Licoflavone B Suppresses Influenza A Virus by Targeting the Viral RNA-Dependent RNA Polymerase (RdRp)
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds and Medicine
2.2. Cells and Viruses
2.3. Virus Inhibition Assay
2.4. Cell Viability Test
2.5. Detection of Licoflavone B’s Inhibition Activity at the Late Stage of Viral Replication
2.6. Validation of Licoflavone B Suppression of Viral RdRp Activity Via Transient Transfection Assay
2.7. Western Blotting
2.8. Immunofluorescence Assay
2.9. Quantitative Real-Time RT-PCR
2.10. Computerized Virtual Docking Licoflavone B with RdRp
3. Results
3.1. Inhibitory Effect and Cytotoxicity of Licoflavone B
3.2. Verification of the Inhibitory Activity of Licoflavone B at Late Stage of Virus Replication
3.3. Validation of the Viral Target
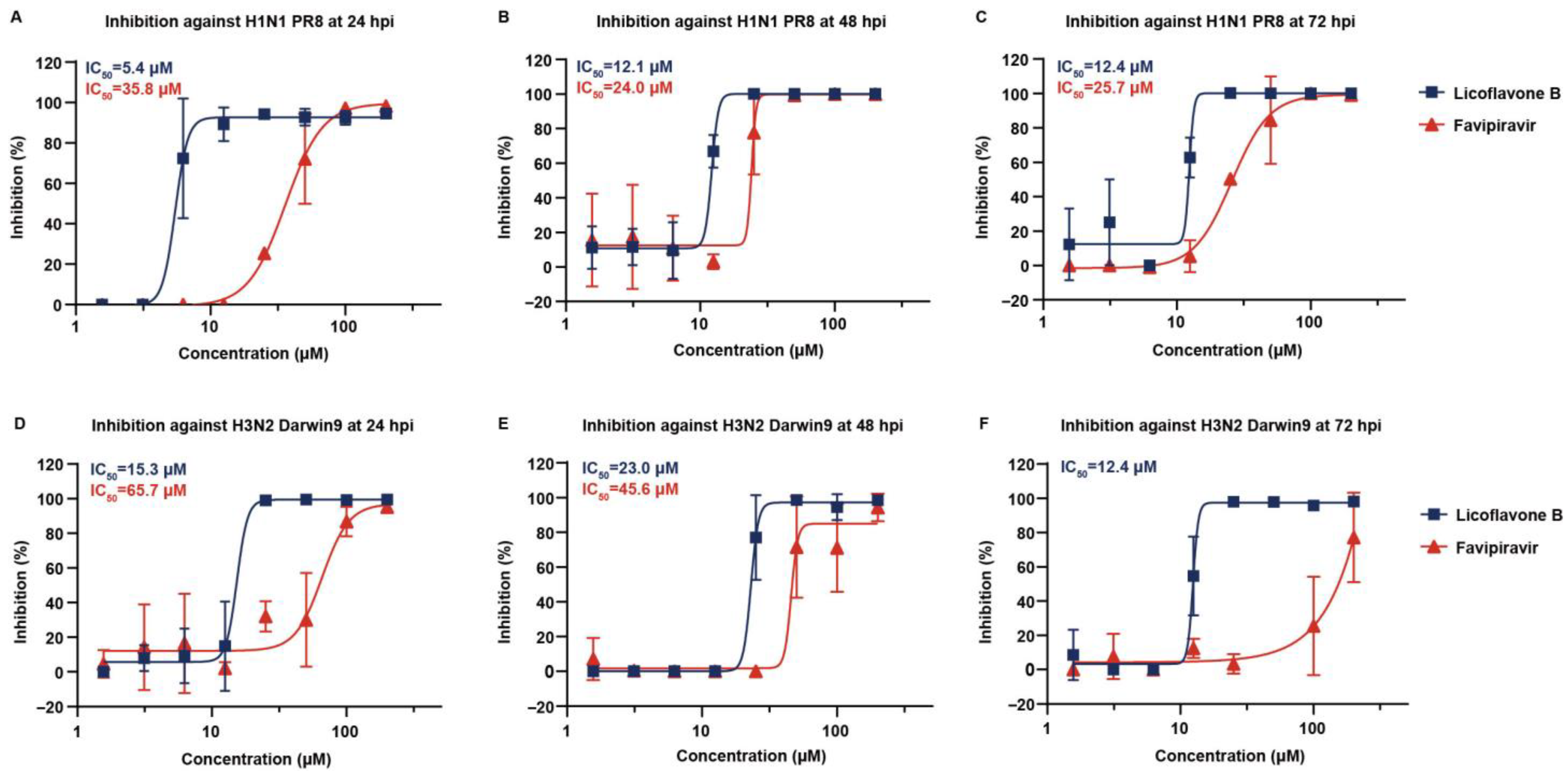
3.4. Broad-Spectrum Antiviral Potency
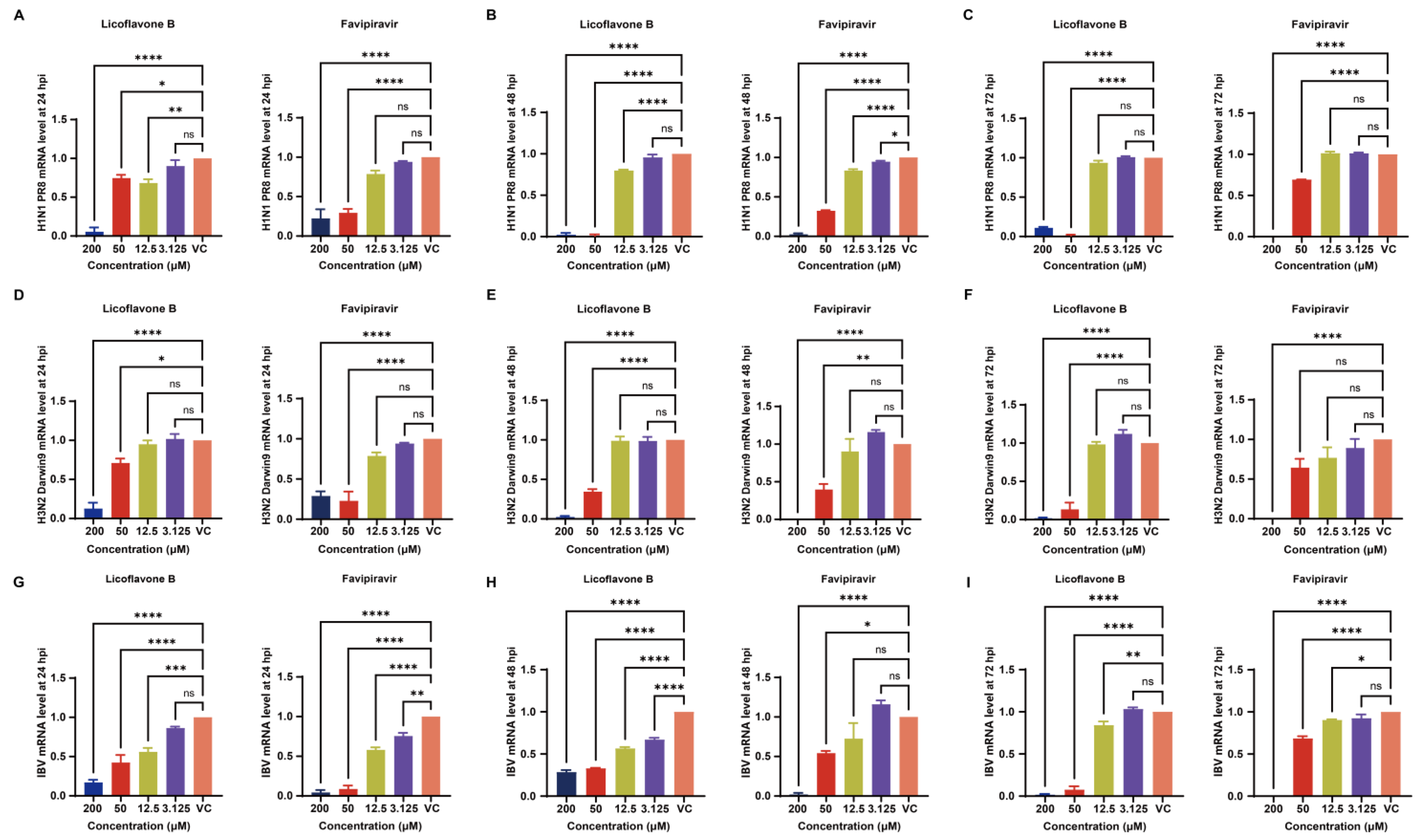
3.5. Viral Protein Expression Analysis Demonstrates Dose Dependent Inhibition by Licoflavone B
3.6. Molecular Docking
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El Zowalaty, M.E.; Bustin, S.A.; Husseiny, M.I.; Ashour, H.M. Avian influenza: Virology, diagnosis and surveillance. Future Microbiol. 2013, 8, 1209–1227. [Google Scholar] [CrossRef]
- Medina, R.A.; García-Sastre, A. Influenza A viruses: New research developments. Nat. Rev. Microbiol. 2011, 9, 590–603. [Google Scholar] [CrossRef]
- Hu, T.; Miles, A.C.; Pond, T.; Boikos, C.; Maleki, F.; Alfred, T.; Lopez, S.M.C.; McGrath, L. Economic burden and secondary complications of influenza-related hospitalization among adults in the US: A retrospective cohort study. J. Med. Econ. 2024, 27, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Kilbourne, E.D. Influenza pandemics of the 20th century. Emerg. Infect. Dis. 2006, 12, 9–14. [Google Scholar] [CrossRef]
