Antiviral Strategies Targeting Enteroviruses: Current Advances and Future Directions
Abstract
1. Introduction
2. Biology and Pathogenesis of Enteroviruses
2.1. Structure and Genome Organization of Enteroviruses
2.2. Life Cycle of Enteroviruses
2.3. Clinical Manifestations
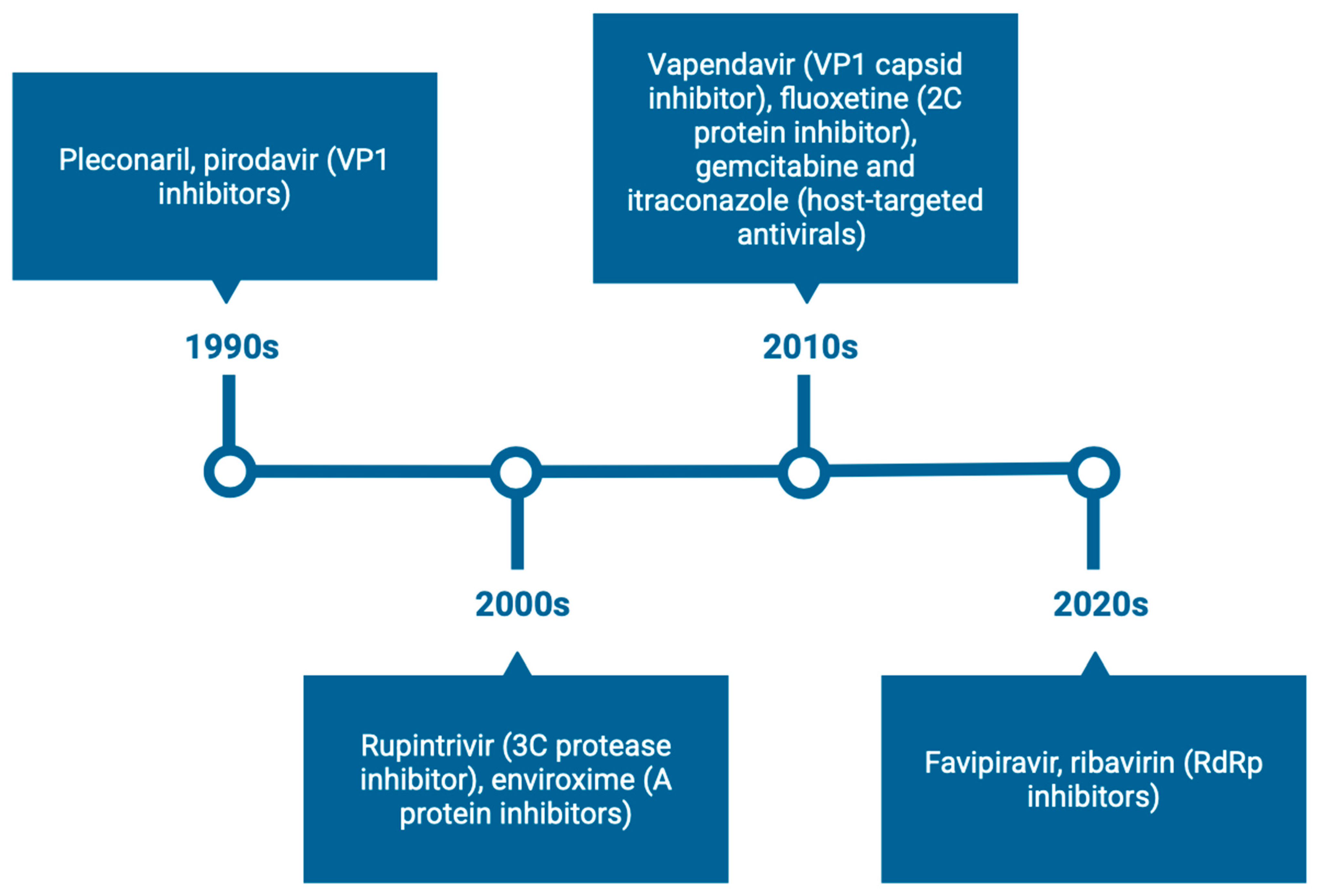
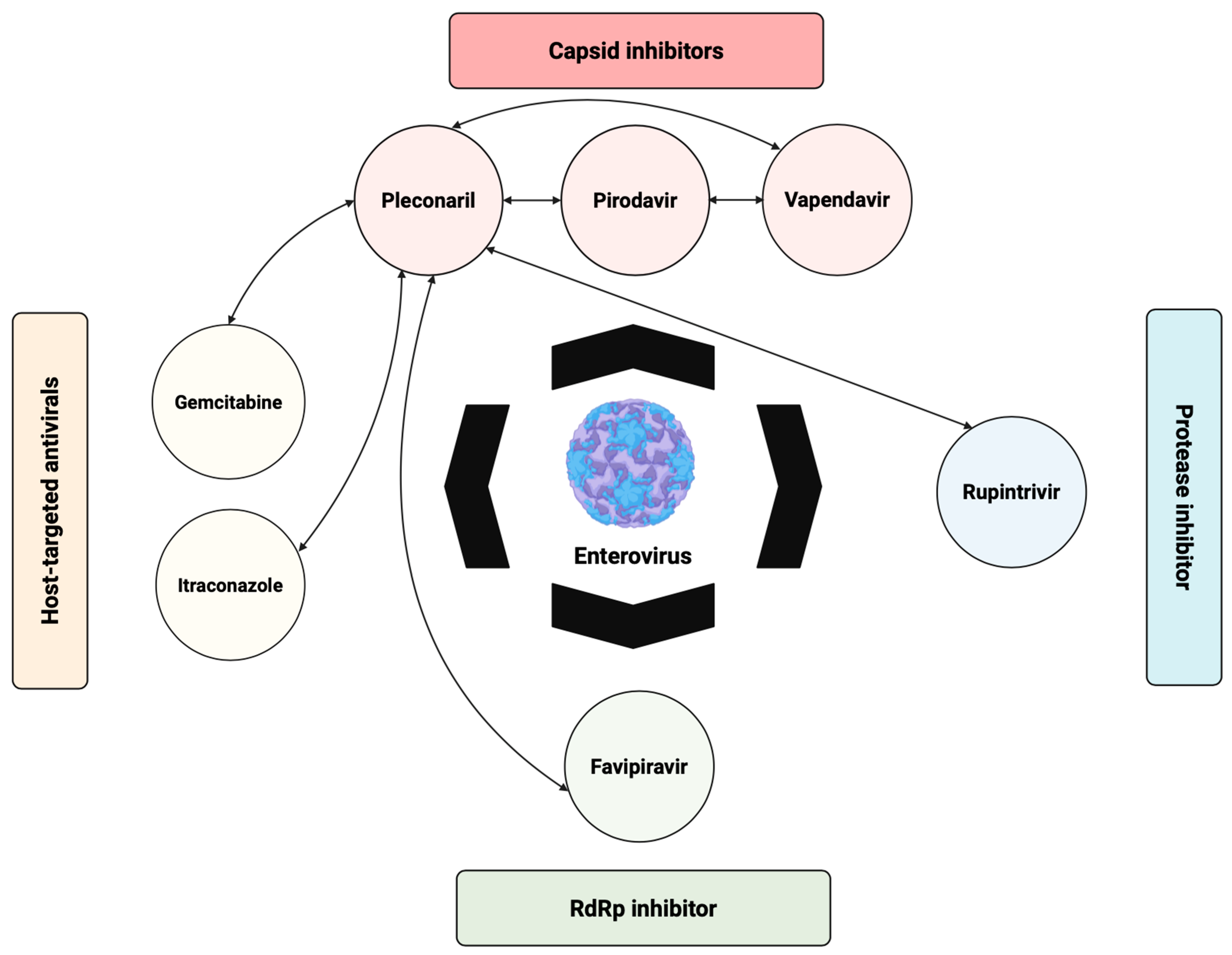
3. Direct-Acting Antivirals (DAAs)
3.1. Capsid Binders
3.1.1. Antivirals Targeting the VP1 Hydrophobic Pocket
3.1.2. Antivirals Targeting the VP1-VP3 Interprotomer Binding Pocket
3.1.3. Antivirals Targeting the Five-Fold Axis of the Capsid
3.2. Non-Structural Protein Inhibitors
3.2.1. 2A Protease Inhibitors
3.2.2. 2B Protein Inhibitors
3.2.3. 2C Protein Inhibitors
3.2.4. 3A Protein Inhibitors
3.2.5. 3C Protease Inhibitors
3.2.6. Three-Dimensional Polymerase Inhibitors
4. Host-Targeting Antivirals
5. Emerging and Experimental Strategies
5.1. Immunotherapeutic Approaches
5.2. RNA Interference (RNAi)
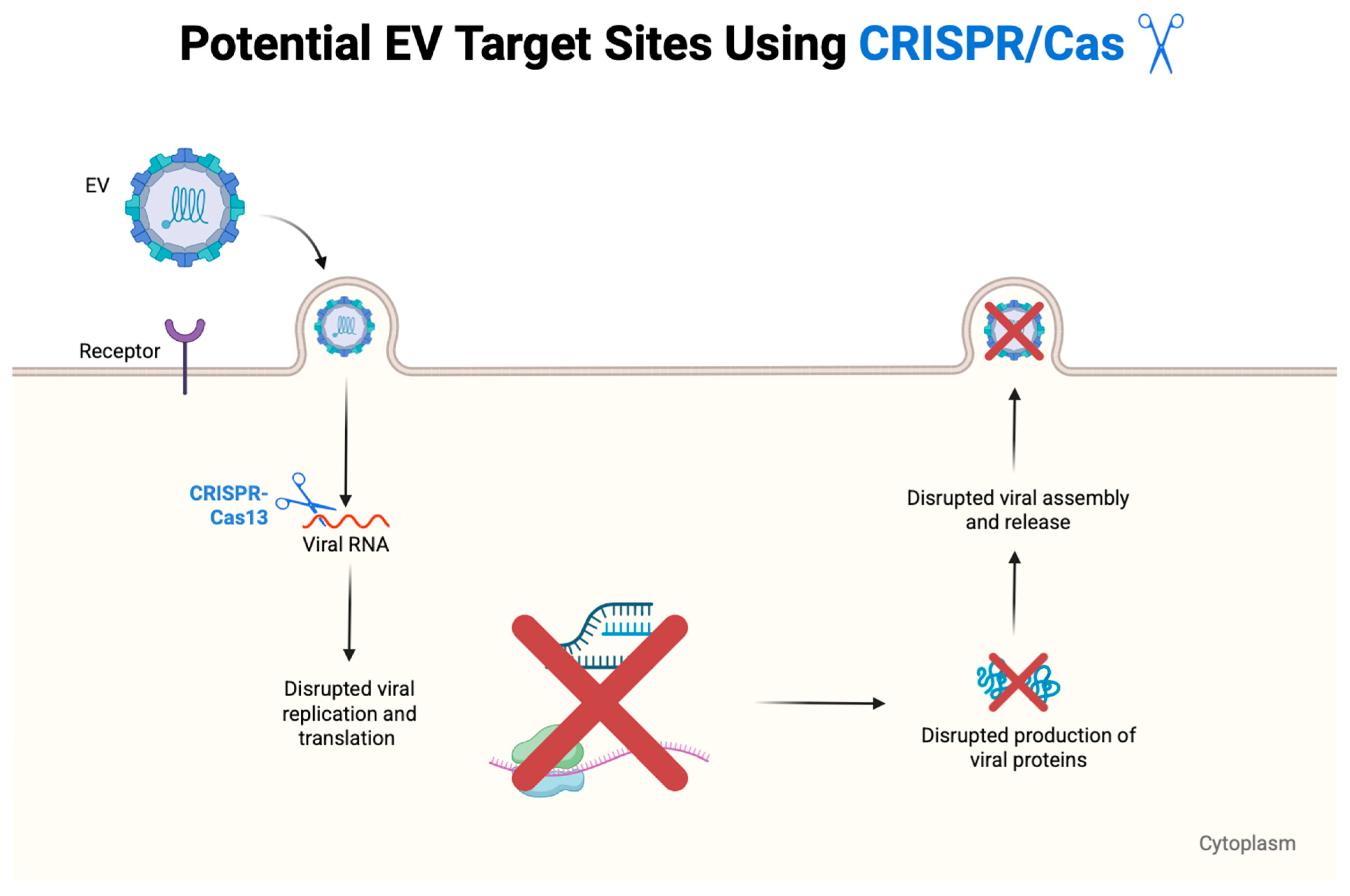
5.3. CRISPR-Based Antivirals
5.4. Peptide-Based Antivirals
6. Challenges in Antiviral Development for the Treatment of EV Infections
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baggen, J.; Thibaut, H.J.; Strating, J.R.P.M.; van Kuppeveld, F.J.M. The life cycle of non-polio enteroviruses and how to target it. Nat. Rev. Microbiol. 2018, 16, 368–381. [Google Scholar] [CrossRef]
- Anasir, M.I.; Poh, C.L. Advances in Antigenic Peptide-Based Vaccine and Neutralizing Antibodies against Viruses Causing Hand, Foot, and Mouth Disease. Int. J. Mol. Sci. 2019, 20, 1256. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Shen, L.; Wu, J.; Zou, X.; Gu, J.; Chen, J.; Mao, L. Enterovirus A71 Proteins: Structure and Function. Front. Microbiol. 2018, 9, 286. [Google Scholar] [CrossRef]
- Yang, B.; Liu, F.; Liao, Q.; Wu, P.; Chang, Z.; Huang, J.; Long, L.; Luo, L.; Li, Y.; Leung, G.M.; et al. Epidemiology of hand, foot and mouth disease in China, 2008 to 2015 prior to the introduction of EV-A71 vaccine. Euro Surveill. 2017, 22, 16-00824. [Google Scholar] [CrossRef] [PubMed]
- Puenpa, J.; Wanlapakorn, N.; Vongpunsawad, S.; Poovorawan, Y. The History of Enterovirus A71 Outbreaks and Molecular Epidemiology in the Asia-Pacific Region. J. Biomed. Sci. 2019, 26, 75. [Google Scholar] [CrossRef]
- Messacar, K.; Abzug, M.J.; Dominguez, S.R. 2014 outbreak of enterovirus D68 in North America. J. Med. Virol. 2016, 88, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.J.; Dass, R.S. Chapter 15—Current therapeutic strategies and novel antiviral compounds for the treatment of nonpolio enteroviruses. In Viral Infections and Antiviral Therapies; Dhara, A.K., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2023. [Google Scholar]
- Lyu, K.; Wang, G.C.; He, Y.L.; Han, J.F.; Ye, Q.; Qin, C.F.; Chen, R. Crystal structures of enterovirus 71 (EV71) recombinant virus particles provide insights into vaccine design. J. Biol. Chem. 2015, 290, 3198–3208. [Google Scholar] [CrossRef]
- Abdelnabi, R.; Geraets, J.A.; Ma, Y.; Mirabelli, C.; Flatt, J.W.; Domanska, A.; Delang, L.; Jochmans, D.; Kumar, T.A.; Jayaprakash, V.; et al. A novel druggable interprotomer pocket in the capsid of rhino- and enteroviruses. PLoS Biol. 2019, 17, e3000281. [Google Scholar] [CrossRef]
- Egorova, A.; Ekins, S.; Schmidtke, M.; Makarov, V. Back to the future: Advances in development of broad-spectrum capsid-binding inhibitors of enteroviruses. Eur. J. Med. Chem. 2019, 178, 606–622. [Google Scholar] [CrossRef]
- Dang, M.; Wang, X.; Wang, Q.; Wang, Y.; Lin, J.; Sun, Y.; Li, X.; Zhang, L.; Lou, Z.; Wang, J.; et al. Molecular mechanism of SCARB2-mediated attachment and uncoating of EV71. Protein Cell 2014, 5, 692–703. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Zheng, M. Enterovirus A71 antivirals: Past, present, and future. Acta Pharm. Sin. B 2022, 12, 1542–1566. [Google Scholar] [CrossRef]
- Wang, S.; Pang, Z.; Fan, H.; Tong, Y. Advances in anti-EV-A71 drug development research. J. Adv. Res. 2024, 56, 137–156. [Google Scholar] [CrossRef]
- Palmenberg, A.C. Proteolytic processing of picornaviral polyprotein. Annu. Rev. Microbiol. 1990, 44, 603–623. [Google Scholar] [CrossRef]
- Laitinen, O.H.; Svedin, E.; Kapell, S.; Nurminen, A.; Hytönen, V.P.; Flodström-Tullberg, M. Enteroviral proteases: Structure, host interactions and pathogenicity. Rev. Med. Virol. 2016, 26, 251–267. [Google Scholar] [CrossRef]
- Bergelson, J.M.; Coyne, C.B. Picornavirus entry. Adv. Exp. Med. Biol. 2013, 790, 24–41. [Google Scholar]
- Anasir, M.I.; Zarif, F.; Poh, C.L. Antivirals blocking entry of enteroviruses and therapeutic potential. J. Biomed. Sci. 2021, 28, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ye, X.; Zhang, X.Y.; Zhu, Z.; Zhang, X.; Xu, Z.; Ding, Z.; Zou, G.; Liu, Q.; Kong, L.; et al. Coxsackievirus A10 atomic structure facilitating the discovery of a broad-spectrum inhibitor against human enteroviruses. Cell Discov. 2019, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhao, Y.; Kotecha, A.; Fry, E.E.; Kelly, J.T.; Wang, X.; Rao, Z.; Rowlands, D.J.; Ren, J.; Stuart, D.I. Unexpected mode of engagement between enterovirus 71 and its receptor SCARB2. Nat. Microbiol. 2019, 4, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Shingler, K.L.; Yoder, J.L.; Carnegie, M.S.; Ashley, R.E.; Makhov, A.M.; Conway, J.F.; Hafenstein, S. The enterovirus 71 A-particle forms a gateway to allow genome release: A cryoEM study of picornavirus uncoating. PLoS Pathog. 2013, 9, e1003240. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, Y.; Ma, H.C.; Paul, A.V.; Wimmer, E. Picornavirus morphogenesis. Microbiol. Mol. Biol. Rev. 2014, 78, 418–437. [Google Scholar] [CrossRef]
- Tan, Y.W.; Hong, W.J.; Chu, J.J. Inhibition of enterovirus VP4 myristoylation is a potential antiviral strategy for hand, foot and mouth disease. Antivir. Res. 2016, 133, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Ao, D.; Sun, S.Q.; Guo, H.C. Topology and biological function of enterovirus non-structural protein 2B as a member of the viroporin family. Vet. Res. 2014, 45, 87. [Google Scholar] [CrossRef]
- Yeager, C.; Carter, G.; Gohara, D.W.; Yennawar, N.H.; Enemark, E.J.; Arnold, J.J.; Cameron, C.E. Enteroviral 2C protein is an RNA-stimulated ATPase and uses a two-step mechanism for binding to RNA and ATP. Nucleic Acids Res. 2022, 50, 11775–11798. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Wang, Z.; Xie, W. Crystal Structure and Thermostability Characterization of Enterovirus D68 3D. J. Virol. 2017, 91, e00876-17. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhong, T.; Wang, Y.; Song, F.M.; Yu, X.F.; Xing, L.P.; Zhang, W.Y.; Yu, J.H.; Hua, S.C. Human Enterovirus 68 Interferes with the Host Cell Cycle to Facilitate Viral Production. Front. Cell. Infect. Microbiol. 2017, 7, 29. [Google Scholar] [CrossRef]
- Jan, S.L.; Fu, Y.C.; Chi, C.S.; Lee, H.F.; Huang, F.L.; Wang, C.C.; Wei, H.J.; Lin, M.C.; Chen, P.Y.; Hwang, B. Catecholamine-Induced Secondary Takotsubo Syndrome in Children With Severe Enterovirus 71 Infection and Acute Heart Failure: A 20-year Experience of a Single Institute. Front. Cardiovasc. Med. 2021, 8, 752232. [Google Scholar] [CrossRef] [PubMed]
- Oermann, C.M.; Schuster, J.E.; Conners, G.P.; Newland, J.G.; Selvarangan, R.; Jackson, M.A. Enterovirus d68. A focused review and clinical highlights from the 2014 U.S. Outbreak. Ann. Am. Thorac. Soc. 2015, 12, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, X.Y.; Yu, X.F. Current Understanding of Human Enterovirus D68. Viruses 2019, 11, 490. [Google Scholar] [CrossRef]
- Tammaro, C.; Guida, M.; Appetecchia, F.; Biava, M.; Consalvi, S.; Poce, G. Direct-Acting Antivirals and Host-Targeting Approaches against Enterovirus B Infections: Recent Advances. Pharmaceuticals 2023, 16, 203. [Google Scholar] [CrossRef]
- Chen, T.C.; Liu, S.C.; Huang, P.N.; Chang, H.Y.; Chern, J.H.; Shih, S.R. Antiviral activity of pyridyl imidazolidinones against enterovirus 71 variants. J. Biomed. Sci. 2008, 15, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.F.; Jheng, J.R.; Lin, G.H.; Chen, Y.L.; Ho, J.Y.; Liu, C.J.; Hsu, K.Y.; Chen, Y.S.; Chan, Y.F.; Yu, H.M.; et al. Rosmarinic acid exhibits broad anti-enterovirus A71 activity by inhibiting the interaction between the five-fold axis of capsid VP1 and cognate sulfated receptors. Emerg. Microbes Infect. 2020, 9, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Yu, Y.J.; Jinn, T.R. Evaluation of the virucidal effects of rosmarinic acid against enterovirus 71 infection via in vitro and in vivo study. Virol. J. 2019, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Hu, Y.; Zhang, J.; Musharrafieh, R.; Wang, J. A Novel Capsid Binding Inhibitor Displays Potent Antiviral Activity against Enterovirus D68. ACS Infect. Dis. 2019, 5, 1952–1962. [Google Scholar] [CrossRef]
- Lacroix, C.; Laconi, S.; Angius, F.; Coluccia, A.; Silvestri, R.; Pompei, R.; Neyts, J.; Leyssen, P. In vitro characterisation of a pleconaril/pirodavir-like compound with potent activity against rhinoviruses. Virol. J. 2015, 12, 106. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, P.; Wang, N.; Zhang, J.; Li, J.; Guo, H.; Yin, X.; Rao, Z.; Wang, X.; Zhang, L. The binding of a monoclonal antibody to the apical region of SCARB2 blocks EV71 infection. Protein Cell 2017, 8, 590–600. [Google Scholar] [CrossRef]
- Ren, P.; Zheng, Y.; Wang, W.; Hong, L.; Delpeyroux, F.; Arenzana-Seisdedos, F.; Altmeyer, R. Suramin interacts with the positively charged region surrounding the 5-fold axis of the EV-A71 capsid and inhibits multiple enterovirus A. Sci. Rep. 2017, 7, 42902. [Google Scholar] [CrossRef]
