Parvovirus RNA Processing: Compact Genomic Organization and Unique Alternative mRNA Processing Mechanisms
Abstract
1. Introduction
2. Parvovirus Replication and Host Interaction
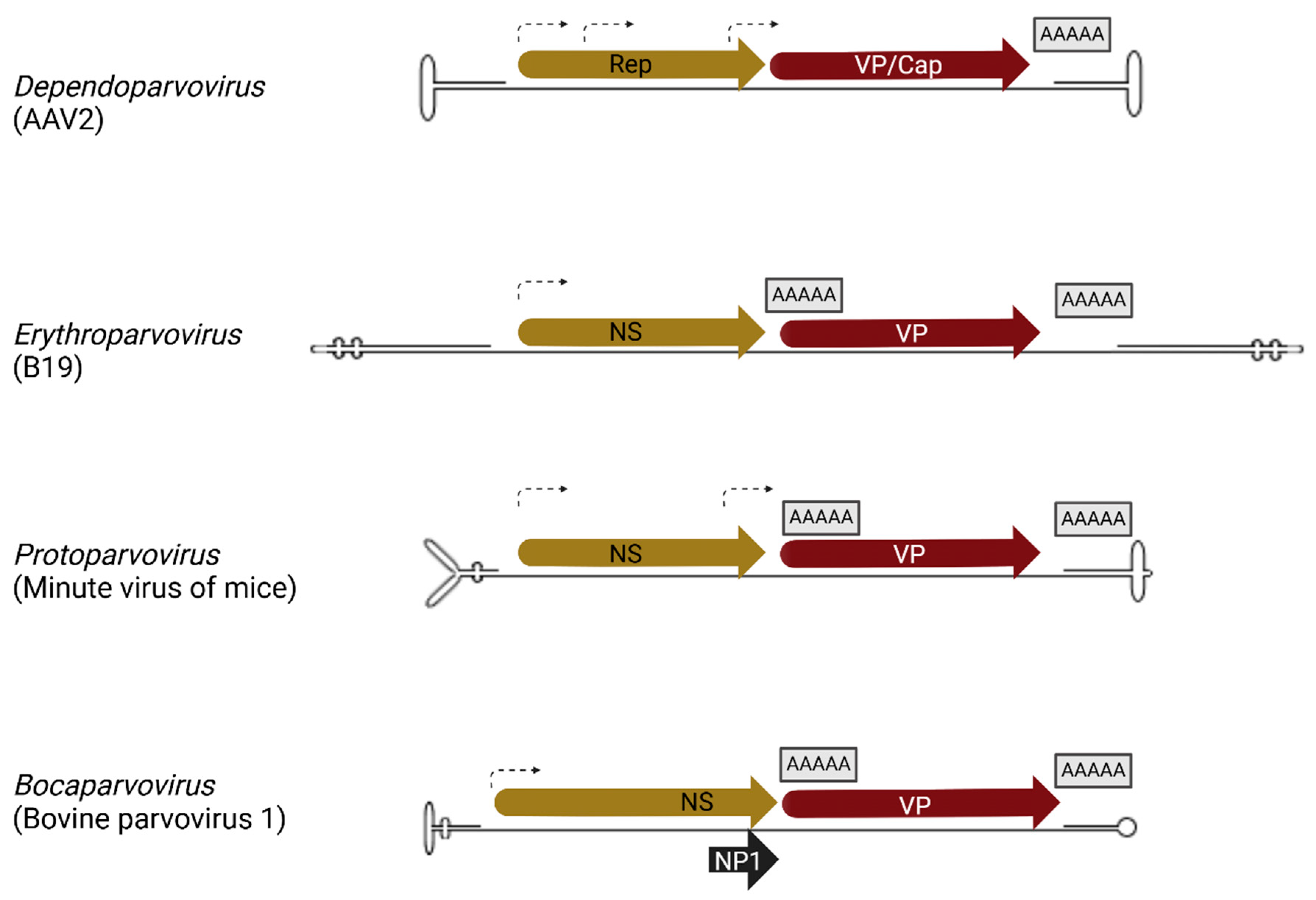
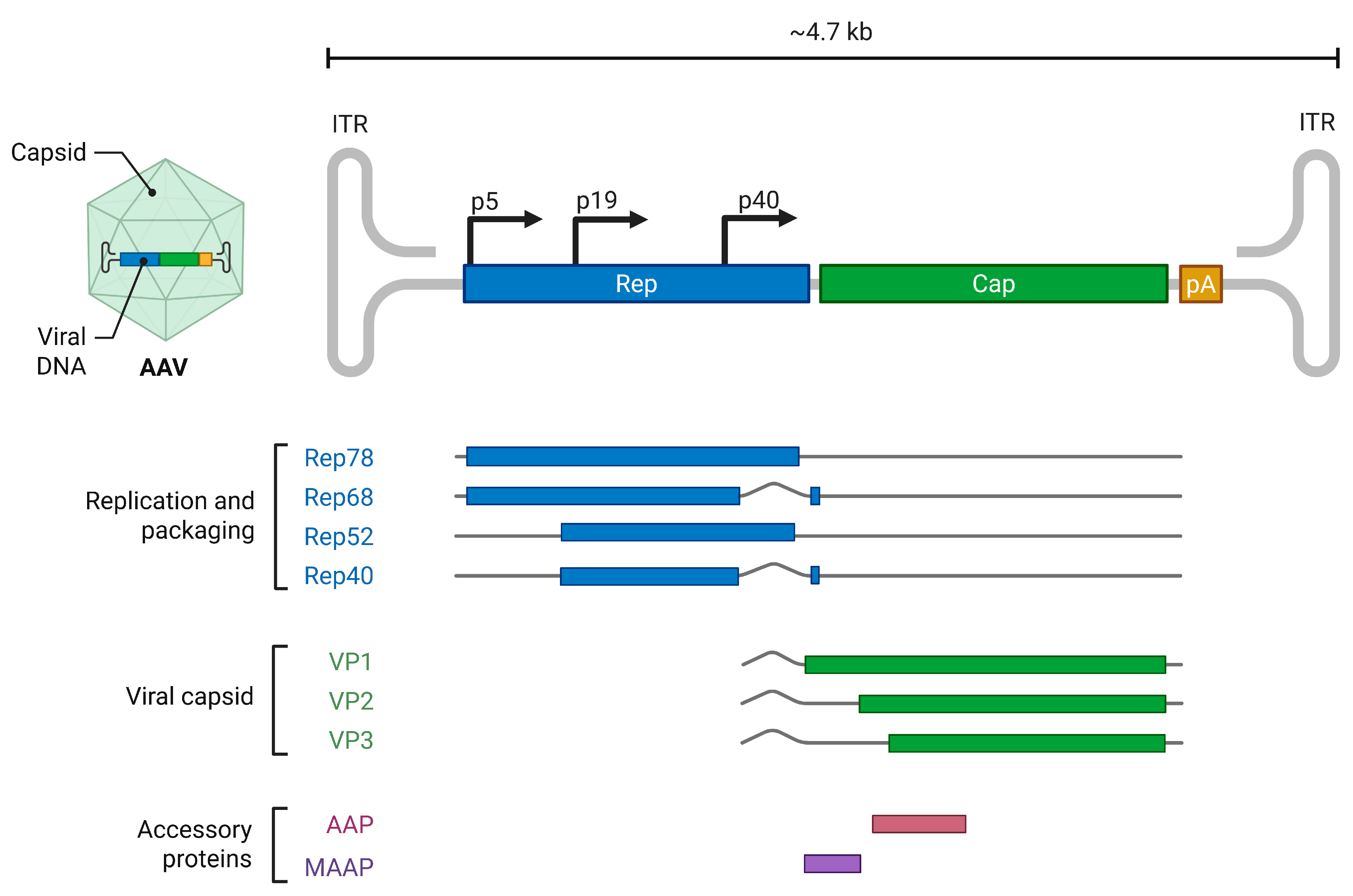
2.1. Dependoparvovirus Adeno-Associated Virus
2.2. Bocaparvovirus Minute Virus of Canines (MVC)
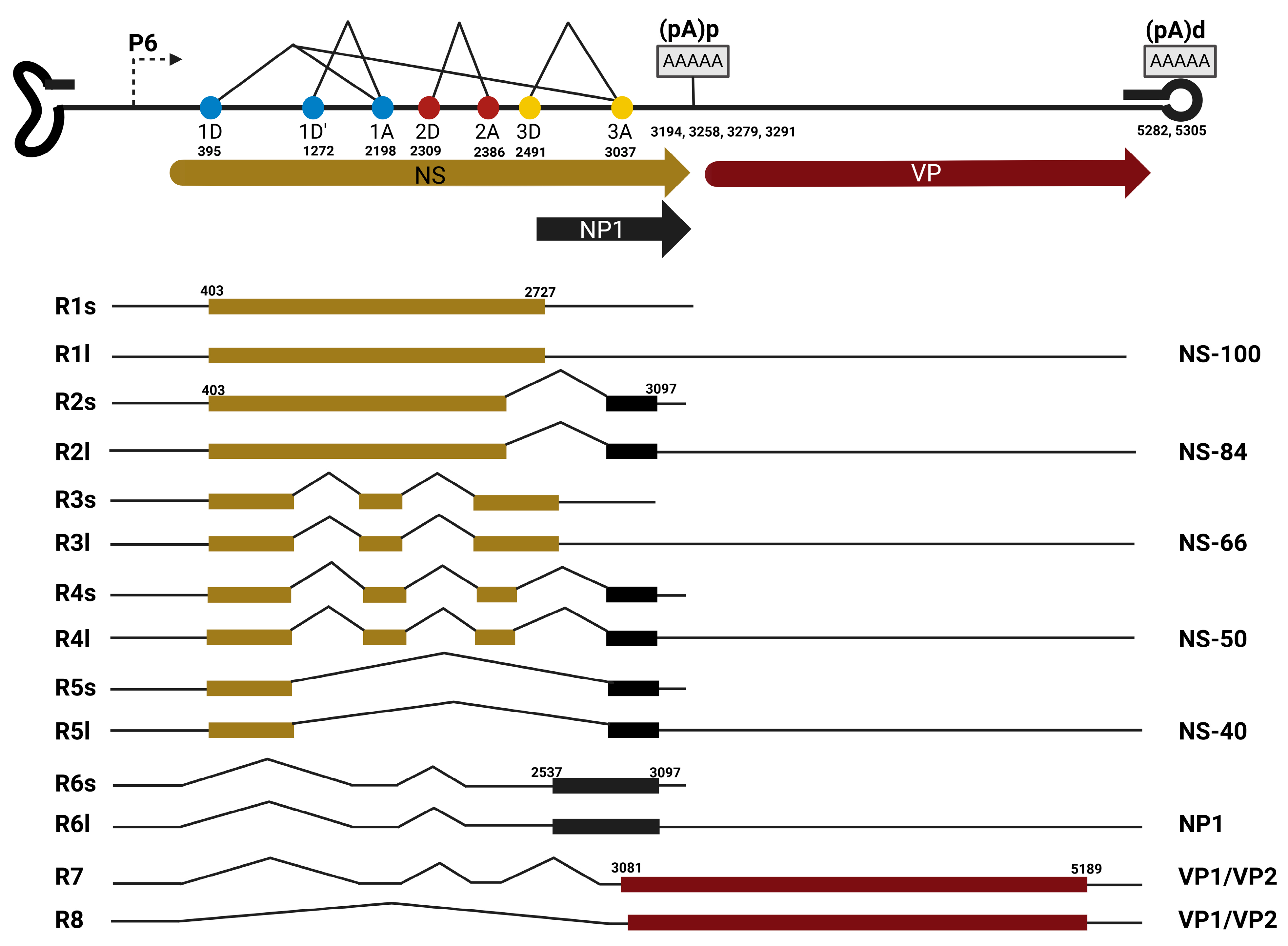
2.2.1. Bocaparvovirus Minute Virus of Canines (MVC) Genomic Organization and Transcription Profile
2.2.2. Bocaparvovirus Minute Virus of Canine (MVC) Replication and Host Cell Interactions
3. Parvoviral mRNA Processing Strategies
3.1. Alternative Splicing
3.1.1. Bocaparvovirus Alternative Splicing
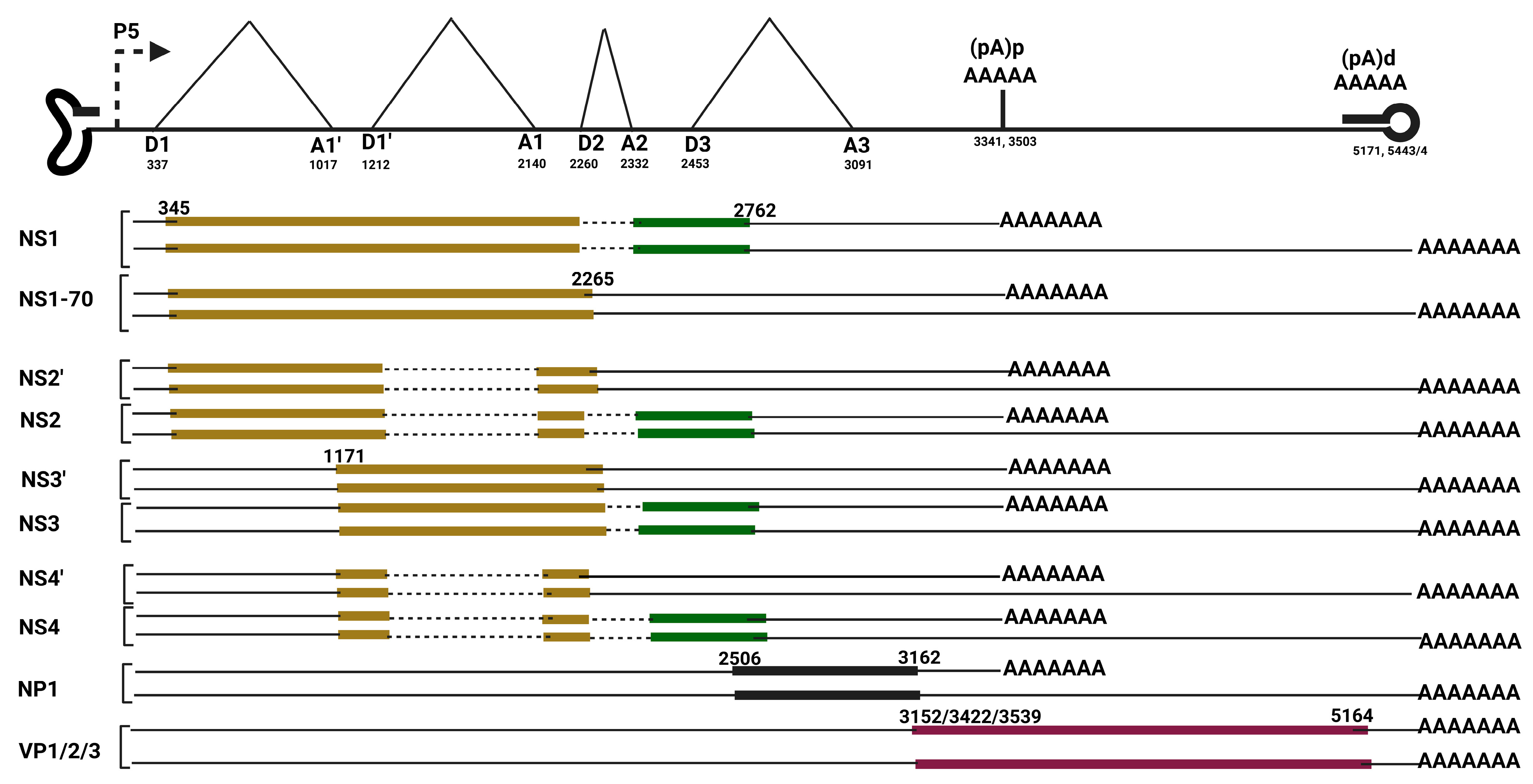
3.1.2. Dependoparvovirus Alternative Splicing
3.2. Alternative Polyadenylation
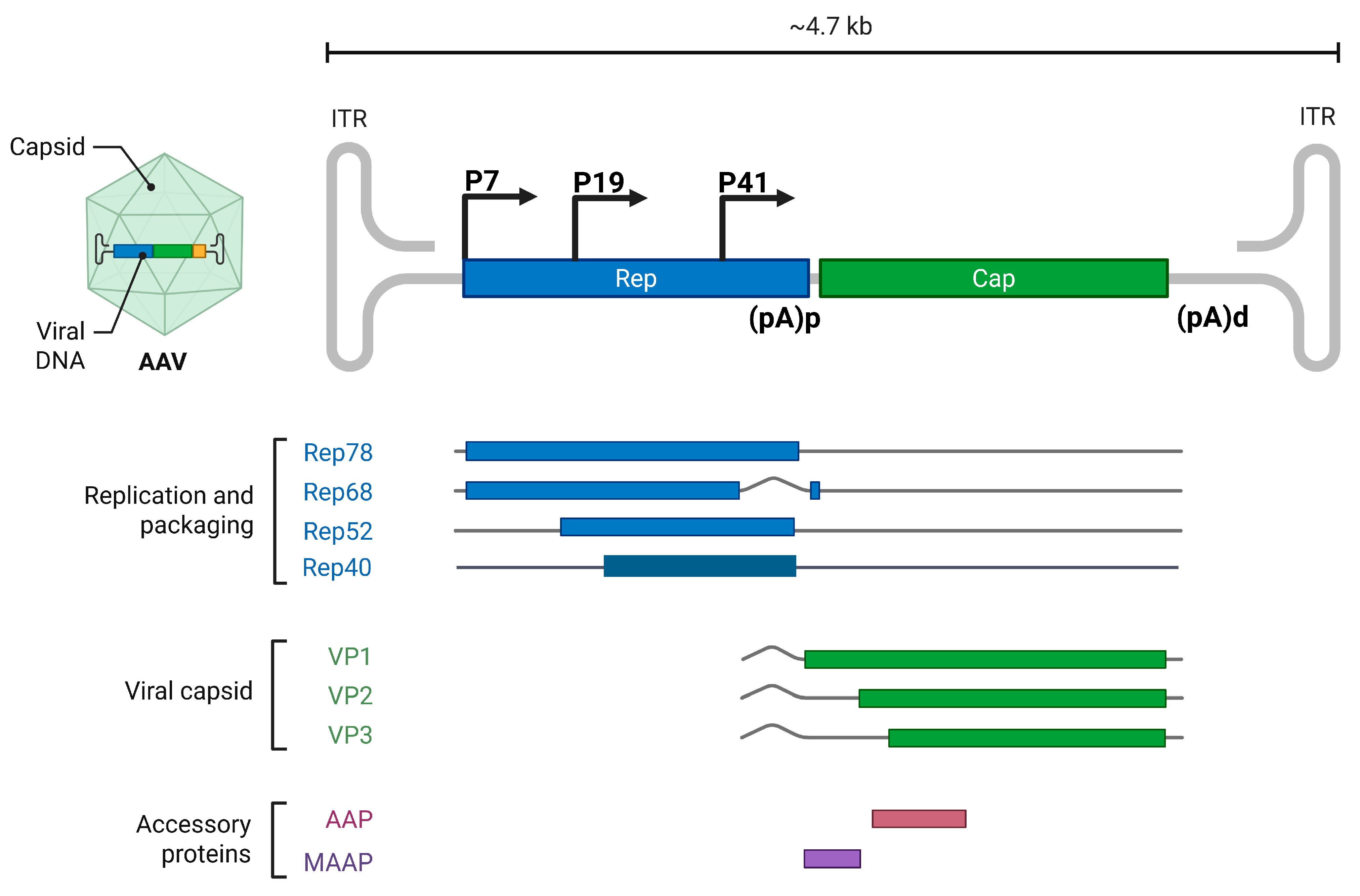
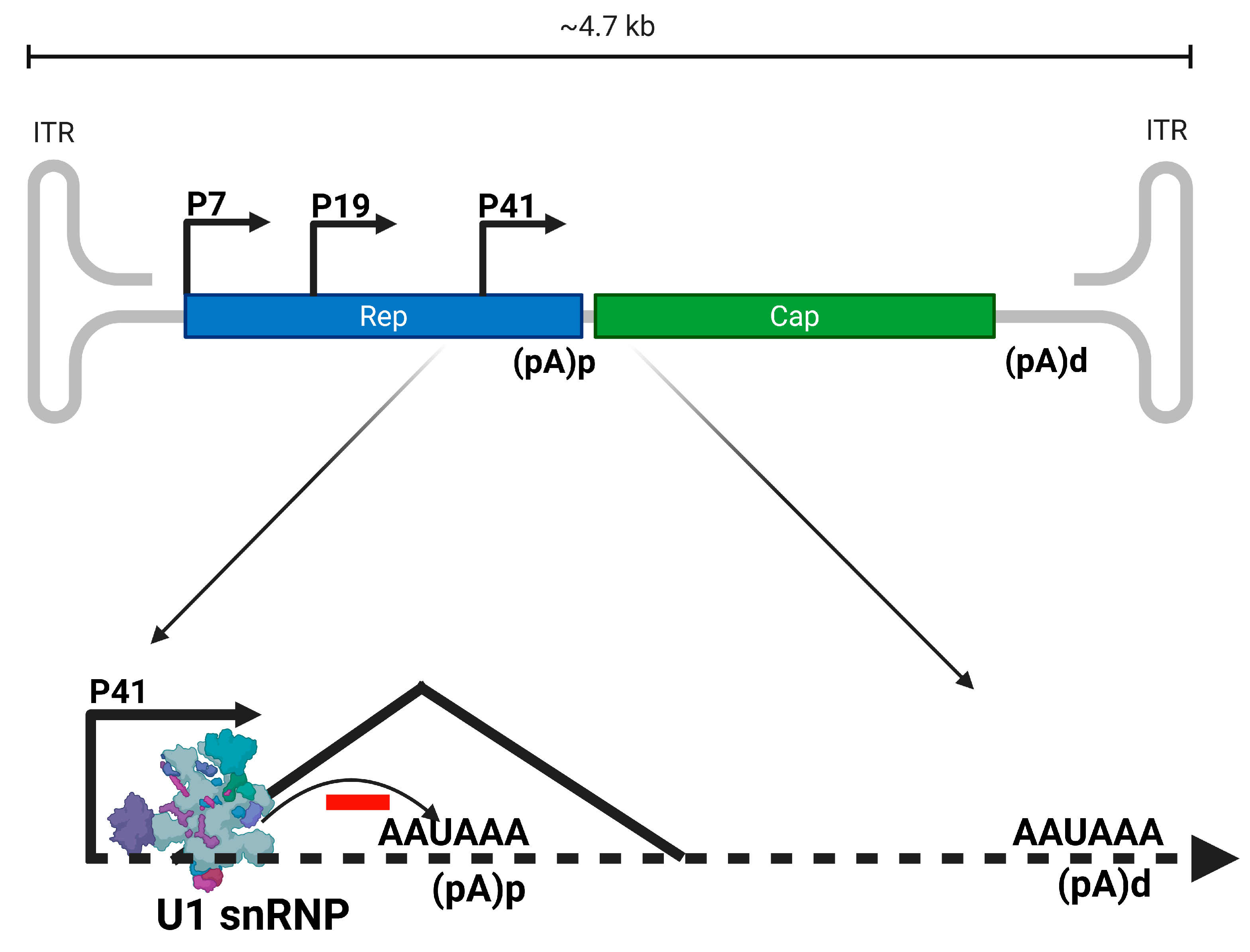
3.2.1. Dependoparvovirus Alternative Polyadenylation
3.2.2. Bocaparvovirus Alternative Polyadenylation
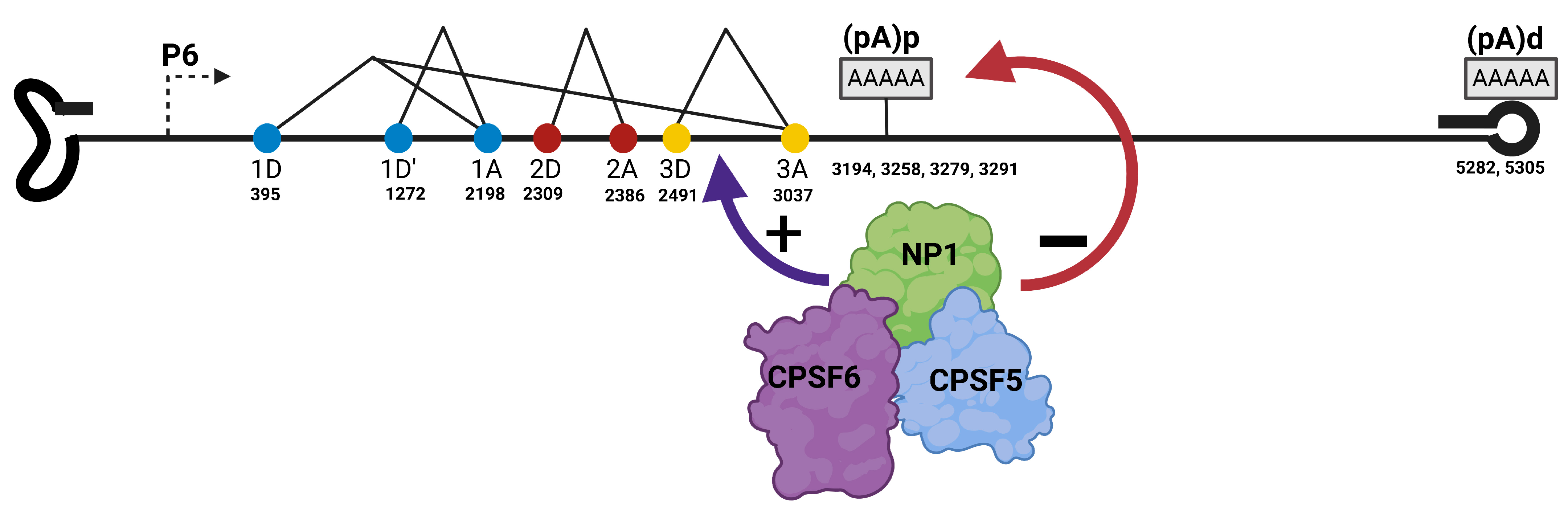
4. Parvovirus RNA Export and Alternative Translation Initiation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AAV | Adeno-associated virus |
| MVC | Minute Virus of Canines |
| HBoV | Human Bocaparvovirus 1 |
| (pA)p | Proximal Polyadenylation |
References
- Jager, M.C.; Tomlinson, J.E.; Lopez-Astacio, R.A.; Parrish, C.R.; Van de Walle, G.R. Small but mighty: Old and new parvoviruses of veterinary significance. Virol. J. 2021, 18, 210. [Google Scholar] [CrossRef] [PubMed]
- Kibenge, F.; Kibenge, M.; Montes de Oca, M.; Godoy, M. Parvoviruses of Aquatic Animals. Pathogens 2024, 13, 625. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Feng, Y.; Parry, N.M.; Annamalai, D.; Carrasco, S.E.; Guo, M.; Muthupalani, S.; Erdman, S.E.; Fox, J.G. Prevalence and Pathologic Characterization of Mouse Kidney Parvovirus in Sentinel CD1 Mice. Comp. Med. 2024, 74, 344–351. [Google Scholar] [CrossRef]
- Tattersall, P. Parvoviruses, 6th ed.; Hodder Arnold: London, UK, 2006. [Google Scholar]
- Cotmore, S.F.; Tattersall, P. Parvovirus diversity and DNA damage responses. Cold Spring Harb. Perspect. Biol. 2013, 5, a012989. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Pintel, D. Processing of adeno-associated virus RNA. Front. Biosci. 2008, 13, 3101–3115. [Google Scholar] [CrossRef]
- Blundell, M.C.; Beard, C.; Astell, C.R. In vitro identification of a B19 parvovirus promoter. Virology 1987, 157, 534–538. [Google Scholar] [CrossRef]
