T-Cell Epitope-Based SARS-CoV-2 DNA Vaccine Encoding an Antigen Fused with Type 1 Herpes Simplex Virus Glycoprotein D (gD)
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Tissue Collection
2.2. Plasmid Design
2.3. In Vitro Expression of the Vaccine Antigens
2.4. Virus Propagation and Titration
2.5. Vaccination and Infection
2.6. Flow Cytometry and Intracellular Cytokine Staining (ICS) Analysis
2.7. IFNγ-ELISpot Assay
2.8. Analysis of Cytokine Production by Cytometric Bead Array
2.9. CD4+/CD8+ T-Cell Depletion
2.10. Quantification of Viral RNA in Tissues
2.11. Statistical Analysis
3. Results
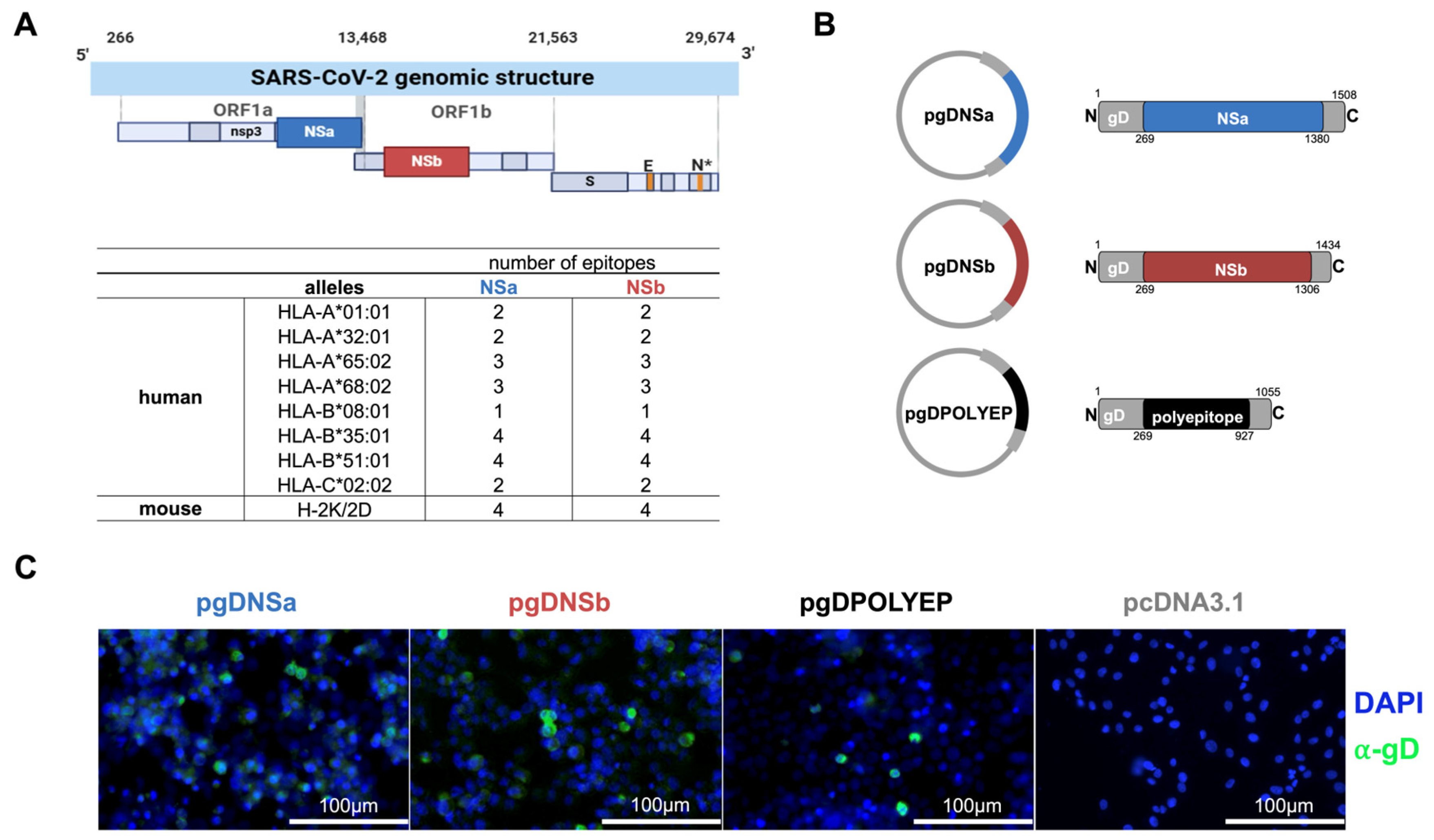
3.1. Design of Vaccine Constructs
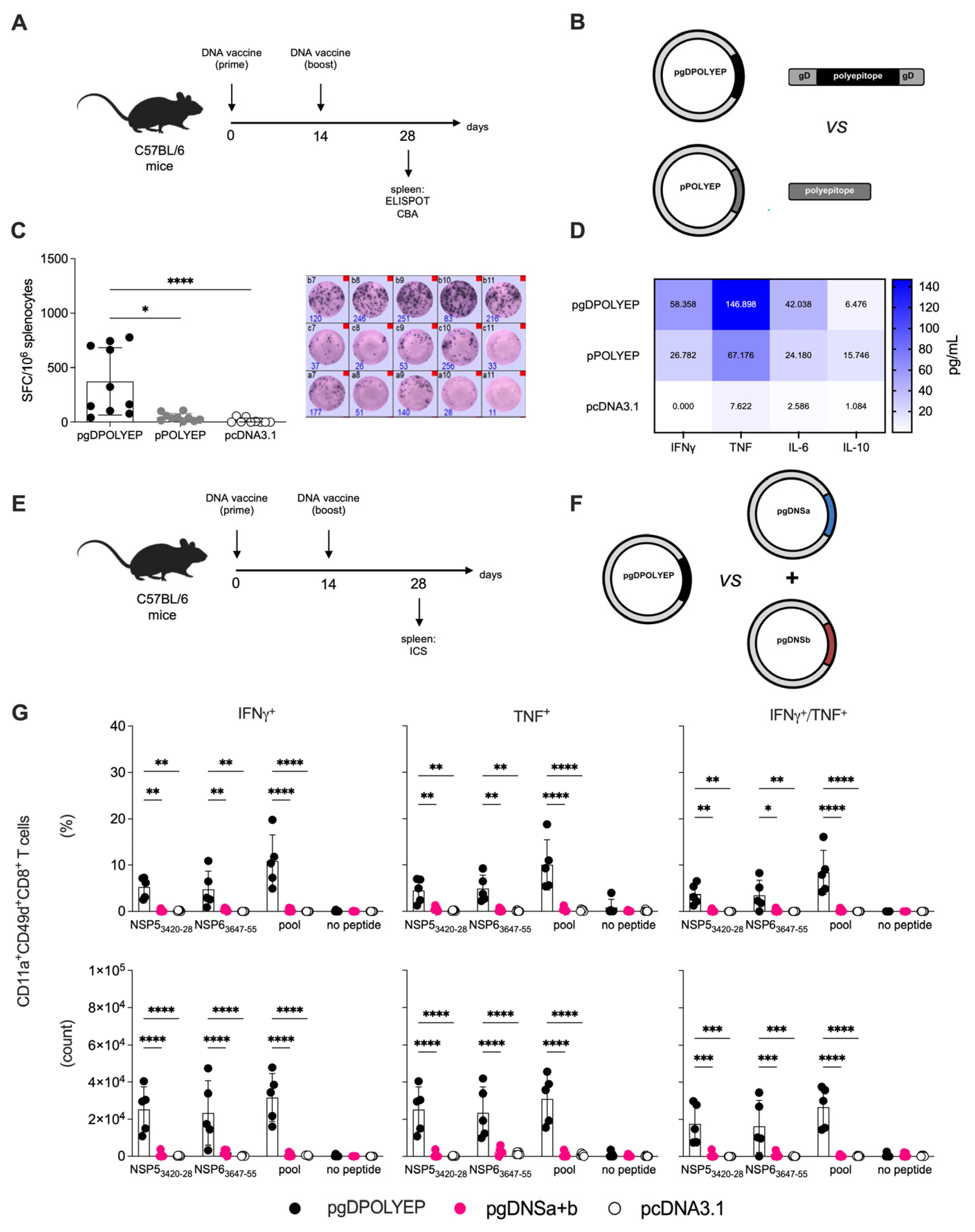
3.2. Evaluation of the Immunomodulatory Role of Glycoprotein D
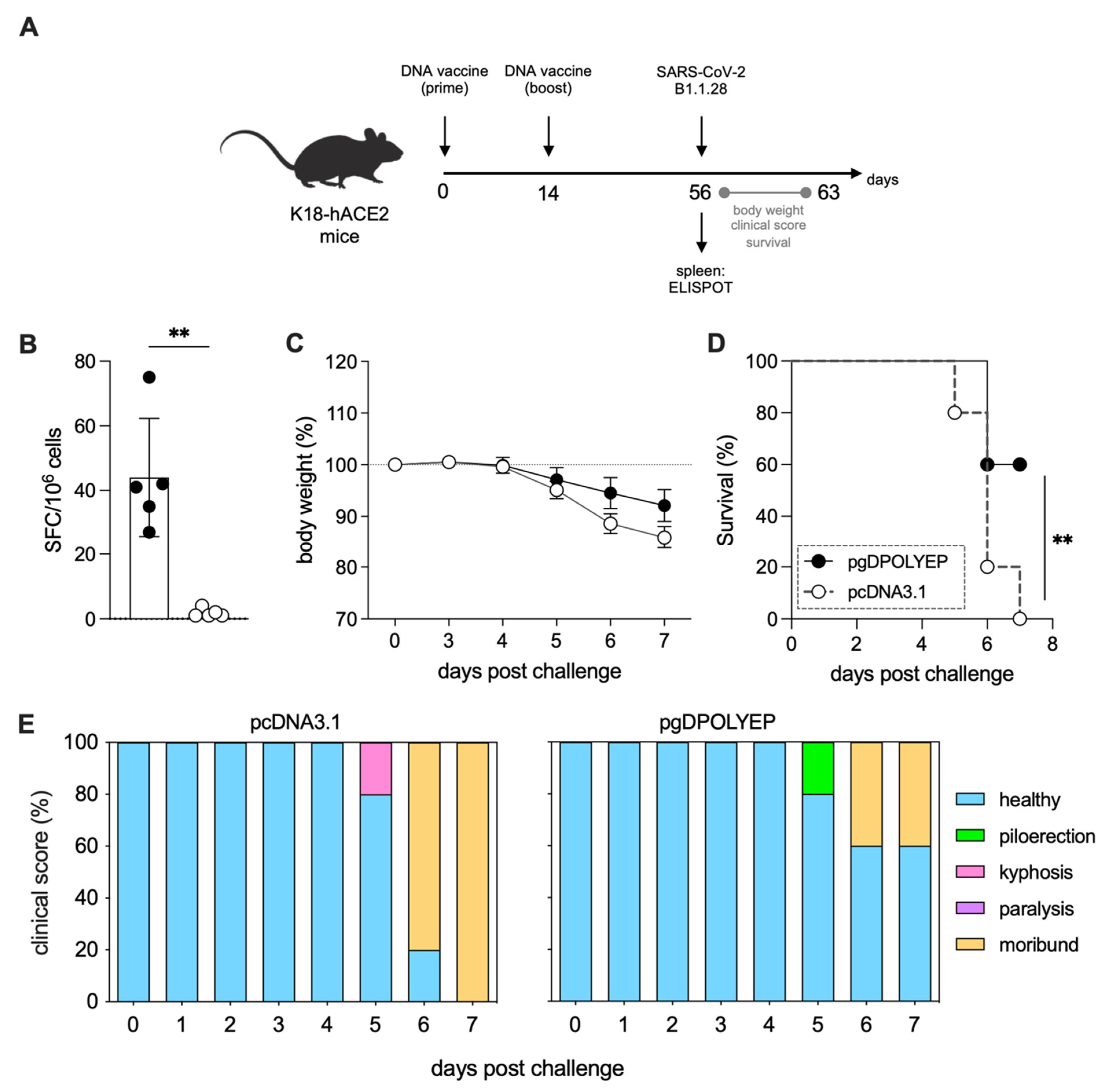
3.3. pgDPOLYEP DNA Vaccine Induced Short-Term Protection to SARS-CoV-2-Challenged K18-hACE2 Transgenic Mice
3.4. pgDPOLYEP Induces Durable T Cell Immunity and Enhanced Protection Against SARS-CoV-2 in K18-hACE2 Transgenic Mice
3.5. T-Cells Are Critical for pgDPOLYEP Induced Protection to SARS-CoV-2 in K18-hACE2 Transgenic Mice
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-Converting Enzyme 2 |
| ANOVA | Analysis of Variance |
| APC | Allophycocyanin |
| BSA | Bovine Serum Albumin |
| BTLA | B and T Lymphocyte Attenuator |
| CD | Cluster of Differentiation |
| CEUA | Ethics Committee on Animal Use |
| CMV | Cytomegalovirus |
| COVID-19 | Coronavirus Disease 2019 |
| DC | Dendritic Cell |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| ELISpot | Enzyme-Linked ImmunoSpot |
| FACS | Fluorescence-Activated Cell Sorting |
| FBS | Fetal Bovine Serum |
| FITC | Fluorescein Isothiocyanate |
| gD | Glycoprotein D |
| HLA | Human Leukocyte Antigen |
| HSV-1 | Herpes Simplex Virus type 1 |
| HVEM | Herpesvirus Entry Mediator |
| ICS | Intracellular Cytokine Staining |
| IEDB | Immune Epitope Database |
| IFN-γ | Interferon Gamma |
| IgG | Immunoglobulin G |
| IL | Interleukin |
| IM | Intramuscular |