- Reshamwala, D.; Shroff, S.; Sheik Amamuddy, O.; Laquintana, V.; Denora, N.; Zacheo, A.; Lampinen, V.; Hytonen, V.P.; Tastan Bishop, Ö.; Krol, S.; et al. Polyphenols Epigallocatechin Gallate and Resveratrol, and Polyphenol-Functionalized Nanoparticles Prevent Enterovirus Infection through Clustering and Stabilization of the Viruses. Pharmaceutics 2021, 13, 1182. [Google Scholar] [CrossRef]
- Huang, Y.L.; Lin, T.M.; Wang, S.Y.; Wang, J.R. The role of conserved arginine and proline residues in enterovirus VP1 protein. J. Microbiol. Immunol. Infect. 2022, 55, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Huang, S.W.; Shen, C.Y.; Cheng, D.; Wang, J.R. Conserved Residues Adjacent to ß-Barrel and Loop Intersection among Enterovirus VP1 Affect Viral Replication: Potential Target for Anti-Enteroviral Development. Viruses 2022, 14, 364. [Google Scholar] [CrossRef]
- Plevka, P.; Perera, R.; Yap, M.L.; Cardosa, J.; Kuhn, R.J.; Rossmann, M.G. Structure of human enterovirus 71 in complex with a capsid-binding inhibitor. Proc. Natl. Acad. Sci. USA 2013, 110, 5463–5467. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, F.; Gu, B.; Ding, C.; Feng, D.; Xie, F.; Wang, J.; Zhang, C.; Cao, Q.; Deng, Y.; et al. In vitro and in vivo evaluation of ribavirin and pleconaril antiviral activity against enterovirus 71 infection. Arch. Virol. 2012, 157, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Smee, D.F.; Evans, W.J.; Nicolaou, K.C.; Tarbet, E.B.; Day, C.W. Susceptibilities of enterovirus D68, enterovirus 71, and rhinovirus 87 strains to various antiviral compounds. Antivir. Res. 2016, 131, 61–65. [Google Scholar] [CrossRef]
- Tijsma, A.; Franco, D.; Tucker, S.; Hilgenfeld, R.; Froeyen, M.; Leyssen, P.; Neyts, J. The capsid binder Vapendavir and the novel protease inhibitor SG85 inhibit enterovirus 71 replication. Antimicrob. Agents Chemother. 2014, 58, 6990–6992. [Google Scholar] [CrossRef]
- Kim, J.; Jung, Y.K.; Kim, C.; Shin, J.S.; Scheers, E.; Lee, J.Y.; Han, S.B.; Lee, C.K.; Neyts, J.; Ha, J.D.; et al. A Novel Series of Highly Potent Small Molecule Inhibitors of Rhinovirus Replication. J. Med. Chem. 2017, 60, 5472–5492. [Google Scholar] [CrossRef]
- Ho, J.Y.; Chern, J.H.; Hsieh, C.F.; Liu, S.T.; Liu, C.J.; Wang, Y.S.; Kuo, T.W.; Hsu, S.J.; Yeh, T.K.; Shih, S.R.; et al. In vitro and in vivo studies of a potent capsid-binding inhibitor of enterovirus 71. J. Antimicrob. Chemother. 2016, 71, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- De Colibus, L.; Wang, X.; Spyrou, J.A.B.; Kelly, J.; Ren, J.; Grimes, J.; Puerstinger, G.; Stonehouse, N.; Walter, T.S.; Hu, Z.; et al. More-powerful virus inhibitors from structure-based analysis of HEV71 capsid-binding molecules. Nat. Struct. Mol. Biol. 2014, 21, 282–288. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; He, W.; Sun, Y.; Guo, Y.; Zhong, W.; Gao, Q.; Liao, M.; Wang, X.; Cai, Y.; et al. Design, Synthesis, and Evaluation of Novel Enterovirus 71 Inhibitors as Therapeutic Drug Leads for the Treatment of Human Hand, Foot, and Mouth Disease. J. Med. Chem. 2020, 63, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Chern, J.H.; Lee, C.C.; Chang, C.S.; Lee, Y.C.; Tai, C.L.; Lin, Y.T.; Shia, K.S.; Lee, C.Y.; Shih, S.R. Synthesis and antienteroviral activity of a series of novel, oxime ether-containing pyridyl imidazolidinones. Bioorg Med. Chem. Lett. 2004, 14, 5051–5056. [Google Scholar] [CrossRef]
- Li, P.; Yu, J.; Hao, F.; He, H.; Shi, X.; Hu, J.; Wang, L.; Du, C.; Zhang, X.; Sun, Y.; et al. Discovery of Potent EV71 Capsid Inhibitors for Treatment of HFMD. ACS Med. Chem. Lett. 2017, 8, 841–846. [Google Scholar] [CrossRef]
- Han, X.; Sun, N.; Wu, H.; Guo, D.; Tien, P.; Dong, C.; Wu, S.; Zhou, H.B. Identification and Structure-Activity Relationships of Diarylhydrazides as Novel Potent and Selective Human Enterovirus Inhibitors. J. Med. Chem. 2016, 59, 2139–2150. [Google Scholar] [CrossRef]
- Arita, M.; Fuchino, H.; Kawakami, H.; Ezaki, M.; Kawahara, N. Characterization of a New Antienterovirus D68 Compound Purified from Avocado. ACS Infect. Dis. 2020, 6, 2291–2300. [Google Scholar] [CrossRef]
- Li, G.; Gao, Q.; Yuan, S.; Wang, L.; Altmeyer, R.; Lan, K.; Yin, F.; Zou, G. Characterization of three small molecule inhibitors of enterovirus 71 identified from screening of a library of natural products. Antivir. Res. 2017, 143, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Lacroix, C.; Cabiddu, M.G.; Neyts, J.; Leyssen, P.; Pompei, R. Exploration of the anti-enterovirus activity of a series of pleconaril/pirodavir-like compounds. Antivir. Chem. Chemother. 2015, 24, 56–61. [Google Scholar] [CrossRef]
- Egorova, A.; Kazakova, E.; Jahn, B.; Ekins, S.; Makarov, V.; Schmidtke, M. Novel pleconaril derivatives: Influence of substituents in the isoxazole and phenyl rings on the antiviral activity against enteroviruses. Eur. J. Med. Chem. 2020, 188, 112007. [Google Scholar] [CrossRef]
- Makarov, V.A.; Braun, H.; Richter, M.; Riabova, O.B.; Kirchmair, J.; Kazakova, E.S.; Seidel, N.; Wutzler, P.; Schmidtke, M. Pyrazolopyrimidines: Potent Inhibitors Targeting the Capsid of Rhino- and Enteroviruses. ChemMedChem 2015, 10, 1629–1634. [Google Scholar] [CrossRef]
- Carta, A.; Sanna, G.; Briguglio, I.; Madeddu, S.; Vitale, G.; Piras, S.; Corona, P.; Peana, A.T.; Laurini, E.; Fermeglia, M.; et al. Quinoxaline derivatives as new inhibitors of coxsackievirus B5. Eur. J. Med. Chem. 2018, 145, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, S.; Ibba, R.; Sanna, G.; Piras, S.; Riu, F.; Marongiu, A.; Ambrosino, A.; Caria, P.; Onnis, V.; Franci, G.; et al. Human Enterovirus B: Selective Inhibition by Quinoxaline Derivatives and Bioinformatic RNA-Motif Identification as New Targets. Pharmaceuticals 2022, 15, 181. [Google Scholar] [CrossRef] [PubMed]
- Kalam, N.; Balasubramaniam, V.R.M.T. Emerging Therapeutics in the Fight Against EV-D68: A Review of Current Strategies. Influenza Other Respir. Viruses 2024, 18, e70064. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sheng, J.; Fokine, A.; Meng, G.; Shin, W.H.; Long, F.; Kuhn, R.J.; Kihara, D.; Rossmann, M.G. Structure and inhibition of EV-D68, a virus that causes respiratory illness in children. Science 2015, 347, 71–74. [Google Scholar] [CrossRef]
- Senior, K. FDA panel rejects common cold treatment. Lancet Infect. Dis. 2002, 2, 264. [Google Scholar] [CrossRef]
- Rhoden, E.; Zhang, M.; Nix, W.A.; Oberste, M.S. In Vitro Efficacy of Antiviral Compounds against Enterovirus D68. Antimicrob. Agents Chemother. 2015, 59, 7779–7781. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Fan, S.; Cao, R.; He, X.; Li, W.; Xu, L.; Cheng, T.; Li, H.; Zhong, W. Discovery and Optimization of Quinoline Analogues as Novel Potent Antivirals against Enterovirus D68. J. Med. Chem. 2022, 65, 14792–14808. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Abdelnabi, R.; Delang, L.; Froeyen, M.; Luyten, W.; Neyts, J.; Mirabelli, C. New class of early-stage enterovirus inhibitors with a novel mechanism of action. Antivir. Res. 2017, 147, 67–74. [Google Scholar] [CrossRef]
- Shetnev, A.A.; Volobueva, A.S.; Panova, V.A.; Zarubaev, V.V.; Baykov, S.V. Design of 4-Substituted Sulfonamidobenzoic Acid Derivatives Targeting Coxsackievirus B3. Life 2022, 12, 1832. [Google Scholar] [CrossRef]
- Sun, L.; Lee, H.; Thibaut, H.J.; Lanko, K.; Rivero-Buceta, E.; Bator, C.; Martinez-Gualda, B.; Dallmeier, K.; Delang, L.; Leyssen, P.; et al. Viral engagement with host receptors blocked by a novel class of tryptophan dendrimers that targets the 5-fold-axis of the enterovirus-A71 capsid. PLoS Pathog. 2019, 15, e1007760. [Google Scholar] [CrossRef]
- Martínez-Gualda, B.; Sun, L.; Martí-Marí, O.; Noppen, S.; Abdelnabi, R.; Bator, C.M.; Quesada, E.; Delang, L.; Mirabelli, C.; Lee, H.; et al. Scaffold Simplification Strategy Leads to a Novel Generation of Dual Human Immunodeficiency Virus and Enterovirus-A71 Entry Inhibitors. J. Med. Chem. 2020, 63, 349–368. [Google Scholar] [CrossRef]
- Ren, P.; Zou, G.; Bailly, B.; Xu, S.; Zeng, M.; Chen, X.; Shen, L.; Zhang, Y.; Guillon, P.; Arenzana-Seisdedos, F.; et al. The approved pediatric drug suramin identified as a clinical candidate for the treatment of EV71 infection-suramin inhibits EV71 infection in vitro and in vivo. Emerg. Microbes Infect. 2014, 3, e62. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; McLaughlin, N.P.; Pan, J.; Goldstein, S.; Hafenstein, S.; Shimizu, H.; Winkler, J.D.; Bergelson, J.M. The Suramin Derivative NF449 Interacts with the 5-fold Vertex of the Enterovirus A71 Capsid to Prevent Virus Attachment to PSGL-1 and Heparan Sulfate. PLoS Pathog. 2015, 11, e1005184. [Google Scholar] [CrossRef]
- Meng, T.; Jia, Q.; Wong, S.M.; Chua, K.B. In Vitro and In Vivo Inhibition of the Infectivity of Human Enterovirus 71 by a Sulfonated Food Azo Dye, Brilliant Black BN. J. Virol. 2019, 93, 10-1128. [Google Scholar] [CrossRef]
- Wang, H.; Lei, X.; Xiao, X.; Yang, C.; Lu, W.; Huang, Z.; Leng, Q.; Jin, Q.; He, B.; Meng, G.; et al. Reciprocal Regulation between Enterovirus 71 and the NLRP3 Inflammasome. Cell Rep. 2015, 12, 42–48. [Google Scholar] [CrossRef]
- Wang, B.; Xi, X.; Lei, X.; Zhang, X.; Cui, S.; Wang, J.; Jin, Q.; Zhao, Z. Enterovirus 71 protease 2Apro targets MAVS to inhibit anti-viral type I interferon responses. PLoS Pathog. 2013, 9, e1003231. [Google Scholar] [CrossRef]
- Falah, N.; Montserret, R.; Lelogeais, V.; Schuffenecker, I.; Lina, B.; Cortay, J.C.; Violot, S. Blocking human enterovirus 71 replication by targeting viral 2A protease. J. Antimicrob. Chemother. 2012, 67, 2865–2869. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Huang, A.C.; Hour, M.J.; Huang, S.H.; Kung, S.H.; Chen, C.H.; Chen, I.C.; Chang, Y.S.; Lien, J.C.; Lin, C.W. Antiviral Potential of a Novel Compound CW-33 against Enterovirus A71 via Inhibition of Viral 2A Protease. Viruses 2015, 7, 3155–3171. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Y.; Cao, L.; Wang, P.; Qing, J.; Zheng, Q.; Shang, L.; Yin, Z.; Sun, Y. A Conserved Inhibitory Mechanism of a Lycorine Derivative against Enterovirus and Hepatitis C Virus. Antimicrob. Agents Chemother. 2016, 60, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Hou, X.; Peng, H.; Zhang, L.; Jiang, Q.; Shi, M.; Ji, Y.; Wang, Y.; Shi, W.; et al. Chlorogenic acid inhibits the replication and viability of enterovirus 71 in vitro. PLoS ONE 2013, 8, e76007. [Google Scholar] [CrossRef]
