Abstract
Enteroviruses, a diverse genus within the Picornaviridae family, are responsible for a wide range of human infections, including hand, foot, and mouth disease, respiratory disease, aseptic meningitis, encephalitis, myocarditis, and acute flaccid paralysis. Despite their substantial global health burden and the frequent emergence of outbreaks, no specific antiviral therapies are currently approved for clinical use against non-polio enteroviruses. This review provides a comprehensive overview of the current landscape of antiviral strategies targeting enteroviruses, including direct-acting antivirals such as capsid binders, protease inhibitors, and viral RNA polymerase inhibitors. We also examine the potential of host-targeting agents that interfere with virus–host interactions essential for replication. Emerging strategies such as immunotherapeutic approaches, RNA interference, CRISPR-based antivirals, and peptide-based antivirals are also explored. Furthermore, we address key challenges, including viral diversity, drug resistance, and limitations in preclinical models. By highlighting recent advances and ongoing efforts in antiviral development, this review aims to guide future research and accelerate the discovery of effective therapies against enterovirus infections.
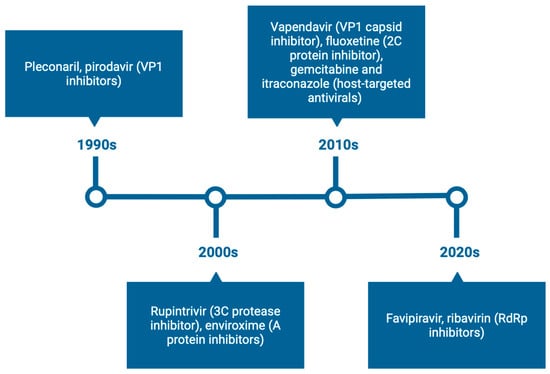
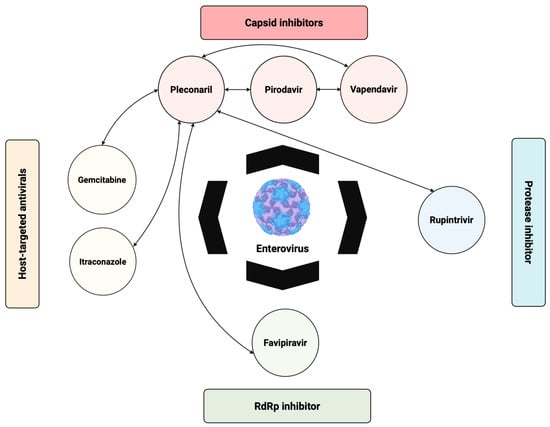
1. Introduction
The Enterovirus (EV) genus, a member of the Picornaviridae family, consists of 13 species, of which 7 are known to infect humans. These include four EV species—EV-A, EV-B, EV-C, and EV-D—as well as three rhinovirus (RV) species—RV-A, RV-B, and RV-C. EVs that can cause human infections include EV-A71, Coxsackievirus A6 (CV-A6), CV-A10, and CV-A16 (EV-A); CV-B3, CV-B5, echoviruses, and CV-A9 (EV-B); poliovirus and CV-A21 (EV-C); and EV-D68 and EV-D70 (EV-D). Collectively, these viruses are responsible for a wide range of clinical manifestations, from mild illnesses to severe complications such as hand, foot, and mouth disease (HFMD), aseptic meningitis, encephalitis, myocarditis, acute flaccid paralysis, and respiratory diseases [1].
The EV genome is a positive-sense single-stranded RNA with a single open reading frame (ORF), flanked by 5′ and 3′ untranslated regions (UTRs) [2]. The ORF is translated into a single polyprotein that is proteolytically cleaved into three regions: P1, P2, and P3. P1 gives rise to the four structural capsid proteins VP1–VP4, which mediate viral entry by interacting with host cell receptors and uncoating the viral genome. P2 and P3 encode non-structural (NS) proteins (2A–2C and 3A–3D), which play essential roles in genome replication, polyprotein processing, and modulation of host cellular pathways [3]. Due to their involvement in critical stages of the viral life cycle, these viral proteins represent attractive targets for antiviral intervention.
Although most EV infections are self-limiting, certain serotypes can cause severe disease, particularly in infants, the elderly, and immunocompromised individuals. Over the past decade, EV-A71 and CV-A16 have caused major HFMD outbreaks across Asia, while EV-D68 has emerged as a respiratory pathogen associated with severe respiratory illness and acute flaccid myelitis, notably during the 2014 outbreak in North America [4,5,6]. These recurring and unpredictable outbreaks underscore the need for effective antiviral therapeutics. However, despite the considerable global health burden posed by non-polio enteroviruses, there are currently no approved antiviral therapies for clinical use.
In this review, we provide a comprehensive overview of the current strategies under investigation for the development of anti-EV therapeutics. We discuss direct-acting antivirals (DAAs), particularly capsid binders, protease inhibitors, and polymerase inhibitors, alongside host-targeting antivirals that disrupt virus–host interactions essential for replication. Additionally, we highlight immunotherapeutic approaches such as neutralizing monoclonal antibodies, intravenous immunoglobulin (IVIG), and type I interferons (IFNs). The emergence of novel technologies including RNA interference, CRISPR-based antivirals, and broad-spectrum antivirals will also be explored. Finally, we address challenges such as viral diversity, resistance, limitations of current preclinical models, and offer perspectives on the development of future therapeutic strategies.
2. Biology and Pathogenesis of Enteroviruses
2.1. Structure and Genome Organization of Enteroviruses
EVs are small, non-enveloped viruses with a positive-sense single-stranded RNA genome [7]. The virion, approximately 30 nm in diameter, possesses a naked icosahedral capsid exhibiting fivefold rotational symmetry and is formed by 60 identical protomers [8]. Each protomer comprises four structural proteins: VP1, VP2, VP3, and VP4. The outer surface of the capsid comprises VP1, VP2, and VP3, while VP4 is located internally, lining the capsid’s inner face in close proximity to the viral RNA. The capsid proteins VP1 to VP3 share a conserved architecture consisting of eight-stranded antiparallel β-barrels flanked by α-helices. Structural variability among different EVs arises primarily from differences in the loops that connect these secondary structures, contributing to serotype-specific antigenic features [1,3].
The surface topology of the EV capsid is characterized by distinctive morphological features. Star-shaped protrusions are formed by five copies of VP1 around the fivefold axes, while a canyon encircles these axes, formed by the junction of the “north rim” (VP1) and the “south rim” (VP2 and VP3). Additional surface structures include a prominent protrusion or “puff” generated by a loop in VP2 and a “knob” formed by a VP3 loop. The capsid also contains large depressions at the twofold axes. A conserved hydrophobic pocket lies beneath the canyon floor in VP1, typically occupied by a lipid-like molecule known as the “pocket factor”, which stabilizes the virion and plays a role in uncoating during cell entry [1,8,9]. While the amino acid residues lining this pocket are highly conserved, the identity of the pocket factor can vary between different EVs and may include sphingosine or other fatty acids, as observed in EV-A71 [10,11,12].
The EV genome spans approximately 7.4 kilobases and carries UTRs at both the 5′ and 3′ ends. The 5′ UTR contains a highly structured internal ribosome entry site (IRES), enabling cap-independent translation, and is covalently linked to a small viral protein, VPg, which is critical for initiating RNA replication. The 3′ UTR ends in a polyadenylated tail that is essential for genome stability and viral infectivity [1]. The viral genome encodes a single large open reading frame, which is translated into a polyprotein of approximately 2193 amino acids. This polyprotein undergoes proteolytic cleavage by viral proteases into three precursor regions: P1, P2, and P3 [13]. The P1 region is further processed into four structural proteins that form the viral capsid. The P2 and P3 regions yield seven NS proteins—2A, 2B, 2C, 3A, 3B (VPg), 3C (protease), and 3D (RNA-dependent RNA polymerase) [14].
These NS proteins are essential for viral replication and pathogenesis. They participate in the assembly of membranous replication organelles, facilitate the hijacking of host cell resources, and suppress host immune responses. Some of these proteins, such as 2A and 3C, also modulate cellular signaling pathways and have been implicated in inducing apoptosis [15]. The genomic and structural organization of EVs reflect their evolutionary adaptation to evade host defenses and establish infection across a wide range of tissues. This intricate architecture and replication strategy offer several conserved viral components, particularly in the capsid and NS regions, as attractive targets for the development of antiviral therapies.
2.2. Life Cycle of Enteroviruses
EVs follow a conserved replication cycle that includes attachment, entry, uncoating, translation, replication, assembly, and release. The infection begins when EVs attach to host cell surface receptors, many of which are members of the immunoglobulin (Ig) superfamily or integrin receptors [16]. The viral capsid has a “canyon” structure that accommodates the apical domain of Ig-like receptors, triggering conformational changes necessary for uncoating [9,17]. In some enteroviruses, including EV-A71 and CV-A16, which have shallower canyons, receptor binding occurs at regions outside of the canyon, specifically, at the VP1 GH and VP2 EF loops [18,19].
In general, receptor binding is also facilitated by cell surface attachment factors such as heparan sulfate or sialic acid, which enhance virus attachment without necessarily mediating entry or uncoating. Once bound, EVs are typically internalized via clathrin-mediated endocytosis, though alternative endocytic pathways such as caveolin-mediated uptake or macropinocytosis may also be employed, depending on the virus and cell type. After endocytosis, acidification of the endosome promotes conformational changes in the capsid that facilitate genome release into the cytoplasm, usually via pore formation or capsid destabilization. There is also evidence suggesting that some EVs may bypass the endosome and release their genome directly at the plasma membrane [20].
The released positive-sense single-stranded RNA genome is directly translated by host ribosomes to produce a large polyprotein, which is then proteolytically cleaved into structural and NS proteins. The structural proteins, VP1 through VP4, assemble into an icosahedral capsid in which VP1, VP2, and VP3 form the external shell, while VP4 lines the internal surface and associates with the viral RNA [21]. VP4 also undergoes myristoylation, a post-translational modification critical for virion assembly and infectivity [22]. NS proteins encoded by the polyprotein, including 2A–2C and 3A–3D, play key roles in RNA replication, host cell remodeling, and immune evasion [1]. For instance, 2B acts as a viroporin that alters membrane permeability and intracellular calcium homeostasis [23], while 2C, an ATPase and RNA-binding protein, is involved in membrane remodeling for replication complex formation and may contribute to genome packaging [24]. The viral proteases 2A and 3C, as well as their precursor 3CD, mediate polyprotein processing and inhibit host transcription and translation to favor viral protein synthesis [15]. Genome replication is driven by the RNA-dependent RNA polymerase 3Dpol, which synthesizes a negative-strand intermediate used to generate new positive-sense genomes. This process is primed by the uridylylated VPg protein [25].
Replication occurs within virus-induced membranous compartments derived from host organelles, which concentrate viral components and shield RNA from host defenses. Encapsidation of progeny genomes occurs concurrently with capsid assembly, and virion maturation is facilitated by proteolytic cleavage events and environmental factors such as low pH. Virus release typically occurs via cell lysis, though some EVs can exit through non-lytic, vesicle-associated secretion pathways that may aid in immune evasion and viral spread [13]. Throughout this cycle, EVs manipulate host cellular processes, including gene expression, membrane trafficking, and immune signaling, to create a favorable environment for replication [26]. Despite strain-specific complexities in receptor usage and entry mechanisms, the core steps of the EV life cycle are conserved, offering opportunities for broad-spectrum antiviral targeting.
2.3. Clinical Manifestations
Most nonpolio enterovirus infections tend to resolve on their own and are typically asymptomatic, mild, and short-lived. However, in individuals with weakened immune systems—such as infants, young children, or immunocompromised persons—the infection can escalate into serious neurological conditions. EV-A71, CV-A16, and, more recently, CV-A6 are recognized as the primary causes of HFMD, a condition commonly affecting neonates and infants. HFMD is marked by fever, maculopapular or erythematous rashes on the limbs, and painful ulcers in the mouth. Due to their pronounced neurotropism, especially in the case of EV-A71, complications like brainstem encephalitis, acute flaccid paralysis, and aseptic meningitis can arise during seasonal outbreaks. In more severe cases, the viruses may spread to other organs, potentially leading to life-threatening outcomes such as pulmonary edema, septic shock in newborns, or heart dysfunction [27].
Unlike other enteroviruses, EV-D68 infections present differently. These viruses are sensitive to acidic environments and thrive in cooler temperatures, making them more suited to infecting the nasal passages of the upper respiratory tract rather than the acidic gastrointestinal tract. EV-D68 is primarily linked to moderate to severe respiratory syndromes, including severe bronchitis and interstitial pneumonia. Nevertheless, similar to other human enteroviruses, EV-D68 can replicate in neurological tissues and has been classified as a high-risk pathogen, especially following a series of outbreaks in 2014 [28,29].
5. Emerging and Experimental Strategies
5.1. Immunotherapeutic Approaches
Human intravenous immunoglobulin (hIVIG), consisting of pooled IgG antibodies from healthy donors, has demonstrated broad neutralizing activity against several EVs, including EV-D68. Studies showed that commercial IVIG preparations contained high titers of neutralizing antibodies, suggesting both shared and unique antigenic determinants when compared to historical EV strains. In animal models, IVIG treatment significantly reduced paralysis incidence and motor deficits, and clinical use in neonates with severe EV infections has led to rapid viral clearance via pathogen-specific antibody responses [59,195].
Monoclonal antibodies (mAbs) could offer a targeted strategy against EVs by binding and neutralizing free viral particles. For EV-D68, multiple mAbs showed protective effects in mice when administered either before or after infection. For instance, antibody EV-D68-228 was able to bind the viral capsid’s five-fold axis, while mAb A61 targeted the VP1 DE loop and prevented interaction with α2,6-linked sialic acid receptors [196,197]. More recently, mAbs 15C5 and 11G1 were found to neutralize EV-D68 through distinct mechanisms, like inducing conformational changes in the viral particle or mimicking receptor interactions to block infection [198].
The innate immune system, particularly type I IFNs, plays a critical role in early antiviral defense. Virus recognition by pattern recognition receptors (PRRs) such as TLR3, TLR7, TLR8, and RIG-I triggers a cascade leading to IFN-β production through IRF3 activation. This initiates a positive feedback loop involving IRF7, resulting in the amplification of IFN-α/β and the expression of ISGs [199]. Several studies have highlighted the importance of IFN signaling in EV infections. For example, mice lacking type I IFN receptors showed significantly reduced survival after CV-A16 infection [180]. Moreover, 3Cpro from EV-A71 was shown to degrade IRF9 and impair IFN signaling [200]. Combination therapy with IFN-α and the protease inhibitor, rupintrivir, showed synergistic suppression of EV-A71 replication [180]. Similarly, EV-D68 3Cpro targeted IRF7 for degradation, further underscoring how viral proteases could evade innate immunity by disrupting IFN responses [201].
Taken together, immunotherapeutic strategies represented a vital and evolving front in the fight against EV infections. Passive immunization using hIVIG and monoclonal antibodies has demonstrated protective efficacy, particularly in severe cases and in vulnerable populations, while offering insights into viral antigenicity and neutralization mechanisms. Concurrently, bolstering the innate immune response through type I IFN signaling provided a promising avenue to counteract EV-mediated immune evasion. However, the heterogeneity among EV species and their diverse strategies to subvert host immunity underscore the need for targeted, virus-specific interventions. Continued investigation into host–pathogen interactions, coupled with advances in antibody engineering and immune modulation, would be crucial for translating these immunotherapeutic approaches into effective clinical treatments for EV-associated diseases.