- Doerig, C.; Beard, P.; Hirt, B. A transcriptional promoter of the human parvovirus B19 active in vitro and in vivo. Virology 1987, 157, 539–542. [Google Scholar] [CrossRef]
- Alexandersen, S.; Bloom, M.E.; Perryman, S. Detailed transcription map of Aleutian mink disease parvovirus. J. Virol. 1988, 62, 3684–3694. [Google Scholar] [CrossRef]
- Summerford, C.; Samulski, R.J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 1998, 72, 1438–1445. [Google Scholar] [CrossRef]
- Kaludov, N.; Brown, K.E.; Walters, R.W.; Zabner, J.; Chiorini, J.A. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J. Virol. 2001, 75, 6884–6893. [Google Scholar] [CrossRef]
- Kern, A.; Schmidt, K.; Leder, C.; Muller, O.J.; Wobus, C.E.; Bettinger, K.; Von der Lieth, C.W.; King, J.A.; Kleinschmidt, J.A. Identification of a heparin-binding motif on adeno-associated virus type 2 capsids. J. Virol. 2003, 77, 11072–11081. [Google Scholar] [CrossRef]
- Girod, A.; Wobus, C.E.; Zadori, Z.; Ried, M.; Leike, K.; Tijssen, P.; Kleinschmidt, J.A.; Hallek, M. The VP1 capsid protein of adeno-associated virus type 2 is carrying a phospholipase A2 domain required for virus infectivity. J. Gen. Virol. 2002, 83, 973–978. [Google Scholar] [CrossRef]
- Zadori, Z.; Szelei, J.; Lacoste, M.C.; Li, Y.; Gariepy, S.; Raymond, P.; Allaire, M.; Nabi, I.R.; Tijssen, P. A viral phospholipase A2 is required for parvovirus infectivity. Dev. Cell 2001, 1, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Porwal, M.; Cohen, S.; Snoussi, K.; Popa-Wagner, R.; Anderson, F.; Dugot-Senant, N.; Wodrich, H.; Dinsart, C.; Kleinschmidt, J.A.; Pante, N.; et al. Parvoviruses cause nuclear envelope breakdown by activating key enzymes of mitosis. PLoS Pathog. 2013, 9, e1003671. [Google Scholar] [CrossRef] [PubMed]
- Bashir, T.; Horlein, R.; Rommelaere, J.; Willwand, K. Cyclin A activates the DNA polymerase delta -dependent elongation machinery in vitro: A parvovirus DNA replication model. Proc. Natl. Acad. Sci. USA 2000, 97, 5522–5527. [Google Scholar] [CrossRef]
- Chang, L.S.; Shi, Y.; Shenk, T. Adeno-associated virus P5 promoter contains an adenovirus E1A-inducible element and a binding site for the major late transcription factor. J. Virol. 1989, 63, 3479–3488. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, M.D.; Fisher, K.J.; Wilson, J.M. Recruitment of wild-type and recombinant adeno-associated virus into adenovirus replication centers. J. Virol. 1996, 70, 1845–1854. [Google Scholar] [CrossRef]
- Cziepluch, C.; Lampel, S.; Grewenig, A.; Grund, C.; Lichter, P.; Rommelaere, J. H-1 parvovirus-associated replication bodies: A distinct virus-induced nuclear structure. J. Virol. 2000, 74, 4807–4815. [Google Scholar] [CrossRef]