- Chen, S.G.; Cheng, M.L.; Chen, K.H.; Horng, J.T.; Liu, C.C.; Wang, S.M.; Sakurai, H.; Leu, Y.L.; Wang, S.D.; Ho, H.Y. Antiviral activities of Schizonepeta tenuifolia Briq. against enterovirus 71 in vitro and in vivo. Sci. Rep. 2017, 7, 935. [Google Scholar] [CrossRef]
- Chen, S.G.; Leu, Y.L.; Cheng, M.L.; Ting, S.C.; Liu, C.C.; Wang, S.D.; Yang, C.H.; Hung, C.Y.; Sakurai, H.; Chen, K.H.; et al. Anti-enterovirus 71 activities of Melissa officinalis extract and its biologically active constituent rosmarinic acid. Sci. Rep. 2017, 7, 12264. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wang, Y.; Pei, X.; Wang, C.; Niu, Y.; Xu, P.; Peng, Y. Substituted 3-benzylcoumarins 13 and 14 suppress enterovirus A71 replication by impairing viral 2A. Antivir. Res. 2018, 160, 10–16. [Google Scholar] [CrossRef]
- Musharrafieh, R.; Ma, C.; Zhang, J.; Hu, Y.; Diesing, J.M.; Marty, M.T.; Wang, J. Validating Enterovirus D68-2A(pro) as an Antiviral Drug Target and the Discovery of Telaprevir as a Potent D68-2A(pro) Inhibitor. J. Virol. 2019, 93, 10-1128. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, W.; Niu, Y.; Wang, S.; Chen, B.; Xiong, R.; Zhang, P.; Luo, Z.; Wu, Y.; Fan, C.; et al. Phosphorylation of enteroviral 2A(pro) at Ser/Thr125 benefits its proteolytic activity and viral pathogenesis. J. Med. Virol. 2023, 95, e28400. [Google Scholar] [CrossRef]
- Cai, Q.; Yameen, M.; Liu, W.; Gao, Z.; Li, Y.; Peng, X.; Cai, Y.; Wu, C.; Zheng, Q.; Li, J.; et al. Conformational plasticity of the 2A proteinase from enterovirus 71. J. Virol. 2013, 87, 7348–7356. [Google Scholar] [CrossRef]
- Xie, S.; Wang, K.; Yu, W.; Lu, W.; Xu, K.; Wang, J.; Ye, B.; Schwarz, W.; Jin, Q.; Sun, B. DIDS blocks a chloride-dependent current that is mediated by the 2B protein of enterovirus 71. Cell Res. 2011, 21, 1271–1275. [Google Scholar] [CrossRef]
- Nieva, J.L.; Madan, V.; Carrasco, L. Viroporins: Structure and biological functions. Nat. Rev. Microbiol. 2012, 10, 563–574. [Google Scholar] [CrossRef]
- Cong, H.; Du, N.; Yang, Y.; Song, L.; Zhang, W.; Tien, P. Enterovirus 71 2B Induces Cell Apoptosis by Directly Inducing the Conformational Activation of the Proapoptotic Protein Bax. J. Virol. 2016, 90, 9862–9877. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Wang, K.; Zhao, K.; Hua, S.C.; Du, J. The Structure, Function, and Mechanisms of Action of Enterovirus Non-structural Protein 2C. Front. Microbiol. 2020, 11, 615965. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Tian, J.; Qin, B.; Wojdyla, J.A.; Wang, B.; Zhao, Z.; Wang, M.; Cui, S. Crystal structure of 2C helicase from enterovirus 71. Sci. Adv. 2017, 3, e1602573. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, C.; Yang, R.; Bai, P.; Zhang, X.Y.; Kong, J.; Yin, L.; Qiu, Y.; Zhou, X. Antiviral Peptides Targeting the Helicase Activity of Enterovirus Nonstructural Protein 2C. J. Virol. 2021, 95, e02324-20. [Google Scholar] [CrossRef] [PubMed]
- Messacar, K.; Sillau, S.; Hopkins, S.E.; Otten, C.; Wilson-Murphy, M.; Wong, B.; Santoro, J.D.; Treister, A.; Bains, H.K.; Torres, A.; et al. Safety, tolerability, and efficacy of fluoxetine as an antiviral for acute flaccid myelitis. Neurology 2019, 92, e2118–e2126. [Google Scholar] [CrossRef]
- Tyler, K.L. Rationale for the evaluation of fluoxetine in the treatment of enterovirus D68-associated acute flaccid myelitis. JAMA Neurol. 2015, 72, 493–494. [Google Scholar] [CrossRef]
- Manganaro, R.; Zonsics, B.; Bauer, L.; Lorenzo Lopez, M.; Donselaar, T.; Zwaagstra, M.; Saporito, F.; Ferla, S.; Strating, J.R.P.M.; Coutard, B.; et al. Synthesis and antiviral effect of novel fluoxetine analogues as enterovirus 2C inhibitors. Antivir. Res. 2020, 178, 104781. [Google Scholar] [CrossRef]
- Bauer, L.; Manganaro, R.; Zonsics, B.; Hurdiss, D.L.; Zwaagstra, M.; Donselaar, T.; Welter, N.G.E.; van Kleef, R.G.D.M.; Lopez, M.L.; Bevilacqua, F.; et al. Rational design of highly potent broad-spectrum enterovirus inhibitors targeting the nonstructural protein 2C. PLoS Biol. 2020, 18, e3000904. [Google Scholar] [CrossRef]
- Ulferts, R.; de Boer, S.M.; van der Linden, L.; Bauer, L.; Lyoo, H.R.; Maté, M.J.; Lichière, J.; Canard, B.; Lelieveld, D.; Omta, W.; et al. Screening of a Library of FDA-Approved Drugs Identifies Several Enterovirus Replication Inhibitors That Target Viral Protein 2C. Antimicrob. Agents Chemother. 2016, 60, 2627–2638. [Google Scholar] [CrossRef] [PubMed]
- Musharrafieh, R.; Zhang, J.; Tuohy, P.; Kitamura, N.; Bellampalli, S.S.; Hu, Y.; Khanna, R.; Wang, J. Discovery of Quinoline Analogues as Potent Antivirals against Enterovirus D68 (EV-D68). J. Med. Chem. 2019, 62, 4074–4090. [Google Scholar] [CrossRef] [PubMed]
- Musharrafieh, R.; Kitamura, N.; Hu, Y.; Wang, J. Development of broad-spectrum enterovirus antivirals based on quinoline scaffold. Bioorg Chem. 2020, 101, 103981. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, Z.; Jin, M.; Shu, T.; Chen, Y.; Feng, L.; Zhang, Q.; Lan, K.; Wu, S.; Zhou, H.B. Identification of dibucaine derivatives as novel potent enterovirus 2C helicase inhibitors: In vitro, in vivo, and combination therapy study. Eur. J. Med. Chem. 2020, 202, 112310. [Google Scholar] [CrossRef]
- Ma, C.; Hu, Y.; Zhang, J.; Wang, J. Pharmacological Characterization of the Mechanism of Action of R523062, a Promising Antiviral for Enterovirus D68. ACS Infect. Dis. 2020, 6, 2260–2270. [Google Scholar] [CrossRef]
- Zuo, J.; Kye, S.; Quinn, K.K.; Cooper, P.; Damoiseaux, R.; Krogstad, P. Discovery of Structurally Diverse Small-Molecule Compounds with Broad Antiviral Activity against Enteroviruses. Antimicrob. Agents Chemother. 2015, 60, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zuo, J.; Krogstad, P.; Jung, M.E. Synthesis and Structure-Activity Relationship (SAR) Studies of Novel Pyrazolopyridine Derivatives as Inhibitors of Enterovirus Replication. J. Med. Chem. 2018, 61, 1688–1703. [Google Scholar] [CrossRef]
- Hu, Y.; Kitamura, N.; Musharrafieh, R.; Wang, J. Discovery of Potent and Broad-Spectrum Pyrazolopyridine-Containing Antivirals against Enteroviruses D68, A71, and Coxsackievirus B3 by Targeting the Viral 2C Protein. J. Med. Chem. 2021, 64, 8755–8774. [Google Scholar] [CrossRef]
- De Palma, A.M.; Heggermont, W.; Lanke, K.; Coutard, B.; Bergmann, M.; Monforte, A.M.; Canard, B.; De Clercq, E.; Chimirri, A.; Pürstinger, G.; et al. The thiazolobenzimidazole TBZE-029 inhibits enterovirus replication by targeting a short region immediately downstream from motif C in the nonstructural protein 2C. J. Virol. 2008, 82, 4720–4730. [Google Scholar] [CrossRef]
- Guan, H.; Tian, J.; Zhang, C.; Qin, B.; Cui, S. Crystal structure of a soluble fragment of poliovirus 2CATPase. PLoS Pathog. 2018, 14, e1007304. [Google Scholar] [CrossRef] [PubMed]
- Hurdiss, D.L.; El Kazzi, P.; Bauer, L.; Papageorgiou, N.; Ferron, F.P.; Donselaar, T.; van Vliet, A.L.W.; Canard, B.; Decroly, E.; Brancale, A.; et al. Fluoxetine targets an allosteric site in the enterovirus 2C AAA+ ATPase and stabilizes the hexameric complex. Sci. Adv. 2022, 8, eabj7615. [Google Scholar] [CrossRef]
- Li, Y.; Jian, X.; Yin, P.; Zhu, G.; Zhang, L. Elucidating the Host Interactome of EV-A71 2C Reveals Viral Dependency Factors. Front. Microbiol. 2019, 10, 636. [Google Scholar] [CrossRef] [PubMed]
- Horova, V.; Lyoo, H.; Różycki, B.; Chalupska, D.; Smola, M.; Humpolickova, J.; Strating, J.R.P.M.; van Kuppeveld, F.J.M.; Boura, E.; Klima, M.; et al. Convergent evolution in the mechanisms of ACBD3 recruitment to picornavirus replication sites. PLoS Pathog. 2019, 15, e1007962. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Cheng, A.; Wen, X.; Ou, X.; Mao, S.; Gao, Q.; Sun, D.; Jia, R.; Yang, Q.; et al. Enterovirus Replication Organelles and Inhibitors of Their Formation. Front. Microbiol. 2020, 11, 1817. [Google Scholar] [CrossRef]
- Nagy, P.D.; Strating, J.R.; van Kuppeveld, F.J. Building Viral Replication Organelles: Close Encounters of the Membrane Types. PLoS Pathog. 2016, 12, e1005912. [Google Scholar] [CrossRef] [PubMed]
- Lyoo, H.; van der Schaar, H.M.; Dorobantu, C.M.; Rabouw, H.H.; Strating, J.R.P.M.; van Kuppeveld, F.J.M. ACBD3 Is an Essential Pan-enterovirus Host Factor That Mediates the Interaction between Viral 3A Protein and Cellular Protein PI4KB. mBio 2019, 10, e02742-18. [Google Scholar] [CrossRef]
- Miller, F.D.; Monto, A.S.; DeLong, D.C.; Exelby, A.; Bryan, E.R.; Srivastava, S. Controlled trial of enviroxime against natural rhinovirus infections in a community. Antimicrob. Agents Chemother. 1985, 27, 102–106. [Google Scholar] [CrossRef]
- Heinz, B.A.; Vance, L.M. The antiviral compound enviroxime targets the 3A coding region of rhinovirus and poliovirus. J. Virol. 1995, 69, 4189–4197. [Google Scholar] [CrossRef]
- Arita, M.; Wakita, T.; Shimizu, H. Characterization of pharmacologically active compounds that inhibit poliovirus and enterovirus 71 infectivity. J. Gen. Virol. 2008, 89, 2518–2530. [Google Scholar] [CrossRef]
- Arita, M.; Wakita, T.; Shimizu, H. Cellular kinase inhibitors that suppress enterovirus replication have a conserved target in viral protein 3A similar to that of enviroxime. J. Gen. Virol. 2009, 90 Pt 8, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Takebe, Y.; Wakita, T.; Shimizu, H. A bifunctional anti-enterovirus compound that inhibits replication and the early stage of enterovirus 71 infection. J. Gen. Virol. 2010, 91, 2734–2744. [Google Scholar] [CrossRef] [PubMed]
