Subtype-Specific HIV-1 Protease and the Role of Hinge and Flap Dynamics in Drug Resistance: A Subtype C Narrative
Abstract
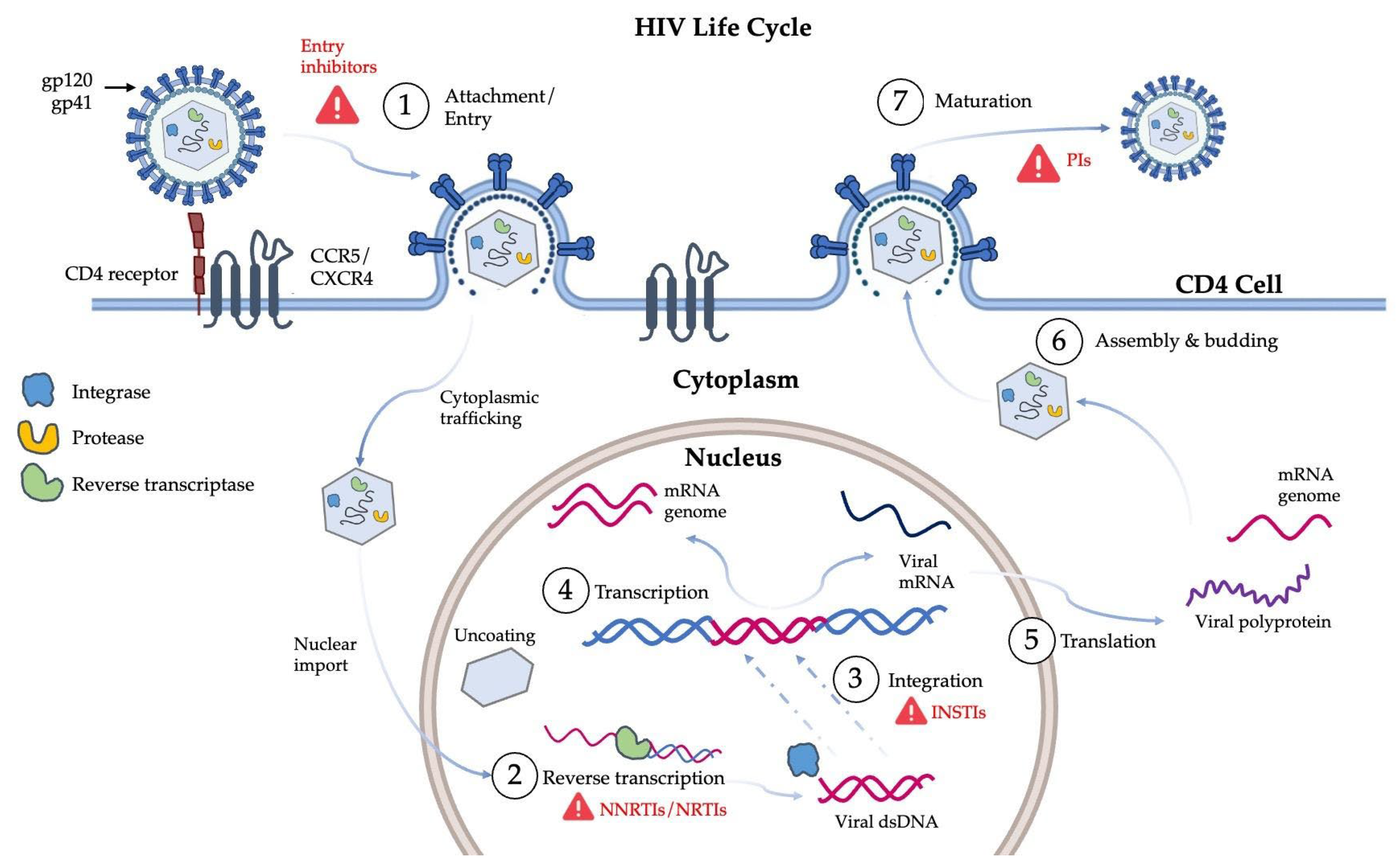
1. Introduction
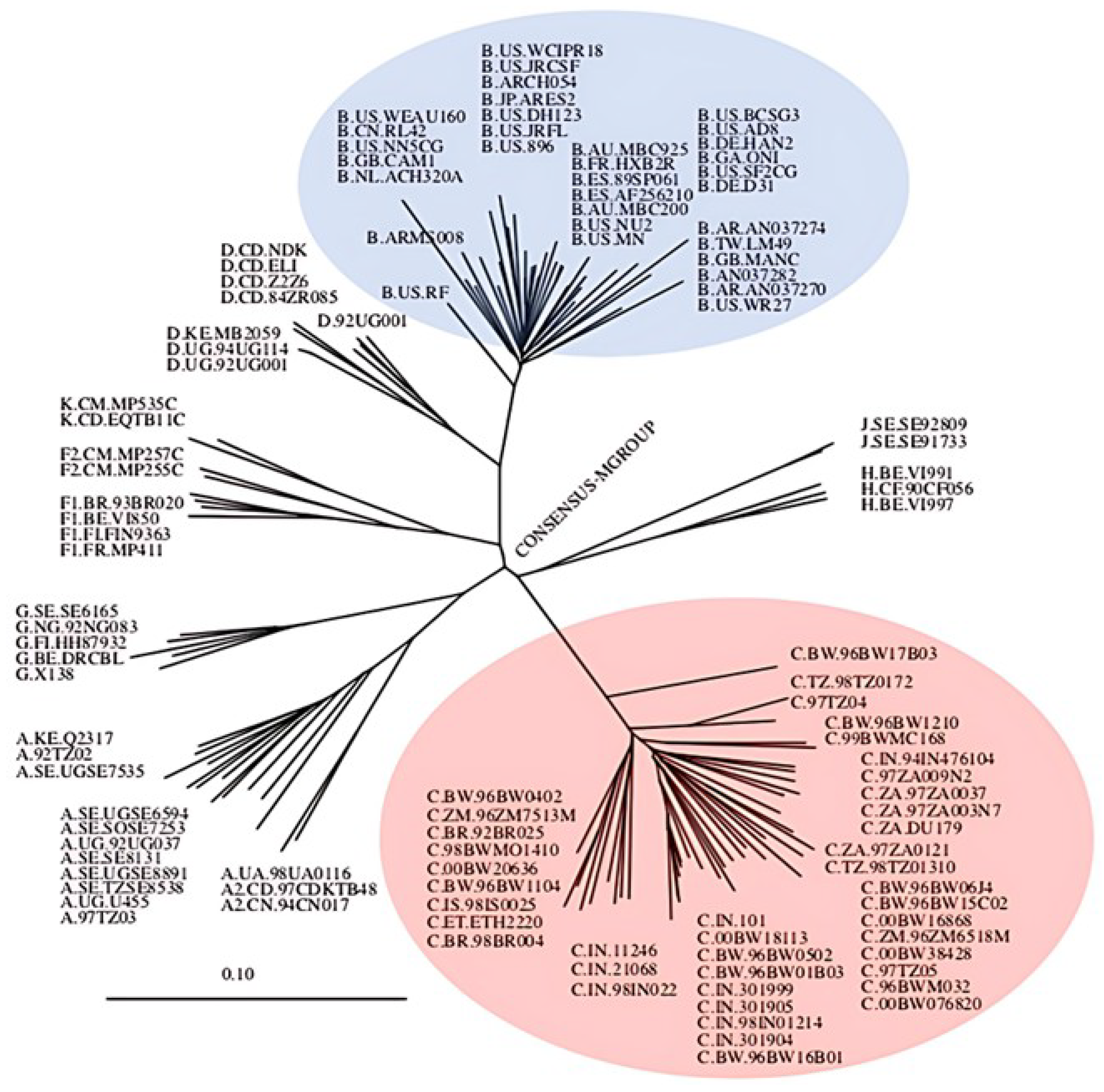
1.1. Genetic Diversity of HIV-1
1.2. The Global Predominating Subtype: HIV-1 Subtype C
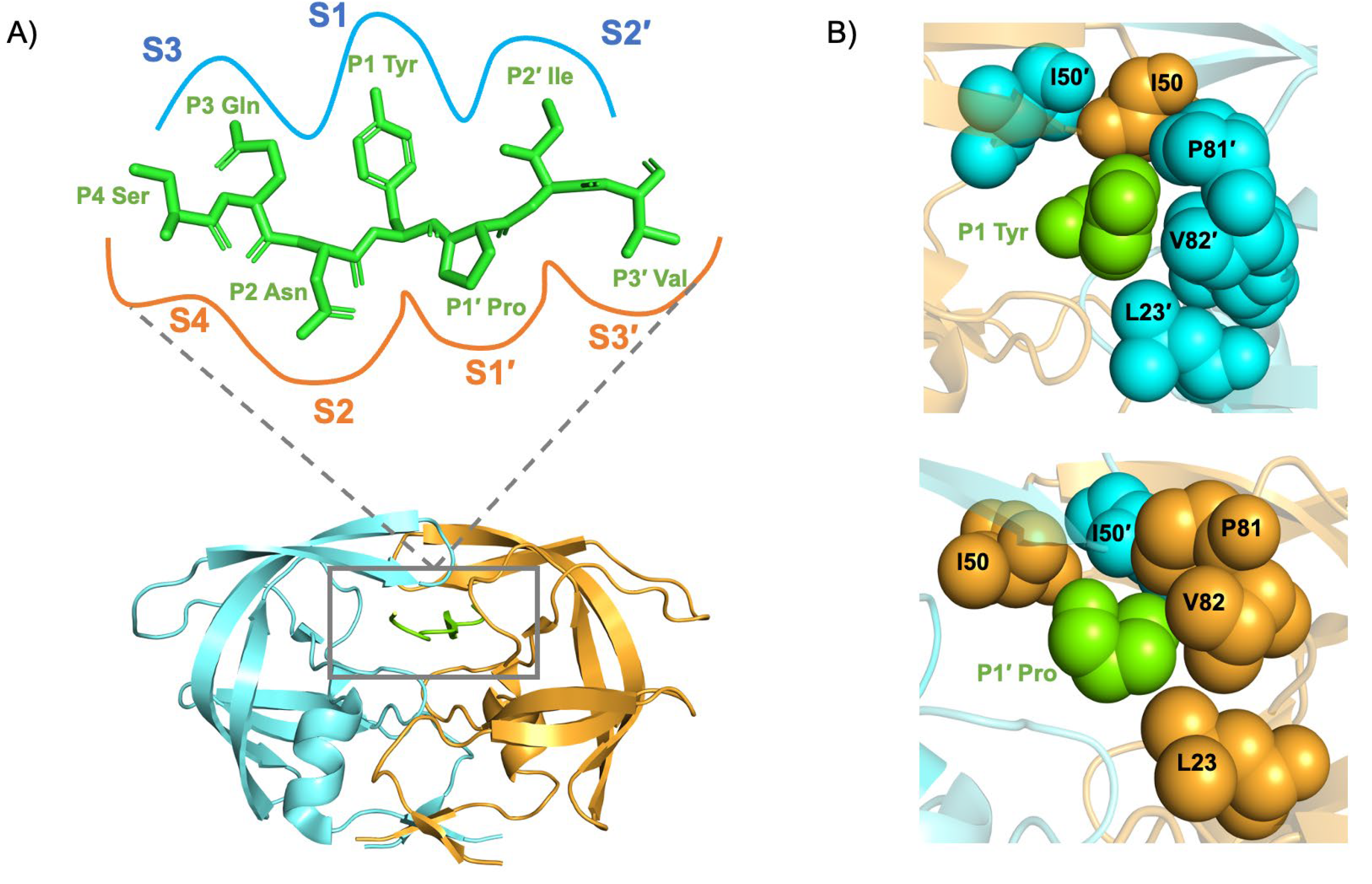
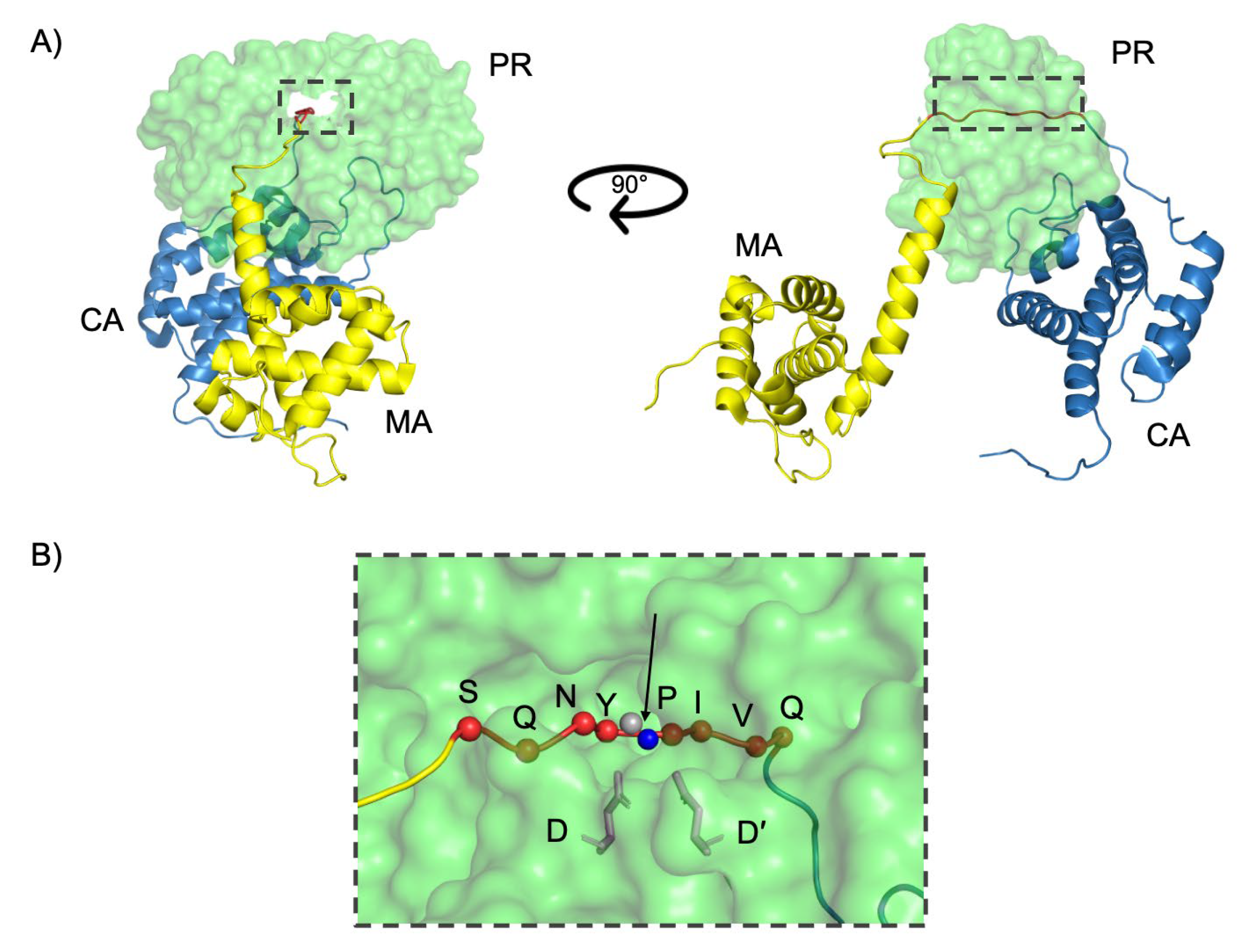
1.3. Structure of the HIV-1 Subtype C Protease
1.4. Function of the HIV-1 Subtype C Protease
1.5. Dynamics of the HIV-1 Subtype C Protease
| NOP | Location in Protease | Functional Effects | References |
|---|---|---|---|
| T12S | Fulcrum | May influence local packing and backbone flexibility; proximal to hydrophobic core; and potential impact on flap dynamics. | [11,12,32] |
| I15V | Fulcrum/hydrophobic core | Reduces side-chain bulk, disrupts hydrophobic packing; alters anchoring of hinge/cantilever; and increases flap flexibility. | [11,12,33] |