- Adeyemi, R.O.; Landry, S.; Davis, M.E.; Weitzman, M.D.; Pintel, D.J. Parvovirus minute virus of mice induces a DNA damage response that facilitates viral replication. PLoS Pathog. 2010, 6, e1001141. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, A.Y.; Qiu, J. Bocavirus infection induces a DNA damage response that facilitates viral DNA replication and mediates cell death. J. Virol. 2011, 85, 133–145. [Google Scholar] [CrossRef]
- Lou, S.; Luo, Y.; Cheng, F.; Huang, Q.; Shen, W.; Kleiboeker, S.; Tisdale, J.F.; Liu, Z.; Qiu, J. Human parvovirus B19 DNA replication induces a DNA damage response that is dispensable for cell cycle arrest at phase G2/M. J. Virol. 2012, 86, 10748–10758. [Google Scholar] [CrossRef]
- Schwartz, R.A.; Carson, C.T.; Schuberth, C.; Weitzman, M.D. Adeno-associated virus replication induces a DNA damage response coordinated by DNA-dependent protein kinase. J. Virol. 2009, 83, 6269–6278. [Google Scholar] [CrossRef] [PubMed]
- Rommelaere, J.; Geletneky, K.; Angelova, A.L.; Daeffler, L.; Dinsart, C.; Kiprianova, I.; Schlehofer, J.R.; Raykov, Z. Oncolytic parvoviruses as cancer therapeutics. Cytokine Growth Factor Rev. 2010, 21, 185–195. [Google Scholar] [CrossRef]
- Mousset, S.; Ouadrhiri, Y.; Caillet-Fauquet, P.; Rommelaere, J. The cytotoxicity of the autonomous parvovirus minute virus of mice nonstructural proteins in FR3T3 rat cells depends on oncogene expression. J. Virol. 1994, 68, 6446–6453. [Google Scholar] [CrossRef]
- Atchison, R.W.; Casto, B.C.; Hammon, W.M. Adenovirus-Associated Defective Virus Particles. Science 1965, 149, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Agbandje-McKenna, M.; Kleinschmidt, J. AAV capsid structure and cell interactions. Methods Mol. Biol. 2011, 807, 47–92. [Google Scholar] [CrossRef] [PubMed]
- Yalkinoglu, A.O.; Heilbronn, R.; Burkle, A.; Schlehofer, J.R.; zur Hausen, H. DNA amplification of adeno-associated virus as a response to cellular genotoxic stress. Cancer Res. 1988, 48, 3123–3129. [Google Scholar]
- Weindler, F.W.; Heilbronn, R. A subset of herpes simplex virus replication genes provides helper functions for productive adeno-associated virus replication. J. Virol. 1991, 65, 2476–2483. [Google Scholar] [CrossRef]
- Kotin, R.M.; Siniscalco, M.; Samulski, R.J.; Zhu, X.D.; Hunter, L.; Laughlin, C.A.; McLaughlin, S.; Muzyczka, N.; Rocchi, M.; Berns, K.I. Site-specific integration by adeno-associated virus. Proc. Natl. Acad. Sci. USA 1990, 87, 2211–2215. [Google Scholar] [CrossRef]