- De Palma, A.M.; Thibaut, H.J.; van der Linden, L.; Lanke, K.; Heggermont, W.; Ireland, S.; Andrews, R.; Arimilli, M.; Al-Tel, T.H.; De Clercq, E.; et al. Mutations in the nonstructural protein 3A confer resistance to the novel enterovirus replication inhibitor TTP-8307. Antimicrob. Agents Chemother. 2009, 53, 1850–1857. [Google Scholar] [CrossRef]
- Albulescu, L.; Bigay, J.; Biswas, B.; Weber-Boyvat, M.; Dorobantu, C.M.; Delang, L.; van der Schaar, H.M.; Jung, Y.S.; Neyts, J.; Olkkonen, V.M.; et al. Uncovering oxysterol-binding protein (OSBP) as a target of the anti-enteroviral compound TTP-8307. Antivir. Res. 2017, 140, 37–44. [Google Scholar] [CrossRef]
- Gao, Q.; Yuan, S.; Zhang, C.; Wang, Y.; He, G.; Zhang, S.; Altmeyer, R.; Zou, G. Discovery of itraconazole with broad-spectrum in vitro antienterovirus activity that targets nonstructural protein 3A. Antimicrob. Agents Chemother. 2015, 59, 2654–2665. [Google Scholar] [CrossRef]
- Rhoden, E.; Ng, T.F.F.; Campagnoli, R.; Nix, W.A.; Konopka-Anstadt, J.; Selvarangan, R.; Briesach, L.; Oberste, M.S.; Weldon, W.C. Antifungal Triazole Posaconazole Targets an Early Stage of the Parechovirus A3 Life Cycle. Antimicrob. Agents Chemother. 2020, 64, e02372-19. [Google Scholar] [CrossRef]
- Lu, G.; Qi, J.; Chen, Z.; Xu, X.; Gao, F.; Lin, D.; Qian, W.; Liu, H.; Jiang, H.; Yan, J.; et al. Enterovirus 71 and coxsackievirus A16 3C proteases: Binding to rupintrivir and their substrates and anti-hand, foot, and mouth disease virus drug design. J. Virol. 2011, 85, 10319–10331. [Google Scholar] [CrossRef]
- de Breyne, S.; Bonderoff, J.M.; Chumakov, K.M.; Lloyd, R.E.; Hellen, C.U. Cleavage of eukaryotic initiation factor eIF5B by enterovirus 3C proteases. Virology 2008, 378, 118–122. [Google Scholar] [CrossRef]
- Wen, W.; Qi, Z.; Wang, J. The Function and Mechanism of Enterovirus 71 (EV71) 3C Protease. Curr. Microbiol. 2020, 77, 1968–1975. [Google Scholar] [CrossRef]
- Li, B.; Yue, Y.; Zhang, Y.; Yuan, Z.; Li, P.; Song, N.; Lin, W.; Liu, Y.; Gu, L.; Meng, H. A Novel Enterovirus 71 (EV71) Virulence Determinant: The 69th Residue of 3C Protease Modulates Pathogenicity. Front. Cell. Infect. Microbiol. 2017, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shang, C.; Yang, P.; Li, L.; Zhai, Y.; Yin, Z.; Wang, B.; Shang, L. 4-Iminooxazolidin-2-one as a Bioisostere of the Cyanohydrin Moiety: Inhibitors of Enterovirus 71 3C Protease. J. Med. Chem. 2018, 61, 10333–10339. [Google Scholar] [CrossRef]
- Ma, Y.; Li, L.; He, S.; Shang, C.; Sun, Y.; Liu, N.; Meek, T.D.; Wang, Y.; Shang, L. Application of Dually Activated Michael Acceptor to the Rational Design of Reversible Covalent Inhibitor for Enterovirus 71 3C Protease. J. Med. Chem. 2019, 62, 6146–6162. [Google Scholar] [CrossRef]
- Ma, G.H.; Ye, Y.; Zhang, D.; Xu, X.; Si, P.; Peng, J.L.; Xiao, Y.L.; Cao, R.Y.; Yin, Y.L.; Chen, J.; et al. Identification and biochemical characterization of DC07090 as a novel potent small molecule inhibitor against human enterovirus 71 3C protease by structure-based virtual screening. Eur. J. Med. Chem. 2016, 124, 981–991. [Google Scholar] [CrossRef]
- Hayden, F.G.; Turner, R.B.; Gwaltney, J.M.; Chi-Burris, K.; Gersten, M.; Hsyu, P.; Patick, A.K.; Smith, G.J.; Zalman, L.S. Phase II, randomized, double-blind, placebo-controlled studies of ruprintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers. Antimicrob. Agents Chemother. 2003, 47, 3907–3916. [Google Scholar] [CrossRef] [PubMed]
- Patick, A.K.; Brothers, M.A.; Maldonado, F.; Binford, S.; Maldonado, O.; Fuhrman, S.; Petersen, A.; Smith, G.J.; Zalman, L.S.; Burns-Naas, L.A.; et al. In vitro antiviral activity and single-dose pharmacokinetics in humans of a novel, orally bioavailable inhibitor of human rhinovirus 3C protease. Antimicrob. Agents Chemother. 2005, 49, 2267–2275. [Google Scholar] [CrossRef]
- Kankam, M.K.; Burns, J.M.; Collett, M.S.; Corrado, M.L.; Hincks, J.R. A Phase 1 Study of the Safety, Tolerability, and Pharmacokinetics of Single and Multiple Oral Doses of V-7404 in Healthy Adult Volunteers. Antimicrob. Agents Chemother. 2021, 65, e0102921. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; Zusinaite, E.; Tenson, T.; Oksenych, V.; Wang, W.; Afset, J.E.; Bjørås, M.; Kainov, D.E. Novel Synergistic Anti-Enteroviral Drug Combinations. Viruses 2022, 14, 1866. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lovell, S.; Tiew, K.C.; Mandadapu, S.R.; Alliston, K.R.; Battaile, K.P.; Groutas, W.C.; Chang, K.O. Broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, noroviruses, and coronaviruses. J. Virol. 2012, 86, 11754–11762. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, C.; Szeto, T.; Hurst, B.; Tarbet, B.; Wang, J. Boceprevir, Calpain Inhibitors II and XII, and GC-376 Have Broad-Spectrum Antiviral Activity against Coronaviruses. ACS Infect. Dis. 2021, 7, 586–597. [Google Scholar] [CrossRef]
- Wang, J.; Fan, T.; Yao, X.; Wu, Z.; Guo, L.; Lei, X.; Wang, M.; Jin, Q.; Cui, S. Crystal structures of enterovirus 71 3C protease complexed with rupintrivir reveal the roles of catalytically important residues. J. Virol. 2011, 85, 10021–10030. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.W.; Ang, M.J.; Lau, Q.Y.; Poulsen, A.; Ng, F.M.; Then, S.W.; Peng, J.; Hill, J.; Hong, W.J.; Chia, C.S.; et al. Antiviral activities of peptide-based covalent inhibitors of the Enterovirus 71 3C protease. Sci. Rep. 2016, 6, 33663. [Google Scholar] [CrossRef]
- Schulz, R.; Atef, A.; Becker, D.; Gottschalk, F.; Tauber, C.; Wagner, S.; Arkona, C.; Abdel-Hafez, A.A.; Farag, H.H.; Rademann, J.; et al. Phenylthiomethyl Ketone-Based Fragments Show Selective and Irreversible Inhibition of Enteroviral 3C Proteases. J. Med. Chem. 2018, 61, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, B.; Zhai, Y.; Yin, Z.; Sun, Y.; Rao, Z. Peptidyl aldehyde NK-1.8k suppresses enterovirus 71 and enterovirus 68 infection by targeting protease 3C. Antimicrob. Agents Chemother. 2015, 59, 2636–2646. [Google Scholar] [CrossRef]
- Boras, B.; Jones, R.M.; Anson, B.J.; Arenson, D.; Aschenbrenner, L.; Bakowski, M.A.; Beutler, N.; Binder, J.; Chen, E.; Eng, H.; et al. Discovery of a Novel Inhibitor of Coronavirus 3CL Protease for the Potential Treatment of COVID-19. bioRxiv 2021. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, L.; Zhai, Y.; Yin, Z.; Sun, Y.; Shang, L. Structure of the Enterovirus 71 3C Protease in Complex with NK-1.8k and Indications for the Development of Antienterovirus Protease Inhibitor. Antimicrob. Agents Chemother. 2017, 61, e00298-17. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, D.; Kusov, Y.; Nian, Y.; Ma, Q.; Wang, J.; von Brunn, A.; Leyssen, P.; Lanko, K.; Neyts, J.; et al. α-Ketoamides as Broad-Spectrum Inhibitors of Coronavirus and Enterovirus Replication: Structure-Based Design, Synthesis, and Activity Assessment. J. Med. Chem. 2020, 63, 4562–4578. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Jochmans, D.; Xie, H.; Yang, H.; Li, J.; Su, H.; Chang, D.; Wang, J.; Peng, J.; Zhu, L.; et al. Design, Synthesis, and Biological Evaluation of Peptidomimetic Aldehydes as Broad-Spectrum Inhibitors against Enterovirus and SARS-CoV-2. J. Med. Chem. 2022, 65, 2794–2808. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, S.; Xiao, T.; Li, Y.; Su, Z.; Wei, W.; Hao, F.; Hu, G.; Lin, F.; Chen, X.; et al. Design, synthesis, and evaluation of a novel macrocyclic anti-EV71 agent. Bioorganic Med. Chem. 2020, 28, 115551. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhao, X.; Cui, Z.; Wang, M.; Wang, Y.; Li, L.; Sun, Q.; Yang, X.; Zeng, D.; Liu, Y.; et al. Cyanohydrin as an Anchoring Group for Potent and Selective Inhibitors of Enterovirus 71 3C Protease. J. Med. Chem. 2015, 58, 9414–9420. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, M.; Ma, S.; Ma, Y.; Liu, S.; Shang, L.; Zhu, C.; Ye, S.; Wang, Y. 4-Iminooxazolidin-2-One as a Bioisostere of Cyanohydrin Suppresses EV71 Proliferation by Targeting 3C. Microbiol. Spectr. 2021, 9, e0102521. [Google Scholar] [CrossRef]
- Cao, Z.; Ding, Y.; Ke, Z.; Cao, L.; Li, N.; Ding, G.; Wang, Z.; Xiao, W. Luteoloside Acts as 3C Protease Inhibitor of Enterovirus 71 In Vitro. PLoS ONE 2016, 11, e0148693. [Google Scholar] [CrossRef]
- Kim, B.K.; Ko, H.; Jeon, E.S.; Ju, E.S.; Jeong, L.S.; Kim, Y.C. 2,3,4-Trihydroxybenzyl-hydrazide analogues as novel potent coxsackievirus B3 3C protease inhibitors. Eur. J. Med. Chem. 2016, 120, 202–216. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Q.; Wang, Y.; Pang, Z.; Liu, J.; Yin, Z.; Lou, Z. Activity-Based Protein Profiling Identifies ATG4B as a Key Host Factor for Enterovirus 71 Proliferation. J. Virol. 2019, 93, e01092-19. [Google Scholar] [CrossRef]
- Steuten, K.; Kim, H.; Widen, J.C.; Babin, B.M.; Onguka, O.; Lovell, S.; Bolgi, O.; Cerikan, B.; Neufeldt, C.J.; Cortese, M.; et al. Challenges for Targeting SARS-CoV-2 Proteases as a Therapeutic Strategy for COVID-19. ACS Infect. Dis. 2021, 7, 1457–1468. [Google Scholar] [CrossRef]
- Kitamura, N.; Sacco, M.D.; Ma, C.; Hu, Y.; Townsend, J.A.; Meng, X.; Zhang, F.; Zhang, X.; Ba, M.; Szeto, T.; et al. Expedited Approach toward the Rational Design of Noncovalent SARS-CoV-2 Main Protease Inhibitors. J. Med. Chem. 2022, 65, 2848–2865. [Google Scholar] [CrossRef]