5.2. RNA Interference (RNAi)
RNAi is a gene-silencing process that occurs after transcription and was first observed in pigmented petunias in 1990 [202]. It was first demonstrated that this potent gene-silencing effect was initiated by double-stranded RNA (dsRNA) in Caenorhabditis elegans, leading to the degradation of matching mRNA molecules [203]. The RNAi pathway is driven by Dicer, an RNase III family endonuclease, which generates small RNA molecules that guide the Argonaute (AGO) protein—an essential part of the RNA-induced silencing complex (RISC)—to degrade complementary mRNAs. These small RNAs include microRNAs (miRNAs), derived from hairpin-shaped precursor miRNAs (pre-miRNAs), and small interfering RNAs (siRNAs), processed from long dsRNAs [204].
RNAi is increasingly recognized as a conserved antiviral mechanism in eukaryotes, though its role in mammals has been a subject of debate. This skepticism largely stems from the dominant presence of IFN-mediated responses and adaptive immunity in mammals, which are absent in invertebrates and plants. Some studies suggested a competitive or inhibitory interaction between the RNAi and IFN pathways, with IFN-associated proteins such as LGP2 shown to suppress Dicer, a key enzyme in RNAi [205,206]. Despite this, accumulating evidence supported the existence of functional antiviral RNAi in both undifferentiated and differentiated mammalian cells [207,208]. In mammals, Dicer processes viral dsRNA intermediates into 21–23 nucleotide virus-derived small interfering RNAs (vsiRNAs), which are loaded into AGO2, the only slicing-competent AGO protein, to mediate degradation of complementary viral RNAs [204]. Notably, an alternative isoform of Dicer, termed antiviral Dicer (aviD), was recently discovered. This isoform lacks the Hel2i domain and exhibits enhanced capacity to generate antiviral siRNAs, offering increased protection against RNA viruses such as Zika virus and SARS-CoV-2 [209]. To evade host RNAi responses, many viruses encode viral suppressors of RNAi (VSRs) [210].
CV-B3 has been the most extensively studied EV in the context of RNAi-based antiviral strategies. Early investigations demonstrated that siRNAs targeting conserved regions of the viral 3D polymerase (3Dpol) effectively reduced CV-B3 replication by 80–90% in vitro, with protective effects lasting several days. Additionally, siRNAs against the host cell receptor CAR significantly reduced viral titers, and their combination with viral-targeting siRNAs was proposed as a robust dual-targeting approach [211]. siRNA-2A targeting the viral 2A protease showed potent antiviral effects in vitro and in a susceptible mouse model, reduced viral titers, tissue damage, and improved survival. However, repeated administrations were required for sustained effects due to siRNA degradation [212]. Another potent siRNA, siRNA-4, directed against the 2A region, showed 92% inhibition in vitro and protected cells both pre- and post-infection. This siRNA displayed strand-specific activity, targeting the positive-sense RNA with high specificity and without inducing escape mutants, likely due to targeting at a highly conserved and essential site [213]. Kim et al. (2007) designed shRNAs targeting several genomic regions, including 3Dpol and VP1, with shRNA-2 and shRNA-5 demonstrating in vivo efficacy in reducing viral load and tissue damage in mice [214]. Fechner et al. (2007) demonstrated that stable coxsackievirus–adenovirus receptor (CAR) knockdown via vector-delivered shRNA achieved 97% inhibition of CV-B3 in cardiac cells and improved outcomes when compared to siRNAs or plasmid shRNAs, especially in vivo [215]. Yao et al. (2012) targeted the conserved 2B region using plasmid and lentiviral vectors. Lentiviral delivery (Lenti-2B) was particularly effective in vivo, reducing viral titers, inflammation, and improving survival. The antiviral mechanism included direct RNA degradation and interference with the anti-apoptotic functions of the virus [216]. Additional work explored 5′ UTR targeting using chemically modified siRNAs (siLNAs and gapmers), with LNA-siRNA No. 20 and viral titers were significantly reduced, and cell viability was improved [217,218]. AAV-mediated delivery of shRNAs targeting 3Dpol achieved >1000-fold viral reduction in cardiomyocytes and improved heart function in vivo [219]. Stein et al. (2015) showed that combining AAV-shRNA targeting RdRp with sCAR-Fc further improved cardiac protection and reduced viral load in a myocarditis model [220]. Luan et al. (2012) confirmed that siRNAs targeting 2C and 3C of CV-B3 were more effective than those targeting structural regions like VP1 or the 5′ UTR, identifying siRNA-5 (targeting 2C) as especially potent [221]. Another study developed a Dicer-processed pool of siRNAs from a long dsRNA spanning the 2B-3D regions, achieved a strong cross-inhibition of CV-B3, CV-B4, and CV-A9 without IFN activation [222]. Werk et al. (2009) demonstrated that combining sCAR-Fc with siRNAs targeting 3Dpol synergistically reduced viral load and improved cell survival in a persistent CV-B3 model [223].
For CV-B4, Tan et al. (2010) tested siRNAs targeting conserved non-structural genes (2A, 3C, 3D). siRNA 3C showed the strongest effect, reducing replication significantly, followed by siRNA 3D. No cytotoxicity or IFN activation was observed, and antiviral effects lasted up to 48 h. Combining siRNAs yielded no additive benefit, but the study confirmed the therapeutic potential of siRNA 3C [224]. In the case of echovirus 30, Rothe et al. (2009) used computational modeling to design potent siRNAs against the 3Dpol gene, with several showing strong antiviral activity and one achieving an IC50 of ~1 nM. Additionally, targeting the host factor decay-accelerating factor (DAF) with RNAi partially inhibited infection. Combining shRNAs against both 3Dpol and DAF using adeno-associated virus (AAV) for delivery produced stronger and more durable inhibition [225]. In a related study, they developed a modular AAV-compatible shRNA expression system capable of targeting multiple genes simultaneously. Dual-targeting vectors against RdRp and DAF achieved enhanced suppression of viral replication, even though additive effects were not always observed. This strategy also enabled future expansion to 4–6 shRNAs per vector [226].
For EV-A71, Tan et al. (2007) demonstrated that 19-mer siRNAs and plasmid-expressed shRNAs targeting 3Dpol protected suckling mice from infection and paralysis without inducing IFN responses. In contrast, longer 29-mer shRNAs were potent in vitro but failed in vivo, likely due to processing inefficiency [227]. In a follow-up study, 29-mer shRNAs targeting 3Dpol, 3Cpro, and 2C were evaluated. The shRNA against 3Dpol was most effective (91% inhibition), with no cytotoxicity or IFN activation. Enhanced potency was attributed to better processing or Dicer binding [228]. Deng et al. (2012) evaluated both unmodified and chemically modified siRNAs (2′-O-Me, 2′-F) against the 5′ UTR of EV-A71. These siRNAs suppressed viral RNA, protein expression, and cytopathic effects in RD cells without triggering immune responses, suggesting their clinical promise [229]. Liu et al. (2016) designed three siRNAs against the conserved 2Apro region of EV-A71, all of which showed strong antiviral effects against multiple strains in vitro, highlighting the 2A protease as a viable therapeutic target [230]. Li et al. (2013) uncovered that miR-548 family members negatively regulated IFN-λ1 expression by binding to the 3′ UTR. Inhibition of miR-548 increased IFN-stimulated gene expression and reduced EV-A71 replication, making the miR-548 inhibitor a potential therapeutic option [231]. Fang et al. identified the 3A protein of EV-A71 as a viral suppressor of RNAi (VSR). Peptides like ER-DRI neutralized this VSR function, restored RNAi, and suppressed infection in cells and mice, validating VSR-targeting as a novel antiviral strategy [232].
For EV-70, two siRNAs targeting 3Dpol significantly suppressed viral RNA and protein levels in infected RD cells without activating the IFN pathway. si-3D2 was more effective than si-3D1 and showed both prophylactic and therapeutic effects in vitro [233]. Jun et al. (2011) later developed AHCe-3D-3, a cross-reactive siRNA targeting the 3Dpol gene conserved in both EV-70 and CV-A24, which are the etiological agents of acute hemorrhagic conjunctivitis. AHCe-3D-3 was effective in primary human conjunctival cells and showed cytoprotective effects against both viruses. The accessibility to its target region likely enhanced its potency, and the siRNA retained efficacy regardless of 5′ phosphorylation status [234]. For CV-A16, Wu et al. (2008) screened 30 siRNAs targeting conserved regions of eight viral genes. Thirteen candidates could reduce reporter activity by >80%, and several significantly suppressed viral replication in Vero cells. A pooled siRNA mixture also proved effective and safe, showing dose-dependent inhibition with no cytotoxicity. The results supported the use of multi-siRNA cocktails to prevent resistance and expand antiviral coverage [235].
Collectively, these studies underscore the versatility and promise of RNAi-based strategies against a wide spectrum of EVs. The success of these approaches hinged on selecting conserved, functionally essential target sites, optimizing delivery systems (e.g., AAV, lentivirus), and in some cases, combining viral and host-targeted strategies to improve durability and suppress viral escape. Despite progress, challenges remained in mammalian antiviral RNAi research. These include inconsistent detection of vsiRNAs, limited cleavage efficiency of full-length Dicer, and the lack of a clear phenotype in Dicer-deficient cells, in terms of increased viral replication. Addressing these limitations is essential for advancing RNAi-based antiviral strategies against EVs and other RNA viruses.
5.3. CRISPR-Based Antivirals
The CRISPR-Cas system, originally discovered as a bacterial adaptive immune mechanism, uses RNA-guided nucleases to recognize and cleave specific DNA or RNA sequences. The precision and efficiency of CRISPR-Cas have made it a leading tool for genome editing in mammalian cells [236]. Since the pioneering use of Cas9, the development of Cas12 and Cas13 enzymes has broadened the CRISPR toolkit to include both DNA- and RNA-targeting applications [237]. This versatility renders CRISPR systems especially attractive for antiviral interventions, as viral genomes are composed of either DNA or RNA [238,239]. Beyond editing, catalytically inactive variants like dCas9 can be fused with regulatory domains to modulate gene expression through CRISPR interference (CRISPRi) or activation (CRISPRa) [237]. Compared to conventional gene-silencing methods such as siRNA or shRNA, CRISPR offers enhanced specificity, adaptability, and functional range, making it a powerful platform for developing novel antiviral strategies [240].
Building on these fundamental capabilities, CRISPR-Cas technology has recently been explored as a promising antiviral strategy against EVs. Given the high mutation rates and genetic plasticity of EVs, conventional antivirals often struggle with the rapid emergence of resistance. CRISPR systems, particularly Cas13, provide a unique advantage by directly targeting and degrading viral RNA with high specificity. The RNA-guided RNase activity of Cas13 enables it to cleave single-stranded viral genomes in a sequence-specific manner, without requiring a protospacer adjacent motif (PAM), making it especially suitable for targeting RNA viruses like EV-A71, CV-A16, and CV-B3 [237]. This approach is illustrated in Figure 7, which highlights the RNA-targeting activity of CRISPR-Cas13 and its interference with viral replication and release in EV-infected cells. Recent studies have demonstrated the utility of CRISPR-based screening platforms to identify host dependency factors required for EV infection and replication. These genome-wide knockout or CRISPRi screens could uncover essential cellular receptors, cofactors, and immune modulators that support viral entry or replication. Such discoveries not only illuminate virus–host interactions but also offer opportunities to design host-targeted therapeutics with reduced risk of resistance [241].
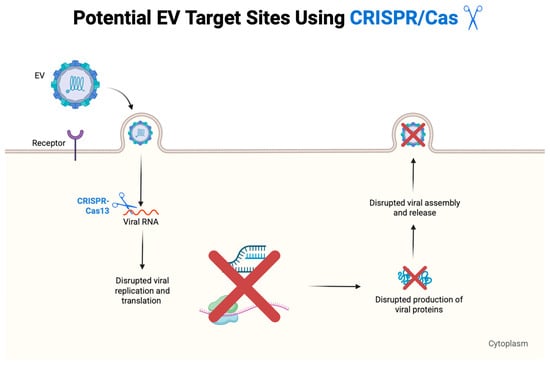
Figure 7.
Antiviral mechanism of CRISPR-Cas13 against EVs. CRISPR-Cas13 targeted and cleaved viral RNA in the cytoplasm, disrupting replication and translation. This blocked the production of viral proteins and interfered with virion assembly and release. This approach offers a sequence-specific strategy to inhibit positive-sense RNA viruses such as EV-A71, CV-B3, and EV-D68 [237]. The figure was created using Biorender.com.
Recent studies have demonstrated the utility of CRISPR-based screening platforms to identify host dependency factors required for EV infection and replication. These genome-wide knockout or CRISPRi screens have uncovered several host proteins that facilitate viral entry and propagation. For instance, Diep et al. (2019) identified SET domain-containing 3 (SETD3), an actin histidine methyltransferase, as a key host factor for rhinovirus, EV-D68, and EV-A71. SETD3 was shown to interact with the viral 2A protease independent of its enzymatic activity—a previously unrecognized proviral role essential for EV replication [242]. Similarly, olfactomedin-like 3 (OLFML3) was found to support rhinovirus infection by suppressing the interferon response, while mannosyl (alpha-1,6-)-glycoprotein beta-1,6-N-acetyl-glucosaminyltransferase (MGAT5) and a component of the oligomeric Golgi complex subunit 1 (COG1) were shown to be critical for EV-D68 infection [243,244].
Beyond initial screens, CRISPR-Cas has proven instrumental in clarifying conflicting findings from earlier RNAi studies. The host protein, Acyl-Coenzyme A Binding Domain Containing 3 (ACBD3), previously proposed to interact with the EV 3A protein and mediate PI4KB recruitment to viral replication organelles, yielded inconsistent results when knocked down by siRNA. Some studies reported inhibition of poliovirus replication, while others observed no impact on viral replication or PI4KB localization [245,246,247,248]. To resolve this, Lyoo et al. (2019) employed CRISPR-Cas9 to generate an ACBD3 knockout cell line and conclusively demonstrated its essential role in supporting replication of EV-A71, poliovirus, and rhinovirus, as well as in PI4KB recruitment [108]. These findings highlighted the superior precision and reliability of complete gene knockout over transient knockdown approaches.
In addition to host gene perturbation, direct targeting of the viral genome using multiplexed guide RNAs has been proposed to enhance therapeutic durability. By simultaneously attacking multiple conserved regions of the viral genome, CRISPR-Cas strategies might prevent the emergence of escape mutants—a critical concern in RNA virus therapeutics [249,250]. Despite these promising developments, several challenges remained. Effective in vivo delivery of CRISPR components, particularly to tissues affected by EVs (e.g., heart, brain, or gastrointestinal tract), remains a technical bottleneck. Additionally, concerns about off-target effects, immunogenicity, and long-term safety must be rigorously addressed. Strategies such as lipid nanoparticle encapsulation, tissue-specific AAV vectors, and high-fidelity Cas variants are currently under investigation to improve delivery precision and minimize unintended consequences [251,252,253].
A recent study demonstrated the potent antiviral potential of CRISPR-Cas13d delivered via AAV against EV-A71, a major cause of HFMD for which no specific antiviral treatment currently exists. Using a custom bioinformatics pipeline called Cas13gRNAtor, researchers designed guide RNAs (gRNAs) that targeted conserved regions across the EV-A71 genome. These gRNAs were packaged with Cas13d into AAV vectors for both prophylactic and therapeutic applications. In in vitro assays, AAV-CRISPR-Cas13d constructs reduced EV-A71 viral titers by more than 99.99%. When administered to lethally infected mice, the treatment significantly inhibited viral replication, prevented clinical symptoms, and dramatically improved survival rates. Notably, the CRISPR-Cas13d system effectively cleared the virus from critical tissues such as the spinal cord and skeletal muscle, areas where siRNA-based approaches previously failed to access. The gRNA pool strategy—targeting multiple conserved sites within the viral 3D polymerase gene—was shown to enhance antiviral efficacy and maintain broad activity across various EV-A71 strains. Importantly, whole transcriptome analysis confirmed that this approach was highly specific, with no detectable off-target effects. Compared to RNAi, the AAV-CRISPR-Cas13d platform offered superior tissue penetration, durability, and viral clearance. This study highlighted the feasibility of using AAV-delivered CRISPR-Cas13 as a DAA modality for RNA viruses like EV-A71, with strong potential for further development into clinically applicable treatments for EV infections [254].