- Cheung, T.K.W.; Poon, L.L.M. Biology of influenza A virus. Ann. N. Y. Acad. Sci. 2007, 1102, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, S.; Kawaoka, Y. The pathogenesis of influenza virus infections: The contributions of virus and host factors. Curr. Opin. Immunol. 2011, 23, 481–486. [Google Scholar] [CrossRef]
- Meier-Ewert, H.; Compans, R.W. Time course of synthesis and assembly of influenza virus proteins. J. Virol. 1974, 14, 1083–1091. [Google Scholar] [CrossRef]
- Area, E.; Martín-Benito, J.; Gastaminza, P.; Torreira, E.; Valpuesta, J.M.; Carrascosa, J.L.; Ortín, J. 3D structure of the influenza virus polymerase complex: Localization of subunit domains. Proc. Natl. Acad. Sci. USA 2004, 101, 308–313. [Google Scholar] [CrossRef]
- Fodor, E.; Te Velthuis, A.J.W. Structure and function of the influenza virus transcription and replication machinery. Cold Spring Harb. Perspect. Med. 2020, 10, a038398. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Antiviral agents active against influenza A viruses. Nat. Rev. Drug Discov. 2006, 5, 1015–1025. [Google Scholar] [CrossRef]
- Liu, X.; Liang, J.; Yu, Y.; Han, X.; Yu, L.; Chen, F.; Xu, Z.; Chen, Q.; Jin, M.; Dong, C.; et al. Discovery of aryl benzoyl hydrazide derivatives as novel potent broad-spectrum inhibitors of influenza A virus RNA-dependent RNA polymerase (RdRp). J. Med. Chem. 2022, 65, 3814–3832. [Google Scholar] [CrossRef]
- Abraham, G.M.; Morton, J.B.; Saravolatz, L.D. Baloxavir: A novel antiviral agent in the treatment of influenza. Clin. Infect. Dis. 2020, 71, 1790–1794. [Google Scholar] [CrossRef]
- Shiraki, K.; Daikoku, T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020, 209, 107512. [Google Scholar] [CrossRef]