- Kotin, R.M.; Menninger, J.C.; Ward, D.C.; Berns, K.I. Mapping and direct visualization of a region-specific viral DNA integration site on chromosome 19q13-qter. Genomics 1991, 10, 831–834. [Google Scholar] [CrossRef]
- Qing, K.; Mah, C.; Hansen, J.; Zhou, S.; Dwarki, V.; Srivastava, A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat. Med. 1999, 5, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Pillay, S.; Zou, W.; Cheng, F.; Puschnik, A.S.; Meyer, N.L.; Ganaie, S.S.; Deng, X.; Wosen, J.E.; Davulcu, O.; Yan, Z.; et al. Adeno-associated Virus (AAV) Serotypes Have Distinctive Interactions with Domains of the Cellular AAV Receptor. J. Virol. 2017, 91, e00391-17. [Google Scholar] [CrossRef] [PubMed]
- Pillay, S.; Meyer, N.L.; Puschnik, A.S.; Davulcu, O.; Diep, J.; Ishikawa, Y.; Jae, L.T.; Wosen, J.E.; Nagamine, C.M.; Chapman, M.S.; et al. An essential receptor for adeno-associated virus infection. Nature 2016, 530, 108–112. [Google Scholar] [CrossRef]
- Zengel, J.; Carette, J.E. Structural and cellular biology of adeno-associated virus attachment and entry. Adv. Virus Res. 2020, 106, 39–84. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Davidson, B.L.; Stein, C.S.; Martins, I.; Scudiero, D.; Monks, A.; Chiorini, J.A. Identification of PDGFR as a receptor for AAV-5 transduction. Nat. Med. 2003, 9, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Nonnenmacher, M.; Weber, T. Adeno-associated virus 2 infection requires endocytosis through the CLIC/GEEC pathway. Cell Host Microbe 2011, 10, 563–576. [Google Scholar] [CrossRef]
- Bartlett, J.S.; Wilcher, R.; Samulski, R.J. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J. Virol. 2000, 74, 2777–2785. [Google Scholar] [CrossRef]
- Weitzman, M.D.; Linden, R.M. Adeno-associated virus biology. Methods Mol. Biol. 2011, 807, 1–23. [Google Scholar] [CrossRef]
- Ward, P.; Dean, F.B.; O’Donnell, M.E.; Berns, K.I. Role of the adenovirus DNA-binding protein in in vitro adeno-associated virus DNA replication. J. Virol. 1998, 72, 420–427. [Google Scholar] [CrossRef]
- Nayak, R.; Pintel, D.J. Positive and negative effects of adenovirus type 5 helper functions on adeno-associated virus type 5 (AAV5) protein accumulation govern AAV5 virus production. J. Virol. 2007, 81, 2205–2212. [Google Scholar] [CrossRef]
- Nayak, R.; Pintel, D.J. Adeno-associated viruses can induce phosphorylation of eIF2alpha via PKR activation, which can be overcome by helper adenovirus type 5 virus-associated RNA. J. Virol. 2007, 81, 11908–11916. [Google Scholar] [CrossRef] [PubMed]