| L19I | Fulcrum/hydrophobic core | Modifies core hydrophobic interactions and destabilizes fulcrum. | [11,12,33] |
| M36I | Hinge | Alters hinge region mobility; linked to increased flap conformational variability; and associated with drug resistance adaptation. | [12,32,43,44] |
| R41K | Hinge | Conserves charge but alters local hydrogen bonding and potential effects on hinge flexibility and dimer interface. | [11,12,31,32] |
| H69K | Cantilever | Increases basicity; affects electrostatics and flap-cantilever interactions; and promotes open flap conformations. | [11,12,39] |
| L89M | 80s Loop/hydrophobic core | Subtle hydrophobic shift and influences PR core stability by increasing stability of hinge, fulcrum, and cantilever regions. | [11,12,40,42] |
| I93L | α-Helix/hydrophobic core | Conservative change; affects α-helix packing; and may influence flap anchoring and hydrophobic sliding mechanism. | [11,33,39] |
2. HIV-1 Protease Inhibition
2.1. NOPs, Drug-Induced Protease Mutations, and Inhibitor Resistance
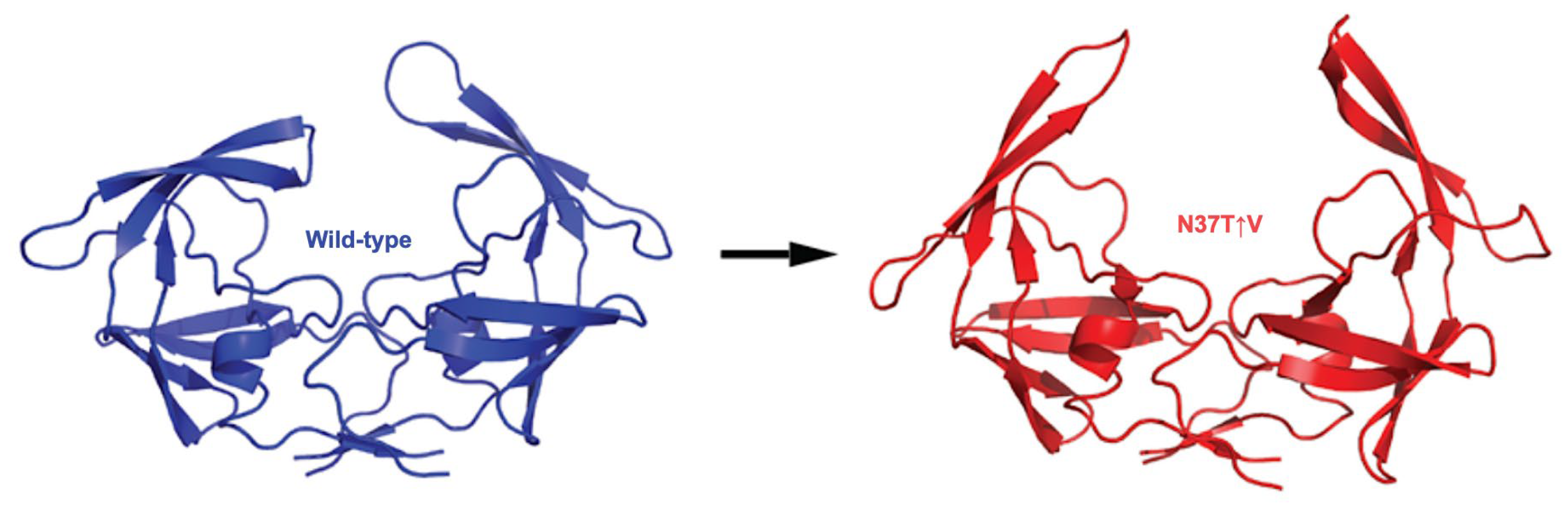
2.2. Effect of Insertion Mutations on the Dynamics of HIV-1 Subtype C Protease
2.3. Clinical Studies Demonstrating Reduced PI Efficacy in Subtype C
3. Novel Paradigms for Drug Development and PR Inhibition
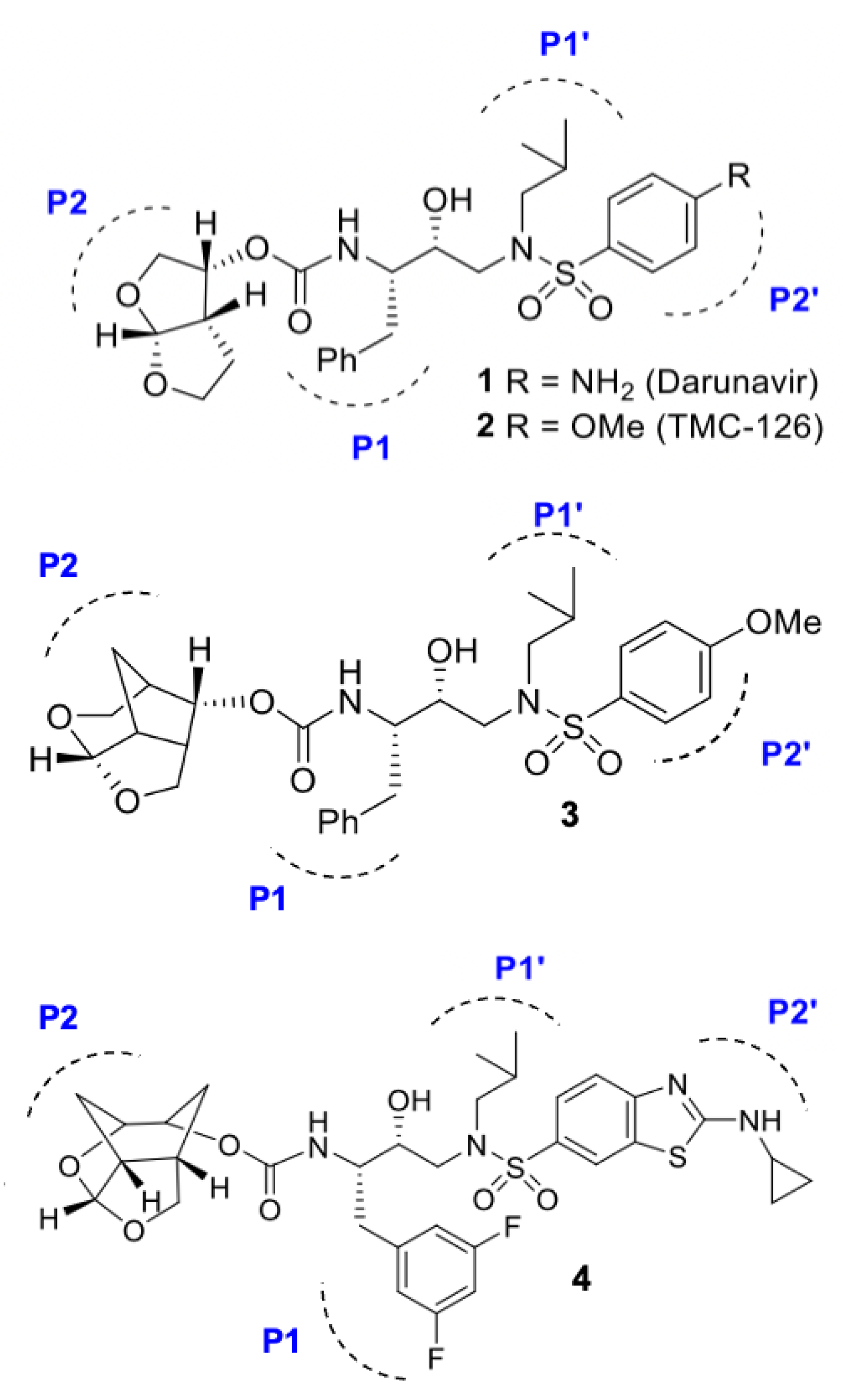
3.1. Darunavir Scaffold-Based Next-Generation Inhibitors
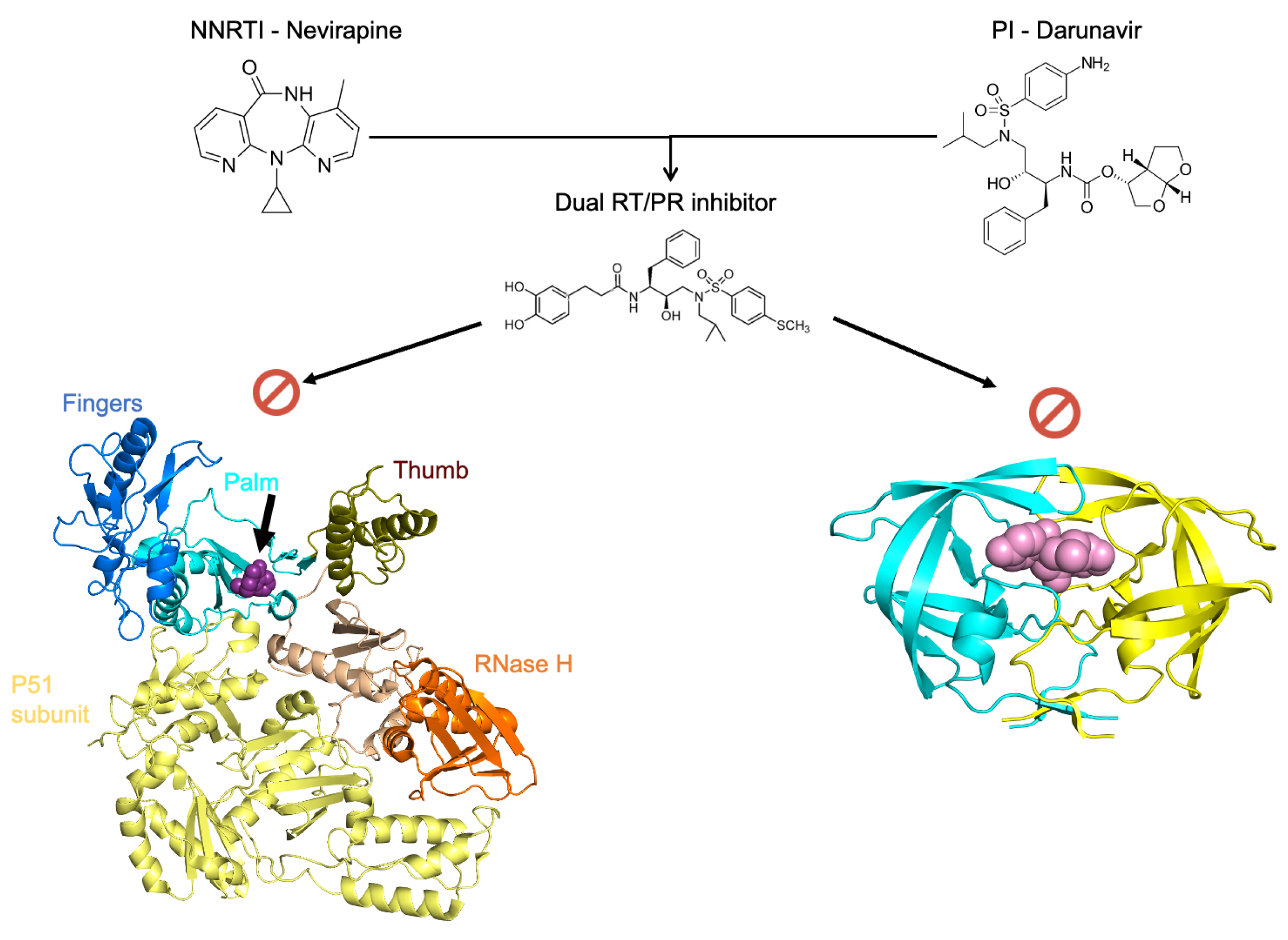
3.2. Dual Action Inhibitors of HIV Reverse Transcriptase and Protease
3.3. Novel Fullerene Derivatives as Dual RT/PR Inhibitors
3.4. Allosteric Inhibitors of the HIV Protease
3.5. Biologics Targeting HIV Protease
3.5.1. RNA Aptamers
3.5.2. Peptide-Based Inhibitors
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNAIDS. Global HIV & AIDS Statistics—Fact Sheet. 2023. Available online: https://www.unaids.org/en/resources/documents/2023/UNAIDS_FactSheet (accessed on 30 December 2023).