| IN | Intranasal |
| mRNA | Messenger Ribonucleic Acid |
| MHC | Major Histocompatibility Complex |
| N | Nucleocapsid Protein |
| NS | Non-Structural |
| ORF | Open Reading Frame |
| PBS | Phosphate-Buffered Saline |
| PFA | Paraformaldehyde |
| PFU | Plaque-Forming Unit |
| RNA | Ribonucleic Acid |
| RT–PCR | Reverse Transcription Polymerase Chain Reaction |
| S | Spike Protein |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SEM | Standard Error of the Mean |
| SFC | Spot-Forming Cell |
| Th1 | T Helper type 1 |
| TNF | Tumor Necrosis Factor |
| VOC | Variant of Concern |
References
- WHO. COVID-19 Epidemiological Update, 169th ed.; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Gavriatopoulou, M.; Korompoki, E.; Fotiou, D.; Ntanasis-Stathopoulos, I.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Organ-specific manifestations of COVID-19 infection. Clin. Exp. Med. 2020, 20, 493–506. [Google Scholar] [CrossRef]
- Robba, C.; Battaglini, D.; Pelosi, P.; Rocco, P.R.M. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev. Respir. Med. 2020, 14, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccin. Immunother. 2022, 18, 2002083. [Google Scholar] [CrossRef]
- Rappuoli, R.; Alter, G.; Pulendran, B. Transforming vaccinology. Cell 2024, 187, 5171–5194. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat. Med. 2021, 27, 1147–1148. [Google Scholar] [CrossRef] [PubMed]
- Carpp, L.N.; Hyrien, O.; Fong, Y.; Benkeser, D.; Roels, S.; Stieh, D.J.; Van Dromme, I.; Van Roey, G.A.; Kenny, A.; Huang, Y.; et al. Neutralizing antibody correlate of protection against severe-critical COVID-19 in the ENSEMBLE single-dose Ad26.COV2.S vaccine efficacy trial. Nat. Commun. 2024, 15, 9785. [Google Scholar] [CrossRef]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Lustig, Y.; Joseph, G.; Gilboa, M.; Barda, N.; Gens, I.; Indenbaum, V.; Halpern, O.; Katz-Likvornik, S.; Levin, T.; et al. Correlates of protection against COVID-19 infection and intensity of symptomatic disease in vaccinated individuals exposed to SARS-CoV-2 in households in Israel (ICoFS): A prospective cohort study. Lancet Microbe 2023, 4, e309–e318. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar] [CrossRef]