- Alazard-Dany, N.; Nicolas, A.; Ploquin, A.; Strasser, R.; Greco, A.; Epstein, A.L.; Fraefel, C.; Salvetti, A. Definition of herpes simplex virus type 1 helper activities for adeno-associated virus early replication events. PLoS Pathog. 2009, 5, e1000340. [Google Scholar] [CrossRef] [PubMed]
- Chandler, M.; de la Cruz, F.; Dyda, F.; Hickman, A.B.; Moncalian, G.; Ton-Hoang, B. Breaking and joining single-stranded DNA: The HUH endonuclease superfamily. Nat. Rev. Microbiol. 2013, 11, 525–538. [Google Scholar] [CrossRef]
- King, J.A.; Dubielzig, R.; Grimm, D.; Kleinschmidt, J.A. DNA helicase-mediated packaging of adeno-associated virus type 2 genomes into preformed capsids. EMBO J. 2001, 20, 3282–3291. [Google Scholar] [CrossRef] [PubMed]
- Fraefel, C.; Bittermann, A.G.; Bueler, H.; Heid, I.; Bachi, T.; Ackermann, M. Spatial and temporal organization of adeno-associated virus DNA replication in live cells. J. Virol. 2004, 78, 389–398. [Google Scholar] [CrossRef][Green Version]
- Binn, L.N.; Lazar, E.C.; Eddy, G.A.; Kajima, M. Recovery and characterization of a minute virus of canines. Infect. Immun. 1970, 1, 503–508. [Google Scholar] [CrossRef]
- Allander, T.; Tammi, M.T.; Eriksson, M.; Bjerkner, A.; Tiveljung-Lindell, A.; Andersson, B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. USA 2005, 102, 12891–12896. [Google Scholar] [CrossRef]
- Bates, R.C.; Storz, J.; Reed, D.E. Isolation and comparison of bovine parvoviruses. J. Infect. Dis. 1972, 126, 531–536. [Google Scholar] [CrossRef]
- Carmichael, L.E.; Schlafer, D.H.; Hashimoto, A. Minute virus of canines (MVC, canine parvovirus type-1): Pathogenicity for pups and seroprevalence estimate. J. Vet. Diagn. Investig. 1994, 6, 165–174. [Google Scholar] [CrossRef]
- Manteufel, J.; Truyen, U. Animal bocaviruses: A brief review. Intervirology 2008, 51, 328–334. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, A.Y.; Cheng, F.; Guan, W.; Johnson, F.B.; Qiu, J. Molecular characterization of infectious clones of the minute virus of canines reveals unique features of bocaviruses. J. Virol. 2009, 83, 3956–3967. [Google Scholar] [CrossRef] [PubMed]
- Lederman, M.; Patton, J.T.; Stout, E.R.; Bates, R.C. Virally coded noncapsid protein associated with bovine parvovirus infection. J. Virol. 1984, 49, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Hashimoto, M.; Hajima, T.; Takiguchi, M.; Hashimoto, A.; Une, Y.; Roerink, F.; Ohshima, T.; Parrish, C.R.; Carmichael, L.E. Virologic and serologic identification of minute virus of canines (canine parvovirus type 1) from dogs in Japan. J. Clin. Microbiol. 2002, 40, 3993–3998. [Google Scholar] [CrossRef]