- Jacobo-Molina, A.; Arnold, E. HIV reverse transcriptase structure-function relationships. Biochemistry 1991, 30, 6351–6356. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.A.G.; Kräusslich, H.-G. The molecular architecture of HIV. J. Mol. Biol. 2011, 410, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Checkley, M.A.; Luttge, B.G.; Freed, E.O. HIV-1 Envelope Glycoprotein Biosynthesis, Trafficking, and Incorporation. J. Mol. Biol. 2011, 410, 582–608. [Google Scholar] [CrossRef] [PubMed]
- Faust, T.B.; Binning, J.M.; Gross, J.D.; Frankel, A.D. Making Sense of Multifunctional Proteins: Human Immunodeficiency Virus Type 1 Accessory and Regulatory Proteins and Connections to Transcription. Annu. Rev. Virol. 2017, 4, 241–260. [Google Scholar] [CrossRef]
- Peeters, M.; Sharp, P.M. Genetic diversity of HIV-1: The moving target. AIDS Lond. Engl. 2000, 14 (Suppl. S3), S129–S140. [Google Scholar]
- Perelson, A.S.; Nelson, P.W. Mathematical Analysis of HIV-I: Dynamics in Vivo. SIAM Rev. 1999, 41, 3–44. [Google Scholar] [CrossRef]
- Bhagavan, N.V.; Ha, C.-E. (Eds.) Chapter 22—DNA Replication, Repair, and Mutagenesis. In Essentials of Medical Biochemistry, 2nd ed.; Academic Press: San Diego, CA, USA, 2015; pp. 401–417. [Google Scholar] [CrossRef]
- Nyamweya, S.; Hegedus, A.; Jaye, A.; Rowland-Jones, S.; Flanagan, K.L.; Macallan, D.C. Comparing HIV-1 and HIV-2 infection: Lessons for viral immunopathogenesis. Rev. Med. Virol. 2013, 23, 221–240. [Google Scholar] [CrossRef]
- Robertson, D.L.; Anderson, J.P.; Bradac, J.A.; Carr, J.K.; Foley, B.; Funkhouser, R.K.; Gao, F.; Hahn, B.H.; Kalish, M.L.; Kuiken, C.; et al. HIV-1 nomenclature proposal. Science 2000, 288, 55–56. [Google Scholar] [CrossRef]
- Mosebi, S.; Morris, L.; Dirr, H.W.; Sayed, Y. Active-site mutations in the South African human immunodeficiency virus type 1 subtype C protease have a significant impact on clinical inhibitor binding: Kinetic and thermodynamic study. J. Virol. 2008, 82, 11476–11479. [Google Scholar] [CrossRef]
- Naicker, P.; Sayed, Y. Non-B HIV-1 subtypes in sub-Saharan Africa: Impact of subtype on protease inhibitor efficacy. Biol. Chem. 2014, 395, 1151–1161. [Google Scholar] [CrossRef]
- Sherry, D.; Worth, R.; Ismail, Z.S.; Sayed, Y. Cantilever-centric mechanism of cooperative non-active site mutations in HIV protease: Implications for flap dynamics. J. Mol. Graph. Model. 2021, 106, 107931. [Google Scholar] [CrossRef] [PubMed]
- Sherry, D.; Worth, R.; Sayed, Y. Elasticity-Associated Functionality and Inhibition of the HIV Protease. Adv. Exp. Med. Biol. 2021, 1371, 79–108. [Google Scholar] [CrossRef]
- Velazquez-Campoy, A.; Vega, S.; Freire, E. Amplification of the effects of drug resistance mutations by background polymorphisms in HIV-1 protease from African subtypes. Biochemistry 2002, 41, 8613–8619. [Google Scholar] [CrossRef] [PubMed]
- Marie, V.; Gordon, M.L. The HIV-1 Gag Protein Displays Extensive Functional and Structural Roles in Virus Replication and Infectivity. Int. J. Mol. Sci. 2022, 23, 7569. [Google Scholar] [CrossRef]
- Mattei, S.; Anders, M.; Konvalinka, J.; Kräusslich, H.-G.; Briggs, J.A.G.; Müller, B. Induced Maturation of Human Immunodeficiency Virus. J. Virol. 2014, 88, 13722–13731. [Google Scholar] [CrossRef]
- Ning, J.; Erdemci-Tandogan, G.; Yufenyuy, E.L.; Wagner, J.; Himes, B.A.; Zhao, G.; Aiken, C.; Zandi, R.; Zhang, P. In vitro protease cleavage and computer simulations reveal the HIV-1 capsid maturation pathway. Nat. Commun. 2016, 7, 13689. [Google Scholar] [CrossRef]
- Parry, C.M.; Kohli, A.; Boinett, C.J.; Towers, G.J.; McCormick, A.L.; Pillay, D. Gag Determinants of Fitness and Drug Susceptibility in Protease Inhibitor-Resistant Human Immunodeficiency Virus Type 1. J. Virol. 2009, 83, 9094–9101. [Google Scholar] [CrossRef]
- Codoñer, F.M.; Peña, R.; Blanch-Lombarte, O.; Jimenez-Moyano, E.; Pino, M.; Vollbrecht, T.; Clotet, B.; Martinez-Picado, J.; Draenert, R.; Prado, J.G. Gag-protease coevolution analyses define novel structural surfaces in the HIV-1 matrix and capsid involved in resistance to Protease Inhibitors. Sci. Rep. 2017, 7, 3717. [Google Scholar] [CrossRef]
- Marie, V.; Gordon, M. Gag-protease coevolution shapes the outcome of lopinavir-inclusive treatment regimens in chronically infected HIV-1 subtype C patients. Bioinforma. Oxf. Engl. 2019, 35, 3219–3223. [Google Scholar] [CrossRef]
- Su, C.T.-T.; Koh, D.W.-S.; Gan, S.K.-E. Reviewing HIV-1 Gag Mutations in Protease Inhibitors Resistance: Insights for Possible Novel Gag Inhibitor Designs. Molecules 2019, 24, 3243. [Google Scholar] [CrossRef]
- Dam, E.; Quercia, R.; Glass, B.; Descamps, D.; Launay, O.; Duval, X.; Kräusslich, H.-G.; Hance, A.J.; Clavel, F.; Group, A. Gag Mutations Strongly Contribute to HIV-1 Resistance to Protease Inhibitors in Highly Drug-Experienced Patients besides Compensating for Fitness Loss. PLOS Pathog. 2009, 5, e1000345. [Google Scholar] [CrossRef]
- Kožíšek, M.; Henke, S.; Šašková, K.G.; Jacobs, G.B.; Schuch, A.; Buchholz, B.; Müller, V.; Kräusslich, H.-G.; Řezáčová, P.; Konvalinka, J.; et al. Mutations in HIV-1 gag and pol Compensate for the Loss of Viral Fitness Caused by a Highly Mutated Protease. Antimicrob. Agents Chemother. 2012, 56, 4320–4330. [Google Scholar] [CrossRef]
- Kiguoya, M.W.; Mann, J.K.; Chopera, D.; Gounder, K.; Lee, G.Q.; Hunt, P.W.; Martin, J.N.; Ball, T.B.; Kimani, J.; Brumme, Z.L.; et al. Subtype-Specific Differences in Gag-Protease-Driven Replication Capacity Are Consistent with Intersubtype Differences in HIV-1 Disease Progression. J. Virol. 2017, 91, e00253-17. [Google Scholar] [CrossRef]
- Marozsan, A.J.; Moore, D.M.; Lobritz, M.A.; Fraundorf, E.; Abraha, A.; Reeves, J.D.; Arts, E.J. Differences in the fitness of two diverse wild-type human immunodeficiency virus type 1 isolates are related to the efficiency of cell binding and entry. J. Virol. 2005, 79, 7121–7134. [Google Scholar] [CrossRef] [PubMed]
- Venner, C.M.; Nankya, I.; Kyeyune, F.; Demers, K.; Kwok, C.; Chen, P.-L.; Rwambuya, S.; Munjoma, M.; Chipato, T.; Byamugisha, J.; et al. Infecting HIV-1 Subtype Predicts Disease Progression in Women of Sub-Saharan Africa. EBioMedicine 2016, 13, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Naicker, P.; Seele, P.; Dirr, H.W.; Sayed, Y. F99 is critical for dimerization and activation of South African HIV-1 subtype C protease. Protein J. 2013, 32, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Mager, P.P. The active site of HIV-1 protease. Med. Res. Rev. 2001, 21, 348–353. [Google Scholar] [CrossRef]