Altogether, CRISPR-Cas technology offers a transformative framework for antiviral intervention against EVs. Its dual capacity to manipulate host factors and directly cleave viral RNA positions it as a next-generation antiviral platform, capable of overcoming limitations associated with traditional RNAi or small-molecule drugs. Continued refinement of delivery systems, guided RNA design, and an understanding of viral escape mechanisms will be essential to translate this potential into clinical application.
5.4. Peptide-Based Antivirals
Peptides are increasingly recognized as viable therapeutic agents, with a growing number under investigation as antimicrobial and antiviral compounds in clinical trials [255]. Antiviral peptides (AVPs) are typically cationic and amphipathic, allowing them to interact with both viral and host components. These physicochemical features support the rational design of peptides that inhibit viral entry or fusion, often by mimicking sequences derived from the virus itself [256,257].
Compared to small molecules, peptides offer advantages such as high specificity, reduced off-target effects, better tolerability, and minimal toxic byproducts, as their degradation products are amino acids. Moreover, peptides are generally less susceptible to resistance since they can target multiple functional regions of the virus across different stages of the life cycle. Combining AVPs that act on different mechanisms may further reduce the likelihood of viral escape [258]. However, only one peptide-based antiviral, enfuvirtide, is currently FDA-approved for HIV, where it inhibits viral fusion with host cells [259]. Other peptide drugs, such as vancomycin, bacitracin, and neosporin, are widely used against bacterial infections [260].
A major challenge in peptide-based antiviral development is the high cost of production and formulation. Issues such as low oral bioavailability, rapid enzymatic degradation, short plasma half-life, and poor systemic delivery limit the therapeutic utility of natural peptides. For instance, enfuvirtide requires twice-daily injections, costing around USD 90/day, which impacts patient adherence. Strategies such as truncating peptides to identify minimal active motifs, recombinant peptide expression, and the use of gold nanoparticles or delivery technologies (e.g., PharmaFilm™) have been explored to overcome these barriers [258].
Peptides also hold promise in combating drug resistance. Small-molecule antivirals often induce resistant mutants, as seen with influenza and RSV. For example, RSV developed escape mutations (P488I/V, D486N, D498Y, L141W) against GS-5806, RV-521, and JNJ-53718678 [261,262,263]. In contrast, peptides such as 3ac targeting RSV’s post-fusion 6B complex retained efficacy against these mutants [264]. Likewise, EV-A71 rapidly develops resistance to small-molecule antivirals like ribavirin (via S264L, G64R/T in 3Dpol) and capsid-binding imidazolidinones (via I113M, V123I in VP1) [265,266].
To bypass such resistance, targeting viral entry via host receptors with short peptides offers a compelling approach. SP40, derived from EV-A71 VP1, was shown to block viral attachment by interacting with the nucleolin receptor [267,268,269]. Another example is RGDS, which inhibits fibronectin receptor-mediated entry of EV-A71 [270]. SP81, a synthetic peptide derived from the VP1 capsid protein of EV-A71, demonstrated potent antiviral activity with low cytotoxicity (CC50: 90.32 µM). It interfered with multiple stages of infection, including viral attachment, entry, and post-entry replication, and also exhibited direct virucidal effects, achieving approximately 95% inactivation within 5 min. The peptide is predicted to interact with viral capsid proteins such as VP1 and may also inhibit IRES-mediated translation. Although in vivo stability remains a limitation, strategies such as D-amino acid substitution or nanocarrier delivery could improve its therapeutic application [271]. These peptides, though promising, still require optimization to improve stability, systemic bioavailability, and delivery before clinical translation. Overall, peptide-based antivirals represent a promising but underdeveloped class of therapeutics for enteroviruses and other RNA viruses. Continued advances in peptide design, delivery technologies, and formulation strategies are expected to enhance their clinical potential.
7. Conclusions
EVs represent a significant public health burden due to their wide range of clinical manifestations, from mild febrile illness to severe neurological and cardiac complications. Despite substantial research efforts, there are still no approved antiviral therapies targeting EV infections. This therapeutic gap is largely driven by challenges such as high viral mutation rates, limited broad-spectrum efficacy, and poor translation from in vitro studies to effective in vivo outcomes.
Numerous small-molecule antivirals have been explored, targeting viral capsid proteins, proteases, and replication complexes. While several compounds have shown potent in vitro activity, many failed to progress due to resistance, toxicity, or narrow strain specificity. Recent advances in structure-based drug design and high-throughput screening have yielded promising leads, but these require further optimization and clinical validation. Targeting host factors presents an alternative strategy with the potential to overcome viral resistance and broaden therapeutic coverage. However, this approach carries the risk of off-target effects and toxicity due to the essential role of host proteins in normal cellular functions. A detailed understanding of virus–host interactions remained critical for identifying viable targets with minimal adverse consequences. Immunotherapeutic interventions, including monoclonal antibodies, IVIG, and modulation of innate immune pathways, have also shown encouraging preclinical efficacy. These approaches highlighted the value of leveraging host immunity as a means to control EV infections. At the same time, emerging technologies such as RNAi and CRISPR-Cas systems offered new modalities that directly target viral RNA with high specificity, demonstrating robust antiviral activity both in vitro and in vivo.
Nonetheless, several overarching challenges continued to impede progress. Animal models often exhibit inconsistent disease phenotypes and limited relevance to human pathology, complicating the evaluation of candidate antivirals. The lack of standardized experimental methodologies further hampered cross-study comparisons and reproducibility. Moreover, the sporadic and geographically dispersed nature of severe EV outbreaks posed logistical difficulties for conducting designed clinical trials. Moving forward, a combination of strategies would be essential. Rational drug design, host-targeted approaches, and gene-editing technologies should be integrated with improved disease models and harmonized evaluation criteria. Combination therapies, designed to enhance efficacy and limit resistance, also hold promise for future development.
Ultimately, bridging the gap between bench and bedside would require interdisciplinary collaboration, sustained investment in antiviral research, and the continued refinement of translational tools. As some EVs remain better studied than others, extending these advances to lesser-known serotypes would be key to achieving comprehensive and durable antiviral solutions across the EV spectrum.
Author Contributions
M.F.L.: Conceptualization, writing—original draft preparation, visualization; S.K.T.: Writing—review and editing. C.L.P.: Conceptualization, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Author Seng Kong Tham and Chit Laa Poh were employed by the company ALPS Global Holding Berhad. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Baggen, J.; Thibaut, H.J.; Strating, J.R.P.M.; van Kuppeveld, F.J.M. The life cycle of non-polio enteroviruses and how to target it. Nat. Rev. Microbiol. 2018, 16, 368–381. [Google Scholar] [CrossRef]
- Anasir, M.I.; Poh, C.L. Advances in Antigenic Peptide-Based Vaccine and Neutralizing Antibodies against Viruses Causing Hand, Foot, and Mouth Disease. Int. J. Mol. Sci. 2019, 20, 1256. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Shen, L.; Wu, J.; Zou, X.; Gu, J.; Chen, J.; Mao, L. Enterovirus A71 Proteins: Structure and Function. Front. Microbiol. 2018, 9, 286. [Google Scholar] [CrossRef]
- Yang, B.; Liu, F.; Liao, Q.; Wu, P.; Chang, Z.; Huang, J.; Long, L.; Luo, L.; Li, Y.; Leung, G.M.; et al. Epidemiology of hand, foot and mouth disease in China, 2008 to 2015 prior to the introduction of EV-A71 vaccine. Euro Surveill. 2017, 22, 16-00824. [Google Scholar] [CrossRef] [PubMed]
- Puenpa, J.; Wanlapakorn, N.; Vongpunsawad, S.; Poovorawan, Y. The History of Enterovirus A71 Outbreaks and Molecular Epidemiology in the Asia-Pacific Region. J. Biomed. Sci. 2019, 26, 75. [Google Scholar] [CrossRef]
- Messacar, K.; Abzug, M.J.; Dominguez, S.R. 2014 outbreak of enterovirus D68 in North America. J. Med. Virol. 2016, 88, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.J.; Dass, R.S. Chapter 15—Current therapeutic strategies and novel antiviral compounds for the treatment of nonpolio enteroviruses. In Viral Infections and Antiviral Therapies; Dhara, A.K., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2023. [Google Scholar]
- Lyu, K.; Wang, G.C.; He, Y.L.; Han, J.F.; Ye, Q.; Qin, C.F.; Chen, R. Crystal structures of enterovirus 71 (EV71) recombinant virus particles provide insights into vaccine design. J. Biol. Chem. 2015, 290, 3198–3208. [Google Scholar] [CrossRef]
- Abdelnabi, R.; Geraets, J.A.; Ma, Y.; Mirabelli, C.; Flatt, J.W.; Domanska, A.; Delang, L.; Jochmans, D.; Kumar, T.A.; Jayaprakash, V.; et al. A novel druggable interprotomer pocket in the capsid of rhino- and enteroviruses. PLoS Biol. 2019, 17, e3000281. [Google Scholar] [CrossRef]
- Egorova, A.; Ekins, S.; Schmidtke, M.; Makarov, V. Back to the future: Advances in development of broad-spectrum capsid-binding inhibitors of enteroviruses. Eur. J. Med. Chem. 2019, 178, 606–622. [Google Scholar] [CrossRef]
- Dang, M.; Wang, X.; Wang, Q.; Wang, Y.; Lin, J.; Sun, Y.; Li, X.; Zhang, L.; Lou, Z.; Wang, J.; et al. Molecular mechanism of SCARB2-mediated attachment and uncoating of EV71. Protein Cell 2014, 5, 692–703. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Zheng, M. Enterovirus A71 antivirals: Past, present, and future. Acta Pharm. Sin. B 2022, 12, 1542–1566. [Google Scholar] [CrossRef]
- Wang, S.; Pang, Z.; Fan, H.; Tong, Y. Advances in anti-EV-A71 drug development research. J. Adv. Res. 2024, 56, 137–156. [Google Scholar] [CrossRef]
- Palmenberg, A.C. Proteolytic processing of picornaviral polyprotein. Annu. Rev. Microbiol. 1990, 44, 603–623. [Google Scholar] [CrossRef]
- Laitinen, O.H.; Svedin, E.; Kapell, S.; Nurminen, A.; Hytönen, V.P.; Flodström-Tullberg, M. Enteroviral proteases: Structure, host interactions and pathogenicity. Rev. Med. Virol. 2016, 26, 251–267. [Google Scholar] [CrossRef]
- Bergelson, J.M.; Coyne, C.B. Picornavirus entry. Adv. Exp. Med. Biol. 2013, 790, 24–41. [Google Scholar]
- Anasir, M.I.; Zarif, F.; Poh, C.L. Antivirals blocking entry of enteroviruses and therapeutic potential. J. Biomed. Sci. 2021, 28, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ye, X.; Zhang, X.Y.; Zhu, Z.; Zhang, X.; Xu, Z.; Ding, Z.; Zou, G.; Liu, Q.; Kong, L.; et al. Coxsackievirus A10 atomic structure facilitating the discovery of a broad-spectrum inhibitor against human enteroviruses. Cell Discov. 2019, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhao, Y.; Kotecha, A.; Fry, E.E.; Kelly, J.T.; Wang, X.; Rao, Z.; Rowlands, D.J.; Ren, J.; Stuart, D.I. Unexpected mode of engagement between enterovirus 71 and its receptor SCARB2. Nat. Microbiol. 2019, 4, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Shingler, K.L.; Yoder, J.L.; Carnegie, M.S.; Ashley, R.E.; Makhov, A.M.; Conway, J.F.; Hafenstein, S. The enterovirus 71 A-particle forms a gateway to allow genome release: A cryoEM study of picornavirus uncoating. PLoS Pathog. 2013, 9, e1003240. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, Y.; Ma, H.C.; Paul, A.V.; Wimmer, E. Picornavirus morphogenesis. Microbiol. Mol. Biol. Rev. 2014, 78, 418–437. [Google Scholar] [CrossRef]
- Tan, Y.W.; Hong, W.J.; Chu, J.J. Inhibition of enterovirus VP4 myristoylation is a potential antiviral strategy for hand, foot and mouth disease. Antivir. Res. 2016, 133, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Ao, D.; Sun, S.Q.; Guo, H.C. Topology and biological function of enterovirus non-structural protein 2B as a member of the viroporin family. Vet. Res. 2014, 45, 87. [Google Scholar] [CrossRef]