- Adeyemi, R.O.; Pintel, D.J. Replication of minute virus of mice in murine cells is facilitated by virally induced depletion of p21. J. Virol. 2012, 86, 8328–8332. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, M.D.; Lilley, C.E.; Chaurushiya, M.S. Genomes in conflict: Maintaining genome integrity during virus infection. Annu. Rev. Microbiol. 2010, 64, 61–81. [Google Scholar] [CrossRef]
- Lilley, C.E.; Schwartz, R.A.; Weitzman, M.D. Using or abusing: Viruses and the cellular DNA damage response. Trends Microbiol. 2007, 15, 119–126. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, L.; Gamez, M.; Imperiale, M.J. Roles of ATM and ATR-mediated DNA damage responses during lytic BK polyomavirus infection. PLoS Pathog. 2012, 8, e1002898. [Google Scholar] [CrossRef]
- Jiang, M.; Imperiale, M.J. Design stars: How small DNA viruses remodel the host nucleus. Future Virol. 2012, 7, 445–459. [Google Scholar] [CrossRef]
- Luo, Y.; Deng, X.; Cheng, F.; Li, Y.; Qiu, J. SMC1-mediated intra-S-phase arrest facilitates bocavirus DNA replication. J. Virol. 2013, 87, 4017–4032. [Google Scholar] [CrossRef]
- Chen, A.Y.; Luo, Y.; Cheng, F.; Sun, Y.; Qiu, J. Bocavirus infection induces mitochondrion-mediated apoptosis and cell cycle arrest at G2/M phase. J. Virol. 2010, 84, 5615–5626. [Google Scholar] [CrossRef]
- Cotmore, S.F.; Tattersall, P. The autonomously replicating parvoviruses of vertebrates. Adv. Virus Res. 1987, 33, 91–174. [Google Scholar]
- Zhang, X.; Guo, J.; Xu, H.; Ding, S.; Liu, L.; Chen, Z.; Yang, J.; Liu, Y.; Hao, H.; Huang, F.; et al. NS1-mediated enhancement of MVC transcription and replication promoted by KAT5/H4K12ac. J. Virol. 2024, 98, e0169523. [Google Scholar] [CrossRef]
- Ayoubi, T.A.; Van De Ven, W.J. Regulation of gene expression by alternative promoters. FASEB J. 1996, 10, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Schwartz, S. Regulation of human papillomavirus gene expression by splicing and polyadenylation. Nat. Rev. Microbiol. 2013, 11, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.; Weisburd, B.; Stern-Ginossar, N.; Mercier, A.; Madrid, A.S.; Bellare, P.; Holdorf, M.; Weissman, J.S.; Ganem, D. KSHV 2.0: A comprehensive annotation of the Kaposi’s sarcoma-associated herpesvirus genome using next-generation sequencing reveals novel genomic and functional features. PLoS Pathog. 2014, 10, e1003847. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Roeder, R.G. Definition of a novel promoter for the major adenovirus-associated virus mRNA. Cell 1980, 22, 231–242. [Google Scholar] [CrossRef]