- Gustchina, A.; Weber, I.T. Comparison of inhibitor binding in HIV-1 protease and in non-viral aspartic proteases: The role of the flap. FEBS Lett. 1990, 269, 269–272. [Google Scholar] [CrossRef]
- Ledwaba, J.; Sayed, Y.; Pillay, V.; Morris, L.; Hunt, G. Low Frequency of Protease Inhibitor Resistance Mutations and Insertions in HIV-1 Subtype C Protease Inhibitor-Naïve Sequences. AIDS Res. Hum. Retroviruses 2019, 35, 673–678. [Google Scholar] [CrossRef]
- Naicker, P.; Achilonu, I.; Fanucchi, S.; Fernandes, M.; Ibrahim, M.A.A.; Dirr, H.W.; Soliman, M.E.S.; Sayed, Y. Structural insights into the South African HIV-1 subtype C protease: Impact of hinge region dynamics and flap flexibility in drug resistance. J. Biomol. Struct. Dyn. 2013, 31, 1370–1380. [Google Scholar] [CrossRef]
- Foulkes-Murzycki, J.E.; Scott, W.R.P.; Schiffer, C.A. Hydrophobic Sliding: A Possible Mechanism for Drug Resistance in Human Immunodeficiency Virus Type 1 Protease. Structure 2007, 15, 225–233. [Google Scholar] [CrossRef]
- Freedberg, D.I.; Ishima, R.; Jacob, J.; Wang, Y.-X.; Kustanovich, I.; Louis, J.M.; Torchia, D.A. Rapid structural fluctuations of the free HIV protease flaps in solution: Relationship to crystal structures and comparison with predictions of dynamics calculations. Protein Sci. Publ. Protein Soc. 2002, 11, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Hornak, V.; Okur, A.; Rizzo, R.C.; Simmerling, C. HIV-1 protease flaps spontaneously close to the correct structure in simulations following manual placement of an inhibitor into the open state. J. Am. Chem. Soc. 2006, 128, 2812–2813. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nicholson, L.K.; Yamazaki, T.; Torchia, D.A.; Grzesiek, S.; Bax, A.; Stahl, S.J.; Kaufman, J.D.; Wingfield, P.T.; Lam, P.Y.; Jadhav, P.K. Flexibility and function in HIV-1 protease. Nat. Struct. Biol. 1995, 2, 274–280. [Google Scholar] [CrossRef]
- Perryman, A.L.; Lin, J.-H.; McCammon, J.A. HIV-1 protease molecular dynamics of a wild-type and of the V82F/I84V mutant: Possible contributions to drug resistance and a potential new target site for drugs. Protein Sci. 2004, 13, 1108–1123. [Google Scholar] [CrossRef]
- Schechter, I.; Berger, A. On the size of the active site in proteases. I. Papain. Biochem. Biophys. Res. Commun. 1967, 27, 157–162. [Google Scholar] [CrossRef]
- Maphumulo, S.I.; Halder, A.K.; Govender, T.; Maseko, S.; Maguire, G.E.M.; Honarparvar, B.; Kruger, H.G. Exploring the flap dynamics of the South African HIV subtype C protease in presence of FDA-approved inhibitors: MD study. Chem. Biol. Drug Des. 2018, 92, 1899–1913. [Google Scholar] [CrossRef]
- Kear, J.L.; Blackburn, M.E.; Veloro, A.M.; Dunn, B.M.; Fanucci, G.E. Subtype Polymorphisms Among HIV-1 Protease Variants Confer Altered Flap Conformations and Flexibility. J. Am. Chem. Soc. 2009, 131, 14650–14651. [Google Scholar] [CrossRef]
- Zondagh, J.; Balakrishnan, V.; Achilonu, I.; Dirr, H.W.; Sayed, Y. Molecular dynamics and ligand docking of a hinge region variant of South African HIV-1 subtype C protease. J. Mol. Graph. Model. 2018, 82, 1–11. [Google Scholar] [CrossRef]
- Coman, R.M.; Robbins, A.H.; Fernandez, M.A.; Gilliland, C.T.; Sochet, A.A.; Goodenow, M.M.; McKenna, R.; Dunn, B.M. The Contribution of Naturally Occurring Polymorphisms in Altering the Biochemical and Structural Characteristics of HIV-1 Subtype C Protease. Biochemistry 2008, 47, 731–743. [Google Scholar] [CrossRef]
- Zondagh, J.; Basson, A.E.; Achilonu, I.; Morris, L.; Dirr, H.W.; Sayed, Y. Drug susceptibility and replication capacity of a rare HIV-1 subtype C protease hinge region variant. Antivir. Ther. 2019, 24, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cajas, J.L.; Pai, N.P.; Klein, M.B.; Wainberg, M.A. Differences in resistance mutations among HIV-1 non-subtype B infections: A systematic review of evidence (1996–2008). J. Int. AIDS Soc. 2009, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Wlodawer, A.; Vondrasek, J. INHIBITORS OF HIV-1 PROTEASE: A Major Success of Structure-Assisted Drug Design1. Annu. Rev. Biophys. 1998, 27, 249–284. [Google Scholar] [CrossRef] [PubMed]
- Partaledis, J.A.; Yamaguchi, K.; Tisdale, M.; Blair, E.E.; Falcione, C.; Maschera, B.; Myers, R.E.; Pazhanisamy, S.; Futer, O.; Cullinan, A.B. In vitro selection and characterization of human immunodeficiency virus type 1 (HIV-1) isolates with reduced sensitivity to hydroxyethylamino sulfonamide inhibitors of HIV-1 aspartyl protease. J. Virol. 1995, 69, 5228–5235. [Google Scholar] [CrossRef]
- Lefebvre, E.; Schiffer, C.A. Resilience to Resistance of HIV-1 Protease Inhibitors: Profile of Darunavir. AIDS Rev. 2008, 10, 131–142. [Google Scholar]
- Thompson, M.A.; Aberg, J.A.; Cahn, P.; Montaner, J.S.G.; Rizzardini, G.; Telenti, A.; Gatell, J.M.; Günthard, H.F.; Hammer, S.M.; Hirsch, M.S.; et al. Antiretroviral Treatment of Adult HIV Infection: 2010 Recommendations of the International AIDS Society–USA Panel. JAMA 2010, 304, 321–333. [Google Scholar] [CrossRef]
- Volberding, P.A.; Deeks, S.G. Antiretroviral therapy and management of HIV infection. Lancet 2010, 376, 49–62. [Google Scholar] [CrossRef]
- Wu, T.D.; Schiffer, C.A.; Gonzales, M.J.; Taylor, J.; Kantor, R.; Chou, S.; Israelski, D.; Zolopa, A.R.; Fessel, W.J.; Shafer, R.W. Mutation Patterns and Structural Correlates in Human Immunodeficiency Virus Type 1 Protease following Different Protease Inhibitor Treatments. J. Virol. 2003, 77, 4836–4847. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Schock, H.B.; Hall, D.; Chen, E.; Kuo, L.C. Three-dimensional structure of a mutant HIV-1 protease displaying cross-resistance to all protease inhibitors in clinical trials. J. Biol. Chem. 1995, 270, 21433–21436. [Google Scholar] [CrossRef]
- Markowitz, M.; Mo, H.; Kempf, D.J.; Norbeck, D.W.; Bhat, T.N.; Erickson, J.W.; Ho, D.D. Selection and analysis of human immunodeficiency virus type 1 variants with increased resistance to ABT-538, a novel protease inhibitor. J. Virol. 1995, 69, 701–706. [Google Scholar] [CrossRef]
- Clavel, F.; Mammano, F. Role of Gag in HIV Resistance to Protease Inhibitors. Viruses 2010, 2, 1411–1426. [Google Scholar] [CrossRef]
- Nijhuis, M.; Schuurman, R.; de Jong, D.; Erickson, J.; Gustchina, E.; Albert, J.; Schipper, P.; Gulnik, S.; Boucher, C.A. Increased fitness of drug resistant HIV-1 protease as a result of acquisition of compensatory mutations during suboptimal therapy. AIDS Lond. Engl. 1999, 13, 2349–2359. [Google Scholar] [CrossRef]
- Velázquez-Campoy, A.; Vega, S.; Fleming, E.; Bacha, U.; Sayed, Y.; Dirr, H.W.; Freire, E. Protease inhibition in African subtypes of HIV-1. AIDS Rev. 2003, 5, 165–171. [Google Scholar]
