, F.; Cavaliere, A.; Ciaccia, A.; Chinellato, A.; Costantini, A.; Dell’Orco, S.; et al. Healthcare Resource Consumption and Related Costs in Patients on Antiretroviral Therapies: Findings from Real-World Data in Italy. Int. J. Environ. Res. Public Health 2023, 20, 3789. [Google Scholar] [CrossRef]
- Papa, N.; Cammarota, S.; Citarella, A.; Atripaldi, L.; Bernardi, F.F.; Fogliasecca, M.; Giugliano, N.; Trama, U.; Spatarella, M. Evolution in Real-World Therapeutic Strategies for HIV Treatment: A Retrospective Study in Southern Italy, 2014–2020. J. Clin. Med. 2021, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Nasi, M.; De Biasi, S.; Gibellini, L.; Bianchini, E.; Pecorini, S.; Bacca, V.; Guaraldi, G.; Mussini, C.; Pinti, M.; Cossarizza, A. Ageing and Inflammation in Patients with HIV Infection. Clin. Exp. Immunol. 2016, 187, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Dorrucci, M.; Regine, V.; Pezzotti, P.; Mammone, A.; Girardi, E.; Suligoi, B.; HIV Surveillance System Group; Italian HIV Surveillance System. Demographic and Socio-Economic Determinants of Poor HIV-Risk Perception at First HIV Diagnosis: Analysis of the HIV Surveillance Data, Italy 2010–2016. Ann. Ist. Super. Sanita 2020, 56, 267–276. [Google Scholar] [CrossRef]
- d’Arminio Monforte, A.; Rodano’, A.; Fanti, I.; Perno, C.; Segala, D.; Santoro, A.; Pasticci, M.; Calza, L.; Carraro, A.; Cingolani, A.; et al. OC-66 Socio-Demographic, Clinical and Therapeutic Features of Persons with HIV (PWH) Currently in Care in Italy: Data from the ICONA Cohort. Sex. Transm. Infect. 2024, 100, A64–A65. [Google Scholar]
- Max, B. Update On HIV Integrase Inhibitors for the Treatment of HIV-1 Infection. Future Virol. 2019, 14, 693–709. [Google Scholar] [CrossRef]
- Eaton, E.F.; Tamhane, A.; Davy-Mendez, T.; Mathews, W.C.; Moore, R.D.; Saag, M.S.; Mugavero, M.J. Trends in Antiretroviral Therapy Prescription, Durability and Modification: New Drugs, More Changes, but Less Failure. AIDS 2018, 32, 347–355. [Google Scholar] [CrossRef]
- Pau, A.K.; George, J.M. Antiretroviral Therapy. Infect. Dis. Clin. N. Am. 2014, 28, 371–402. [Google Scholar] [CrossRef]
- Hruz, P.W.; Murata, H.; Mueckler, M. Adverse Metabolic Consequences of HIV Protease Inhibitor Therapy: The Search for a Central Mechanism. Am. J. Physiol.-Endocrinol. Metab. 2001, 280, E549–E553. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhuang, C.; Chen, F. Druggability Modification Strategies of the Diarylpyrimidine-type Non-nucleoside Reverse Transcriptase Inhibitors. Med. Res. Rev. 2021, 41, 1255–1290. [Google Scholar] [CrossRef] [PubMed]