- Yeager, C.; Carter, G.; Gohara, D.W.; Yennawar, N.H.; Enemark, E.J.; Arnold, J.J.; Cameron, C.E. Enteroviral 2C protein is an RNA-stimulated ATPase and uses a two-step mechanism for binding to RNA and ATP. Nucleic Acids Res. 2022, 50, 11775–11798. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Wang, Z.; Xie, W. Crystal Structure and Thermostability Characterization of Enterovirus D68 3D. J. Virol. 2017, 91, e00876-17. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhong, T.; Wang, Y.; Song, F.M.; Yu, X.F.; Xing, L.P.; Zhang, W.Y.; Yu, J.H.; Hua, S.C. Human Enterovirus 68 Interferes with the Host Cell Cycle to Facilitate Viral Production. Front. Cell. Infect. Microbiol. 2017, 7, 29. [Google Scholar] [CrossRef]
- Jan, S.L.; Fu, Y.C.; Chi, C.S.; Lee, H.F.; Huang, F.L.; Wang, C.C.; Wei, H.J.; Lin, M.C.; Chen, P.Y.; Hwang, B. Catecholamine-Induced Secondary Takotsubo Syndrome in Children With Severe Enterovirus 71 Infection and Acute Heart Failure: A 20-year Experience of a Single Institute. Front. Cardiovasc. Med. 2021, 8, 752232. [Google Scholar] [CrossRef] [PubMed]
- Oermann, C.M.; Schuster, J.E.; Conners, G.P.; Newland, J.G.; Selvarangan, R.; Jackson, M.A. Enterovirus d68. A focused review and clinical highlights from the 2014 U.S. Outbreak. Ann. Am. Thorac. Soc. 2015, 12, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, X.Y.; Yu, X.F. Current Understanding of Human Enterovirus D68. Viruses 2019, 11, 490. [Google Scholar] [CrossRef]
- Tammaro, C.; Guida, M.; Appetecchia, F.; Biava, M.; Consalvi, S.; Poce, G. Direct-Acting Antivirals and Host-Targeting Approaches against Enterovirus B Infections: Recent Advances. Pharmaceuticals 2023, 16, 203. [Google Scholar] [CrossRef]
- Chen, T.C.; Liu, S.C.; Huang, P.N.; Chang, H.Y.; Chern, J.H.; Shih, S.R. Antiviral activity of pyridyl imidazolidinones against enterovirus 71 variants. J. Biomed. Sci. 2008, 15, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.F.; Jheng, J.R.; Lin, G.H.; Chen, Y.L.; Ho, J.Y.; Liu, C.J.; Hsu, K.Y.; Chen, Y.S.; Chan, Y.F.; Yu, H.M.; et al. Rosmarinic acid exhibits broad anti-enterovirus A71 activity by inhibiting the interaction between the five-fold axis of capsid VP1 and cognate sulfated receptors. Emerg. Microbes Infect. 2020, 9, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Yu, Y.J.; Jinn, T.R. Evaluation of the virucidal effects of rosmarinic acid against enterovirus 71 infection via in vitro and in vivo study. Virol. J. 2019, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Hu, Y.; Zhang, J.; Musharrafieh, R.; Wang, J. A Novel Capsid Binding Inhibitor Displays Potent Antiviral Activity against Enterovirus D68. ACS Infect. Dis. 2019, 5, 1952–1962. [Google Scholar] [CrossRef]
- Lacroix, C.; Laconi, S.; Angius, F.; Coluccia, A.; Silvestri, R.; Pompei, R.; Neyts, J.; Leyssen, P. In vitro characterisation of a pleconaril/pirodavir-like compound with potent activity against rhinoviruses. Virol. J. 2015, 12, 106. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, P.; Wang, N.; Zhang, J.; Li, J.; Guo, H.; Yin, X.; Rao, Z.; Wang, X.; Zhang, L. The binding of a monoclonal antibody to the apical region of SCARB2 blocks EV71 infection. Protein Cell 2017, 8, 590–600. [Google Scholar] [CrossRef]
- Ren, P.; Zheng, Y.; Wang, W.; Hong, L.; Delpeyroux, F.; Arenzana-Seisdedos, F.; Altmeyer, R. Suramin interacts with the positively charged region surrounding the 5-fold axis of the EV-A71 capsid and inhibits multiple enterovirus A. Sci. Rep. 2017, 7, 42902. [Google Scholar] [CrossRef]
- Reshamwala, D.; Shroff, S.; Sheik Amamuddy, O.; Laquintana, V.; Denora, N.; Zacheo, A.; Lampinen, V.; Hytonen, V.P.; Tastan Bishop, Ö.; Krol, S.; et al. Polyphenols Epigallocatechin Gallate and Resveratrol, and Polyphenol-Functionalized Nanoparticles Prevent Enterovirus Infection through Clustering and Stabilization of the Viruses. Pharmaceutics 2021, 13, 1182. [Google Scholar] [CrossRef]
- Huang, Y.L.; Lin, T.M.; Wang, S.Y.; Wang, J.R. The role of conserved arginine and proline residues in enterovirus VP1 protein. J. Microbiol. Immunol. Infect. 2022, 55, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Huang, S.W.; Shen, C.Y.; Cheng, D.; Wang, J.R. Conserved Residues Adjacent to ß-Barrel and Loop Intersection among Enterovirus VP1 Affect Viral Replication: Potential Target for Anti-Enteroviral Development. Viruses 2022, 14, 364. [Google Scholar] [CrossRef]
- Plevka, P.; Perera, R.; Yap, M.L.; Cardosa, J.; Kuhn, R.J.; Rossmann, M.G. Structure of human enterovirus 71 in complex with a capsid-binding inhibitor. Proc. Natl. Acad. Sci. USA 2013, 110, 5463–5467. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, F.; Gu, B.; Ding, C.; Feng, D.; Xie, F.; Wang, J.; Zhang, C.; Cao, Q.; Deng, Y.; et al. In vitro and in vivo evaluation of ribavirin and pleconaril antiviral activity against enterovirus 71 infection. Arch. Virol. 2012, 157, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Smee, D.F.; Evans, W.J.; Nicolaou, K.C.; Tarbet, E.B.; Day, C.W. Susceptibilities of enterovirus D68, enterovirus 71, and rhinovirus 87 strains to various antiviral compounds. Antivir. Res. 2016, 131, 61–65. [Google Scholar] [CrossRef]
- Tijsma, A.; Franco, D.; Tucker, S.; Hilgenfeld, R.; Froeyen, M.; Leyssen, P.; Neyts, J. The capsid binder Vapendavir and the novel protease inhibitor SG85 inhibit enterovirus 71 replication. Antimicrob. Agents Chemother. 2014, 58, 6990–6992. [Google Scholar] [CrossRef]
- Kim, J.; Jung, Y.K.; Kim, C.; Shin, J.S.; Scheers, E.; Lee, J.Y.; Han, S.B.; Lee, C.K.; Neyts, J.; Ha, J.D.; et al. A Novel Series of Highly Potent Small Molecule Inhibitors of Rhinovirus Replication. J. Med. Chem. 2017, 60, 5472–5492. [Google Scholar] [CrossRef]
- Ho, J.Y.; Chern, J.H.; Hsieh, C.F.; Liu, S.T.; Liu, C.J.; Wang, Y.S.; Kuo, T.W.; Hsu, S.J.; Yeh, T.K.; Shih, S.R.; et al. In vitro and in vivo studies of a potent capsid-binding inhibitor of enterovirus 71. J. Antimicrob. Chemother. 2016, 71, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- De Colibus, L.; Wang, X.; Spyrou, J.A.B.; Kelly, J.; Ren, J.; Grimes, J.; Puerstinger, G.; Stonehouse, N.; Walter, T.S.; Hu, Z.; et al. More-powerful virus inhibitors from structure-based analysis of HEV71 capsid-binding molecules. Nat. Struct. Mol. Biol. 2014, 21, 282–288. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; He, W.; Sun, Y.; Guo, Y.; Zhong, W.; Gao, Q.; Liao, M.; Wang, X.; Cai, Y.; et al. Design, Synthesis, and Evaluation of Novel Enterovirus 71 Inhibitors as Therapeutic Drug Leads for the Treatment of Human Hand, Foot, and Mouth Disease. J. Med. Chem. 2020, 63, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Chern, J.H.; Lee, C.C.; Chang, C.S.; Lee, Y.C.; Tai, C.L.; Lin, Y.T.; Shia, K.S.; Lee, C.Y.; Shih, S.R. Synthesis and antienteroviral activity of a series of novel, oxime ether-containing pyridyl imidazolidinones. Bioorg Med. Chem. Lett. 2004, 14, 5051–5056. [Google Scholar] [CrossRef]
- Li, P.; Yu, J.; Hao, F.; He, H.; Shi, X.; Hu, J.; Wang, L.; Du, C.; Zhang, X.; Sun, Y.; et al. Discovery of Potent EV71 Capsid Inhibitors for Treatment of HFMD. ACS Med. Chem. Lett. 2017, 8, 841–846. [Google Scholar] [CrossRef]
- Han, X.; Sun, N.; Wu, H.; Guo, D.; Tien, P.; Dong, C.; Wu, S.; Zhou, H.B. Identification and Structure-Activity Relationships of Diarylhydrazides as Novel Potent and Selective Human Enterovirus Inhibitors. J. Med. Chem. 2016, 59, 2139–2150. [Google Scholar] [CrossRef]
- Arita, M.; Fuchino, H.; Kawakami, H.; Ezaki, M.; Kawahara, N. Characterization of a New Antienterovirus D68 Compound Purified from Avocado. ACS Infect. Dis. 2020, 6, 2291–2300. [Google Scholar] [CrossRef]
- Li, G.; Gao, Q.; Yuan, S.; Wang, L.; Altmeyer, R.; Lan, K.; Yin, F.; Zou, G. Characterization of three small molecule inhibitors of enterovirus 71 identified from screening of a library of natural products. Antivir. Res. 2017, 143, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Lacroix, C.; Cabiddu, M.G.; Neyts, J.; Leyssen, P.; Pompei, R. Exploration of the anti-enterovirus activity of a series of pleconaril/pirodavir-like compounds. Antivir. Chem. Chemother. 2015, 24, 56–61. [Google Scholar] [CrossRef]
- Egorova, A.; Kazakova, E.; Jahn, B.; Ekins, S.; Makarov, V.; Schmidtke, M. Novel pleconaril derivatives: Influence of substituents in the isoxazole and phenyl rings on the antiviral activity against enteroviruses. Eur. J. Med. Chem. 2020, 188, 112007. [Google Scholar] [CrossRef]
- Makarov, V.A.; Braun, H.; Richter, M.; Riabova, O.B.; Kirchmair, J.; Kazakova, E.S.; Seidel, N.; Wutzler, P.; Schmidtke, M. Pyrazolopyrimidines: Potent Inhibitors Targeting the Capsid of Rhino- and Enteroviruses. ChemMedChem 2015, 10, 1629–1634. [Google Scholar] [CrossRef]
- Carta, A.; Sanna, G.; Briguglio, I.; Madeddu, S.; Vitale, G.; Piras, S.; Corona, P.; Peana, A.T.; Laurini, E.; Fermeglia, M.; et al. Quinoxaline derivatives as new inhibitors of coxsackievirus B5. Eur. J. Med. Chem. 2018, 145, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, S.; Ibba, R.; Sanna, G.; Piras, S.; Riu, F.; Marongiu, A.; Ambrosino, A.; Caria, P.; Onnis, V.; Franci, G.; et al. Human Enterovirus B: Selective Inhibition by Quinoxaline Derivatives and Bioinformatic RNA-Motif Identification as New Targets. Pharmaceuticals 2022, 15, 181. [Google Scholar] [CrossRef] [PubMed]
- Kalam, N.; Balasubramaniam, V.R.M.T. Emerging Therapeutics in the Fight Against EV-D68: A Review of Current Strategies. Influenza Other Respir. Viruses 2024, 18, e70064. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sheng, J.; Fokine, A.; Meng, G.; Shin, W.H.; Long, F.; Kuhn, R.J.; Kihara, D.; Rossmann, M.G. Structure and inhibition of EV-D68, a virus that causes respiratory illness in children. Science 2015, 347, 71–74. [Google Scholar] [CrossRef]
- Senior, K. FDA panel rejects common cold treatment. Lancet Infect. Dis. 2002, 2, 264. [Google Scholar] [CrossRef]
- Rhoden, E.; Zhang, M.; Nix, W.A.; Oberste, M.S. In Vitro Efficacy of Antiviral Compounds against Enterovirus D68. Antimicrob. Agents Chemother. 2015, 59, 7779–7781. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Fan, S.; Cao, R.; He, X.; Li, W.; Xu, L.; Cheng, T.; Li, H.; Zhong, W. Discovery and Optimization of Quinoline Analogues as Novel Potent Antivirals against Enterovirus D68. J. Med. Chem. 2022, 65, 14792–14808. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Abdelnabi, R.; Delang, L.; Froeyen, M.; Luyten, W.; Neyts, J.; Mirabelli, C. New class of early-stage enterovirus inhibitors with a novel mechanism of action. Antivir. Res. 2017, 147, 67–74. [Google Scholar] [CrossRef]
- Shetnev, A.A.; Volobueva, A.S.; Panova, V.A.; Zarubaev, V.V.; Baykov, S.V. Design of 4-Substituted Sulfonamidobenzoic Acid Derivatives Targeting Coxsackievirus B3. Life 2022, 12, 1832. [Google Scholar] [CrossRef]
- Sun, L.; Lee, H.; Thibaut, H.J.; Lanko, K.; Rivero-Buceta, E.; Bator, C.; Martinez-Gualda, B.; Dallmeier, K.; Delang, L.; Leyssen, P.; et al. Viral engagement with host receptors blocked by a novel class of tryptophan dendrimers that targets the 5-fold-axis of the enterovirus-A71 capsid. PLoS Pathog. 2019, 15, e1007760. [Google Scholar] [CrossRef]
- Martínez-Gualda, B.; Sun, L.; Martí-Marí, O.; Noppen, S.; Abdelnabi, R.; Bator, C.M.; Quesada, E.; Delang, L.; Mirabelli, C.; Lee, H.; et al. Scaffold Simplification Strategy Leads to a Novel Generation of Dual Human Immunodeficiency Virus and Enterovirus-A71 Entry Inhibitors. J. Med. Chem. 2020, 63, 349–368. [Google Scholar] [CrossRef]
- Ren, P.; Zou, G.; Bailly, B.; Xu, S.; Zeng, M.; Chen, X.; Shen, L.; Zhang, Y.; Guillon, P.; Arenzana-Seisdedos, F.; et al. The approved pediatric drug suramin identified as a clinical candidate for the treatment of EV71 infection-suramin inhibits EV71 infection in vitro and in vivo. Emerg. Microbes Infect. 2014, 3, e62. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; McLaughlin, N.P.; Pan, J.; Goldstein, S.; Hafenstein, S.; Shimizu, H.; Winkler, J.D.; Bergelson, J.M. The Suramin Derivative NF449 Interacts with the 5-fold Vertex of the Enterovirus A71 Capsid to Prevent Virus Attachment to PSGL-1 and Heparan Sulfate. PLoS Pathog. 2015, 11, e1005184. [Google Scholar] [CrossRef]
- Meng, T.; Jia, Q.; Wong, S.M.; Chua, K.B. In Vitro and In Vivo Inhibition of the Infectivity of Human Enterovirus 71 by a Sulfonated Food Azo Dye, Brilliant Black BN. J. Virol. 2019, 93, 10-1128. [Google Scholar] [CrossRef]
- Wang, H.; Lei, X.; Xiao, X.; Yang, C.; Lu, W.; Huang, Z.; Leng, Q.; Jin, Q.; He, B.; Meng, G.; et al. Reciprocal Regulation between Enterovirus 71 and the NLRP3 Inflammasome. Cell Rep. 2015, 12, 42–48. [Google Scholar] [CrossRef]
- Wang, B.; Xi, X.; Lei, X.; Zhang, X.; Cui, S.; Wang, J.; Jin, Q.; Zhao, Z. Enterovirus 71 protease 2Apro targets MAVS to inhibit anti-viral type I interferon responses. PLoS Pathog. 2013, 9, e1003231. [Google Scholar] [CrossRef]