- Goldfarb, N.E.; Ohanessian, M.; Biswas, S.; McGee, T.D., Jr.; Mahon, B.P.; Ostrov, D.A.; Garcia, J.; Tang, Y.; McKenna, R.; Roitberg, A.; et al. Defective Hydrophobic Sliding Mechanism and Active Site Expansion in HIV-1 Protease Drug Resistant Variant Gly48Thr/Leu89Met: Mechanisms for the Loss of Saquinavir Binding Potency. Biochemistry 2015, 54, 422–433. [Google Scholar] [CrossRef]
- Mittal, S.; Cai, Y.; Nalam, M.N.L.; Bolon, D.N.A.; Schiffer, C.A. Hydrophobic Core Flexibility Modulates Enzyme Activity in HIV-1 Protease. J. Am. Chem. Soc. 2012, 134, 4163–4168. [Google Scholar] [CrossRef]
- Sankaran, S.V.; Krishnan, S.R.; Sayed, Y.; Gromiha, M.M. Mechanism of drug resistance in HIV-1 protease subtype C in the presence of Atazanavir. Curr. Res. Struct. Biol. 2024, 7, 100132. [Google Scholar] [CrossRef] [PubMed]
- Hornak, V.; Okur, A.; Rizzo, R.C.; Simmerling, C. HIV-1 protease flaps spontaneously open and reclose in molecular dynamics simulations. Proc. Natl. Acad. Sci. USA 2006, 103, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Özer, N.; Özen, A.; Schiffer, C.A.; Haliloğlu, T. Drug-resistant HIV-1 protease regains functional dynamics through cleavage site coevolution. Evol. Appl. 2015, 8, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Basson, A.; Achilonu, I.; Dirr, H.W.; Morris, L.; Sayed, Y. Double trouble? Gag in conjunction with double insert in HIV protease contributes to reduced DRV susceptibility. Biochem. J. 2019, 476, 375–384. [Google Scholar] [CrossRef]
- Prabu-Jeyabalan, M.; Nalivaika, E.A.; King, N.M.; Schiffer, C.A. Structural Basis for Coevolution of a Human Immunodeficiency Virus Type 1 Nucleocapsid-p1 Cleavage Site with a V82A Drug-Resistant Mutation in Viral Protease. J. Virol. 2004, 78, 12446–12454. [Google Scholar] [CrossRef]
- King, N.M.; Prabu-Jeyabalan, M.; Nalivaika, E.A.; Wigerinck, P.; de Béthune, M.-P.; Schiffer, C.A. Structural and Thermodynamic Basis for the Binding of TMC114, a Next-Generation Human Immunodeficiency Virus Type 1 Protease Inhibitor. J. Virol. 2004, 78, 12012–12021. [Google Scholar] [CrossRef] [PubMed]
- Myint, L.; Matsuda, M.; Matsuda, Z.; Yokomaku, Y.; Chiba, T.; Okano, A.; Yamada, K.; Sugiura, W. Gag non-cleavage site mutations contribute to full recovery of viral fitness in protease inhibitor-resistant human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 2004, 48, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Giandhari, J.; Basson, A.E.; Coovadia, A.; Kuhn, L.; Abrams, E.J.; Strehlau, R.; Morris, L.; Hunt, G.M. Genetic Changes in HIV-1 Gag-Protease Associated with Protease Inhibitor-Based Therapy Failure in Pediatric Patients. AIDS Res. Hum. Retroviruses 2015, 31, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, L.; Louis, J.M.; Ghirlando, R.; Clore, G.M. Transient HIV-1 Gag–protease interactions revealed by paramagnetic NMR suggest origins of compensatory drug resistance mutations. Proc. Natl. Acad. Sci. USA 2016, 113, 12456–12461. [Google Scholar] [CrossRef]
- Li, G.; Verheyen, J.; Theys, K.; Piampongsant, S. Van Laethem, and A.-M. Vandamme. HIV-1 Gag C-terminal amino acid substitutions emerging under selective pressure of protease inhibitors in patient populations infected with different HIV-1 subtypes. Retrovirology 2014, 11, 79. [Google Scholar] [CrossRef][Green Version]
- Kozísek, M.; Sasková, K.G.; Rezácová, P.; Brynda, J.; van Maarseveen, N.M.; De Jong, D.; Boucher, C.A.; Kagan, R.M.; Nijhuis, M.; Konvalinka, J. Ninety-nine is not enough: Molecular characterization of inhibitor-resistant human immunodeficiency virus type 1 protease mutants with insertions in the flap region. J. Virol. 2008, 82, 5869–5878. [Google Scholar] [CrossRef]
- Pereira-Vaz, J.; Duque, V.; Trindade, L.; Saraiva-da-Cunha, J.; Meliço-Silvestre, A. Detection of the protease codon 35 amino acid insertion in sequences from treatment-naïve HIV-1 subtype C infected individuals in the Central Region of Portugal. J. Clin. Virol. 2009, 46, 169–172. [Google Scholar] [CrossRef]
- Bessong, P.O.; Mphahlele, J.; Choge, I.A.; Obi, L.C.; Morris, L.; Hammarskjold, M.-L.; Rekosh, D.M. Resistance Mutational Analysis of HIV Type 1 Subtype C among Rural South African Drug-Naive Patients Prior to Large-Scale Availability of Antiretrovirals. AIDS Res. Hum. Retroviruses 2006, 22, 1306–1312. [Google Scholar] [CrossRef]
- Maseko, S.B.; Padayachee, E.; Govender, T.; Sayed, Y.; Kruger, G.; Maguire, G.E.M.; Lin, J. I36T↑T mutation in South African subtype C (C-SA) HIV-1 protease significantly alters protease-drug interactions. Biol. Chem. 2017, 398, 1109–1117. [Google Scholar] [CrossRef]
- Sanusi, Z.K.; Govender, T.; Maguire, G.E.M.; Maseko, S.B.; Lin, J.; Kruger, H.G.; Honarparvar, B. An insight to the molecular interactions of the FDA approved HIV PR drugs against L38L↑N↑L PR mutant. J. Comput. Aided Mol. Des. 2018, 32, 459–471. [Google Scholar] [CrossRef]
- Mokhantso, T.; Sherry, D.; Worth, R.; Pandian, R.; Achilonu, I.; Sayed, Y. Contrasting the effect of hinge region insertions and non-active site mutations on HIV protease-inhibitor interactions: Insights from altered flap dynamics. J. Mol. Graph. Model. 2024, 133, 108850. [Google Scholar] [CrossRef]
- Lockhat, H.A.; Silva, J.R.A.; Alves, C.N.; Govender, T.; Lameira, J.; Maguire, G.E.M.; Sayed, Y.; Kruger, H.G. Binding Free Energy Calculations of Nine FDA-approved Protease Inhibitors Against HIV-1 Subtype C I36T↑T Containing 100 Amino Acids Per Monomer. Chem. Biol. Drug Des. 2016, 87, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.S.; Worth, R.; Mosebi, S.; Sayed, Y. HIV Protease Hinge Region Insertions at Codon 38 Affect Enzyme Kinetics, Conformational Stability and Dynamics. Protein J. 2023, 42, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.L.; Mellors, J.W.; Venter, W.D.F.; Sanne, I.; Stevens, W. Protease Inhibitor Resistance Is Uncommon in HIV-1 Subtype C Infected Patients on Failing Second-Line Lopinavir/r-Containing Antiretroviral Therapy in South Africa. AIDS Res. Treat. 2011, 2011, 769627. [Google Scholar] [CrossRef] [PubMed]
- Levison, J.H.; Orrell, C.; Gallien, S.; Kuritzkes, D.R.; Fu, N.; Losina, E.; Freedberg, K.A.; Wood, R. Virologic Failure of Protease Inhibitor-Based Second-Line Antiretroviral Therapy without Resistance in a Large HIV Treatment Program in South Africa. PLoS ONE 2012, 7, e32144. [Google Scholar] [CrossRef]
- White, E.; Smit, E.; Churchill, D.; Collins, S.; Booth, C.; Tostevin, A.; Sabin, C.; Pillay, D.; Dunn, D.T. No Evidence That HIV-1 Subtype C Infection Compromises the Efficacy of Tenofovir-Containing Regimens: Cohort Study in the United Kingdom. J. Infect. Dis. 2016, 214, 1302–1308. [Google Scholar] [CrossRef]