- Fragkou, P.C.; Moschopoulos, C.D.; Dimopoulou, D.; Triantafyllidi, H.; Birmpa, D.; Benas, D.; Tsiodras, S.; Kavatha, D.; Antoniadou, A.; Papadopoulos, A. Cardiovascular Disease and Risk Assessment in People Living with HIV: Current Practices and Novel Perspectives. Hell. J. Cardiol. 2023, 71, 42–54. [Google Scholar] [CrossRef]
- Clement, M.E.; Park, L.P.; Navar, A.M.; Okeke, N.L.; Pencina, M.J.; Douglas, P.S.; Naggie, S. Statin Utilization and Recommendations Among HIV- and HCV-Infected Veterans: A Cohort Study. Clin. Infect. Dis. 2016, 63, 407–413. [Google Scholar] [CrossRef]
- Todd, J.V.; Cole, S.R.; Wohl, D.A.; Simpson, R.J.; Funk, M.J.; Brookhart, M.A.; Cocohoba, J.; Merenstein, D.; Sharma, A.; Lazar, J.; et al. Underutilization of Statins When Indicated in HIV-Seropositive and Seronegative Women. AIDS Patient Care STDs 2017, 31, 447–454. [Google Scholar] [CrossRef]
- Al-Kindi, S.G.; Zidar, D.A.; McComsey, G.A.; Longenecker, C.T. Gender Differences in Statin Prescription Rate Among Patients Living With HIV and Hepatitis C Virus. Clin. Infect. Dis. 2016, 63, 993–994. [Google Scholar] [CrossRef]
- Ladapo, J.A.; Richards, A.K.; DeWitt, C.M.; Harawa, N.T.; Shoptaw, S.; Cunningham, W.E.; Mafi, J.N. Disparities in the Quality of Cardiovascular Care Between HIV-Infected Versus HIV-Uninfected Adults in the United States: A Cross-Sectional Study. J. Am. Heart Assoc. 2017, 6, e007107. [Google Scholar] [CrossRef]
- Stolbach, A.; Paziana, K.; Heverling, H.; Pham, P. A Review of the Toxicity of HIV Medications II: Interactions with Drugs and Complementary and Alternative Medicine Products. J. Med. Toxicol. 2015, 11, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Dusina, A.; Lombardi, F.; Tamburrini, E.; Onorati, F.; Petrucci, M.; Di Giambenedetto, S. Home Care Assistance: Has Covid-19 Had an Impact on the Complex Management of HIV Patients? AIDS Behav. 2023, 27, 1173–1181. [Google Scholar] [CrossRef]
- Puskas, C.M.; Kaida, A.; Miller, C.L.; Zhang, W.; Yip, B.; Pick, N.; Montaner, J.S.G.; Hogg, R.S. The Adherence Gap: A Longitudinal Examination of Men’s and Women’s Antiretroviral Therapy Adherence in British Columbia, 2000–2014. AIDS 2017, 31, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Puskas, C.M.; Forrest, J.I.; Parashar, S.; Salters, K.A.; Cescon, A.M.; Kaida, A.; Miller, C.L.; Bangsberg, D.R.; Hogg, R.S. Women and Vulnerability to HAART Non-Adherence: A Literature Review of Treatment Adherence by Gender from 2000 to 2011. Curr. HIV/AIDS Rep. 2011, 8, 277–287. [Google Scholar] [CrossRef]