- Falah, N.; Montserret, R.; Lelogeais, V.; Schuffenecker, I.; Lina, B.; Cortay, J.C.; Violot, S. Blocking human enterovirus 71 replication by targeting viral 2A protease. J. Antimicrob. Chemother. 2012, 67, 2865–2869. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Huang, A.C.; Hour, M.J.; Huang, S.H.; Kung, S.H.; Chen, C.H.; Chen, I.C.; Chang, Y.S.; Lien, J.C.; Lin, C.W. Antiviral Potential of a Novel Compound CW-33 against Enterovirus A71 via Inhibition of Viral 2A Protease. Viruses 2015, 7, 3155–3171. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Y.; Cao, L.; Wang, P.; Qing, J.; Zheng, Q.; Shang, L.; Yin, Z.; Sun, Y. A Conserved Inhibitory Mechanism of a Lycorine Derivative against Enterovirus and Hepatitis C Virus. Antimicrob. Agents Chemother. 2016, 60, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Hou, X.; Peng, H.; Zhang, L.; Jiang, Q.; Shi, M.; Ji, Y.; Wang, Y.; Shi, W.; et al. Chlorogenic acid inhibits the replication and viability of enterovirus 71 in vitro. PLoS ONE 2013, 8, e76007. [Google Scholar] [CrossRef]
- Chen, S.G.; Cheng, M.L.; Chen, K.H.; Horng, J.T.; Liu, C.C.; Wang, S.M.; Sakurai, H.; Leu, Y.L.; Wang, S.D.; Ho, H.Y. Antiviral activities of Schizonepeta tenuifolia Briq. against enterovirus 71 in vitro and in vivo. Sci. Rep. 2017, 7, 935. [Google Scholar] [CrossRef]
- Chen, S.G.; Leu, Y.L.; Cheng, M.L.; Ting, S.C.; Liu, C.C.; Wang, S.D.; Yang, C.H.; Hung, C.Y.; Sakurai, H.; Chen, K.H.; et al. Anti-enterovirus 71 activities of Melissa officinalis extract and its biologically active constituent rosmarinic acid. Sci. Rep. 2017, 7, 12264. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wang, Y.; Pei, X.; Wang, C.; Niu, Y.; Xu, P.; Peng, Y. Substituted 3-benzylcoumarins 13 and 14 suppress enterovirus A71 replication by impairing viral 2A. Antivir. Res. 2018, 160, 10–16. [Google Scholar] [CrossRef]
- Musharrafieh, R.; Ma, C.; Zhang, J.; Hu, Y.; Diesing, J.M.; Marty, M.T.; Wang, J. Validating Enterovirus D68-2A(pro) as an Antiviral Drug Target and the Discovery of Telaprevir as a Potent D68-2A(pro) Inhibitor. J. Virol. 2019, 93, 10-1128. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, W.; Niu, Y.; Wang, S.; Chen, B.; Xiong, R.; Zhang, P.; Luo, Z.; Wu, Y.; Fan, C.; et al. Phosphorylation of enteroviral 2A(pro) at Ser/Thr125 benefits its proteolytic activity and viral pathogenesis. J. Med. Virol. 2023, 95, e28400. [Google Scholar] [CrossRef]
- Cai, Q.; Yameen, M.; Liu, W.; Gao, Z.; Li, Y.; Peng, X.; Cai, Y.; Wu, C.; Zheng, Q.; Li, J.; et al. Conformational plasticity of the 2A proteinase from enterovirus 71. J. Virol. 2013, 87, 7348–7356. [Google Scholar] [CrossRef]
- Xie, S.; Wang, K.; Yu, W.; Lu, W.; Xu, K.; Wang, J.; Ye, B.; Schwarz, W.; Jin, Q.; Sun, B. DIDS blocks a chloride-dependent current that is mediated by the 2B protein of enterovirus 71. Cell Res. 2011, 21, 1271–1275. [Google Scholar] [CrossRef]
- Nieva, J.L.; Madan, V.; Carrasco, L. Viroporins: Structure and biological functions. Nat. Rev. Microbiol. 2012, 10, 563–574. [Google Scholar] [CrossRef]
- Cong, H.; Du, N.; Yang, Y.; Song, L.; Zhang, W.; Tien, P. Enterovirus 71 2B Induces Cell Apoptosis by Directly Inducing the Conformational Activation of the Proapoptotic Protein Bax. J. Virol. 2016, 90, 9862–9877. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Wang, K.; Zhao, K.; Hua, S.C.; Du, J. The Structure, Function, and Mechanisms of Action of Enterovirus Non-structural Protein 2C. Front. Microbiol. 2020, 11, 615965. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Tian, J.; Qin, B.; Wojdyla, J.A.; Wang, B.; Zhao, Z.; Wang, M.; Cui, S. Crystal structure of 2C helicase from enterovirus 71. Sci. Adv. 2017, 3, e1602573. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, C.; Yang, R.; Bai, P.; Zhang, X.Y.; Kong, J.; Yin, L.; Qiu, Y.; Zhou, X. Antiviral Peptides Targeting the Helicase Activity of Enterovirus Nonstructural Protein 2C. J. Virol. 2021, 95, e02324-20. [Google Scholar] [CrossRef] [PubMed]
- Messacar, K.; Sillau, S.; Hopkins, S.E.; Otten, C.; Wilson-Murphy, M.; Wong, B.; Santoro, J.D.; Treister, A.; Bains, H.K.; Torres, A.; et al. Safety, tolerability, and efficacy of fluoxetine as an antiviral for acute flaccid myelitis. Neurology 2019, 92, e2118–e2126. [Google Scholar] [CrossRef]
- Tyler, K.L. Rationale for the evaluation of fluoxetine in the treatment of enterovirus D68-associated acute flaccid myelitis. JAMA Neurol. 2015, 72, 493–494. [Google Scholar] [CrossRef]
- Manganaro, R.; Zonsics, B.; Bauer, L.; Lorenzo Lopez, M.; Donselaar, T.; Zwaagstra, M.; Saporito, F.; Ferla, S.; Strating, J.R.P.M.; Coutard, B.; et al. Synthesis and antiviral effect of novel fluoxetine analogues as enterovirus 2C inhibitors. Antivir. Res. 2020, 178, 104781. [Google Scholar] [CrossRef]
- Bauer, L.; Manganaro, R.; Zonsics, B.; Hurdiss, D.L.; Zwaagstra, M.; Donselaar, T.; Welter, N.G.E.; van Kleef, R.G.D.M.; Lopez, M.L.; Bevilacqua, F.; et al. Rational design of highly potent broad-spectrum enterovirus inhibitors targeting the nonstructural protein 2C. PLoS Biol. 2020, 18, e3000904. [Google Scholar] [CrossRef]
- Ulferts, R.; de Boer, S.M.; van der Linden, L.; Bauer, L.; Lyoo, H.R.; Maté, M.J.; Lichière, J.; Canard, B.; Lelieveld, D.; Omta, W.; et al. Screening of a Library of FDA-Approved Drugs Identifies Several Enterovirus Replication Inhibitors That Target Viral Protein 2C. Antimicrob. Agents Chemother. 2016, 60, 2627–2638. [Google Scholar] [CrossRef] [PubMed]
- Musharrafieh, R.; Zhang, J.; Tuohy, P.; Kitamura, N.; Bellampalli, S.S.; Hu, Y.; Khanna, R.; Wang, J. Discovery of Quinoline Analogues as Potent Antivirals against Enterovirus D68 (EV-D68). J. Med. Chem. 2019, 62, 4074–4090. [Google Scholar] [CrossRef] [PubMed]
- Musharrafieh, R.; Kitamura, N.; Hu, Y.; Wang, J. Development of broad-spectrum enterovirus antivirals based on quinoline scaffold. Bioorg Chem. 2020, 101, 103981. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, Z.; Jin, M.; Shu, T.; Chen, Y.; Feng, L.; Zhang, Q.; Lan, K.; Wu, S.; Zhou, H.B. Identification of dibucaine derivatives as novel potent enterovirus 2C helicase inhibitors: In vitro, in vivo, and combination therapy study. Eur. J. Med. Chem. 2020, 202, 112310. [Google Scholar] [CrossRef]
- Ma, C.; Hu, Y.; Zhang, J.; Wang, J. Pharmacological Characterization of the Mechanism of Action of R523062, a Promising Antiviral for Enterovirus D68. ACS Infect. Dis. 2020, 6, 2260–2270. [Google Scholar] [CrossRef]
- Zuo, J.; Kye, S.; Quinn, K.K.; Cooper, P.; Damoiseaux, R.; Krogstad, P. Discovery of Structurally Diverse Small-Molecule Compounds with Broad Antiviral Activity against Enteroviruses. Antimicrob. Agents Chemother. 2015, 60, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zuo, J.; Krogstad, P.; Jung, M.E. Synthesis and Structure-Activity Relationship (SAR) Studies of Novel Pyrazolopyridine Derivatives as Inhibitors of Enterovirus Replication. J. Med. Chem. 2018, 61, 1688–1703. [Google Scholar] [CrossRef]
- Hu, Y.; Kitamura, N.; Musharrafieh, R.; Wang, J. Discovery of Potent and Broad-Spectrum Pyrazolopyridine-Containing Antivirals against Enteroviruses D68, A71, and Coxsackievirus B3 by Targeting the Viral 2C Protein. J. Med. Chem. 2021, 64, 8755–8774. [Google Scholar] [CrossRef]
- De Palma, A.M.; Heggermont, W.; Lanke, K.; Coutard, B.; Bergmann, M.; Monforte, A.M.; Canard, B.; De Clercq, E.; Chimirri, A.; Pürstinger, G.; et al. The thiazolobenzimidazole TBZE-029 inhibits enterovirus replication by targeting a short region immediately downstream from motif C in the nonstructural protein 2C. J. Virol. 2008, 82, 4720–4730. [Google Scholar] [CrossRef]
- Guan, H.; Tian, J.; Zhang, C.; Qin, B.; Cui, S. Crystal structure of a soluble fragment of poliovirus 2CATPase. PLoS Pathog. 2018, 14, e1007304. [Google Scholar] [CrossRef] [PubMed]
- Hurdiss, D.L.; El Kazzi, P.; Bauer, L.; Papageorgiou, N.; Ferron, F.P.; Donselaar, T.; van Vliet, A.L.W.; Canard, B.; Decroly, E.; Brancale, A.; et al. Fluoxetine targets an allosteric site in the enterovirus 2C AAA+ ATPase and stabilizes the hexameric complex. Sci. Adv. 2022, 8, eabj7615. [Google Scholar] [CrossRef]
- Li, Y.; Jian, X.; Yin, P.; Zhu, G.; Zhang, L. Elucidating the Host Interactome of EV-A71 2C Reveals Viral Dependency Factors. Front. Microbiol. 2019, 10, 636. [Google Scholar] [CrossRef] [PubMed]
- Horova, V.; Lyoo, H.; Różycki, B.; Chalupska, D.; Smola, M.; Humpolickova, J.; Strating, J.R.P.M.; van Kuppeveld, F.J.M.; Boura, E.; Klima, M.; et al. Convergent evolution in the mechanisms of ACBD3 recruitment to picornavirus replication sites. PLoS Pathog. 2019, 15, e1007962. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Cheng, A.; Wen, X.; Ou, X.; Mao, S.; Gao, Q.; Sun, D.; Jia, R.; Yang, Q.; et al. Enterovirus Replication Organelles and Inhibitors of Their Formation. Front. Microbiol. 2020, 11, 1817. [Google Scholar] [CrossRef]
- Nagy, P.D.; Strating, J.R.; van Kuppeveld, F.J. Building Viral Replication Organelles: Close Encounters of the Membrane Types. PLoS Pathog. 2016, 12, e1005912. [Google Scholar] [CrossRef] [PubMed]
- Lyoo, H.; van der Schaar, H.M.; Dorobantu, C.M.; Rabouw, H.H.; Strating, J.R.P.M.; van Kuppeveld, F.J.M. ACBD3 Is an Essential Pan-enterovirus Host Factor That Mediates the Interaction between Viral 3A Protein and Cellular Protein PI4KB. mBio 2019, 10, e02742-18. [Google Scholar] [CrossRef]
- Miller, F.D.; Monto, A.S.; DeLong, D.C.; Exelby, A.; Bryan, E.R.; Srivastava, S. Controlled trial of enviroxime against natural rhinovirus infections in a community. Antimicrob. Agents Chemother. 1985, 27, 102–106. [Google Scholar] [CrossRef]
- Heinz, B.A.; Vance, L.M. The antiviral compound enviroxime targets the 3A coding region of rhinovirus and poliovirus. J. Virol. 1995, 69, 4189–4197. [Google Scholar] [CrossRef]
- Arita, M.; Wakita, T.; Shimizu, H. Characterization of pharmacologically active compounds that inhibit poliovirus and enterovirus 71 infectivity. J. Gen. Virol. 2008, 89, 2518–2530. [Google Scholar] [CrossRef]
- Arita, M.; Wakita, T.; Shimizu, H. Cellular kinase inhibitors that suppress enterovirus replication have a conserved target in viral protein 3A similar to that of enviroxime. J. Gen. Virol. 2009, 90 Pt 8, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Takebe, Y.; Wakita, T.; Shimizu, H. A bifunctional anti-enterovirus compound that inhibits replication and the early stage of enterovirus 71 infection. J. Gen. Virol. 2010, 91, 2734–2744. [Google Scholar] [CrossRef] [PubMed]
- De Palma, A.M.; Thibaut, H.J.; van der Linden, L.; Lanke, K.; Heggermont, W.; Ireland, S.; Andrews, R.; Arimilli, M.; Al-Tel, T.H.; De Clercq, E.; et al. Mutations in the nonstructural protein 3A confer resistance to the novel enterovirus replication inhibitor TTP-8307. Antimicrob. Agents Chemother. 2009, 53, 1850–1857. [Google Scholar] [CrossRef]
- Albulescu, L.; Bigay, J.; Biswas, B.; Weber-Boyvat, M.; Dorobantu, C.M.; Delang, L.; van der Schaar, H.M.; Jung, Y.S.; Neyts, J.; Olkkonen, V.M.; et al. Uncovering oxysterol-binding protein (OSBP) as a target of the anti-enteroviral compound TTP-8307. Antivir. Res. 2017, 140, 37–44. [Google Scholar] [CrossRef]
- Gao, Q.; Yuan, S.; Zhang, C.; Wang, Y.; He, G.; Zhang, S.; Altmeyer, R.; Zou, G. Discovery of itraconazole with broad-spectrum in vitro antienterovirus activity that targets nonstructural protein 3A. Antimicrob. Agents Chemother. 2015, 59, 2654–2665. [Google Scholar] [CrossRef]
- Rhoden, E.; Ng, T.F.F.; Campagnoli, R.; Nix, W.A.; Konopka-Anstadt, J.; Selvarangan, R.; Briesach, L.; Oberste, M.S.; Weldon, W.C. Antifungal Triazole Posaconazole Targets an Early Stage of the Parechovirus A3 Life Cycle. Antimicrob. Agents Chemother. 2020, 64, e02372-19. [Google Scholar] [CrossRef]
- Lu, G.; Qi, J.; Chen, Z.; Xu, X.; Gao, F.; Lin, D.; Qian, W.; Liu, H.; Jiang, H.; Yan, J.; et al. Enterovirus 71 and coxsackievirus A16 3C proteases: Binding to rupintrivir and their substrates and anti-hand, foot, and mouth disease virus drug design. J. Virol. 2011, 85, 10319–10331. [Google Scholar] [CrossRef]
- de Breyne, S.; Bonderoff, J.M.; Chumakov, K.M.; Lloyd, R.E.; Hellen, C.U. Cleavage of eukaryotic initiation factor eIF5B by enterovirus 3C proteases. Virology 2008, 378, 118–122. [Google Scholar] [CrossRef]
- Wen, W.; Qi, Z.; Wang, J. The Function and Mechanism of Enterovirus 71 (EV71) 3C Protease. Curr. Microbiol. 2020, 77, 1968–1975. [Google Scholar] [CrossRef]
- Li, B.; Yue, Y.; Zhang, Y.; Yuan, Z.; Li, P.; Song, N.; Lin, W.; Liu, Y.; Gu, L.; Meng, H. A Novel Enterovirus 71 (EV71) Virulence Determinant: The 69th Residue of 3C Protease Modulates Pathogenicity. Front. Cell. Infect. Microbiol. 2017, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shang, C.; Yang, P.; Li, L.; Zhai, Y.; Yin, Z.; Wang, B.; Shang, L. 4-Iminooxazolidin-2-one as a Bioisostere of the Cyanohydrin Moiety: Inhibitors of Enterovirus 71 3C Protease. J. Med. Chem. 2018, 61, 10333–10339. [Google Scholar] [CrossRef]
- Ma, Y.; Li, L.; He, S.; Shang, C.; Sun, Y.; Liu, N.; Meek, T.D.; Wang, Y.; Shang, L. Application of Dually Activated Michael Acceptor to the Rational Design of Reversible Covalent Inhibitor for Enterovirus 71 3C Protease. J. Med. Chem. 2019, 62, 6146–6162. [Google Scholar] [CrossRef]
- Ma, G.H.; Ye, Y.; Zhang, D.; Xu, X.; Si, P.; Peng, J.L.; Xiao, Y.L.; Cao, R.Y.; Yin, Y.L.; Chen, J.; et al. Identification and biochemical characterization of DC07090 as a novel potent small molecule inhibitor against human enterovirus 71 3C protease by structure-based virtual screening. Eur. J. Med. Chem. 2016, 124, 981–991. [Google Scholar] [CrossRef]