- Zazzi, M. High genetic barrier antiretroviral drugs in human immunodeficiency virus-positive pregnancy. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2010, 50, 895–897. [Google Scholar] [CrossRef]
- Weber, I.T.; Kneller, D.W.; Wong-Sam, A. Highly resistant HIV-1 proteases and strategies for their inhibition. Future Med. Chem. 2015, 7, 1023–1038. [Google Scholar] [CrossRef]
- Weber, I.T.; Agniswamy, J. HIV-1 Protease: Structural Perspectives on Drug Resistance. Viruses 2009, 1, 1110–1136. [Google Scholar] [CrossRef]
- Sherry, D.; Pandian, R.; Sayed, Y. Non-active site mutations in the HIV protease: Diminished drug binding affinity is achieved through modulating the hydrophobic sliding mechanism. Int. J. Biol. Macromol. 2022, 217, 27–41. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Dawson, Z.L.; Mitsuya, H. Darunavir, a conceptually new HIV-1 protease inhibitor for the treatment of drug-resistant HIV. Bioorg. Med. Chem. 2007, 15, 7576–7580. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Yedidi, R.S.; Dewdney, T.G.; Reiter, S.J.; Brunzelle, J.S.; Kovari, I.A.; Kovari, L.C. Conserved hydrogen bonds and water molecules in MDR HIV-1 protease substrate complexes. Biochem. Biophys. Res. Commun. 2013, 430, 1022–1027. [Google Scholar] [CrossRef]
- Agniswamy, J.; Kneller, D.W.; Ghosh, A.K.; Weber, I.T. Novel HIV PR inhibitors with C4-substituted bis-THF and bis-fluoro-benzyl target the two active site mutations of highly drug resistant mutant PRS17. Biochem. Biophys. Res. Commun. 2021, 566, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Xia, Z.; Kovela, S.; Robinson, W.L.; Johnson, M.E.; Kneller, D.W.; Wang, Y.-F.; Aoki, M.; Takamatsu, Y.; Weber, I.T.; et al. Potent HIV-1 Protease Inhibitors Containing Carboxylic and Boronic Acids: Effect on Enzyme Inhibition and Antiviral Activity and Protein-Ligand X-ray Structural Studies. ChemMedChem 2019, 14, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yang, Z.; Zhu, M.; Dong, B.; Zhou, L.; Zhang, G.; Wang, J.; Wang, Y. Design and synthesis of potent HIV-1 protease inhibitors with (S)-tetrahydrofuran-tertiary amine-acetamide as P2−ligand: Structure−activity studies and biological evaluation. Eur. J. Med. Chem. 2017, 137, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Zhu, M.; Dong, B.; Wang, J.-X.; Zhang, G.-N.; Zhang, F.; Wang, Y.-C. Design, synthesis and biological evaluation of HIV-1 protease inhibitors with morpholine derivatives as P2 ligands in combination with cyclopropyl as P1’ ligand. Bioorg. Med. Chem. Lett. 2020, 30, 127019. [Google Scholar] [CrossRef]
- Hohlfeld, K.; Wegner, J.K.; Kesteleyn, B.; Linclau, B.; Unge, J. Disubstituted Bis-THF Moieties as New P2 Ligands in Nonpeptidal HIV-1 Protease Inhibitors (II). J. Med. Chem. 2015, 58, 4029–4038. [Google Scholar] [CrossRef]
- Hohlfeld, K.; Tomassi, C.; Wegner, J.K.; Kesteleyn, B.; Linclau, B. Disubstituted Bis-THF Moieties as New P2 Ligands in Nonpeptidal HIV-1 Protease Inhibitors. ACS Med. Chem. Lett. 2011, 2, 461–465. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Kovela, S.; Osswald, H.L.; Amano, M.; Aoki, M.; Agniswamy, J.; Wang, Y.-F.; Weber, I.T.; Mitsuya, H. Structure-Based Design of Highly Potent HIV-1 Protease Inhibitors Containing New Tricyclic Ring P2-Ligands: Design, Synthesis, Biological, and X-ray Structural Studies. J. Med. Chem. 2020, 63, 4867–4879. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Rao, K.V.; Nyalapatla, P.R.; Kovela, S.; Brindisi, M.; Osswald, H.L.; Sekhara Reddy, B.; Agniswamy, J.; Wang, Y.-F.; Aoki, M.; et al. Design of Highly Potent, Dual-Acting and Central-Nervous-System-Penetrating HIV-1 Protease Inhibitors with Excellent Potency against Multidrug-Resistant HIV-1 Variants. ChemMedChem 2018, 13, 803–815. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Rao, K.V.; Nyalapatla, P.R.; Osswald, H.L.; Martyr, C.D.; Aoki, M.; Hayashi, H.; Agniswamy, J.; Wang, Y.-F.; Bulut, H.; et al. Design and Development of Highly Potent HIV-1 Protease Inhibitors with a Crown-Like Oxotricyclic Core as the P2-Ligand to Combat Multidrug-Resistant HIV Variants. J. Med. Chem. 2017, 60, 4267–4278. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Shahabi, D.; Kipfmiller, M.; Ghosh, A.K.; Johnson, M.; Wang, Y.-F.; Agniswamy, J.; Amano, M.; Weber, I.T.; Mitsuya, H. Evaluation of darunavir-derived HIV-1 protease inhibitors incorporating P2′ amide-derivatives: Synthesis, biological evaluation and structural studies. Bioorg. Med. Chem. Lett. 2023, 83, 129168. [Google Scholar] [CrossRef]
- Aoki, M.; Hayashi, H.; Rao, K.V.; Das, D.; Higashi-Kuwata, N.; Bulut, H.; Aoki-Ogata, H.; Takamatsu, Y.; Yedidi, R.S.; Davis, D.A.; et al. A novel central nervous system-penetrating protease inhibitor overcomes human immunodeficiency virus 1 resistance with unprecedented aM to pM potency. eLife 2017, 6, e28020. [Google Scholar] [CrossRef]
- Kohlstaedt, L.A.; Wang, J.; Friedman, J.M.; Rice, P.A.; Steitz, T.A. Crystal Structure at 3.5 Å Resolution of HIV-1 Reverse Transcriptase Complexed with an Inhibitor. Science 1992, 256, 1783–1790. [Google Scholar] [CrossRef]
- Mishra, S.; Pandey, A.; Manvati, S. Coumarin: An emerging antiviral agent. Heliyon 2020, 6, e03217. [Google Scholar] [CrossRef] [PubMed]
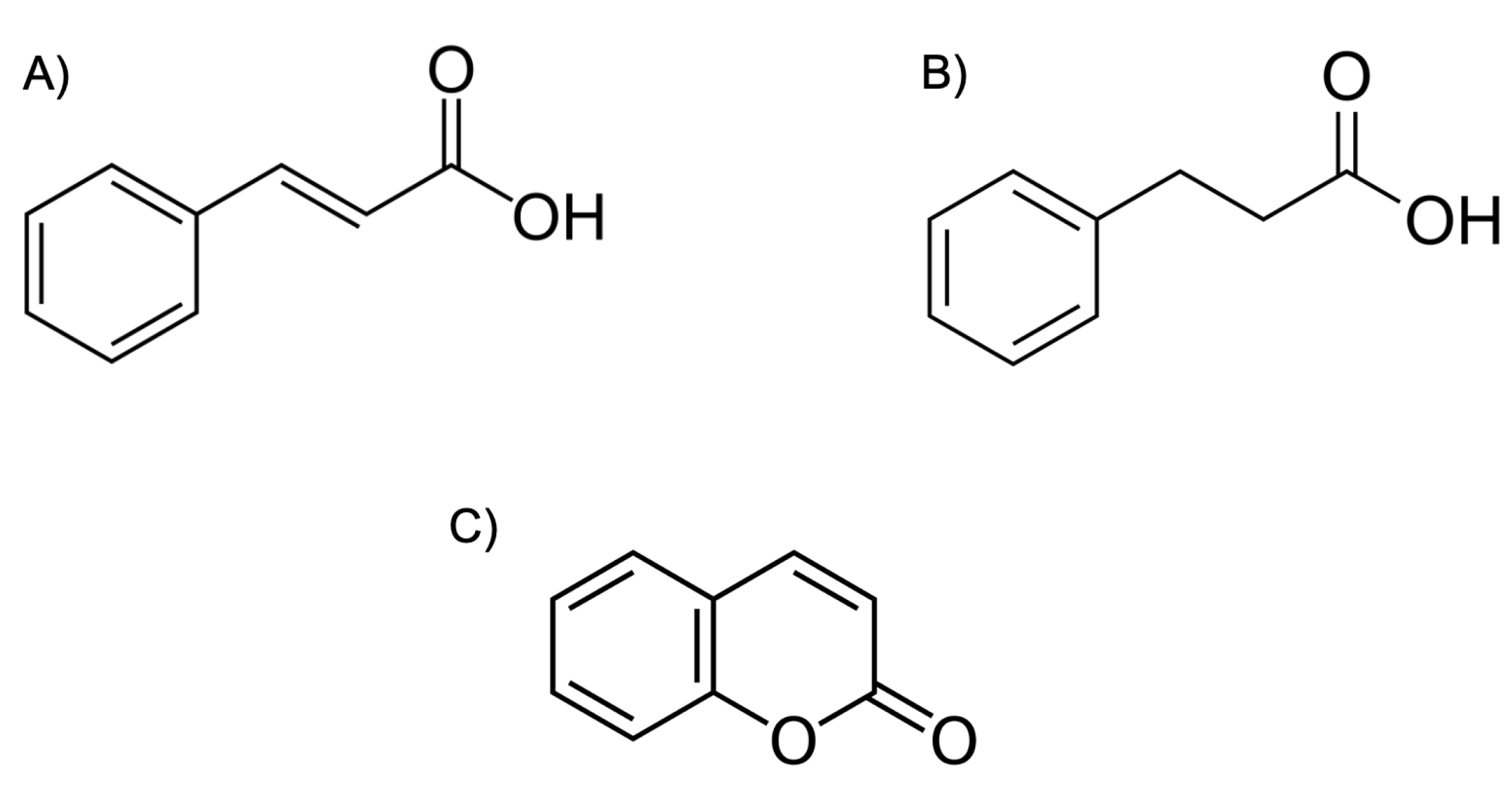
- Olomola, T.O.; Klein, R.; Mautsa, N.; Sayed, Y.; Kaye, P.T. Synthesis and evaluation of coumarin derivatives as potential dual-action HIV-1 protease and reverse transcriptase inhibitors. Bioorg. Med. Chem. 2013, 21, 1964–1971. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Shan, Q.; Ma, L.; Wen, J.; Dong, B.; Zhang, G.; Wang, M.; Wang, J.; Zhou, J.; Cen, S.; et al. Design and biological evaluation of cinnamic and phenylpropionic amide derivatives as novel dual inhibitors of HIV-1 protease and reverse transcriptase. Eur. J. Med. Chem. 2021, 220, 113498. [Google Scholar] [CrossRef] [PubMed]
- Mashino, T.; Nishikawa, D.; Takahashi, K.; Usui, N.; Yamori, T.; Seki, M.; Endo, T.; Mochizuki, M. Antibacterial and antiproliferative activity of cationic fullerene derivatives. Bioorg. Med. Chem. Lett. 2003, 13, 4395–4397. [Google Scholar] [CrossRef]
- Yasuno, T.; Ohe, T.; Kataoka, H.; Hashimoto, K.; Ishikawa, Y.; Furukawa, K.; Tateishi, Y.; Kobayashi, T.; Takahashi, K.; Nakamura, S.; et al. Fullerene derivatives as dual inhibitors of HIV-1 reverse transcriptase and protease. Bioorg. Med. Chem. Lett. 2021, 31, 127675. [Google Scholar] [CrossRef]