- McComsey, G.A.; Lingohr-Smith, M.; Rogers, R.; Lin, J.; Donga, P. Real-World Adherence to Antiretroviral Therapy Among HIV-1 Patients Across the United States. Adv. Ther. 2021, 38, 4961–4974. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.; Donga, P.; Côté-Sergent, A.; Rossi, C.; Lefebvre, P.; Lafeuille, M.-H.; Hardy, H.; Emond, B. Treatment Patterns and Predictors of Adherence in HIV Patients Receiving Single- or Multiple-Tablet Darunavir, Cobicistat, Emtricitabine, and Tenofovir Alafenamide. Patient Prefer. Adherence 2020, 14, 2315–2326. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, E.; Park, B.-J.; Bang, J.H.; Lee, J.Y. Adherence to Antiretroviral Therapy and Factors Affecting Low Medication Adherence among Incident HIV-Infected Individuals during 2009–2016: A Nationwide Study. Sci. Rep. 2018, 8, 3133. [Google Scholar] [CrossRef] [PubMed]
- Dorrucci, M.; Regine, V.; Pugliese, L.; Suligoi, B. Impact of COVID-19 Epidemic on Temporal Pattern of New HIV Diagnoses in Italy, 2021 Database. Eur. J. Public Health 2023, 33, 1171–1176. [Google Scholar] [CrossRef]

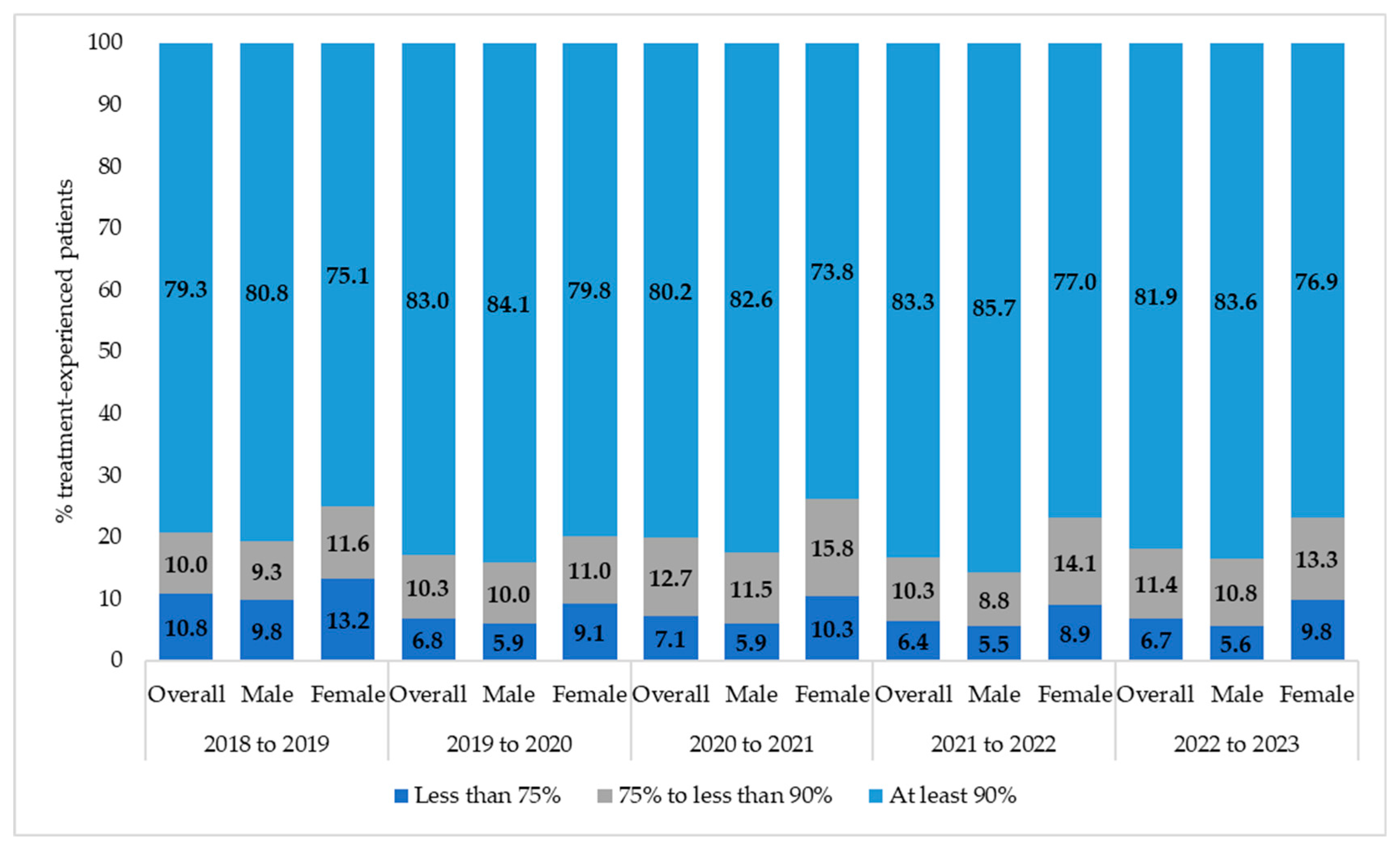
| 2018 N = 2781 | 2019 N = 2957 | 2020 N = 3016 | 2021 N = 3102 | 2022 N = 3003 | 2023 N = 2797 | CAGR% | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | |||
| Male | 2029 | 73.0 | 2159 | 73.0 | 2199 | 72.9 | 2262 | 72.9 | 2212 | 73.7 | 2060 | 73.7 | 0.2 | 0.963 |
| Age group | ||||||||||||||
| 18–34 | 368 | 13.2 | 400 | 13.5 | 379 | 12.6 | 367 | 11.8 | 283 | 9.4 | 222 | 7.9 | −9.7 | <0.0001 |
| 35–44 | 521 | 18.7 | 554 | 18.7 | 543 | 18.0 | 539 | 17.4 | 482 | 16.1 | 423 | 15.1 | −4.2 | |
| 45–54 | 1092 | 39.3 | 1036 | 35.0 | 982 | 32.6 | 938 | 30.2 | 866 | 28.8 | 755 | 27.0 | −7.2 | |
| 55–64 | 634 | 22.8 | 767 | 25.9 | 892 | 29.6 | 999 | 32.2 | 1078 | 35.9 | 1072 | 38.3 | 10.9 | |
| ≥65 | 166 | 6.0 | 200 | 6.8 | 220 | 7.3 | 259 | 8.3 | 294 | 9.8 | 325 | 11.6 | 14.3 | |
| Non-Italian origin | 124 | 4.5 | 123 | 4.2 | 123 | 4.1 | 141 | 4.5 | 110 | 3.7 | 89 | 3.2 | −6.5 | 0.082 |
| STR | 1253 | 45.1 | 1551 | 52.5 | 1883 | 62.4 | 2197 | 70.8 | 2282 | 76.0 | 2227 | 79.6 | 12.1 | <0.0001 |
| MTR | 1528 | 54.9 | 1406 | 47.5 | 1133 | 37.6 | 905 | 29.2 | 721 | 24.0 | 570 | 20.4 | −18.0 | |
| NRTI backbones | ||||||||||||||
| TAF/FTC | 1495 | 53.8 | 1866 | 63.1 | 2015 | 66.8 | 2054 | 66.2 | 1888 | 62.9 | 1694 | 60.6 | 2.4 | <0.0001 |
| ABC/3TC | 583 | 21.0 | 558 | 18.9 | 478 | 15.8 | 416 | 13.4 | 263 | 8.8 | 134 | 4.8 | −25.6 | <0.0001 |
| TDF/FTC | 209 | 7.5 | 69 | 2.3 | 51 | 1.7 | 41 | 1.3 | 34 | 1.1 | 32 | 1.1 | −31.4 | <0.0001 |
| None | 494 | 17.8 | 464 | 16.7 | 472 | 17.0 | 591 | 21.3 | 818 | 29.4 | 937 | 33.7 | 13.6 | <0.0001 |
| ART regimen category | ||||||||||||||
| INSTI-based | 1445 | 52.0 | 1675 | 56.6 | 1803 | 59.8 | 1933 | 62.3 | 1995 | 66.4 | 1930 | 69.0 | 5.8 | <0.0001 |
| PI-based | 925 | 33.3 | 846 | 28.6 | 780 | 25.9 | 760 | 24.5 | 655 | 21.8 | 507 | 18.1 | −11.4 | <0.0001 |