- Sun, J.; Yogarajah, T.; Lee, R.C.H.; Kaur, P.; Inoue, M.; Tan, Y.W.; Chu, J.J.H. Drug repurposing of pyrimidine analogs as potent antiviral compounds against human enterovirus A71 infection with potential clinical applications. Sci. Rep. 2020, 10, 8159. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, C.; Kim, D.E.; Song, J.H.; Choi, M.; Choi, K.; Kang, M.; Lee, K.; Kim, H.S.; Shin, J.S.; et al. Synergistic antiviral activity of gemcitabine and ribavirin against enteroviruses. Antivir. Res. 2015, 124, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, D.E.; Jang, K.S.; Kim, S.J.; Cho, S.; Kim, C. Gemcitabine, a broad-spectrum antiviral drug, suppresses enterovirus infections through innate immunity induced by the inhibition of pyrimidine biosynthesis and nucleotide depletion. Oncotarget 2017, 8, 115315–115325. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Yang, J.; Zheng, B.; Zhang, Y.; Cao, Y.; Huan, C.; Wang, S.; Chang, J.; Zhang, W. The Pyrimidine Analog FNC Potently Inhibits the Replication of Multiple Enteroviruses. J. Virol. 2020, 94, e00204-20. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Yuan, S.; Gao, Q.; Lan, K.; Altmeyer, R.; Zou, G. In Vitro Assessment of Combinations of Enterovirus Inhibitors against Enterovirus 71. Antimicrob. Agents Chemother. 2016, 60, 5357–5367. [Google Scholar] [CrossRef]
- Shang, L.; Wang, Y.; Qing, J.; Shu, B.; Cao, L.; Lou, Z.; Gong, P.; Sun, Y.; Yin, Z. An adenosine nucleoside analogue NITD008 inhibits EV71 proliferation. Antivir. Res. 2014, 112, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, Y.L.; Schul, W.; Wang, Q.Y.; Gu, F.; Duraiswamy, J.; Kondreddi, R.R.; Niyomrattanakit, P.; Lakshminarayana, S.B.; Goh, A.; et al. An adenosine nucleoside inhibitor of dengue virus. Proc. Natl. Acad. Sci. USA 2009, 106, 20435–20439. [Google Scholar] [CrossRef]
- Deng, C.L.; Yeo, H.; Ye, H.Q.; Liu, S.Q.; Shang, B.D.; Gong, P.; Alonso, S.; Shi, P.Y.; Zhang, B. Inhibition of enterovirus 71 by adenosine analog NITD008. J. Virol. 2014, 88, 11915–11923. [Google Scholar] [CrossRef]
- Yin, W.; Mao, C.; Luan, X.; Shen, D.D.; Shen, Q.; Su, H.; Wang, X.; Zhou, F.; Zhao, W.; Gao, M.; et al. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science 2020, 368, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Tosh, D.K.; Toti, K.S.; Hurst, B.L.; Julander, J.G.; Jacobson, K.A. Structure activity relationship of novel antiviral nucleosides against Enterovirus A71. Bioorg Med. Chem. Lett. 2020, 30, 127599. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Chang, H.Y.; Lin, P.F.; Chern, J.H.; Hsu, J.T.; Chang, C.Y.; Shih, S.R. Novel antiviral agent DTriP-22 targets RNA-dependent RNA polymerase of enterovirus 71. Antimicrob. Agents Chemother. 2009, 53, 2740–2747. [Google Scholar] [CrossRef]
- Hung, H.C.; Chen, T.C.; Fang, M.Y.; Yen, K.J.; Shih, S.R.; Hsu, J.T.; Tseng, C.P. Inhibition of enterovirus 71 replication and the viral 3D polymerase by aurintricarboxylic acid. J. Antimicrob. Chemother. 2010, 65, 676–683. [Google Scholar] [CrossRef]
- van der Linden, L.; Vives-Adrián, L.; Selisko, B.; Ferrer-Orta, C.; Liu, X.; Lanke, K.; Ulferts, R.; De Palma, A.M.; Tanchis, F.; Goris, N.; et al. The RNA template channel of the RNA-dependent RNA polymerase as a target for development of antiviral therapy of multiple genera within a virus family. PLoS Pathog. 2015, 11, e1004733. [Google Scholar] [CrossRef]
- Velu, A.B.; Chen, G.W.; Hsieh, P.T.; Horng, J.T.; Hsu, J.T.; Hsieh, H.P.; Chen, T.C.; Weng, K.F.; Shih, S.R. BPR-3P0128 inhibits RNA-dependent RNA polymerase elongation and VPg uridylylation activities of Enterovirus 71. Antivir. Res. 2014, 112, 18–25. [Google Scholar] [CrossRef]
- Wu, K.X.; Chu, J.J. Antiviral screen identifies EV71 inhibitors and reveals camptothecin-target, DNA topoisomerase 1 as a novel EV71 host factor. Antivir. Res. 2017, 143, 122–133. [Google Scholar] [CrossRef]
- Li, W.; Ross-Smith, N.; Proud, C.G.; Belsham, G.J. Cleavage of translation initiation factor 4AI (eIF4AI) but not eIF4AII by foot-and-mouth disease virus 3C protease: Identification of the eIF4AI cleavage site. FEBS Lett. 2001, 507, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.R.; Stollar, V.; Li, M.L. Host factors in enterovirus 71 replication. J. Virol. 2011, 85, 9658–9666. [Google Scholar] [CrossRef] [PubMed]
- Arita, M. Phosphatidylinositol-4 kinase III beta and oxysterol-binding protein accumulate unesterified cholesterol on poliovirus-induced membrane structure. Microbiol. Immunol. 2014, 58, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Roulin, P.S.; Lötzerich, M.; Torta, F.; Tanner, L.B.; van Kuppeveld, F.J.; Wenk, M.R.; Greber, U.F. Rhinovirus uses a phosphatidylinositol 4-phosphate/cholesterol counter-current for the formation of replication compartments at the ER-Golgi interface. Cell Host Microbe 2014, 16, 677–690. [Google Scholar] [CrossRef]
- Albulescu, L.; Strating, J.R.; Thibaut, H.J.; van der Linden, L.; Shair, M.D.; Neyts, J.; van Kuppeveld, F.J. Broad-range inhibition of enterovirus replication by OSW-1, a natural compound targeting OSBP. Antivir. Res. 2015, 117, 110–114. [Google Scholar] [CrossRef]
- Burgett, A.W.; Poulsen, T.B.; Wangkanont, K.; Anderson, D.R.; Kikuchi, C.; Shimada, K.; Okubo, S.; Fortner, K.C.; Mimaki, Y.; Kuroda, M.; et al. Natural products reveal cancer cell dependence on oxysterol-binding proteins. Nat. Chem. Biol. 2011, 7, 639–647. [Google Scholar] [CrossRef]
- Roberts, B.L.; Severance, Z.C.; Bensen, R.C.; Le, A.T.; Kothapalli, N.R.; Nuñez, J.I.; Ma, H.; Wu, S.; Standke, S.J.; Yang, Z.; et al. Transient Compound Treatment Induces a Multigenerational Reduction of Oxysterol-Binding Protein (OSBP) Levels and Prophylactic Antiviral Activity. ACS Chem. Biol. 2019, 14, 276–287. [Google Scholar] [CrossRef]
- Strating, J.R.; van der Linden, L.; Albulescu, L.; Bigay, J.; Arita, M.; Delang, L.; Leyssen, P.; van der Schaar, H.M.; Lanke, K.H.; Thibaut, H.J.; et al. Itraconazole inhibits enterovirus replication by targeting the oxysterol-binding protein. Cell Rep. 2015, 10, 600–615. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.L.; Severance, Z.C.; Bensen, R.C.; Le-McClain, A.T.; Malinky, C.A.; Mettenbrink, E.M.; Nuñez, J.I.; Reddig, W.J.; Blewett, E.L.; Burgett, A.W.G. Differing activities of oxysterol-binding protein (OSBP) targeting anti-viral compounds. Antivir. Res. 2019, 170, 104548. [Google Scholar] [CrossRef]
- Arita, M.; Dobrikov, G.; Pürstinger, G.; Galabov, A.S. Allosteric Regulation of Phosphatidylinositol 4-Kinase III Beta by an Antipicornavirus Compound MDL-860. ACS Infect. Dis. 2017, 3, 585–594. [Google Scholar] [CrossRef]
- Mejdrová, I.; Chalupská, D.; Plačková, P.; Müller, C.; Šála, M.; Klíma, M.; Baumlová, A.; Hřebabecký, H.; Procházková, E.; Dejmek, M.; et al. Rational Design of Novel Highly Potent and Selective Phosphatidylinositol 4-Kinase IIIβ (PI4KB) Inhibitors as Broad-Spectrum Antiviral Agents and Tools for Chemical Biology. J. Med. Chem. 2017, 60, 100–118. [Google Scholar] [CrossRef]
- Nikolova, I.; Slavchev, I.; Ravutsov, M.; Dangalov, M.; Nikolova, Y.; Zagranyarska, I.; Stoyanova, A.; Nikolova, N.; Mukova, L.; Grozdanov, P.; et al. Anti-enteroviral activity of new MDL-860 analogues: Synthesis, in vitro/in vivo studies and QSAR analysis. Bioorganic Chem. 2019, 85, 487–497. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, L.; Gao, H.; Wu, Y.; Wang, Y.; Fang, F.; Lan, T.; Lou, Z.; Rao, Y. Discovery, Optimization, and Target Identification of Novel Potent Broad-Spectrum Antiviral Inhibitors. J. Med. Chem. 2019, 62, 4056–4073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Das, P.; Schmolke, M.; Manicassamy, B.; Wang, Y.; Deng, X.; Cai, L.; Tu, B.P.; Forst, C.V.; Roth, M.G.; et al. Inhibition of pyrimidine synthesis reverses viral virulence factor-mediated block of mRNA nuclear export. J. Cell Biol. 2012, 196, 315–326. [Google Scholar] [CrossRef]
- Hoffmann, H.H.; Kunz, A.; Simon, V.A.; Palese, P.; Shaw, M.L. Broad-spectrum antiviral that interferes with de novo pyrimidine biosynthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 5777–5782. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.N.; Lai, K.K.; Dai, J.; Kok, K.H.; Chen, H.; Chan, K.H.; Yuen, K.Y.; Kao, R.Y.T. Broad-spectrum inhibition of common respiratory RNA viruses by a pyrimidine synthesis inhibitor with involvement of the host antiviral response. J. Gen. Virol. 2017, 98, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Liou, A.T.; Liao, C.C.; Chou, S.F.; Chang, Y.S.; Chang, C.S.; Shih, C. Hypoxia and therapeutic treatment of EV-A71 with an immune modulator TLR7 agonist in a new immunocompetent mouse model. J. Biomed. Sci. 2019, 26, 93. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, B.; Chen, X.; Song, N.; Wu, J.; Li, G.; Yu, P.; Han, Y.; Liu, J.; Qin, C. GS-9620 inhibits enterovirus 71 replication mainly through the NF-κB and PI3K-AKT signaling pathways. Antivir. Res. 2018, 153, 39–48. [Google Scholar] [CrossRef]
- Hung, H.C.; Wang, H.C.; Shih, S.R.; Teng, I.F.; Tseng, C.P.; Hsu, J.T. Synergistic inhibition of enterovirus 71 replication by interferon and rupintrivir. J. Infect. Dis. 2011, 203, 1784–1790. [Google Scholar] [CrossRef]
- Mohamed, A.; Bakir, T.; Al-Hawel, H.; Al-Sharif, I.; Bakheet, R.; Kouser, L.; Murugaiah, V.; Al-Mozaini, M. HIV-2 Vpx neutralizes host restriction factor SAMHD1 to promote viral pathogenesis. Sci. Rep. 2021, 11, 20984. [Google Scholar] [CrossRef]
- Li, Z.; Huan, C.; Wang, H.; Liu, Y.; Liu, X.; Su, X.; Yu, J.; Zhao, Z.; Yu, X.F.; Zheng, B.; et al. TRIM21-mediated proteasomal degradation of SAMHD1 regulates its antiviral activity. EMBO Rep. 2020, 21, e47528. [Google Scholar] [CrossRef]
- Li, Z.; Yang, X.; Zhao, Z.; Liu, X.; Zhang, W. Host Restriction Factor A3G Inhibits the Replication of Enterovirus D68 by Competitively Binding the 5’ Untranslated Region with PCBP1. J. Virol. 2022, 96, e0170821. [Google Scholar] [CrossRef]