- Friedman, S.H.; Ganapathi, P.S.; Rubin, Y.; Kenyon, G.L. Optimizing the Binding of Fullerene Inhibitors of the HIV-1 Protease through Predicted Increases in Hydrophobic Desolvation. J. Med. Chem. 1998, 41, 2424–2429. [Google Scholar] [CrossRef]
- Ibrahim, M.; Saleh, N.A.; Hameed, A.J.; Elshemey, W.M.; Elsayed, A.A. Structural and electronic properties of new fullerene derivatives and their possible application as HIV-1 protease inhibitors. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2010, 75, 702–709. [Google Scholar] [CrossRef]
- Souffrant, M.; Yao, X.-Q.; Hamelberg, D. Evolving Mutational Buildup in HIV-1 Protease Shifts Conformational Dynamics to Gain Drug Resistance. J. Chem. Inf. Model. 2023, 63, 3892–3902. [Google Scholar] [CrossRef] [PubMed]
- Wong-Sam, A.; Wang, Y.-F.; Kneller, D.W.; Kovalevsky, A.Y.; Ghosh, A.K.; Harrison, R.W.; Weber, I.T. HIV-1 protease with 10 lopinavir and darunavir resistance mutations exhibits altered inhibition, structural rearrangements and extreme dynamics. J. Mol. Graph. Model. 2022, 117, 108315. [Google Scholar] [CrossRef] [PubMed]
- Leidner, F.; Yilmaz, N.K.; Schiffer, C.A. Deciphering Complex Mechanisms of Resistance and Loss of Potency through Coupled Molecular Dynamics and Machine Learning. J. Chem. Theory Comput. 2021, 17, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Perryman, A.L.; Zhang, Q.; Soutter, H.H.; Rosenfeld, R.; McRee, D.E.; Olson, A.J.; Elder, J.E.; David Stout, C. Fragment-Based Screen against HIV Protease. Chem. Biol. Drug Des. 2010, 75, 257–268. [Google Scholar] [CrossRef]
- Tiefenbrunn, T.; Forli, S.; Baksh, M.M.; Chang, M.W.; Happer, M.; Lin, Y.-C.; Perryman, A.L.; Rhee, J.-K.; Torbett, B.E.; Olson, A.J.; et al. Small Molecule Regulation of Protein Conformation by Binding in the Flap of HIV Protease. ACS Chem. Biol. 2013, 8, 1223–1231. [Google Scholar] [CrossRef]
- Tiefenbrunn, T.; Forli, S.; Happer, M.; Gonzalez, A.; Tsai, Y.; Soltis, M.; Elder, J.H.; Olson, A.J.; Stout, C.D. Crystallographic Fragment-Based Drug Discovery: Use of a Brominated Fragment Library Targeting HIV Protease. Chem. Biol. Drug Des. 2014, 83, 141–148. [Google Scholar] [CrossRef]
- Luchi, A.; Angelina, E.; Bogado, L.; Forli, S.; Olson, A.; Peruchena, N. Flap-site Fragment Restores Back Wild-type Behaviour in Resistant Form of HIV Protease. Mol. Inform. 2018, 37, 1800053. [Google Scholar] [CrossRef]
- Agniswamy, J.; Shen, C.-H.; Aniana, A.; Sayer, J.M.; Louis, J.M.; Weber, I.T. HIV-1 Protease with 20 Mutations Exhibits Extreme Resistance to Clinical Inhibitors through Coordinated Structural Rearrangements. Biochemistry 2012, 51, 2819–2828. [Google Scholar] [CrossRef][Green Version]
- Mittal, S.; Bandaranayake, R.M.; King, N.M.; Prabu-Jeyabalan, M.; Nalam, M.N.L.; Nalivaika, E.A.; Kurt Yilmaz, N.; Schiffer, C.A. Structural and Thermodynamic Basis of Amprenavir/Darunavir and Atazanavir Resistance in HIV-1 Protease with Mutations at Residue 50. J. Virol. 2013, 87, 4176–4184. [Google Scholar] [CrossRef]
- Damm, K.L.; Ung, P.M.U.; Quintero, J.J.; Gestwicki, J.E.; Carlson, H.A. A poke in the eye: Inhibiting HIV-1 protease through its flap-recognition pocket. Biopolymers 2008, 89, 643–652. [Google Scholar] [CrossRef]
- Ung, P.M.-U.; Dunbar, J.B.; Gestwicki, J.E.; Carlson, H.A. An Allosteric Modulator of HIV-1 Protease Shows Equipotent Inhibition of Wild-Type and Drug-Resistant Proteases. J. Med. Chem. 2014, 57, 6468–6478. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Ajamgard, M.; Sardroodi, J.J.; Ebrahimzadeh, A.R. A Molecular Dynamics Study of the Inhibition of Monomeric HIV-1 Protease as An Alternative to Overcome Drug Resistance by RNA Aptamers as A Therapeutic Tool. ChemistrySelect 2020, 5, 9086–9096. [Google Scholar] [CrossRef]
- Qureshi, A.; Thakur, N.; Kumar, M. HIPdb: A Database of Experimentally Validated HIV Inhibiting Peptides. PLoS ONE 2013, 8, e54908. [Google Scholar] [CrossRef] [PubMed]
- Babé, L.M.; Rosé, J.; Craik, C.S. Synthetic ‘interface’ peptides alter dimeric assembly of the HIV 1 and 2 proteases. Protein Sci. 1992, 1, 1244–1253. [Google Scholar] [CrossRef]
- Schramm, H.J.; Boetzel, J.; Büttner, J.; Fritsche, E.; Göhring, W.; Jaeger, E.; König, S.; Thumfart, O.; Wenger, T.; Nagel, N.E.; et al. The inhibition of human immunodeficiency virus proteases by ‘interface peptides’. Antiviral Res. 1996, 30, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Broglia, R.A.; Tiana, G. Thermodynamics of strongly allosteric inhibition: A model study of HIV-1 protease. Eur. Biophys. J. EBJ 2012, 41, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Broglia, R.; Levy, Y.; Tiana, G. HIV-1 protease folding and the design of drugs which do not create resistance. Curr. Opin. Struct. Biol. 2008, 18, 60–66. [Google Scholar] [CrossRef]
- Broglia, R.A.; Tiana, G.; Sutto, L.; Provasi, D.; Simona, F. Design of HIV-1-PR inhibitors that do not create resistance: Blocking the folding of single monomers. Protein Sci. 2005, 14, 2668–2681. [Google Scholar] [CrossRef]

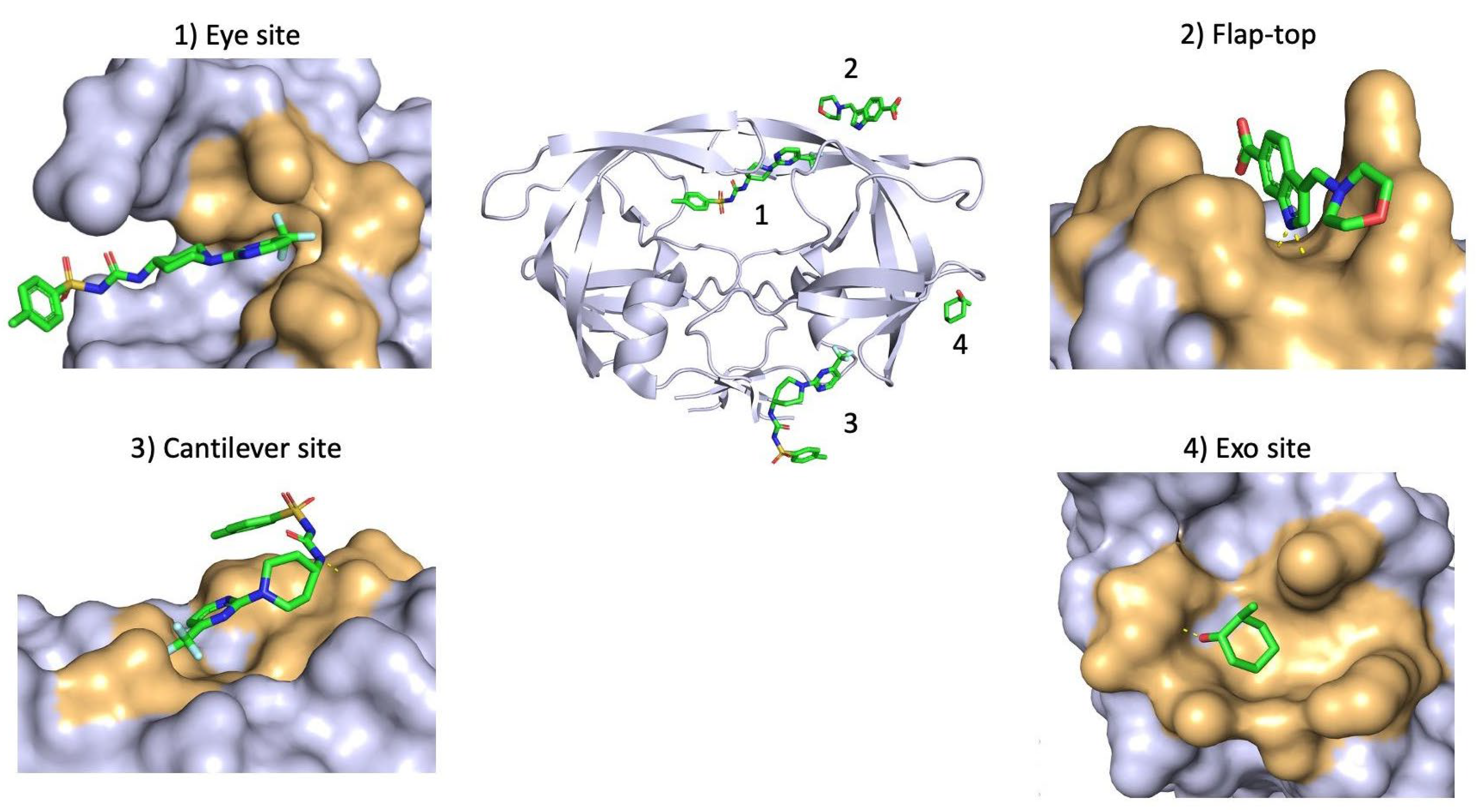

| Binding site | Associated Residues | Allosteric Binder | PDB ID | Reference |
|---|---|---|---|---|
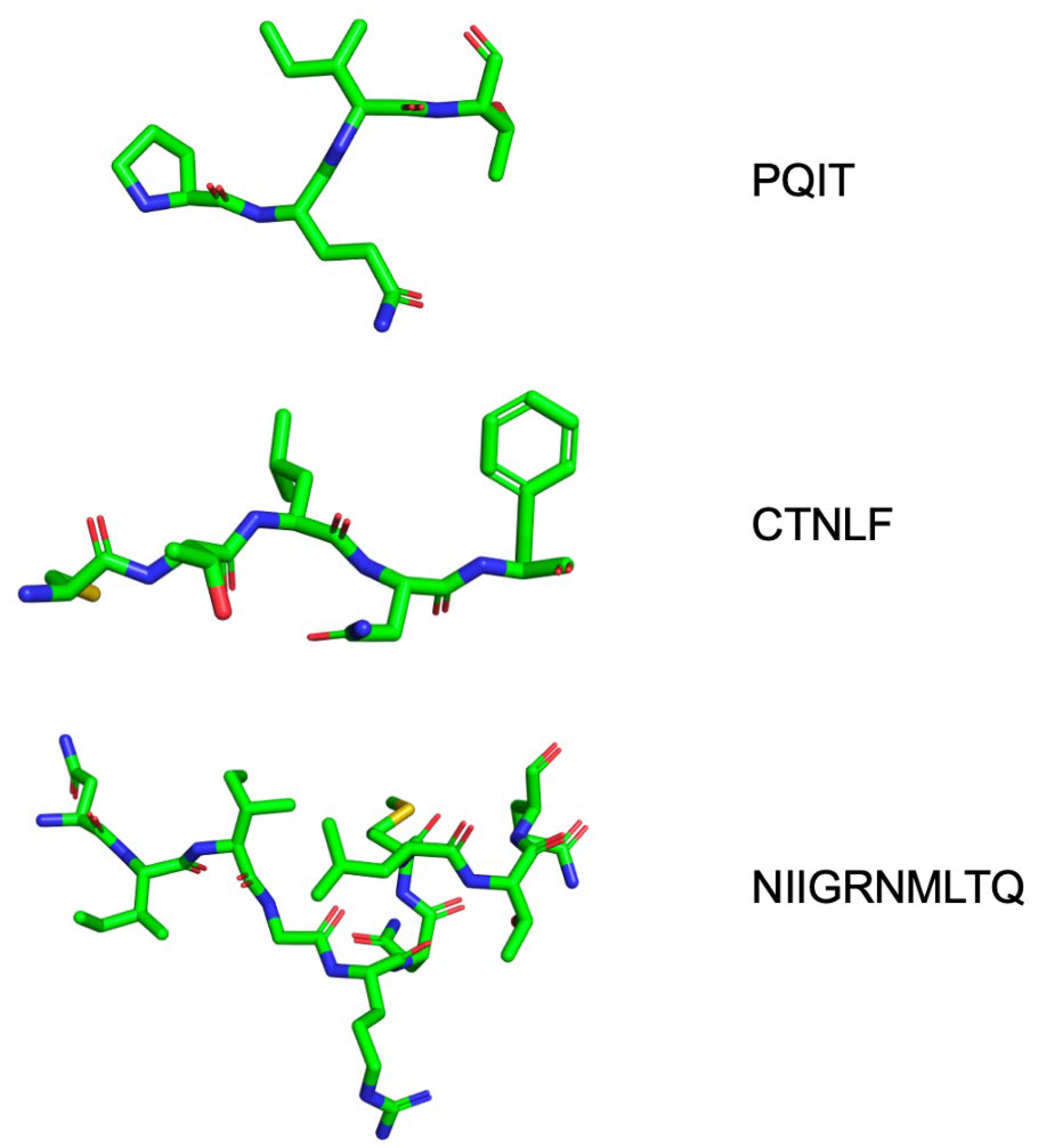
| Exo site | K14 G16 G17 N18 L63 E65 L70 | 4D9 | 3KFN | [107] |
| Br7 | N/A | [108] | ||
| Flap-top pocket | Y42 P44 M46 K55 R57 P79 | 1F1-N | 4EJK | [109] |
| AK-2097 | 4TVG | [109] | ||
| Br6 | 2AZC | [110] | ||
| DRV | 3UCB | [111] | ||
| ATV | 3OXV | [112] | ||
| Eye Site | V32 I47 G48 G49 I50 I54 V56 G78 P79 T80 P81 I84 | DHQB | N/A | [113] |
| HIVE 9 | 5VJ3 | N/A | ||
| NIT | N/A | [114] | ||
| 5NI | N/A | [107] | ||
| Cantilever site | P1 Q2 I3 K7 T12 C67 | HIVE 9 | 5W5W | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherry, D.; Sheik Ismail, Z.; Mokhantso, T.; Sayed, Y. Subtype-Specific HIV-1 Protease and the Role of Hinge and Flap Dynamics in Drug Resistance: A Subtype C Narrative. Viruses 2025, 17, 1044. https://doi.org/10.3390/v17081044
Sherry D, Sheik Ismail Z, Mokhantso T, Sayed Y. Subtype-Specific HIV-1 Protease and the Role of Hinge and Flap Dynamics in Drug Resistance: A Subtype C Narrative. Viruses. 2025; 17(8):1044. https://doi.org/10.3390/v17081044
Chicago/Turabian StyleSherry, Dean, Zaahida Sheik Ismail, Tshele Mokhantso, and Yasien Sayed. 2025. "Subtype-Specific HIV-1 Protease and the Role of Hinge and Flap Dynamics in Drug Resistance: A Subtype C Narrative" Viruses 17, no. 8: 1044. https://doi.org/10.3390/v17081044
APA StyleSherry, D., Sheik Ismail, Z., Mokhantso, T., & Sayed, Y. (2025). Subtype-Specific HIV-1 Protease and the Role of Hinge and Flap Dynamics in Drug Resistance: A Subtype C Narrative. Viruses, 17(8), 1044. https://doi.org/10.3390/v17081044