- Gaitonde, D.Y.; Moore, F.C.; Morgan, M.K. Influenza: Diagnosis and treatment. Am. Fam. Physician 2019, 100, 751–758. [Google Scholar] [PubMed]
- Gubareva, L.V.; Mishin, V.P.; Patel, M.C.; Chesnokov, A.; Nguyen, H.T.; De La Cruz, J.; Spencer, S.; Campbell, A.P.; Sinner, M.; Reid, H.; et al. Assessing baloxavir susceptibility of influenza viruses circulating in the United States during the 2016/17 and 2017/18 seasons. Euro Surveill. 2019, 24, 1800666. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.J.; Price, G.E.; Barnett, J.M.; Hiscox, S.A.; Smith, H.; Sweet, C. Role of neuraminidase in influenza virus-induced apoptosis. J. Gen. Virol. 1999, 80, 137–146. [Google Scholar] [CrossRef]
- Das, K. Antivirals targeting influenza A virus. J. Med. Chem. 2012, 55, 6263–6277. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Jones, J.C.; Wong, S.S.; Zanin, M. Antivirals targeting the surface glycoproteins of influenza virus: Mechanisms of action and resistance. Viruses 2021, 13, 624. [Google Scholar] [CrossRef]
- Dong, G.; Peng, C.; Luo, J.; Wang, C.; Han, L.; Wu, B.; Ji, G.; He, H. Adamantane-resistant influenza A viruses in the world (1902–2013): Frequency and distribution of M2 gene mutations. PLoS ONE 2015, 10, e0119115. [Google Scholar] [CrossRef]
- Collins, P.J.; Haire, L.F.; Lin, Y.P.; Liu, J.; Russell, R.J.; Walker, P.A.; Skehel, J.J.; Martin, S.R.; Hay, A.J.; Gamblin, S.J. Crystal structures of oseltamivir-resistant influenza virus neuraminidase mutants. Nature 2008, 453, 1258–1261. [Google Scholar] [CrossRef]
- WHO. Laboratory Methodologies for Testing the Antiviral Susceptibility of Influenza Viruses. Available online: https://www.who.int/teams/global-influenza-programme/laboratory-network/quality-assurance/antiviral-susceptibility-influenza (accessed on 26 July 2025).