- Li, Y.L.; Langley, C.A.; Azumaya, C.M.; Echeverria, I.; Chesarino, N.M.; Emerman, M.; Cheng, Y.; Gross, J.D. The structural basis for HIV-1 Vif antagonism of human APOBEC3G. Nature 2023, 615, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Poh, C.L.; Sam, I.C.; Chan, Y.F. Enterovirus 71 uses cell surface heparan sulfate glycosaminoglycan as an attachment receptor. J. Virol. 2013, 87, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Earley, D.F.; Bailly, B.; Maggioni, A.; Kundur, A.R.; Thomson, R.J.; Chang, C.W.; von Itzstein, M. Efficient Blocking of Enterovirus 71 Infection by Heparan Sulfate Analogues Acting as Decoy Receptors. ACS Infect. Dis. 2019, 5, 1708–1717. [Google Scholar] [CrossRef]
- Pourianfar, H.R.; Poh, C.L.; Fecondo, J.; Grollo, L. In vitro evaluation of the antiviral activity of heparan sulfate mimetic compounds against Enterovirus 71. Virus Res. 2012, 169, 22–29. [Google Scholar] [CrossRef]
- Weng, T.Y.; Chen, L.C.; Shyu, H.W.; Chen, S.H.; Wang, J.R.; Yu, C.K.; Lei, H.Y.; Yeh, T.M. Lactoferrin inhibits enterovirus 71 infection by binding to VP1 protein and host cells. Antivir. Res. 2005, 67, 31–37. [Google Scholar] [CrossRef]
- Yuan, S.; Chu, H.; Huang, J.; Zhao, X.; Ye, Z.W.; Lai, P.M.; Wen, L.; Cai, J.P.; Mo, Y.; Cao, J.; et al. Viruses harness YxxØ motif to interact with host AP2M1 for replication: A vulnerable broad-spectrum antiviral target. Sci. Adv. 2020, 6, eaba7910. [Google Scholar] [CrossRef]
- Hao, T.; Li, Y.; Fan, S.; Li, W.; Wang, S.; Li, S.; Cao, R.; Zhong, W. Design, synthesis and pharmacological evaluation of a novel mTOR-targeted anti-EV71 agent. Eur. J. Med. Chem. 2019, 175, 172–186. [Google Scholar] [CrossRef]
- Wang, H.; Guo, T.; Yang, Y.; Yu, L.; Pan, X.; Li, Y. Lycorine Derivative LY-55 Inhibits EV71 and CVA16 Replication Through Downregulating Autophagy. Front. Cell. Infect. Microbiol. 2019, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, S.; Liu, S.; Chen, Y.; Liu, H.; Su, Y.; Liu, R.; Cui, Y.; Song, Y.; Teng, Y.; et al. Transcriptomic Profiling Reveals a Role for TREM-1 Activation in Enterovirus D68 Infection-Induced Proinflammatory Responses. Front. Immunol. 2021, 12, 749618. [Google Scholar] [CrossRef]
- Qing, J.; Wang, Y.; Sun, Y.; Huang, J.; Yan, W.; Wang, J.; Su, D.; Ni, C.; Li, J.; Rao, Z.; et al. Cyclophilin A associates with enterovirus-71 virus capsid and plays an essential role in viral infection as an uncoating regulator. PLoS Pathog. 2014, 10, e1004422. [Google Scholar] [CrossRef]
- Yan, W.; Qing, J.; Mei, H.; Nong, J.; Huang, J.; Zhu, J.; Jiang, H.; Liu, L.; Zhang, L.; Li, J. Identification, synthesis and pharmacological evaluation of novel anti-EV71 agents via cyclophilin A inhibition. Bioorg Med. Chem. Lett. 2015, 25, 5682–5686. [Google Scholar] [CrossRef]
- Hixon, A.M.; Clarke, P.; Tyler, K.L. Evaluating Treatment Efficacy in a Mouse Model of Enterovirus D68-Associated Paralytic Myelitis. J. Infect. Dis. 2017, 216, 1245–1253. [Google Scholar] [CrossRef]
- Vogt, M.R.; Fu, J.; Kose, N.; Williamson, L.E.; Bombardi, R.; Setliff, I.; Georgiev, I.S.; Klose, T.; Rossmann, M.G.; Bochkov, Y.A.; et al. Human antibodies neutralize enterovirus D68 and protect against infection and paralytic disease. Sci. Immunol. 2020, 5, eaba4902. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, J.; Li, B.; Guo, L.; Li, H.; Song, J.; Yang, Z.; Fan, H.; Huang, X.; Long, H.; et al. A Novel Neutralizing Antibody Specific to the DE Loop of VP1 Can Inhibit EV-D68 Infection in Mice. J. Immunol. 2018, 201, 2557–2569. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhu, R.; Xu, L.; He, M.; Yan, X.; Liu, D.; Yin, Z.; Wu, Y.; Li, Y.; Yang, L.; et al. Atomic structures of enterovirus D68 in complex with two monoclonal antibodies define distinct mechanisms of viral neutralization. Nat. Microbiol. 2019, 4, 124–133. [Google Scholar] [CrossRef]
- Yang, J.; Yang, C.; Guo, N.; Zhu, K.; Luo, K.; Zhang, N.; Zhao, H.; Cui, Y.; Chen, L.; Wang, H.; et al. Type I Interferons Triggered through the Toll-Like Receptor 3-TRIF Pathway Control Coxsackievirus A16 Infection in Young Mice. J. Virol. 2015, 89, 10860–10867. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, J.; Lu, N.; Zhang, C. Enterovirus 71 Antagonizes Antiviral Effects of Type III Interferon and Evades the Clearance of Intestinal Intraepithelial Lymphocytes. Front. Microbiol. 2021, 12, 806084. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Liu, L.; Lei, X.; Zhou, Z.; He, B.; Wang, J. 3C Protease of Enterovirus D68 Inhibits Cellular Defense Mediated by Interferon Regulatory Factor 7. J. Virol. 2016, 90, 1613–1621. [Google Scholar] [CrossRef]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y. Current advances in antiviral RNA interference in mammals. FEBS J. 2024, 291, 208–216. [Google Scholar] [CrossRef]
- Takahashi, T.; Ui-Tei, K. Mutual Regulation of RNA Silencing and the IFN Response as an Antiviral Defense System in Mammalian Cells. Int. J. Mol. Sci. 2020, 21, 1348. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Dai, Y.; Li, Z.; Wang, J.; Ye, Z.; Ren, Y.; Wang, H.; Li, W.X.; Lu, J.; et al. Efficient Dicer processing of virus-derived double-stranded RNAs and its modulation by RIG-I-like receptor LGP2. PLoS Pathog. 2021, 17, e1009790. [Google Scholar] [CrossRef]
- Han, Q.; Chen, G.; Wang, J.; Jee, D.; Li, W.X.; Lai, E.C.; Ding, S.W. Mechanism and Function of Antiviral RNA Interference in Mice. mBio 2020, 11, e03278-19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Ye, Z.; Xu, Y.; Wang, B.; Wang, C.; Dai, Y.; Lu, J.; Lu, B.; Zhang, W.; et al. The activation of antiviral RNA interference not only exists in neural progenitor cells but also in somatic cells in mammals. Emerg. Microbes Infect. 2020, 9, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Poirier, E.Z.; Buck, M.D.; Chakravarty, P.; Carvalho, J.; Frederico, B.; Cardoso, A.; Healy, L.; Ulferts, R.; Beale, R.; Reis e Sousa, C. An isoform of Dicer protects mammalian stem cells against multiple RNA viruses. Science 2021, 373, 231–236. [Google Scholar] [CrossRef]
- Li, W.X.; Ding, S.W. Mammalian viral suppressors of RNA interference. Trends Biochem. Sci. 2022, 47, 978–988. [Google Scholar] [CrossRef]
- Werk, D.; Schubert, S.; Lindig, V.; Grunert, H.P.; Zeichhardt, H.; Erdmann, V.A.; Kurreck, J. Developing an effective RNA interference strategy against a plus-strand RNA virus: Silencing of coxsackievirus B3 and its cognate coxsackievirus-adenovirus receptor. Biol. Chem. 2005, 386, 857–863. [Google Scholar] [CrossRef]
- Merl, S.; Michaelis, C.; Jaschke, B.; Vorpahl, M.; Seidl, S.; Wessely, R. Targeting 2A protease by RNA interference attenuates coxsackieviral cytopathogenicity and promotes survival in highly susceptible mice. Circulation 2005, 111, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Cheung, P.K.; Zhang, H.M.; Chau, D.; Yang, D. Inhibition of coxsackievirus B3 replication by small interfering RNAs requires perfect sequence match in the central region of the viral positive strand. J. Virol. 2005, 79, 2151–2159. [Google Scholar] [CrossRef]
- Kim, J.Y.; Chung, S.K.; Hwang, H.Y.; Kim, H.; Kim, J.H.; Nam, J.H.; Park, S.I. Expression of short hairpin RNAs against the coxsackievirus B3 exerts potential antiviral effects in Cos-7 cells and in mice. Virus Res. 2007, 125, 9–13. [Google Scholar] [CrossRef]
- Fechner, H.; Pinkert, S.; Wang, X.; Sipo, I.; Suckau, L.; Kurreck, J.; Dörner, A.; Sollerbrant, K.; Zeichhardt, H.; Grunert, H.P.; et al. Coxsackievirus B3 and adenovirus infections of cardiac cells are efficiently inhibited by vector-mediated RNA interference targeting their common receptor. Gene Ther. 2007, 14, 960–971. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, Y.; He, F.; Wang, C.; Xiao, Z.; Zou, J.; Wang, F.; Liu, Z. Short hairpin RNA targeting 2B gene of coxsackievirus B3 exhibits potential antiviral effects both in vitro and in vivo. BMC Infect. Dis. 2012, 12, 177. [Google Scholar] [CrossRef]
- Dutkiewicz, M.; Grunert, H.P.; Zeichhardt, H.; Lena, S.W.; Wengel, J.; Kurreck, J. Design of LNA-modified siRNAs against the highly structured 5’ UTR of coxsackievirus B3. FEBS Lett. 2008, 582, 3061–3066. [Google Scholar] [CrossRef]
- Rothe, D.; Werk, D.; Dutkiewicz, M.; Schubert, S.; Grunert, H.P.; Zeichhardt, H.; Erdmann, V.A.; Fechner, H.; Kurreck, J. Inhibition of picornaviruses by means of RNA interference. Nucleic Acids Symp. Ser. 2008, 52, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Fechner, H.; Sipo, I.; Westermann, D.; Pinkert, S.; Wang, X.; Suckau, L.; Kurreck, J.; Zeichhardt, H.; Müller, O.; Vetter, R.; et al. Cardiac-targeted RNA interference mediated by an AAV9 vector improves cardiac function in coxsackievirus B3 cardiomyopathy. J. Mol. Med. 2008, 86, 987–997. [Google Scholar] [CrossRef]
- Stein, E.A.; Pinkert, S.; Becher, P.M.; Geisler, A.; Zeichhardt, H.; Klopfleisch, R.; Poller, W.; Tschöpe, C.; Lassner, D.; Fechner, H.; et al. Combination of RNA interference and virus receptor trap exerts additive antiviral activity in coxsackievirus B3-induced myocarditis in mice. J. Infect. Dis. 2015, 211, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Dai, H.L.; Yang, D.; Zhu, L.; Gao, T.L.; Shao, H.J.; Peng, X.; Jin, Z.F. Small interfering RNA against the 2C genomic region of coxsackievirus B3 exerts potential antiviral effects in permissive HeLa cells. Virus Res. 2012, 163, 183–189. [Google Scholar] [CrossRef]
- Nygårdas, M.; Vuorinen, T.; Aalto, A.P.; Bamford, D.H.; Hukkanen, V. Inhibition of coxsackievirus B3 and related enteroviruses by antiviral short interfering RNA pools produced using phi6 RNA-dependent RNA polymerase. J. Gen. Virol. 2009, 90, 2468–2473. [Google Scholar] [CrossRef]
- Werk, D.; Pinkert, S.; Heim, A.; Zeichhardt, H.; Grunert, H.P.; Poller, W.; Erdmann, V.A.; Fechner, H.; Kurreck, J. Combination of soluble coxsackievirus-adenovirus receptor and anti-coxsackievirus siRNAs exerts synergistic antiviral activity against coxsackievirus B3. Antivir. Res. 2009, 83, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.L.; Wong, A.P.; Poh, C.L. Development of potential antiviral strategy against coxsackievirus B4. Virus Res. 2010, 150, 85–92. [Google Scholar] [CrossRef]