- Adamo, S.; Michler, J.; Zurbuchen, Y.; Cervia, C.; Taeschler, P.; Raeber, M.E.; Baghai Sain, S.; Nilsson, J.; Moor, A.E.; Boyman, O. Signature of long-lived memory CD8(+) T cells in acute SARS-CoV-2 infection. Nature 2022, 602, 148–155. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Liu, X.; Hu, P. T cell epitopes are largely conserved in the SARS-CoV-2 Omicron subvariant (BA.1, BA.2, BA.3, and GKA). J. Med. Virol. 2022, 94, 4591–4592. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e1415. [Google Scholar] [CrossRef]
- Arieta, C.M.; Xie, Y.J.; Rothenberg, D.A.; Diao, H.; Harjanto, D.; Meda, S.; Marquart, K.; Koenitzer, B.; Sciuto, T.E.; Lobo, A.; et al. The T-cell-directed vaccine BNT162b4 encoding conserved non-spike antigens protects animals from severe SARS-CoV-2 infection. Cell 2023, 186, 2392–2409.e2321. [Google Scholar] [CrossRef]
- Oyarzun, P.; Kashyap, M.; Fica, V.; Salas-Burgos, A.; Gonzalez-Galarza, F.F.; McCabe, A.; Jones, A.R.; Middleton, D.; Kobe, B. A Proteome-Wide Immunoinformatics Tool to Accelerate T-Cell Epitope Discovery and Vaccine Design in the Context of Emerging Infectious Diseases: An Ethnicity-Oriented Approach. Front. Immunol. 2021, 12, 598778. [Google Scholar] [CrossRef]
- Adelusi, T.I.; Ogunlana, A.T.; Oyewole, M.P.; Ojo, T.O.; Olaoba, O.T.; Oladipo, E.K.; Akash, S.; Ibenmoussa, S.; Bourhia, M.; Jardan, Y.A.B.; et al. Designing of an innovative conserved multiepitope subunit vaccine targeting SARS-CoV-2 glycoprotein and nucleoprotein through immunoinformatic. Sci. Rep. 2025, 15, 2563. [Google Scholar] [CrossRef]
- Zhou, H.; Leng, P.; Wang, Y.; Yang, K.; Li, C.; Ojcius, D.M.; Wang, P.; Jiang, S. Development of T cell antigen-based human coronavirus vaccines against nAb-escaping SARS-CoV-2 variants. Sci. Bull. 2024, 69, 2456–2470. [Google Scholar] [CrossRef]