- Hayden, F.G.; Turner, R.B.; Gwaltney, J.M.; Chi-Burris, K.; Gersten, M.; Hsyu, P.; Patick, A.K.; Smith, G.J.; Zalman, L.S. Phase II, randomized, double-blind, placebo-controlled studies of ruprintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers. Antimicrob. Agents Chemother. 2003, 47, 3907–3916. [Google Scholar] [CrossRef] [PubMed]
- Patick, A.K.; Brothers, M.A.; Maldonado, F.; Binford, S.; Maldonado, O.; Fuhrman, S.; Petersen, A.; Smith, G.J.; Zalman, L.S.; Burns-Naas, L.A.; et al. In vitro antiviral activity and single-dose pharmacokinetics in humans of a novel, orally bioavailable inhibitor of human rhinovirus 3C protease. Antimicrob. Agents Chemother. 2005, 49, 2267–2275. [Google Scholar] [CrossRef]
- Kankam, M.K.; Burns, J.M.; Collett, M.S.; Corrado, M.L.; Hincks, J.R. A Phase 1 Study of the Safety, Tolerability, and Pharmacokinetics of Single and Multiple Oral Doses of V-7404 in Healthy Adult Volunteers. Antimicrob. Agents Chemother. 2021, 65, e0102921. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; Zusinaite, E.; Tenson, T.; Oksenych, V.; Wang, W.; Afset, J.E.; Bjørås, M.; Kainov, D.E. Novel Synergistic Anti-Enteroviral Drug Combinations. Viruses 2022, 14, 1866. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lovell, S.; Tiew, K.C.; Mandadapu, S.R.; Alliston, K.R.; Battaile, K.P.; Groutas, W.C.; Chang, K.O. Broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, noroviruses, and coronaviruses. J. Virol. 2012, 86, 11754–11762. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, C.; Szeto, T.; Hurst, B.; Tarbet, B.; Wang, J. Boceprevir, Calpain Inhibitors II and XII, and GC-376 Have Broad-Spectrum Antiviral Activity against Coronaviruses. ACS Infect. Dis. 2021, 7, 586–597. [Google Scholar] [CrossRef]
- Wang, J.; Fan, T.; Yao, X.; Wu, Z.; Guo, L.; Lei, X.; Wang, M.; Jin, Q.; Cui, S. Crystal structures of enterovirus 71 3C protease complexed with rupintrivir reveal the roles of catalytically important residues. J. Virol. 2011, 85, 10021–10030. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.W.; Ang, M.J.; Lau, Q.Y.; Poulsen, A.; Ng, F.M.; Then, S.W.; Peng, J.; Hill, J.; Hong, W.J.; Chia, C.S.; et al. Antiviral activities of peptide-based covalent inhibitors of the Enterovirus 71 3C protease. Sci. Rep. 2016, 6, 33663. [Google Scholar] [CrossRef]
- Schulz, R.; Atef, A.; Becker, D.; Gottschalk, F.; Tauber, C.; Wagner, S.; Arkona, C.; Abdel-Hafez, A.A.; Farag, H.H.; Rademann, J.; et al. Phenylthiomethyl Ketone-Based Fragments Show Selective and Irreversible Inhibition of Enteroviral 3C Proteases. J. Med. Chem. 2018, 61, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, B.; Zhai, Y.; Yin, Z.; Sun, Y.; Rao, Z. Peptidyl aldehyde NK-1.8k suppresses enterovirus 71 and enterovirus 68 infection by targeting protease 3C. Antimicrob. Agents Chemother. 2015, 59, 2636–2646. [Google Scholar] [CrossRef]
- Boras, B.; Jones, R.M.; Anson, B.J.; Arenson, D.; Aschenbrenner, L.; Bakowski, M.A.; Beutler, N.; Binder, J.; Chen, E.; Eng, H.; et al. Discovery of a Novel Inhibitor of Coronavirus 3CL Protease for the Potential Treatment of COVID-19. bioRxiv 2021. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, L.; Zhai, Y.; Yin, Z.; Sun, Y.; Shang, L. Structure of the Enterovirus 71 3C Protease in Complex with NK-1.8k and Indications for the Development of Antienterovirus Protease Inhibitor. Antimicrob. Agents Chemother. 2017, 61, e00298-17. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, D.; Kusov, Y.; Nian, Y.; Ma, Q.; Wang, J.; von Brunn, A.; Leyssen, P.; Lanko, K.; Neyts, J.; et al. α-Ketoamides as Broad-Spectrum Inhibitors of Coronavirus and Enterovirus Replication: Structure-Based Design, Synthesis, and Activity Assessment. J. Med. Chem. 2020, 63, 4562–4578. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Jochmans, D.; Xie, H.; Yang, H.; Li, J.; Su, H.; Chang, D.; Wang, J.; Peng, J.; Zhu, L.; et al. Design, Synthesis, and Biological Evaluation of Peptidomimetic Aldehydes as Broad-Spectrum Inhibitors against Enterovirus and SARS-CoV-2. J. Med. Chem. 2022, 65, 2794–2808. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, S.; Xiao, T.; Li, Y.; Su, Z.; Wei, W.; Hao, F.; Hu, G.; Lin, F.; Chen, X.; et al. Design, synthesis, and evaluation of a novel macrocyclic anti-EV71 agent. Bioorganic Med. Chem. 2020, 28, 115551. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhao, X.; Cui, Z.; Wang, M.; Wang, Y.; Li, L.; Sun, Q.; Yang, X.; Zeng, D.; Liu, Y.; et al. Cyanohydrin as an Anchoring Group for Potent and Selective Inhibitors of Enterovirus 71 3C Protease. J. Med. Chem. 2015, 58, 9414–9420. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, M.; Ma, S.; Ma, Y.; Liu, S.; Shang, L.; Zhu, C.; Ye, S.; Wang, Y. 4-Iminooxazolidin-2-One as a Bioisostere of Cyanohydrin Suppresses EV71 Proliferation by Targeting 3C. Microbiol. Spectr. 2021, 9, e0102521. [Google Scholar] [CrossRef]
- Cao, Z.; Ding, Y.; Ke, Z.; Cao, L.; Li, N.; Ding, G.; Wang, Z.; Xiao, W. Luteoloside Acts as 3C Protease Inhibitor of Enterovirus 71 In Vitro. PLoS ONE 2016, 11, e0148693. [Google Scholar] [CrossRef]
- Kim, B.K.; Ko, H.; Jeon, E.S.; Ju, E.S.; Jeong, L.S.; Kim, Y.C. 2,3,4-Trihydroxybenzyl-hydrazide analogues as novel potent coxsackievirus B3 3C protease inhibitors. Eur. J. Med. Chem. 2016, 120, 202–216. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Q.; Wang, Y.; Pang, Z.; Liu, J.; Yin, Z.; Lou, Z. Activity-Based Protein Profiling Identifies ATG4B as a Key Host Factor for Enterovirus 71 Proliferation. J. Virol. 2019, 93, e01092-19. [Google Scholar] [CrossRef]
- Steuten, K.; Kim, H.; Widen, J.C.; Babin, B.M.; Onguka, O.; Lovell, S.; Bolgi, O.; Cerikan, B.; Neufeldt, C.J.; Cortese, M.; et al. Challenges for Targeting SARS-CoV-2 Proteases as a Therapeutic Strategy for COVID-19. ACS Infect. Dis. 2021, 7, 1457–1468. [Google Scholar] [CrossRef]
- Kitamura, N.; Sacco, M.D.; Ma, C.; Hu, Y.; Townsend, J.A.; Meng, X.; Zhang, F.; Zhang, X.; Ba, M.; Szeto, T.; et al. Expedited Approach toward the Rational Design of Noncovalent SARS-CoV-2 Main Protease Inhibitors. J. Med. Chem. 2022, 65, 2848–2865. [Google Scholar] [CrossRef]
- Sun, J.; Yogarajah, T.; Lee, R.C.H.; Kaur, P.; Inoue, M.; Tan, Y.W.; Chu, J.J.H. Drug repurposing of pyrimidine analogs as potent antiviral compounds against human enterovirus A71 infection with potential clinical applications. Sci. Rep. 2020, 10, 8159. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, C.; Kim, D.E.; Song, J.H.; Choi, M.; Choi, K.; Kang, M.; Lee, K.; Kim, H.S.; Shin, J.S.; et al. Synergistic antiviral activity of gemcitabine and ribavirin against enteroviruses. Antivir. Res. 2015, 124, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, D.E.; Jang, K.S.; Kim, S.J.; Cho, S.; Kim, C. Gemcitabine, a broad-spectrum antiviral drug, suppresses enterovirus infections through innate immunity induced by the inhibition of pyrimidine biosynthesis and nucleotide depletion. Oncotarget 2017, 8, 115315–115325. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Yang, J.; Zheng, B.; Zhang, Y.; Cao, Y.; Huan, C.; Wang, S.; Chang, J.; Zhang, W. The Pyrimidine Analog FNC Potently Inhibits the Replication of Multiple Enteroviruses. J. Virol. 2020, 94, e00204-20. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Yuan, S.; Gao, Q.; Lan, K.; Altmeyer, R.; Zou, G. In Vitro Assessment of Combinations of Enterovirus Inhibitors against Enterovirus 71. Antimicrob. Agents Chemother. 2016, 60, 5357–5367. [Google Scholar] [CrossRef]
- Shang, L.; Wang, Y.; Qing, J.; Shu, B.; Cao, L.; Lou, Z.; Gong, P.; Sun, Y.; Yin, Z. An adenosine nucleoside analogue NITD008 inhibits EV71 proliferation. Antivir. Res. 2014, 112, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, Y.L.; Schul, W.; Wang, Q.Y.; Gu, F.; Duraiswamy, J.; Kondreddi, R.R.; Niyomrattanakit, P.; Lakshminarayana, S.B.; Goh, A.; et al. An adenosine nucleoside inhibitor of dengue virus. Proc. Natl. Acad. Sci. USA 2009, 106, 20435–20439. [Google Scholar] [CrossRef]
- Deng, C.L.; Yeo, H.; Ye, H.Q.; Liu, S.Q.; Shang, B.D.; Gong, P.; Alonso, S.; Shi, P.Y.; Zhang, B. Inhibition of enterovirus 71 by adenosine analog NITD008. J. Virol. 2014, 88, 11915–11923. [Google Scholar] [CrossRef]
- Yin, W.; Mao, C.; Luan, X.; Shen, D.D.; Shen, Q.; Su, H.; Wang, X.; Zhou, F.; Zhao, W.; Gao, M.; et al. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science 2020, 368, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Tosh, D.K.; Toti, K.S.; Hurst, B.L.; Julander, J.G.; Jacobson, K.A. Structure activity relationship of novel antiviral nucleosides against Enterovirus A71. Bioorg Med. Chem. Lett. 2020, 30, 127599. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Chang, H.Y.; Lin, P.F.; Chern, J.H.; Hsu, J.T.; Chang, C.Y.; Shih, S.R. Novel antiviral agent DTriP-22 targets RNA-dependent RNA polymerase of enterovirus 71. Antimicrob. Agents Chemother. 2009, 53, 2740–2747. [Google Scholar] [CrossRef]
- Hung, H.C.; Chen, T.C.; Fang, M.Y.; Yen, K.J.; Shih, S.R.; Hsu, J.T.; Tseng, C.P. Inhibition of enterovirus 71 replication and the viral 3D polymerase by aurintricarboxylic acid. J. Antimicrob. Chemother. 2010, 65, 676–683. [Google Scholar] [CrossRef]
- van der Linden, L.; Vives-Adrián, L.; Selisko, B.; Ferrer-Orta, C.; Liu, X.; Lanke, K.; Ulferts, R.; De Palma, A.M.; Tanchis, F.; Goris, N.; et al. The RNA template channel of the RNA-dependent RNA polymerase as a target for development of antiviral therapy of multiple genera within a virus family. PLoS Pathog. 2015, 11, e1004733. [Google Scholar] [CrossRef]
- Velu, A.B.; Chen, G.W.; Hsieh, P.T.; Horng, J.T.; Hsu, J.T.; Hsieh, H.P.; Chen, T.C.; Weng, K.F.; Shih, S.R. BPR-3P0128 inhibits RNA-dependent RNA polymerase elongation and VPg uridylylation activities of Enterovirus 71. Antivir. Res. 2014, 112, 18–25. [Google Scholar] [CrossRef]
- Wu, K.X.; Chu, J.J. Antiviral screen identifies EV71 inhibitors and reveals camptothecin-target, DNA topoisomerase 1 as a novel EV71 host factor. Antivir. Res. 2017, 143, 122–133. [Google Scholar] [CrossRef]
- Li, W.; Ross-Smith, N.; Proud, C.G.; Belsham, G.J. Cleavage of translation initiation factor 4AI (eIF4AI) but not eIF4AII by foot-and-mouth disease virus 3C protease: Identification of the eIF4AI cleavage site. FEBS Lett. 2001, 507, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.R.; Stollar, V.; Li, M.L. Host factors in enterovirus 71 replication. J. Virol. 2011, 85, 9658–9666. [Google Scholar] [CrossRef] [PubMed]
- Arita, M. Phosphatidylinositol-4 kinase III beta and oxysterol-binding protein accumulate unesterified cholesterol on poliovirus-induced membrane structure. Microbiol. Immunol. 2014, 58, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Roulin, P.S.; Lötzerich, M.; Torta, F.; Tanner, L.B.; van Kuppeveld, F.J.; Wenk, M.R.; Greber, U.F. Rhinovirus uses a phosphatidylinositol 4-phosphate/cholesterol counter-current for the formation of replication compartments at the ER-Golgi interface. Cell Host Microbe 2014, 16, 677–690. [Google Scholar] [CrossRef]
- Albulescu, L.; Strating, J.R.; Thibaut, H.J.; van der Linden, L.; Shair, M.D.; Neyts, J.; van Kuppeveld, F.J. Broad-range inhibition of enterovirus replication by OSW-1, a natural compound targeting OSBP. Antivir. Res. 2015, 117, 110–114. [Google Scholar] [CrossRef]
- Burgett, A.W.; Poulsen, T.B.; Wangkanont, K.; Anderson, D.R.; Kikuchi, C.; Shimada, K.; Okubo, S.; Fortner, K.C.; Mimaki, Y.; Kuroda, M.; et al. Natural products reveal cancer cell dependence on oxysterol-binding proteins. Nat. Chem. Biol. 2011, 7, 639–647. [Google Scholar] [CrossRef]
- Roberts, B.L.; Severance, Z.C.; Bensen, R.C.; Le, A.T.; Kothapalli, N.R.; Nuñez, J.I.; Ma, H.; Wu, S.; Standke, S.J.; Yang, Z.; et al. Transient Compound Treatment Induces a Multigenerational Reduction of Oxysterol-Binding Protein (OSBP) Levels and Prophylactic Antiviral Activity. ACS Chem. Biol. 2019, 14, 276–287. [Google Scholar] [CrossRef]
- Strating, J.R.; van der Linden, L.; Albulescu, L.; Bigay, J.; Arita, M.; Delang, L.; Leyssen, P.; van der Schaar, H.M.; Lanke, K.H.; Thibaut, H.J.; et al. Itraconazole inhibits enterovirus replication by targeting the oxysterol-binding protein. Cell Rep. 2015, 10, 600–615. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.L.; Severance, Z.C.; Bensen, R.C.; Le-McClain, A.T.; Malinky, C.A.; Mettenbrink, E.M.; Nuñez, J.I.; Reddig, W.J.; Blewett, E.L.; Burgett, A.W.G. Differing activities of oxysterol-binding protein (OSBP) targeting anti-viral compounds. Antivir. Res. 2019, 170, 104548. [Google Scholar] [CrossRef]
- Arita, M.; Dobrikov, G.; Pürstinger, G.; Galabov, A.S. Allosteric Regulation of Phosphatidylinositol 4-Kinase III Beta by an Antipicornavirus Compound MDL-860. ACS Infect. Dis. 2017, 3, 585–594. [Google Scholar] [CrossRef]