| NNRTI-based | 764 | 27.5 | 758 | 25.6 | 763 | 25.3 | 782 | 25.2 | 823 | 27.4 | 795 | 28.4 | 0.7 | 0.016 |
| Overall | ||
|---|---|---|
| n = 708 | ||
| n | % | |
| Male | 523 | 73.8 |
| Age group | ||
| 18–34 | 212 | 29.9 |
| 35–44 | 205 | 28.9 |
| 45–54 | 168 | 23.7 |
| 55–64 | 105 | 14.8 |
| ≥65 | 19 | 2.7 |
| Non-Italian origin | 133 | 18.8 |
| STR | 499 | 70.5 |
| MTR | 209 | 29.5 |
| NRTI backbones | ||
| TAF/FTC | 526 | 74.2 |
| ABC/3TC | 57 | 8.0 |
| TDF/FTC | 22 | 3.1 |
| None | 104 | 14.7 |
| ART regimen category | ||
| INSTI-based | 452 | 63.8 |
| PI-based | 171 | 24.2 |
| NNRTI-based | 117 | 16.5 |
| Concomitant medications during 1-year follow-up | ||
| Antihypertensives | 122 | 17.2 |
| Statins | 49 | 6.9 |
| Antidiabetics | 24 | 3.4 |
| Overall | Sex | |||||
|---|---|---|---|---|---|---|
| Male | Female | |||||
| n | % | n | % | n | % | |
| PDC category | ||||||
| <75% | 131 | 18.5 | 82 | 15.7 | 49 | 26.4 |
| 75–90% | 74 | 10.5 | 49 | 9.4 | 25 | 13.4 |
| ≥90% | 503 | 71.0 | 391 | 74.9 | 112 | 60.2 |
| Total | 708 | 100.0 | 522 | 100.0 | 186 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trisolini, P.; Cammarota, S.; Citarella, A.; Fogliasecca, M.; Alicchio, V.; Antonacci, S.; Giannini, R.; Lombardi, R.; Piccoli, M.; Pomarico, F.; et al. Antiretroviral Adherence and Use of Antihypertensives, Statins, and Antidiabetics Among Elderly People with HIV: A 5-Year Real-World Study in Southern Italy. Viruses 2025, 17, 1212. https://doi.org/10.3390/v17091212
Trisolini P, Cammarota S, Citarella A, Fogliasecca M, Alicchio V, Antonacci S, Giannini R, Lombardi R, Piccoli M, Pomarico F, et al. Antiretroviral Adherence and Use of Antihypertensives, Statins, and Antidiabetics Among Elderly People with HIV: A 5-Year Real-World Study in Southern Italy. Viruses. 2025; 17(9):1212. https://doi.org/10.3390/v17091212
Chicago/Turabian StyleTrisolini, Pietro, Simona Cammarota, Anna Citarella, Marianna Fogliasecca, Viviana Alicchio, Stefania Antonacci, Romina Giannini, Renato Lombardi, Mariantonietta Piccoli, Francesco Pomarico, and et al. 2025. "Antiretroviral Adherence and Use of Antihypertensives, Statins, and Antidiabetics Among Elderly People with HIV: A 5-Year Real-World Study in Southern Italy" Viruses 17, no. 9: 1212. https://doi.org/10.3390/v17091212
APA StyleTrisolini, P., Cammarota, S., Citarella, A., Fogliasecca, M., Alicchio, V., Antonacci, S., Giannini, R., Lombardi, R., Piccoli, M., Pomarico, F., Procacci, C., Siniscalco, A., Spennato, S., Saracino, A., & Lo Caputo, S. (2025). Antiretroviral Adherence and Use of Antihypertensives, Statins, and Antidiabetics Among Elderly People with HIV: A 5-Year Real-World Study in Southern Italy. Viruses, 17(9), 1212. https://doi.org/10.3390/v17091212