- Porchia, B.; Aps, L.; Moreno, A.C.R.; da Silva, J.R.; Silva, M.O.; Sales, N.S.; Alves, R.; Rocha, C.R.R.; Silva, M.M.; Rodrigues, K.B.; et al. Active immunization combined with cisplatin confers enhanced therapeutic protection and prevents relapses of HPV-induced tumors at different anatomical sites. Int. J. Biol. Sci. 2022, 18, 15–29. [Google Scholar] [CrossRef]
- Ramos da Silva, J.; Bitencourt Rodrigues, K.; Formoso Pelegrin, G.; Silva Sales, N.; Muramatsu, H.; de Oliveira Silva, M.; Porchia, B.; Moreno, A.C.R.; Aps, L.; Venceslau-Carvalho, A.A.; et al. Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci. Transl. Med. 2023, 15, eabn3464. [Google Scholar] [CrossRef] [PubMed]
- Ramos da Silva, J.; Ramos Moreno, A.C.; Silva Sales, N.; de Oliveira Silva, M.; Aps, L.; Porchia, B.; Bitencourt Rodrigues, K.; Cestari Moreno, N.; Venceslau-Carvalho, A.A.; Menck, C.F.M.; et al. A therapeutic DNA vaccine and gemcitabine act synergistically to eradicate HPV-associated tumors in a preclinical model. Oncoimmunology 2021, 10, 1949896. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.H.; Sidney, J.; Dinh, K.; Southwood, S.; Newman, M.J.; Sette, A. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinform. 2006, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Alves, R.P.; Timis, J.; Miller, R.; Valentine, K.; Pinto, P.B.A.; Gonzalez, A.; Regla-Nava, J.A.; Maule, E.; Nguyen, M.N.; Shafee, N.; et al. Human coronavirus OC43-elicited CD4(+) T cells protect against SARS-CoV-2 in HLA transgenic mice. Nat. Commun. 2024, 15, 787. [Google Scholar] [CrossRef]
- Kumari, P.; Rothan, H.A.; Natekar, J.P.; Stone, S.; Pathak, H.; Strate, P.G.; Arora, K.; Brinton, M.A.; Kumar, M. Neuroinvasion and Encephalitis Following Intranasal Inoculation of SARS-CoV-2 in K18-hACE2 Mice. Viruses 2021, 13, 132. [Google Scholar] [CrossRef]