- Mejdrová, I.; Chalupská, D.; Plačková, P.; Müller, C.; Šála, M.; Klíma, M.; Baumlová, A.; Hřebabecký, H.; Procházková, E.; Dejmek, M.; et al. Rational Design of Novel Highly Potent and Selective Phosphatidylinositol 4-Kinase IIIβ (PI4KB) Inhibitors as Broad-Spectrum Antiviral Agents and Tools for Chemical Biology. J. Med. Chem. 2017, 60, 100–118. [Google Scholar] [CrossRef]
- Nikolova, I.; Slavchev, I.; Ravutsov, M.; Dangalov, M.; Nikolova, Y.; Zagranyarska, I.; Stoyanova, A.; Nikolova, N.; Mukova, L.; Grozdanov, P.; et al. Anti-enteroviral activity of new MDL-860 analogues: Synthesis, in vitro/in vivo studies and QSAR analysis. Bioorganic Chem. 2019, 85, 487–497. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, L.; Gao, H.; Wu, Y.; Wang, Y.; Fang, F.; Lan, T.; Lou, Z.; Rao, Y. Discovery, Optimization, and Target Identification of Novel Potent Broad-Spectrum Antiviral Inhibitors. J. Med. Chem. 2019, 62, 4056–4073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Das, P.; Schmolke, M.; Manicassamy, B.; Wang, Y.; Deng, X.; Cai, L.; Tu, B.P.; Forst, C.V.; Roth, M.G.; et al. Inhibition of pyrimidine synthesis reverses viral virulence factor-mediated block of mRNA nuclear export. J. Cell Biol. 2012, 196, 315–326. [Google Scholar] [CrossRef]
- Hoffmann, H.H.; Kunz, A.; Simon, V.A.; Palese, P.; Shaw, M.L. Broad-spectrum antiviral that interferes with de novo pyrimidine biosynthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 5777–5782. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.N.; Lai, K.K.; Dai, J.; Kok, K.H.; Chen, H.; Chan, K.H.; Yuen, K.Y.; Kao, R.Y.T. Broad-spectrum inhibition of common respiratory RNA viruses by a pyrimidine synthesis inhibitor with involvement of the host antiviral response. J. Gen. Virol. 2017, 98, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Liou, A.T.; Liao, C.C.; Chou, S.F.; Chang, Y.S.; Chang, C.S.; Shih, C. Hypoxia and therapeutic treatment of EV-A71 with an immune modulator TLR7 agonist in a new immunocompetent mouse model. J. Biomed. Sci. 2019, 26, 93. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, B.; Chen, X.; Song, N.; Wu, J.; Li, G.; Yu, P.; Han, Y.; Liu, J.; Qin, C. GS-9620 inhibits enterovirus 71 replication mainly through the NF-κB and PI3K-AKT signaling pathways. Antivir. Res. 2018, 153, 39–48. [Google Scholar] [CrossRef]
- Hung, H.C.; Wang, H.C.; Shih, S.R.; Teng, I.F.; Tseng, C.P.; Hsu, J.T. Synergistic inhibition of enterovirus 71 replication by interferon and rupintrivir. J. Infect. Dis. 2011, 203, 1784–1790. [Google Scholar] [CrossRef]
- Mohamed, A.; Bakir, T.; Al-Hawel, H.; Al-Sharif, I.; Bakheet, R.; Kouser, L.; Murugaiah, V.; Al-Mozaini, M. HIV-2 Vpx neutralizes host restriction factor SAMHD1 to promote viral pathogenesis. Sci. Rep. 2021, 11, 20984. [Google Scholar] [CrossRef]
- Li, Z.; Huan, C.; Wang, H.; Liu, Y.; Liu, X.; Su, X.; Yu, J.; Zhao, Z.; Yu, X.F.; Zheng, B.; et al. TRIM21-mediated proteasomal degradation of SAMHD1 regulates its antiviral activity. EMBO Rep. 2020, 21, e47528. [Google Scholar] [CrossRef]
- Li, Z.; Yang, X.; Zhao, Z.; Liu, X.; Zhang, W. Host Restriction Factor A3G Inhibits the Replication of Enterovirus D68 by Competitively Binding the 5’ Untranslated Region with PCBP1. J. Virol. 2022, 96, e0170821. [Google Scholar] [CrossRef]
- Li, Y.L.; Langley, C.A.; Azumaya, C.M.; Echeverria, I.; Chesarino, N.M.; Emerman, M.; Cheng, Y.; Gross, J.D. The structural basis for HIV-1 Vif antagonism of human APOBEC3G. Nature 2023, 615, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Poh, C.L.; Sam, I.C.; Chan, Y.F. Enterovirus 71 uses cell surface heparan sulfate glycosaminoglycan as an attachment receptor. J. Virol. 2013, 87, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Earley, D.F.; Bailly, B.; Maggioni, A.; Kundur, A.R.; Thomson, R.J.; Chang, C.W.; von Itzstein, M. Efficient Blocking of Enterovirus 71 Infection by Heparan Sulfate Analogues Acting as Decoy Receptors. ACS Infect. Dis. 2019, 5, 1708–1717. [Google Scholar] [CrossRef]
- Pourianfar, H.R.; Poh, C.L.; Fecondo, J.; Grollo, L. In vitro evaluation of the antiviral activity of heparan sulfate mimetic compounds against Enterovirus 71. Virus Res. 2012, 169, 22–29. [Google Scholar] [CrossRef]
- Weng, T.Y.; Chen, L.C.; Shyu, H.W.; Chen, S.H.; Wang, J.R.; Yu, C.K.; Lei, H.Y.; Yeh, T.M. Lactoferrin inhibits enterovirus 71 infection by binding to VP1 protein and host cells. Antivir. Res. 2005, 67, 31–37. [Google Scholar] [CrossRef]
- Yuan, S.; Chu, H.; Huang, J.; Zhao, X.; Ye, Z.W.; Lai, P.M.; Wen, L.; Cai, J.P.; Mo, Y.; Cao, J.; et al. Viruses harness YxxØ motif to interact with host AP2M1 for replication: A vulnerable broad-spectrum antiviral target. Sci. Adv. 2020, 6, eaba7910. [Google Scholar] [CrossRef]
- Hao, T.; Li, Y.; Fan, S.; Li, W.; Wang, S.; Li, S.; Cao, R.; Zhong, W. Design, synthesis and pharmacological evaluation of a novel mTOR-targeted anti-EV71 agent. Eur. J. Med. Chem. 2019, 175, 172–186. [Google Scholar] [CrossRef]
- Wang, H.; Guo, T.; Yang, Y.; Yu, L.; Pan, X.; Li, Y. Lycorine Derivative LY-55 Inhibits EV71 and CVA16 Replication Through Downregulating Autophagy. Front. Cell. Infect. Microbiol. 2019, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, S.; Liu, S.; Chen, Y.; Liu, H.; Su, Y.; Liu, R.; Cui, Y.; Song, Y.; Teng, Y.; et al. Transcriptomic Profiling Reveals a Role for TREM-1 Activation in Enterovirus D68 Infection-Induced Proinflammatory Responses. Front. Immunol. 2021, 12, 749618. [Google Scholar] [CrossRef]
- Qing, J.; Wang, Y.; Sun, Y.; Huang, J.; Yan, W.; Wang, J.; Su, D.; Ni, C.; Li, J.; Rao, Z.; et al. Cyclophilin A associates with enterovirus-71 virus capsid and plays an essential role in viral infection as an uncoating regulator. PLoS Pathog. 2014, 10, e1004422. [Google Scholar] [CrossRef]
- Yan, W.; Qing, J.; Mei, H.; Nong, J.; Huang, J.; Zhu, J.; Jiang, H.; Liu, L.; Zhang, L.; Li, J. Identification, synthesis and pharmacological evaluation of novel anti-EV71 agents via cyclophilin A inhibition. Bioorg Med. Chem. Lett. 2015, 25, 5682–5686. [Google Scholar] [CrossRef]
- Hixon, A.M.; Clarke, P.; Tyler, K.L. Evaluating Treatment Efficacy in a Mouse Model of Enterovirus D68-Associated Paralytic Myelitis. J. Infect. Dis. 2017, 216, 1245–1253. [Google Scholar] [CrossRef]
- Vogt, M.R.; Fu, J.; Kose, N.; Williamson, L.E.; Bombardi, R.; Setliff, I.; Georgiev, I.S.; Klose, T.; Rossmann, M.G.; Bochkov, Y.A.; et al. Human antibodies neutralize enterovirus D68 and protect against infection and paralytic disease. Sci. Immunol. 2020, 5, eaba4902. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, J.; Li, B.; Guo, L.; Li, H.; Song, J.; Yang, Z.; Fan, H.; Huang, X.; Long, H.; et al. A Novel Neutralizing Antibody Specific to the DE Loop of VP1 Can Inhibit EV-D68 Infection in Mice. J. Immunol. 2018, 201, 2557–2569. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhu, R.; Xu, L.; He, M.; Yan, X.; Liu, D.; Yin, Z.; Wu, Y.; Li, Y.; Yang, L.; et al. Atomic structures of enterovirus D68 in complex with two monoclonal antibodies define distinct mechanisms of viral neutralization. Nat. Microbiol. 2019, 4, 124–133. [Google Scholar] [CrossRef]
- Yang, J.; Yang, C.; Guo, N.; Zhu, K.; Luo, K.; Zhang, N.; Zhao, H.; Cui, Y.; Chen, L.; Wang, H.; et al. Type I Interferons Triggered through the Toll-Like Receptor 3-TRIF Pathway Control Coxsackievirus A16 Infection in Young Mice. J. Virol. 2015, 89, 10860–10867. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, J.; Lu, N.; Zhang, C. Enterovirus 71 Antagonizes Antiviral Effects of Type III Interferon and Evades the Clearance of Intestinal Intraepithelial Lymphocytes. Front. Microbiol. 2021, 12, 806084. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Liu, L.; Lei, X.; Zhou, Z.; He, B.; Wang, J. 3C Protease of Enterovirus D68 Inhibits Cellular Defense Mediated by Interferon Regulatory Factor 7. J. Virol. 2016, 90, 1613–1621. [Google Scholar] [CrossRef]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y. Current advances in antiviral RNA interference in mammals. FEBS J. 2024, 291, 208–216. [Google Scholar] [CrossRef]
- Takahashi, T.; Ui-Tei, K. Mutual Regulation of RNA Silencing and the IFN Response as an Antiviral Defense System in Mammalian Cells. Int. J. Mol. Sci. 2020, 21, 1348. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Dai, Y.; Li, Z.; Wang, J.; Ye, Z.; Ren, Y.; Wang, H.; Li, W.X.; Lu, J.; et al. Efficient Dicer processing of virus-derived double-stranded RNAs and its modulation by RIG-I-like receptor LGP2. PLoS Pathog. 2021, 17, e1009790. [Google Scholar] [CrossRef]
- Han, Q.; Chen, G.; Wang, J.; Jee, D.; Li, W.X.; Lai, E.C.; Ding, S.W. Mechanism and Function of Antiviral RNA Interference in Mice. mBio 2020, 11, e03278-19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Ye, Z.; Xu, Y.; Wang, B.; Wang, C.; Dai, Y.; Lu, J.; Lu, B.; Zhang, W.; et al. The activation of antiviral RNA interference not only exists in neural progenitor cells but also in somatic cells in mammals. Emerg. Microbes Infect. 2020, 9, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Poirier, E.Z.; Buck, M.D.; Chakravarty, P.; Carvalho, J.; Frederico, B.; Cardoso, A.; Healy, L.; Ulferts, R.; Beale, R.; Reis e Sousa, C. An isoform of Dicer protects mammalian stem cells against multiple RNA viruses. Science 2021, 373, 231–236. [Google Scholar] [CrossRef]
- Li, W.X.; Ding, S.W. Mammalian viral suppressors of RNA interference. Trends Biochem. Sci. 2022, 47, 978–988. [Google Scholar] [CrossRef]
- Werk, D.; Schubert, S.; Lindig, V.; Grunert, H.P.; Zeichhardt, H.; Erdmann, V.A.; Kurreck, J. Developing an effective RNA interference strategy against a plus-strand RNA virus: Silencing of coxsackievirus B3 and its cognate coxsackievirus-adenovirus receptor. Biol. Chem. 2005, 386, 857–863. [Google Scholar] [CrossRef]
- Merl, S.; Michaelis, C.; Jaschke, B.; Vorpahl, M.; Seidl, S.; Wessely, R. Targeting 2A protease by RNA interference attenuates coxsackieviral cytopathogenicity and promotes survival in highly susceptible mice. Circulation 2005, 111, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Cheung, P.K.; Zhang, H.M.; Chau, D.; Yang, D. Inhibition of coxsackievirus B3 replication by small interfering RNAs requires perfect sequence match in the central region of the viral positive strand. J. Virol. 2005, 79, 2151–2159. [Google Scholar] [CrossRef]
- Kim, J.Y.; Chung, S.K.; Hwang, H.Y.; Kim, H.; Kim, J.H.; Nam, J.H.; Park, S.I. Expression of short hairpin RNAs against the coxsackievirus B3 exerts potential antiviral effects in Cos-7 cells and in mice. Virus Res. 2007, 125, 9–13. [Google Scholar] [CrossRef]
- Fechner, H.; Pinkert, S.; Wang, X.; Sipo, I.; Suckau, L.; Kurreck, J.; Dörner, A.; Sollerbrant, K.; Zeichhardt, H.; Grunert, H.P.; et al. Coxsackievirus B3 and adenovirus infections of cardiac cells are efficiently inhibited by vector-mediated RNA interference targeting their common receptor. Gene Ther. 2007, 14, 960–971. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, Y.; He, F.; Wang, C.; Xiao, Z.; Zou, J.; Wang, F.; Liu, Z. Short hairpin RNA targeting 2B gene of coxsackievirus B3 exhibits potential antiviral effects both in vitro and in vivo. BMC Infect. Dis. 2012, 12, 177. [Google Scholar] [CrossRef]
- Dutkiewicz, M.; Grunert, H.P.; Zeichhardt, H.; Lena, S.W.; Wengel, J.; Kurreck, J. Design of LNA-modified siRNAs against the highly structured 5’ UTR of coxsackievirus B3. FEBS Lett. 2008, 582, 3061–3066. [Google Scholar] [CrossRef]
- Rothe, D.; Werk, D.; Dutkiewicz, M.; Schubert, S.; Grunert, H.P.; Zeichhardt, H.; Erdmann, V.A.; Fechner, H.; Kurreck, J. Inhibition of picornaviruses by means of RNA interference. Nucleic Acids Symp. Ser. 2008, 52, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Fechner, H.; Sipo, I.; Westermann, D.; Pinkert, S.; Wang, X.; Suckau, L.; Kurreck, J.; Zeichhardt, H.; Müller, O.; Vetter, R.; et al. Cardiac-targeted RNA interference mediated by an AAV9 vector improves cardiac function in coxsackievirus B3 cardiomyopathy. J. Mol. Med. 2008, 86, 987–997. [Google Scholar] [CrossRef]
- Stein, E.A.; Pinkert, S.; Becher, P.M.; Geisler, A.; Zeichhardt, H.; Klopfleisch, R.; Poller, W.; Tschöpe, C.; Lassner, D.; Fechner, H.; et al. Combination of RNA interference and virus receptor trap exerts additive antiviral activity in coxsackievirus B3-induced myocarditis in mice. J. Infect. Dis. 2015, 211, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Dai, H.L.; Yang, D.; Zhu, L.; Gao, T.L.; Shao, H.J.; Peng, X.; Jin, Z.F. Small interfering RNA against the 2C genomic region of coxsackievirus B3 exerts potential antiviral effects in permissive HeLa cells. Virus Res. 2012, 163, 183–189. [Google Scholar] [CrossRef]
- Nygårdas, M.; Vuorinen, T.; Aalto, A.P.; Bamford, D.H.; Hukkanen, V. Inhibition of coxsackievirus B3 and related enteroviruses by antiviral short interfering RNA pools produced using phi6 RNA-dependent RNA polymerase. J. Gen. Virol. 2009, 90, 2468–2473. [Google Scholar] [CrossRef]