- Samson, M.; Pizzorno, A.; Abed, Y.; Boivin, G. Influenza virus resistance to neuraminidase inhibitors. Antivir. Res. 2013, 98, 174–185. [Google Scholar] [CrossRef]
- Nagata, T.; Lefor, A.K.; Hasegawa, M.; Ishii, M. Favipiravir: A new medication for the ebola virus disease pandemic. Disaster Med. Public Health Prep. 2015, 9, 79–81. [Google Scholar] [CrossRef]
- Zaraket, H.; Saito, R. Japanese surveillance systems and treatment for influenza. Curr. Treat. Options Infect. Dis. 2016, 8, 311–328. [Google Scholar] [CrossRef]
- Takashita, E.; Morita, H.; Ogawa, R.; Nakamura, K.; Fujisaki, S.; Shirakura, M.; Kuwahara, T.; Kishida, N.; Watanabe, S.; Odagiri, T. Susceptibility of Influenza Viruses to the Novel Cap-Dependent Endonuclease Inhibitor Baloxavir Marboxil. Front. Microbiol. 2018, 9, 3026. [Google Scholar] [CrossRef]
- Uyeki, T.M. High-risk Groups for Influenza Complications. JAMA 2020, 324, 2334. [Google Scholar] [CrossRef]
- Xu, H.; Li, S.; Liu, J.; Cheng, J.; Kang, L.; Li, W.; Zhong, Y.; Wei, C.; Fu, L.; Qi, J.; et al. Bioactive compounds from Huashi Baidu decoction possess both antiviral and anti-inflammatory effects against COVID-19. Proc. Natl. Acad. Sci. USA 2023, 120, e2301775120. [Google Scholar] [CrossRef]
- China NHCotPsRo. Influenza Diagnosis and Treatment Plan; China OotNHCotPsRo, Ed.; Office of the National Health Commission of the People’s Republic of China: Beijing, China, 2025.
- Zhang, J.; Xu, X.; Li, N.; Cao, L.; Sun, Y.; Wang, J.; He, S.; Si, J.; Qing, D. Licoflavone B, an isoprene flavonoid derived from licorice residue, relieves dextran sodium sulfate-induced ulcerative colitis by rebuilding the gut barrier and regulating intestinal microflora. Eur. J. Pharmacol. 2022, 916, 174730. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Geng, X.; Zhang, J.; Li, S.; Gao, J. Structure-based discovery of Licoflavone B and ginkgetin targeting c-Myc G-quadruplex to suppress c-Myc transcription and myeloma growth. Chem. Biol. Drug Des. 2022, 100, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Aleixo de Carvalho, L.S.; Geraldo, R.B.; de Moraes, J.; Silva Pinto, P.L.; de Faria Pinto, P.; Pereira Odos, S.; Da Silva Filho, A.A. Schistosomicidal activity and docking of Schistosoma mansoni ATPDase 1 with licoflavone B isolated from Glycyrrhiza inflata (Fabaceae). Exp. Parasitol. 2015, 159, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.; Dyall, J.; Olivo, P.D.; Pekosz, A. Virus-inducible reporter genes as a tool for detecting and quantifying influenza A virus replication. J. Virol. Methods 2005, 126, 13–20. [Google Scholar] [CrossRef]
- Li, Y.; Larrimer, A.; Curtiss, T.; Kim, J.; Jones, A.; Baird-Tomlinson, H.; Pekosz, A.; Olivo, P.D. Influenza virus assays based on virus-inducible reporter cell lines. Influenza Other Respir. Viruses 2009, 3, 241–251. [Google Scholar] [CrossRef]