- Karosiene, E.; Lundegaard, C.; Lund, O.; Nielsen, M. NetMHCcons: A consensus method for the major histocompatibility complex class I predictions. Immunogenetics 2012, 64, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Franco, L.; Oliveira Vidal, P.; Amorim, J.H. In silico design of a Zika virus non-structural protein 5 aiming vaccine protection against zika and dengue in different human populations. J. Biomed. Sci. 2017, 24, 88. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.L.; Shen, W.C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Lasaro, M.O.; Tatsis, N.; Hensley, S.E.; Whitbeck, J.C.; Lin, S.W.; Rux, J.J.; Wherry, E.J.; Cohen, G.H.; Eisenberg, R.J.; Ertl, H.C. Targeting of antigen to the herpesvirus entry mediator augments primary adaptive immune responses. Nat. Med. 2008, 14, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Porchia, B.; Moreno, A.C.R.; Ramos, R.N.; Diniz, M.O.; de Andrade, L.; Rosa, D.S.; Barbuto, J.A.M.; Boscardin, S.B.; Ferreira, L.C.S. Herpes Simplex Virus Glycoprotein D Targets a Specific Dendritic Cell Subset and Improves the Performance of Vaccines to Human Papillomavirus-Associated Tumors. Mol. Cancer Ther. 2017, 16, 1922–1933. [Google Scholar] [CrossRef]
- Lasaro, M.O.; Diniz, M.O.; Reyes-Sandoval, A.; Ertl, H.C.; Ferreira, L.C. Anti-tumor DNA vaccines based on the expression of human papillomavirus-16 E6/E7 oncoproteins genetically fused with the glycoprotein D from herpes simplex virus-1. Microbes Infect. 2005, 7, 1541–1550. [Google Scholar] [CrossRef]
- McCray, P.B., Jr.; Pewe, L.; Wohlford-Lenane, C.; Hickey, M.; Manzel, L.; Shi, L.; Netland, J.; Jia, H.P.; Halabi, C.; Sigmund, C.D.; et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007, 81, 813–821. [Google Scholar] [CrossRef]
- Steinberg, M.W.; Cheung, T.C.; Ware, C.F. The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation. Immunol. Rev. 2011, 244, 169–187. [Google Scholar] [CrossRef]
- Boquett, J.A.; Bisso-Machado, R.; Zagonel-Oliveira, M.; Schuler-Faccini, L.; Fagundes, N.J.R. HLA diversity in Brazil. HLA 2020, 95, 3–14. [Google Scholar] [CrossRef]
- Ramesh, S.; Govindarajulu, M.; Parise, R.S.; Neel, L.; Shankar, T.; Patel, S.; Lowery, P.; Smith, F.; Dhanasekaran, M.; Moore, T. Emerging SARS-CoV-2 Variants: A Review of Its Mutations, Its Implications and Vaccine Efficacy. Vaccines 2021, 9, 1195. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e1019. [Google Scholar] [CrossRef]