- Rothe, D.; Werk, D.; Niedrig, S.; Horbelt, D.; Grunert, H.P.; Zeichhardt, H.; Erdmann, V.A.; Kurreck, J. Antiviral activity of highly potent siRNAs against echovirus 30 and its receptor. J. Virol. Methods 2009, 157, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Rothe, D.; Wajant, G.; Grunert, H.P.; Zeichhardt, H.; Fechner, H.; Kurreck, J. Rapid construction of adeno-associated virus vectors expressing multiple short hairpin RNAs with high antiviral activity against echovirus 30. Oligonucleotides 2010, 20, 191–198. [Google Scholar] [CrossRef]
- Tan, E.L.; Tan, T.M.; Tak Kwong Chow, V.; Poh, C.L. Inhibition of enterovirus 71 in virus-infected mice by RNA interference. Mol. Ther. 2007, 15, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.L.; Tan, T.M.; Chow, V.T.; Poh, C.L. Enhanced potency and efficacy of 29-mer shRNAs in inhibition of Enterovirus 71. Antivir. Res. 2007, 74, 9–15. [Google Scholar] [CrossRef]
- Deng, J.X.; Nie, X.J.; Lei, Y.F.; Ma, C.F.; Xu, D.L.; Li, B.; Xu, Z.K.; Zhang, G.C. The highly conserved 5′ untranslated region as an effective target towards the inhibition of Enterovirus 71 replication by unmodified and appropriate 2′-modified siRNAs. J. Biomed. Sci. 2012, 19, 73. [Google Scholar] [CrossRef]
- Liu, H.; Qin, Y.; Kong, Z.; Shao, Q.; Su, Z.; Wang, S.; Chen, J. siRNA Targeting the 2Apro Genomic Region Prevents Enterovirus 71 Replication In Vitro. PLoS ONE 2016, 11, e0149470. [Google Scholar] [CrossRef]
- Li, Y.; Xie, J.; Xu, X.; Wang, J.; Ao, F.; Wan, Y.; Zhu, Y. MicroRNA-548 down-regulates host antiviral response via direct targeting of IFN-λ1. Protein Cell 2013, 4, 130–141. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Z.; Qiu, Y.; Kong, J.; Fu, Y.; Liu, Y.; Wang, C.; Quan, J.; Wang, Q.; Xu, W.; et al. Inhibition of viral suppressor of RNAi proteins by designer peptides protects from enteroviral infection in vivo. Immunity 2021, 54, 2231–2244.e2236. [Google Scholar] [CrossRef]
- Tan, E.L.; Marcus, K.F.; Poh, C.L. Development of RNA interference (RNAi) as potential antiviral strategy against enterovirus 70. J. Med. Virol. 2008, 80, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Jun, E.J.; Won, M.A.; Ahn, J.; Ko, A.; Moon, H.; Tchah, H.; Kim, Y.K.; Lee, H. An antiviral small-interfering RNA simultaneously effective against the most prevalent enteroviruses causing acute hemorrhagic conjunctivitis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Gao, Y.; Sun, L.; Tien, P.; Jin, Q. Quick identification of effective small interfering RNAs that inhibit the replication of coxsackievirus A16. Antivir. Res. 2008, 80, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Doudna, J.A. CRISPR technology: A decade of genome editing is only the beginning. Science 2023, 379, eadd8643. [Google Scholar] [CrossRef] [PubMed]
- Pacesa, M.; Pelea, O.; Jinek, M. Past, present, and future of CRISPR genome editing technologies. Cell 2024, 187, 1076–1100. [Google Scholar] [CrossRef]
- Kaminski, M.M.; Abudayyeh, O.O.; Gootenberg, J.S.; Zhang, F.; Collins, J.J. CRISPR-based diagnostics. Nat. Biomed. Eng. 2021, 5, 643–656. [Google Scholar] [CrossRef]
- Chavez, M.; Chen, X.; Finn, P.B.; Qi, L.S. Advances in CRISPR therapeutics. Nat. Rev. Nephrol. 2023, 19, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Aliyari, S.; Zhu, Z.; Zheng, H.; Cheng, G.; Zhang, S. CRISPR-Cas: A game-changer in vaccine development and the fight against viral infections. Trends Microbiol. 2025, 33, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.S.; O’Garra, A.; Sher, A.; Wack, A. Host-directed immunotherapy of viral and bacterial infections: Past, present and future. Nat. Rev. Immunol. 2023, 23, 121–133. [Google Scholar] [CrossRef]
- Diep, J.; Ooi, Y.S.; Wilkinson, A.W.; Peters, C.E.; Foy, E.; Johnson, J.R.; Zengel, J.; Ding, S.; Weng, K.F.; Laufman, O.; et al. Enterovirus pathogenesis requires the host methyltransferase SETD3. Nat. Microbiol. 2019, 4, 2523–2537. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, K.; Bae, S.; Park, J.; Lee, C.K.; Kim, M.; Kim, E.; Kim, S.; Kim, C.; Kim, J.S. CRISPR/Cas9-mediated gene knockout screens and target identification via whole-genome sequencing uncover host genes required for picornavirus infection. J. Biol. Chem. 2017, 292, 10664–10671. [Google Scholar] [CrossRef]
- Mei, H.; Zha, Z.; Wang, W.; Xie, Y.; Huang, Y.; Li, W.; Wei, D.; Zhang, X.; Qu, J.; Liu, J. Surfaceome CRISPR screen identifies OLFML3 as a rhinovirus-inducible IFN antagonist. Genome Biol. 2021, 22, 297. [Google Scholar]
- Greninger, A.L.; Knudsen, G.M.; Betegon, M.; Burlingame, A.L.; Derisi, J.L. The 3A protein from multiple picornaviruses utilizes the golgi adaptor protein ACBD3 to recruit PI4KIIIβ. J. Virol. 2012, 86, 3605–3616. [Google Scholar] [CrossRef]
- Téoulé, F.; Brisac, C.; Pelletier, I.; Vidalain, P.O.; Jégouic, S.; Mirabelli, C.; Bessaud, M.; Combelas, N.; Autret, A.; Tangy, F.; et al. The Golgi protein ACBD3, an interactor for poliovirus protein 3A, modulates poliovirus replication. J. Virol. 2013, 87, 11031–11046. [Google Scholar] [CrossRef]
- Dorobantu, C.M.; van der Schaar, H.M.; Ford, L.A.; Strating, J.R.; Ulferts, R.; Fang, Y.; Belov, G.; van Kuppeveld, F.J. Recruitment of PI4KIIIβ to coxsackievirus B3 replication organelles is independent of ACBD3, GBF1, and Arf1. J. Virol. 2014, 88, 2725–2736. [Google Scholar] [CrossRef] [PubMed]
- Dorobantu, C.M.; Ford-Siltz, L.A.; Sittig, S.P.; Lanke, K.H.; Belov, G.A.; van Kuppeveld, F.J.; van der Schaar, H.M. GBF1- and ACBD3-independent recruitment of PI4KIIIβ to replication sites by rhinovirus 3A proteins. J. Virol. 2015, 89, 1913–1918. [Google Scholar] [CrossRef]
- Najafi, S.; Tan, S.C.; Aghamiri, S.; Raee, P.; Ebrahimi, Z.; Jahromi, Z.K.; Rahmati, Y.; Sadri Nahand, J.; Piroozmand, A.; Jajarmi, V.; et al. Therapeutic potentials of CRISPR-Cas genome editing technology in human viral infections. Biomed. Pharmacother. 2022, 148, 112743. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, R.; Tinker-Kulberg, R.; Salehin, M.; Supakar, T.; Chamberlain, S.; Ligaba-Osena, A.; Josephs, E.A. Polyvalent guide RNAs for CRISPR antivirals. iScience 2022, 25, 105333. [Google Scholar] [CrossRef] [PubMed]
- Raguram, A.; Banskota, S.; Liu, D.R. Therapeutic in vivo delivery of gene editing agents. Cell 2022, 185, 2806–2827. [Google Scholar] [CrossRef]
- Saito, M.; Xu, P.; Faure, G.; Maguire, S.; Kannan, S.; Altae-Tran, H.; Vo, S.; Desimone, A.; Macrae, R.K.; Zhang, F. Fanzor is a eukaryotic programmable RNA-guided endonuclease. Nature 2023, 620, 660–668. [Google Scholar] [CrossRef]
- Raghavan, R.; Friedrich, M.J.; King, I.; Chau-Duy-Tam Vo, S.; Strebinger, D.; Lash, B.; Kilian, M.; Platten, M.; Macrae, R.K.; Song, Y.; et al. Rational engineering of minimally immunogenic nucleases for gene therapy. Nat. Commun. 2025, 16, 105. [Google Scholar] [CrossRef]
- Keng, C.T.; Yogarajah, T.; Lee, R.C.H.; Muhammad, I.B.H.; Chia, B.S.; Vasandani, S.R.; Lim, D.S.; Guo, K.; Wong, Y.H.; Mok, C.K.; et al. AAV-CRISPR-Cas13 eliminates human enterovirus and prevents death of infected mice. EBioMedicine 2023, 93, 104682. [Google Scholar] [CrossRef]
- Albericio, F.; Kruger, H.G. Therapeutic peptides. Future Med. Chem. 2012, 4, 1527–1531. [Google Scholar] [CrossRef]
- Vilas Boas, L.C.P.; Campos, M.L.; Berlanda, R.L.A.; de Carvalho Neves, N.; Franco, O.L. Antiviral peptides as promising therapeutic drugs. Cell. Mol. Life Sci. 2019, 76, 3525–3542. [Google Scholar] [CrossRef]
- Xia, S.; Yan, L.; Xu, W.; Agrawal, A.S.; Algaissi, A.; Tseng, C.K.; Wang, Q.; Du, L.; Tan, W.; Wilson, I.A.; et al. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci. Adv. 2019, 5, eaav4580. [Google Scholar] [CrossRef]
- Lalani, S.; Gew, L.T.; Poh, C.L. Antiviral peptides against Enterovirus A71 causing hand, foot and mouth disease. Peptides 2021, 136, 170443. [Google Scholar] [CrossRef] [PubMed]
- Clotet, B.; Raffi, F.; Cooper, D.; Delfraissy, J.F.; Lazzarin, A.; Moyle, G.; Rockstroh, J.; Soriano, V.; Schapiro, J. Clinical management of treatment-experienced, HIV-infected patients with the fusion inhibitor enfuvirtide: Consensus recommendations. AIDS 2004, 18, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E. Cationic antimicrobial peptides: Towards clinical applications. Expert. Opin. Investig. Drugs 2000, 9, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Battles, M.B.; Langedijk, J.P.; Furmanova-Hollenstein, P.; Chaiwatpongsakorn, S.; Costello, H.M.; Kwanten, L.; Vranckx, L.; Vink, P.; Jaensch, S.; Jonckers, T.H.; et al. Molecular mechanism of respiratory syncytial virus fusion inhibitors. Nat. Chem. Biol. 2016, 12, 87–93. [Google Scholar] [CrossRef]
- Roymans, D.; Alnajjar, S.S.; Battles, M.B.; Sitthicharoenchai, P.; Furmanova-Hollenstein, P.; Rigaux, P.; Berg, J.V.D.; Kwanten, L.; Ginderen, M.V.; Verheyen, N.; et al. Therapeutic efficacy of a respiratory syncytial virus fusion inhibitor. Nat. Commun. 2017, 8, 167. [Google Scholar] [CrossRef]
- Jordan, R.; Stray, K.; Anderson, F.; Perron, M.; Mackman, R.; Miller, M.; Mo, H.; Svarovskaia, E.; Martin, R.; Xin, Y.; et al. Analysis of GS-5806 Resistance Emergence in Human Healthy Adult Subjects Experimentally Infected With Respiratory Syncytial Virus (RSV). Open Forum Infect. Dis. 2015, 2 (Suppl. S1), 1189. [Google Scholar] [CrossRef]
- Gaillard, V.; Galloux, M.; Garcin, D.; Eléouët, J.F.; Le Goffic, R.; Larcher, T.; Rameix-Welti, M.A.; Boukadiri, A.; Héritier, J.; Segura, J.M.; et al. A Short Double-Stapled Peptide Inhibits Respiratory Syncytial Virus Entry and Spreading. Antimicrob. Agents Chemother. 2017, 61, e02241-16. [Google Scholar] [CrossRef]