- Hossain, M.J.; Perez, S.; Guo, Z.; Chen, L.M.; Donis, R.O. Establishment and characterization of a Madin-Darby canine kidney reporter cell line for influenza A virus assays. J. Clin. Microbiol. 2010, 48, 2515–2523. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, W.; Liao, L.; Li, J.; Zhang, S.; Kang, X.; Li, Y.; Zhao, L.; Dong, B.; Wang, H.; et al. Development of a Universal Type A Influenza Viral RdRp-Induced Reporter System with Potential for Antiviral Drug Screening. ACS Infect. Dis. 2025, 11, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Walker, A.P.; Carrique, L.; Keown, J.R.; Serna Martin, I.; Karia, D.; Sharps, J.; Hengrung, N.; Pardon, E.; Steyaert, J.; et al. Structures of influenza A virus RNA polymerase offer insight into viral genome replication. Nature 2019, 573, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Samakkarnthai, P.; Saul, D.; Zhang, L.; Aversa, Z.; Doolittle, M.L.; Sfeir, J.G.; Kaur, J.; Atkinson, E.J.; Edwards, J.R.; Russell, G.G.; et al. In vitro and in vivo effects of zoledronic acid on senescence and senescence-associated secretory phenotype markers. Aging 2023, 15, 3331–3355. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar] [CrossRef]
- Grienke, U.; Braun, H.; Seidel, N.; Kirchmair, J.; Richter, M.; Krumbholz, A.; von Grafenstein, S.; Liedl, K.R.; Schmidtke, M.; Rollinger, J.M. Computer-guided approach to access the anti-influenza activity of licorice constituents. J. Nat. Prod. 2014, 77, 563–570. [Google Scholar] [CrossRef]
- Lei, R.; Kim, W.; Lv, H.; Mou, Z.; Scherm, M.J.; Schmitz, A.J.; Turner, J.S.; Tan, T.J.C.; Wang, Y.; Ouyang, W.O.; et al. Leveraging vaccination-induced protective antibodies to define conserved epitopes on influenza N2 neuraminidase. Immunity 2023, 56, 2621–2634.e6. [Google Scholar] [CrossRef]
- AbuBakar, U.; Low, Z.X.; Aris, M.Z.M.; Lani, R.; Abidin, S.A.Z.; Abdullah-Zawawi, M.R.; Hassandarvish, P.; Karsani, S.A.; Khairat, J.E. Antiviral potential of diosmin against influenza A virus. Sci. Rep. 2025, 15, 17192. [Google Scholar] [CrossRef]



| Influenza A virus primers | |
| Forward | 5′-GACCRATCCTGTCACCTCTGAC-3′ |
| Reverse | 5′- GGGCATTYTGGACAAAKCGTCTACG-3′ |
| Probe | 5′-[FAM]TGCAGTCCTCGCTCACTGGGCACG[BHQ-1]-3′ |
| Influenza B virus primers | |
| Forward | 5′-TCCTCAACTCACTCTTCGAGCG-3′ |
| Reverse | 5′-CGGTGCTCTTGACCAAATTGG-3′ |
| Probe | 5′-[FAM]CCAATTCGAGCAGCTGAAACTGCGGTG[BHQ-1]-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, P.; Lv, P.; Zhang, S.; Zhu, Z.; Qian, K.; Han, J.; Cui, Y.; Feng, Y.; Li, Z.; Qiang, L.; et al. Licoflavone B Suppresses Influenza A Virus by Targeting the Viral RNA-Dependent RNA Polymerase (RdRp). Viruses 2025, 17, 1157. https://doi.org/10.3390/v17091157
Fan P, Lv P, Zhang S, Zhu Z, Qian K, Han J, Cui Y, Feng Y, Li Z, Qiang L, et al. Licoflavone B Suppresses Influenza A Virus by Targeting the Viral RNA-Dependent RNA Polymerase (RdRp). Viruses. 2025; 17(9):1157. https://doi.org/10.3390/v17091157
Chicago/Turabian StyleFan, Pu, Peng Lv, Sen Zhang, Zheng Zhu, Kewen Qian, Jin Han, Yue Cui, Ye Feng, Zeya Li, Li Qiang, and et al. 2025. "Licoflavone B Suppresses Influenza A Virus by Targeting the Viral RNA-Dependent RNA Polymerase (RdRp)" Viruses 17, no. 9: 1157. https://doi.org/10.3390/v17091157
APA StyleFan, P., Lv, P., Zhang, S., Zhu, Z., Qian, K., Han, J., Cui, Y., Feng, Y., Li, Z., Qiang, L., Dong, Y., Fang, T., Jiang, T., Yu, C., & Chi, X. (2025). Licoflavone B Suppresses Influenza A Virus by Targeting the Viral RNA-Dependent RNA Polymerase (RdRp). Viruses, 17(9), 1157. https://doi.org/10.3390/v17091157