- Dimeglio, C.; Herin, F.; Da-Silva, I.; Gernigon, C.; Porcheron, M.; Chapuy-Regaud, S.; Izopet, J. Decreased Efficiency of Neutralizing Antibodies from Previously Infected or Vaccinated Individuals against the B.1.617.2 (Delta) SARS-CoV-2 Variant. Microbiol. Spectr. 2022, 10, e0270621. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, M.; Nishina, N.; Moriyama, S.; Takahashi, Y.; Ishii, M.; Saya, H.; Kondo, Y.; Kaneko, Y.; Suzuki, K.; Fukunaga, K.; et al. Immune evasion and chronological decrease in titer of neutralizing antibody against SARS-CoV-2 and its variants of concerns in COVID-19 patients. Clin. Immunol. 2022, 238, 108999. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859.e811. [Google Scholar] [CrossRef]
- Geers, D.; Gommers, L.; Tan, N.H.; Bogers, S.; van Baarle, D.; Grifoni, A.; Sette, A.; Boerma, A.; Visscher, F.; Richard, M.; et al. Profiling the SARS-CoV-2-specific T-cell response. Lancet Infect. Dis. 2024, 24, e477–e478. [Google Scholar] [CrossRef]
- Grifoni, A.; Sidney, J.; Vita, R.; Peters, B.; Crotty, S.; Weiskopf, D.; Sette, A. SARS-CoV-2 human T cell epitopes: Adaptive immune response against COVID-19. Cell Host Microbe 2022, 30, 1788. [Google Scholar] [CrossRef]
- Mateus, J.; Grifoni, A.; Tarke, A.; Sidney, J.; Ramirez, S.I.; Dan, J.M.; Burger, Z.C.; Rawlings, S.A.; Smith, D.M.; Phillips, E.; et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 2020, 370, 89–94. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.K.; DeVico, A.L.; Gallo, R.C. Antibody persistence and T-cell balance: Two key factors confronting HIV vaccine development. Proc. Natl. Acad. Sci. USA 2014, 111, 15614–15621. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Valentine, K.M.; Croft, M.; Shresta, S. Protection against dengue virus requires a sustained balance of antibody and T cell responses. Curr. Opin. Virol. 2020, 43, 22–27. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Stacey, H.D.; Garin-Ortega, L.; Lopez, P.G.; Ramezani-Rad, P.; Ramirez, S.I.; Faraji, F.; Bhavsar, D.; Levi, G.; Krammer, F.; Crotty, S. Local B-cell immunity and durable memory following live-attenuated influenza intranasal vaccination of humans. bioRxiv 2025. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aps, L.R.d.M.M.; Venceslau-Carvalho, A.A.; Freitas, C.L.d.; Porchia, B.F.M.M.; Silva, M.d.O.; Pereira, L.R.; Sales, N.S.; Pelegrin, G.F.; Segabinazi, E.; Rodrigues, K.B.; et al. T-Cell Epitope-Based SARS-CoV-2 DNA Vaccine Encoding an Antigen Fused with Type 1 Herpes Simplex Virus Glycoprotein D (gD). Viruses 2025, 17, 1191. https://doi.org/10.3390/v17091191
Aps LRdMM, Venceslau-Carvalho AA, Freitas CLd, Porchia BFMM, Silva MdO, Pereira LR, Sales NS, Pelegrin GF, Segabinazi E, Rodrigues KB, et al. T-Cell Epitope-Based SARS-CoV-2 DNA Vaccine Encoding an Antigen Fused with Type 1 Herpes Simplex Virus Glycoprotein D (gD). Viruses. 2025; 17(9):1191. https://doi.org/10.3390/v17091191
Chicago/Turabian StyleAps, Luana Raposo de Melo Moraes, Aléxia Adrianne Venceslau-Carvalho, Carla Longo de Freitas, Bruna Felício Milazzotto Maldonado Porchia, Mariângela de Oliveira Silva, Lennon Ramos Pereira, Natiely Silva Sales, Guilherme Formoso Pelegrin, Ethiane Segabinazi, Karine Bitencourt Rodrigues, and et al. 2025. "T-Cell Epitope-Based SARS-CoV-2 DNA Vaccine Encoding an Antigen Fused with Type 1 Herpes Simplex Virus Glycoprotein D (gD)" Viruses 17, no. 9: 1191. https://doi.org/10.3390/v17091191
APA StyleAps, L. R. d. M. M., Venceslau-Carvalho, A. A., Freitas, C. L. d., Porchia, B. F. M. M., Silva, M. d. O., Pereira, L. R., Sales, N. S., Pelegrin, G. F., Segabinazi, E., Rodrigues, K. B., Silva, J. R. d., Almeida, B. d. S., Farias, J. P., Castro-Amarante, M. F., Minoprio, P. M. C., Ferreira, L. C. d. S., & dos Santos Alves, R. P. (2025). T-Cell Epitope-Based SARS-CoV-2 DNA Vaccine Encoding an Antigen Fused with Type 1 Herpes Simplex Virus Glycoprotein D (gD). Viruses, 17(9), 1191. https://doi.org/10.3390/v17091191