- Werk, D.; Pinkert, S.; Heim, A.; Zeichhardt, H.; Grunert, H.P.; Poller, W.; Erdmann, V.A.; Fechner, H.; Kurreck, J. Combination of soluble coxsackievirus-adenovirus receptor and anti-coxsackievirus siRNAs exerts synergistic antiviral activity against coxsackievirus B3. Antivir. Res. 2009, 83, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.L.; Wong, A.P.; Poh, C.L. Development of potential antiviral strategy against coxsackievirus B4. Virus Res. 2010, 150, 85–92. [Google Scholar] [CrossRef]
- Rothe, D.; Werk, D.; Niedrig, S.; Horbelt, D.; Grunert, H.P.; Zeichhardt, H.; Erdmann, V.A.; Kurreck, J. Antiviral activity of highly potent siRNAs against echovirus 30 and its receptor. J. Virol. Methods 2009, 157, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Rothe, D.; Wajant, G.; Grunert, H.P.; Zeichhardt, H.; Fechner, H.; Kurreck, J. Rapid construction of adeno-associated virus vectors expressing multiple short hairpin RNAs with high antiviral activity against echovirus 30. Oligonucleotides 2010, 20, 191–198. [Google Scholar] [CrossRef]
- Tan, E.L.; Tan, T.M.; Tak Kwong Chow, V.; Poh, C.L. Inhibition of enterovirus 71 in virus-infected mice by RNA interference. Mol. Ther. 2007, 15, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.L.; Tan, T.M.; Chow, V.T.; Poh, C.L. Enhanced potency and efficacy of 29-mer shRNAs in inhibition of Enterovirus 71. Antivir. Res. 2007, 74, 9–15. [Google Scholar] [CrossRef]
- Deng, J.X.; Nie, X.J.; Lei, Y.F.; Ma, C.F.; Xu, D.L.; Li, B.; Xu, Z.K.; Zhang, G.C. The highly conserved 5′ untranslated region as an effective target towards the inhibition of Enterovirus 71 replication by unmodified and appropriate 2′-modified siRNAs. J. Biomed. Sci. 2012, 19, 73. [Google Scholar] [CrossRef]
- Liu, H.; Qin, Y.; Kong, Z.; Shao, Q.; Su, Z.; Wang, S.; Chen, J. siRNA Targeting the 2Apro Genomic Region Prevents Enterovirus 71 Replication In Vitro. PLoS ONE 2016, 11, e0149470. [Google Scholar] [CrossRef]
- Li, Y.; Xie, J.; Xu, X.; Wang, J.; Ao, F.; Wan, Y.; Zhu, Y. MicroRNA-548 down-regulates host antiviral response via direct targeting of IFN-λ1. Protein Cell 2013, 4, 130–141. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Z.; Qiu, Y.; Kong, J.; Fu, Y.; Liu, Y.; Wang, C.; Quan, J.; Wang, Q.; Xu, W.; et al. Inhibition of viral suppressor of RNAi proteins by designer peptides protects from enteroviral infection in vivo. Immunity 2021, 54, 2231–2244.e2236. [Google Scholar] [CrossRef]
- Tan, E.L.; Marcus, K.F.; Poh, C.L. Development of RNA interference (RNAi) as potential antiviral strategy against enterovirus 70. J. Med. Virol. 2008, 80, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Jun, E.J.; Won, M.A.; Ahn, J.; Ko, A.; Moon, H.; Tchah, H.; Kim, Y.K.; Lee, H. An antiviral small-interfering RNA simultaneously effective against the most prevalent enteroviruses causing acute hemorrhagic conjunctivitis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Gao, Y.; Sun, L.; Tien, P.; Jin, Q. Quick identification of effective small interfering RNAs that inhibit the replication of coxsackievirus A16. Antivir. Res. 2008, 80, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Doudna, J.A. CRISPR technology: A decade of genome editing is only the beginning. Science 2023, 379, eadd8643. [Google Scholar] [CrossRef] [PubMed]
- Pacesa, M.; Pelea, O.; Jinek, M. Past, present, and future of CRISPR genome editing technologies. Cell 2024, 187, 1076–1100. [Google Scholar] [CrossRef]
- Kaminski, M.M.; Abudayyeh, O.O.; Gootenberg, J.S.; Zhang, F.; Collins, J.J. CRISPR-based diagnostics. Nat. Biomed. Eng. 2021, 5, 643–656. [Google Scholar] [CrossRef]
- Chavez, M.; Chen, X.; Finn, P.B.; Qi, L.S. Advances in CRISPR therapeutics. Nat. Rev. Nephrol. 2023, 19, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Aliyari, S.; Zhu, Z.; Zheng, H.; Cheng, G.; Zhang, S. CRISPR-Cas: A game-changer in vaccine development and the fight against viral infections. Trends Microbiol. 2025, 33, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.S.; O’Garra, A.; Sher, A.; Wack, A. Host-directed immunotherapy of viral and bacterial infections: Past, present and future. Nat. Rev. Immunol. 2023, 23, 121–133. [Google Scholar] [CrossRef]
- Diep, J.; Ooi, Y.S.; Wilkinson, A.W.; Peters, C.E.; Foy, E.; Johnson, J.R.; Zengel, J.; Ding, S.; Weng, K.F.; Laufman, O.; et al. Enterovirus pathogenesis requires the host methyltransferase SETD3. Nat. Microbiol. 2019, 4, 2523–2537. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, K.; Bae, S.; Park, J.; Lee, C.K.; Kim, M.; Kim, E.; Kim, S.; Kim, C.; Kim, J.S. CRISPR/Cas9-mediated gene knockout screens and target identification via whole-genome sequencing uncover host genes required for picornavirus infection. J. Biol. Chem. 2017, 292, 10664–10671. [Google Scholar] [CrossRef]
- Mei, H.; Zha, Z.; Wang, W.; Xie, Y.; Huang, Y.; Li, W.; Wei, D.; Zhang, X.; Qu, J.; Liu, J. Surfaceome CRISPR screen identifies OLFML3 as a rhinovirus-inducible IFN antagonist. Genome Biol. 2021, 22, 297. [Google Scholar]
- Greninger, A.L.; Knudsen, G.M.; Betegon, M.; Burlingame, A.L.; Derisi, J.L. The 3A protein from multiple picornaviruses utilizes the golgi adaptor protein ACBD3 to recruit PI4KIIIβ. J. Virol. 2012, 86, 3605–3616. [Google Scholar] [CrossRef]
- Téoulé, F.; Brisac, C.; Pelletier, I.; Vidalain, P.O.; Jégouic, S.; Mirabelli, C.; Bessaud, M.; Combelas, N.; Autret, A.; Tangy, F.; et al. The Golgi protein ACBD3, an interactor for poliovirus protein 3A, modulates poliovirus replication. J. Virol. 2013, 87, 11031–11046. [Google Scholar] [CrossRef]
- Dorobantu, C.M.; van der Schaar, H.M.; Ford, L.A.; Strating, J.R.; Ulferts, R.; Fang, Y.; Belov, G.; van Kuppeveld, F.J. Recruitment of PI4KIIIβ to coxsackievirus B3 replication organelles is independent of ACBD3, GBF1, and Arf1. J. Virol. 2014, 88, 2725–2736. [Google Scholar] [CrossRef] [PubMed]
- Dorobantu, C.M.; Ford-Siltz, L.A.; Sittig, S.P.; Lanke, K.H.; Belov, G.A.; van Kuppeveld, F.J.; van der Schaar, H.M. GBF1- and ACBD3-independent recruitment of PI4KIIIβ to replication sites by rhinovirus 3A proteins. J. Virol. 2015, 89, 1913–1918. [Google Scholar] [CrossRef]
- Najafi, S.; Tan, S.C.; Aghamiri, S.; Raee, P.; Ebrahimi, Z.; Jahromi, Z.K.; Rahmati, Y.; Sadri Nahand, J.; Piroozmand, A.; Jajarmi, V.; et al. Therapeutic potentials of CRISPR-Cas genome editing technology in human viral infections. Biomed. Pharmacother. 2022, 148, 112743. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, R.; Tinker-Kulberg, R.; Salehin, M.; Supakar, T.; Chamberlain, S.; Ligaba-Osena, A.; Josephs, E.A. Polyvalent guide RNAs for CRISPR antivirals. iScience 2022, 25, 105333. [Google Scholar] [CrossRef] [PubMed]
- Raguram, A.; Banskota, S.; Liu, D.R. Therapeutic in vivo delivery of gene editing agents. Cell 2022, 185, 2806–2827. [Google Scholar] [CrossRef]
- Saito, M.; Xu, P.; Faure, G.; Maguire, S.; Kannan, S.; Altae-Tran, H.; Vo, S.; Desimone, A.; Macrae, R.K.; Zhang, F. Fanzor is a eukaryotic programmable RNA-guided endonuclease. Nature 2023, 620, 660–668. [Google Scholar] [CrossRef]
- Raghavan, R.; Friedrich, M.J.; King, I.; Chau-Duy-Tam Vo, S.; Strebinger, D.; Lash, B.; Kilian, M.; Platten, M.; Macrae, R.K.; Song, Y.; et al. Rational engineering of minimally immunogenic nucleases for gene therapy. Nat. Commun. 2025, 16, 105. [Google Scholar] [CrossRef]
- Keng, C.T.; Yogarajah, T.; Lee, R.C.H.; Muhammad, I.B.H.; Chia, B.S.; Vasandani, S.R.; Lim, D.S.; Guo, K.; Wong, Y.H.; Mok, C.K.; et al. AAV-CRISPR-Cas13 eliminates human enterovirus and prevents death of infected mice. EBioMedicine 2023, 93, 104682. [Google Scholar] [CrossRef]
- Albericio, F.; Kruger, H.G. Therapeutic peptides. Future Med. Chem. 2012, 4, 1527–1531. [Google Scholar] [CrossRef]
- Vilas Boas, L.C.P.; Campos, M.L.; Berlanda, R.L.A.; de Carvalho Neves, N.; Franco, O.L. Antiviral peptides as promising therapeutic drugs. Cell. Mol. Life Sci. 2019, 76, 3525–3542. [Google Scholar] [CrossRef]
- Xia, S.; Yan, L.; Xu, W.; Agrawal, A.S.; Algaissi, A.; Tseng, C.K.; Wang, Q.; Du, L.; Tan, W.; Wilson, I.A.; et al. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci. Adv. 2019, 5, eaav4580. [Google Scholar] [CrossRef]
- Lalani, S.; Gew, L.T.; Poh, C.L. Antiviral peptides against Enterovirus A71 causing hand, foot and mouth disease. Peptides 2021, 136, 170443. [Google Scholar] [CrossRef] [PubMed]
- Clotet, B.; Raffi, F.; Cooper, D.; Delfraissy, J.F.; Lazzarin, A.; Moyle, G.; Rockstroh, J.; Soriano, V.; Schapiro, J. Clinical management of treatment-experienced, HIV-infected patients with the fusion inhibitor enfuvirtide: Consensus recommendations. AIDS 2004, 18, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E. Cationic antimicrobial peptides: Towards clinical applications. Expert. Opin. Investig. Drugs 2000, 9, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Battles, M.B.; Langedijk, J.P.; Furmanova-Hollenstein, P.; Chaiwatpongsakorn, S.; Costello, H.M.; Kwanten, L.; Vranckx, L.; Vink, P.; Jaensch, S.; Jonckers, T.H.; et al. Molecular mechanism of respiratory syncytial virus fusion inhibitors. Nat. Chem. Biol. 2016, 12, 87–93. [Google Scholar] [CrossRef]
- Roymans, D.; Alnajjar, S.S.; Battles, M.B.; Sitthicharoenchai, P.; Furmanova-Hollenstein, P.; Rigaux, P.; Berg, J.V.D.; Kwanten, L.; Ginderen, M.V.; Verheyen, N.; et al. Therapeutic efficacy of a respiratory syncytial virus fusion inhibitor. Nat. Commun. 2017, 8, 167. [Google Scholar] [CrossRef]
- Jordan, R.; Stray, K.; Anderson, F.; Perron, M.; Mackman, R.; Miller, M.; Mo, H.; Svarovskaia, E.; Martin, R.; Xin, Y.; et al. Analysis of GS-5806 Resistance Emergence in Human Healthy Adult Subjects Experimentally Infected With Respiratory Syncytial Virus (RSV). Open Forum Infect. Dis. 2015, 2 (Suppl. S1), 1189. [Google Scholar] [CrossRef]
- Gaillard, V.; Galloux, M.; Garcin, D.; Eléouët, J.F.; Le Goffic, R.; Larcher, T.; Rameix-Welti, M.A.; Boukadiri, A.; Héritier, J.; Segura, J.M.; et al. A Short Double-Stapled Peptide Inhibits Respiratory Syncytial Virus Entry and Spreading. Antimicrob. Agents Chemother. 2017, 61, e02241-16. [Google Scholar] [CrossRef]
- Sadeghipour, S.; Bek, E.J.; McMinn, P.C. Ribavirin-resistant mutants of human enterovirus 71 express a high replication fidelity phenotype during growth in cell culture. J. Virol. 2013, 87, 1759–1769. [Google Scholar] [CrossRef]
- Kelly, J.T.; De Colibus, L.; Elliott, L.; Fry, E.E.; Stuart, D.I.; Rowlands, D.J.; Stonehouse, N.J. Potent antiviral agents fail to elicit genetically-stable resistance mutations in either enterovirus 71 or Coxsackievirus A16. Antivir. Res. 2015, 124, 77–82. [Google Scholar] [CrossRef]
- Tan, C.W.; Chan, Y.F.; Sim, K.M.; Tan, E.L.; Poh, C.L. Inhibition of enterovirus 71 (EV-71) infections by a novel antiviral peptide derived from EV-71 capsid protein VP1. PLoS ONE 2012, 7, e34589. [Google Scholar] [CrossRef] [PubMed]
- Lalani, S.; Masomian, M.; Poh, C.L. Functional Insights into Silymarin as an Antiviral Agent against Enterovirus A71 (EV-A71). Int. J. Mol. Sci. 2021, 22, 8757. [Google Scholar] [CrossRef] [PubMed]
- Lalani, S.; Tan, S.H.; Tan, K.O.; Lim, H.X.; Ong, K.C.; Wong, K.T.; Poh, C.L. Molecular mechanism of L-SP40 peptide and in vivo efficacy against EV-A71 in neonatal mice. Life Sci. 2021, 287, 120097. [Google Scholar] [CrossRef]
- He, Q.Q.; Ren, S.; Xia, Z.C.; Cheng, Z.K.; Peng, N.F.; Zhu, Y. Fibronectin Facilitates Enterovirus 71 Infection by Mediating Viral Entry. J. Virol. 2018, 92, e02251-17. [Google Scholar] [CrossRef]
- Abd-Aziz, N.; Lee, M.F.; Ong, S.K.; Poh, C.L. Antiviral activity of SP81 peptide against Enterovirus A71 (EV-A71). Virology 2024, 589, 109941. [Google Scholar] [CrossRef]
- Evans, W.J.; Hurst, B.L.; Peterson, C.J.; Van Wettere, A.J.; Day, C.W.; Smee, D.F.; Tarbet, E.B. Development of a respiratory disease model for enterovirus D68 in 4-week-old mice for evaluation of antiviral therapies. Antivir. Res. 2019, 162, 61–70. [Google Scholar] [CrossRef]
- Patel, M.C.; Wang, W.; Pletneva, L.M.; Rajagopala, S.V.; Tan, Y.; Hartert, T.V.; Boukhvalova, M.S.; Vogel, S.N.; Das, S.R.; Blanco, J.C. Enterovirus D-68 Infection, Prophylaxis, and Vaccination in a Novel Permissive Animal Model, the Cotton Rat (Sigmodon hispidus). PLoS ONE 2016, 11, e0166336. [Google Scholar] [CrossRef] [PubMed]
- Vermillion, M.S.; Dearing, J.; Zhang, Y.; Adney, D.R.; Scheuermann, R.H.; Pekosz, A.; Tarbet, E.B. Animal Models of Enterovirus D68 Infection and Disease. J. Virol. 2022, 96, e0083322. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Liu, J.; Fu, L.; He, Y.; Cui, G.; Huang, B. Animal models for enterovirus 71: Mechanisms, immunity, and applications. Hum. Vaccin. Immunother. 2025, 21, 2523109. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Zhu, R.; Wu, Y.; Xia, N.; Xu, L.; Cheng, T. Research progress and application prospects of animal models of group B Coxsackievirus infections. Emerg. Microbes Infect. 2025, 14, 2441391. [Google Scholar] [CrossRef]
- Du, S.; Hu, X.; Menéndez-Arias, L.; Zhan, P.; Liu, X. Target-based drug design strategies to overcome resistance to antiviral agents: Opportunities and challenges. Drug Resist. Updates 2024, 73, 101053. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).