- Sharp, P.A. Speculations on RNA splicing. Cell 1981, 23, 643–646. [Google Scholar] [CrossRef]
- Barash, Y.; Calarco, J.A.; Gao, W.; Pan, Q.; Wang, X.; Shai, O.; Blencowe, B.J.; Frey, B.J. Deciphering the splicing code. Nature 2010, 465, 53–59. [Google Scholar] [CrossRef]
- Kornblihtt, A.R.; Schor, I.E.; Allo, M.; Dujardin, G.; Petrillo, E.; Munoz, M.J. Alternative splicing: A pivotal step between eukaryotic transcription and translation. Nat. Rev. Mol. Cell Biol. 2013, 14, 153–165. [Google Scholar] [CrossRef]
- Ward, A.J.; Cooper, T.A. The pathobiology of splicing. J. Pathol. 2010, 220, 152–163. [Google Scholar] [CrossRef]
- Pimentel, H.; Parra, M.; Gee, S.; Ghanem, D.; An, X.; Li, J.; Mohandas, N.; Pachter, L.; Conboy, J.G. A dynamic alternative splicing program regulates gene expression during terminal erythropoiesis. Nucleic Acids Res. 2014, 42, 4031–4042. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lawrence, M.S.; Wan, Y.; Stojanov, P.; Sougnez, C.; Stevenson, K.; Werner, L.; Sivachenko, A.; DeLuca, D.S.; Zhang, L.; et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N. Engl. J. Med. 2011, 365, 2497–2506. [Google Scholar] [CrossRef]
- Bentley, D.L. Coupling mRNA processing with transcription in time and space. Nat. Rev. Genet. 2014, 15, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Luco, R.F.; Pan, Q.; Tominaga, K.; Blencowe, B.J.; Pereira-Smith, O.M.; Misteli, T. Regulation of alternative splicing by histone modifications. Science 2010, 327, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Graveley, B.R. Alternative splicing: Increasing diversity in the proteomic world. Trends Genet. 2001, 17, 100–107. [Google Scholar] [CrossRef]
- Fasina, O.O.; Dong, Y.; Pintel, D.J. NP1 Protein of the Bocaparvovirus Minute Virus of Canines Controls Access to the Viral Capsid Genes via Its Role in RNA Processing. J. Virol. 2016, 90, 1718–1728. [Google Scholar] [CrossRef]
- Fasina, O.O.; Stupps, S.; Figueroa-Cuilan, W.; Pintel, D.J. Minute Virus of Canines NP1 Protein Governs the Expression of a Subset of Essential Nonstructural Proteins via Its Role in RNA Processing. J. Virol. 2017, 91, e00260-17. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, S.; Huang, F.; Liu, H.; Wang, J.; Chen, Z.; Hao, H.; Ding, S.; Liu, L.; Yu, B.; et al. N4-acetylcytidine coordinates with NP1 and CPSF5 to facilitate alternative RNA processing during the replication of minute virus of canines. Nucleic Acids Res. 2025, 53, gkaf229. [Google Scholar] [CrossRef]