- Sadeghipour, S.; Bek, E.J.; McMinn, P.C. Ribavirin-resistant mutants of human enterovirus 71 express a high replication fidelity phenotype during growth in cell culture. J. Virol. 2013, 87, 1759–1769. [Google Scholar] [CrossRef]
- Kelly, J.T.; De Colibus, L.; Elliott, L.; Fry, E.E.; Stuart, D.I.; Rowlands, D.J.; Stonehouse, N.J. Potent antiviral agents fail to elicit genetically-stable resistance mutations in either enterovirus 71 or Coxsackievirus A16. Antivir. Res. 2015, 124, 77–82. [Google Scholar] [CrossRef]
- Tan, C.W.; Chan, Y.F.; Sim, K.M.; Tan, E.L.; Poh, C.L. Inhibition of enterovirus 71 (EV-71) infections by a novel antiviral peptide derived from EV-71 capsid protein VP1. PLoS ONE 2012, 7, e34589. [Google Scholar] [CrossRef] [PubMed]
- Lalani, S.; Masomian, M.; Poh, C.L. Functional Insights into Silymarin as an Antiviral Agent against Enterovirus A71 (EV-A71). Int. J. Mol. Sci. 2021, 22, 8757. [Google Scholar] [CrossRef] [PubMed]
- Lalani, S.; Tan, S.H.; Tan, K.O.; Lim, H.X.; Ong, K.C.; Wong, K.T.; Poh, C.L. Molecular mechanism of L-SP40 peptide and in vivo efficacy against EV-A71 in neonatal mice. Life Sci. 2021, 287, 120097. [Google Scholar] [CrossRef]
- He, Q.Q.; Ren, S.; Xia, Z.C.; Cheng, Z.K.; Peng, N.F.; Zhu, Y. Fibronectin Facilitates Enterovirus 71 Infection by Mediating Viral Entry. J. Virol. 2018, 92, e02251-17. [Google Scholar] [CrossRef]
- Abd-Aziz, N.; Lee, M.F.; Ong, S.K.; Poh, C.L. Antiviral activity of SP81 peptide against Enterovirus A71 (EV-A71). Virology 2024, 589, 109941. [Google Scholar] [CrossRef]
- Evans, W.J.; Hurst, B.L.; Peterson, C.J.; Van Wettere, A.J.; Day, C.W.; Smee, D.F.; Tarbet, E.B. Development of a respiratory disease model for enterovirus D68 in 4-week-old mice for evaluation of antiviral therapies. Antivir. Res. 2019, 162, 61–70. [Google Scholar] [CrossRef]
- Patel, M.C.; Wang, W.; Pletneva, L.M.; Rajagopala, S.V.; Tan, Y.; Hartert, T.V.; Boukhvalova, M.S.; Vogel, S.N.; Das, S.R.; Blanco, J.C. Enterovirus D-68 Infection, Prophylaxis, and Vaccination in a Novel Permissive Animal Model, the Cotton Rat (Sigmodon hispidus). PLoS ONE 2016, 11, e0166336. [Google Scholar] [CrossRef] [PubMed]
- Vermillion, M.S.; Dearing, J.; Zhang, Y.; Adney, D.R.; Scheuermann, R.H.; Pekosz, A.; Tarbet, E.B. Animal Models of Enterovirus D68 Infection and Disease. J. Virol. 2022, 96, e0083322. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Liu, J.; Fu, L.; He, Y.; Cui, G.; Huang, B. Animal models for enterovirus 71: Mechanisms, immunity, and applications. Hum. Vaccin. Immunother. 2025, 21, 2523109. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Zhu, R.; Wu, Y.; Xia, N.; Xu, L.; Cheng, T. Research progress and application prospects of animal models of group B Coxsackievirus infections. Emerg. Microbes Infect. 2025, 14, 2441391. [Google Scholar] [CrossRef]
- Du, S.; Hu, X.; Menéndez-Arias, L.; Zhan, P.; Liu, X. Target-based drug design strategies to overcome resistance to antiviral agents: Opportunities and challenges. Drug Resist. Updates 2024, 73, 101053. [Google Scholar] [CrossRef]

| Target Site | Representative Compounds | EV Species | Mechanism of Action | Efficacy (In Vitro) | Notable Insights | References |
|---|---|---|---|---|---|---|
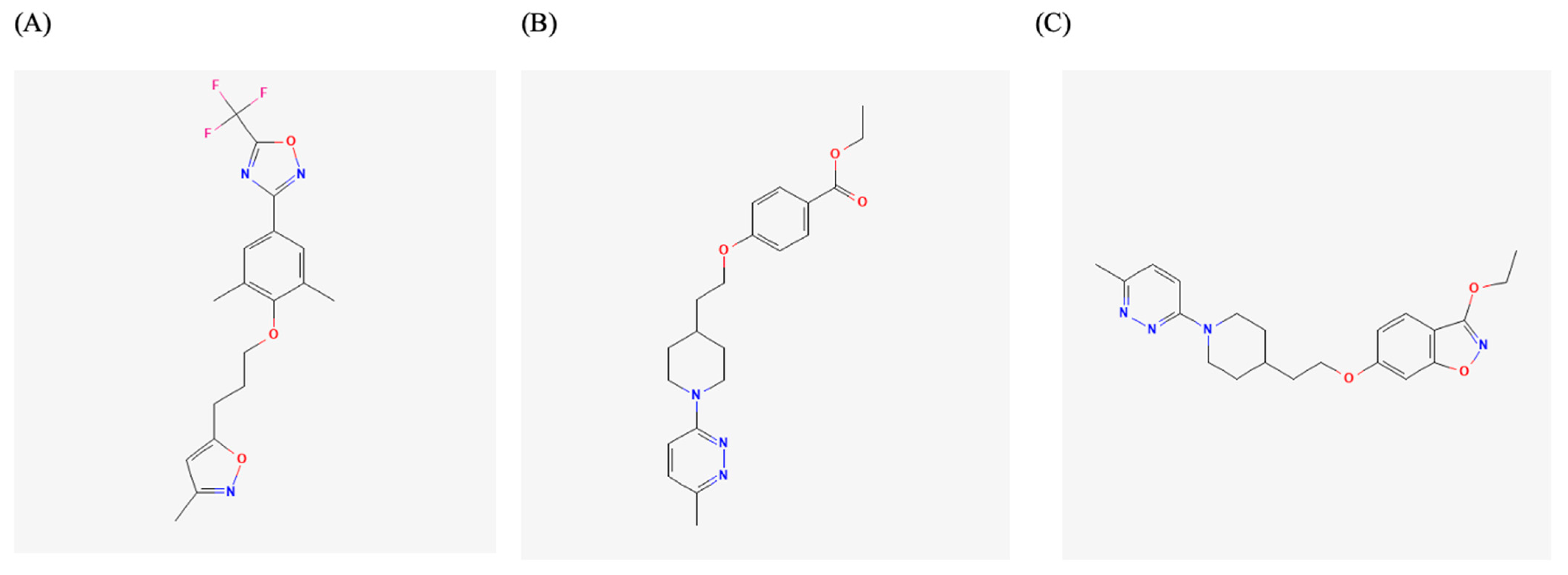
| VP1 hydrophobic pocket | Pleconaril, Vapendavir, PR66, BPROZ-101, NLD-22, VP1-6g | EV-A71, CV-B3, Echoviruses, EV-D68 | Capsid stabilization; blocked uncoating | EC50/IC50: nM–μM | High resistance risk (Taiwan EV-A71 strains), good breadth for EV-A71, poor for EV-D68 | [10,31,42,43,44,46,47,48,49] |
| Compound 36, 10 g | CV-B3, CV-B4 | Effective against pleconaril-resistant strains; orally bioavailable | IC50: 0.01–1 μM | Stable; protective in mice | [55,56] | |
| Compound 19 (quinoline analog) | EV-D68 | Inhibited uncoating | EC50: 0.4–4 μM | Broad strain coverage; good pharmacokinetics profile | [34,63] | |
| VP1-VP3 interprotomer binding pocket | 4EDMAB, Compound 1, 17, 7a | CV-B3, CV-B1/B4/B5/B6, CV-A9 | Stabilized virion; blocked uncoating | EC50: 0.7–9.4 μM | Effective across EV-B and some EV-C/D; resistance mapping ongoing | [9,64,65] |
| Five-fold axis of the capsid | MADL385, CB-30 | EV-A71 | Blocked receptor attachment | EC50: 0.2–353 nM | Resistance mutations (e.g., VP1-S184T); dendrimeric design | [66,67] |
| Rosmarinic acid, Suramin, E151 | EV-A71, CV-A16, CV-A6 | Entry inhibition; blocked receptor interaction | EC50: 31–114 μM | Suramin effective in animal models, but limited by non-specificity | [32,37,68,69,70] | |
| NF110, NM16 (Suramin analogs) | EV-A71 | Blocked binding to sulfated receptors (e.g., PSGL-1) | EC50/IC50: low to sub-μM | Site-specific resistance (e.g., K244R) | [69] |
| Target Protein | Representative Compounds | EV Species | Mechanism of Action | Notable Insights | References |
|---|---|---|---|---|---|
| 2A protease | Z-LVLQTM-FMK, CW33, Telaprevir, Chlorogenic acid | EV-A71, EV-D68 | Inhibited viral polyprotein processing; blocked host translation/immune responses | Limited potency; structure-based studies emerging | [71,72,73,74,75,76,77,78,79,80,81,82] |
| 2B protein | DIDS | EV-A71 | Blocked chloride ion channel; viroporin inhibition | Unclear specificity; selective inhibitors needed | [83,84,85] |
| 2C protein | Fluoxetine, compound 12b, dibucaine, 6aw, JX040, R523062 | EV-A71, EV-D68, CV-B3, Poliovirus | Inhibited ATPase/oligomerization; disrupted replication/encapsidation | Broad-spectrum potential; structural studies advancing | [86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103] |
| 3A protein | Enviroxime, AN-12-H5, itraconazole, TTP-8307 | EV-A71, EV-D68, CV-B3 | Disrupted replication organelle formation | No confirmed direct binders; promising polypharmacology | [105,106,107,108,109,110,111,112,113,114,115,116,117,161] |
| 3C protease | Rupintrivir, AG7404, compound 18p, NK-1.9k | EV-A71, EV-D68, CV-B3, Echoviruses | Inhibited viral proteolytic processing; immune evasion | Potent in vitro activity; resistance and delivery issues remained | [118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,162] |
| 3D polymerase | Gemcitabine, LY2334737, FNC, GPC-N114, BPR-3P0128, DTriP-22 | EV-A71, CV-B3, EV-D68, Poliovirus | Chain termination; RdRp inhibition; blocked RNA elongation | Broad-spectrum potential; nucleoside analogs in clinical use | [147,148,149,150,151,152,154,156,157,158,159,160] |
| Host Target | Representative Compounds/Strategies | Mechanism of Action | Antiviral Effects | References |
|---|---|---|---|---|
| OSBP (Oxysterol-binding protein) | OSW-1, Itraconazole, TTP-8307, T-00127-HEV2 | Disrupted cholesterol transport; impaired viral replication complex formation | Potent EV-A71 inhibition; nanomolar EC50 values; reduced OSBP levels by ~90% | [166,167,168,169,170] |
| PI4KB (Phosphatidylinositol 4-kinase IIIβ) | MDL-860, Compound 10, PIK93, T-00127-HEV1 | Inhibited PI4KB function and lipid kinase activity; blocked replication organelle formation | Nanomolar activity against CV-B3 and EV-A71; MDL-860 analog showed 50% protection in neonatal mice | [171,172,173] |
| DHODH (Dihydroorotate dehydrogenase) | RYL-634, FA-613 | Inhibited pyrimidine biosynthesis; disrupted viral RNA synthesis | RYL-634: EC50 ~4 nM (EV-A71); FA-613: Broad activity against RSV, EVs, CoVs | [174,175,176,177] |
| Innate Immune Modulators (e.g., TLR7, IFN pathways) | R837 (TLR7 agonist), IFN-α, GS-9620 | Activated antiviral IFN responses; reduced viral replication/inflammation | Improved survival in EV-A71 mouse models; synergistic with rupintrivir | [178,179,180] |
| Restriction Factors (e.g., SAMHD1, APOBEC3G) | Endogenous upregulation or stabilization | SAMHD1 blocked VP1–VP2 interaction; A3G disrupted 5′ UTR translation initiation. | Inhibited EV-A71, CV-A16, and EV-D68 | [181,182,183,184] |
| Attachment Factors (e.g., HSPGs) | HTA-22, heparin, lactoferrin | Blocked viral attachment | Inhibited EV-A71, CV-A16, and PV1 | [185,186,187,188] |
| hNMT1 (N-myristoyltransferase 1) | siRNA knockdown, Compound 4O | Prevented VP4 myristoylation; impaired capsid precursor processing. | Inhibited EV-A71 replication | [22] |
| AP2M1 (Adaptor protein complex 2 subunit mu) | ACA (N-(p-amylcinnamoyl)anthranilic acid) | Disrupted 2C localization; inhibited YxxØ motif | Inhibited EV-A71, influenza, Zika, and MERS-CoV. | [189] |
| mTOR signaling and autophagy | Torin2, LY-55 | Inhibited mTOR-mediated autophagy | Inhibited EV-A71 replication with low IC50 values; synergistic with 3-MA. | [190,191] |
| TREM-1–NF-κB–MAPK axis | LP17 peptide | Suppressed virus-induced inflammatory cytokine signaling. | Reduced IL-6, IL-8, TNF-α release in EV-D68-infected cells. | [192] |
| Cyclophilin A (CypA) | Cyclosporine A, HL051001P2, CypA-11 | Blocked VP1–CypA interaction, impaired uncoating. | Exhibited submicromolar activity against EV-A71; synergistic with 3Cpro inhibitors. | [193,194] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.F.; Tham, S.K.; Poh, C.L. Antiviral Strategies Targeting Enteroviruses: Current Advances and Future Directions. Viruses 2025, 17, 1178. https://doi.org/10.3390/v17091178
Lee MF, Tham SK, Poh CL. Antiviral Strategies Targeting Enteroviruses: Current Advances and Future Directions. Viruses. 2025; 17(9):1178. https://doi.org/10.3390/v17091178
Chicago/Turabian StyleLee, Michelle Felicia, Seng Kong Tham, and Chit Laa Poh. 2025. "Antiviral Strategies Targeting Enteroviruses: Current Advances and Future Directions" Viruses 17, no. 9: 1178. https://doi.org/10.3390/v17091178
APA StyleLee, M. F., Tham, S. K., & Poh, C. L. (2025). Antiviral Strategies Targeting Enteroviruses: Current Advances and Future Directions. Viruses, 17(9), 1178. https://doi.org/10.3390/v17091178