- Mihaylov, I.S.; Cotmore, S.F.; Tattersall, P. Complementation for an essential ancillary non-structural protein function across parvovirus genera. Virology 2014, 468–470, 226–237. [Google Scholar] [CrossRef]
- Qiu, J.; Cheng, F.; Pintel, D.J. Expression profiles of bovine adeno-associated virus and avian adeno-associated virus display significant similarity to that of adeno-associated virus type 5. J. Virol. 2006, 80, 5482–5493. [Google Scholar] [CrossRef]
- Berns, K.I.; Giraud, C. Biology of adeno-associated virus. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 1996; Volume 218, pp. 1–23. [Google Scholar]
- Mouw, M.B.; Pintel, D.J. Adeno-associated virus RNAs appear in a temporal order and their splicing is stimulated during coinfection with adenovirus. J. Virol. 2000, 74, 9878–9888. [Google Scholar] [CrossRef]
- Trempe, J.P.; Carter, B.J. Regulation of adeno-associated virus gene expression in 293 cells: Control of mRNA abundance and translation. J. Virol. 1988, 62, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Pintel, D.J. The adeno-associated virus type 2 Rep protein regulates RNA processing via interaction with the transcription template. Mol. Cell. Biol. 2002, 22, 3639–3652. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Nayak, R.; Tullis, G.E.; Pintel, D.J. Characterization of the transcription profile of adeno-associated virus type 5 reveals a number of unique features compared to previously characterized adeno-associated viruses. J. Virol. 2002, 76, 12435–12447. [Google Scholar] [CrossRef] [PubMed]
- Farris, K.D.; Pintel, D.J. Adeno-associated virus type 5 utilizes alternative translation initiation to encode a small Rep40-like protein. J. Virol. 2010, 84, 1193–1197. [Google Scholar] [CrossRef][Green Version]
- Moore, M.J.; Proudfoot, N.J. Pre-mRNA processing reaches back to transcription and ahead to translation. Cell 2009, 136, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Hollerer, I.; Grund, K.; Hentze, M.W.; Kulozik, A.E. mRNA 3′end processing: A tale of the tail reaches the clinic. EMBO Mol. Med. 2014, 6, 16–26. [Google Scholar] [CrossRef]
- Lutz, C.S. Alternative polyadenylation: A twist on mRNA 3′end formation. ACS Chem. Biol. 2008, 3, 609–617. [Google Scholar] [CrossRef]
- Mayr, C.; Bartel, D.P. Widespread shortening of 3′UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell 2009, 138, 673–684. [Google Scholar] [CrossRef]
- Guan, W.; Huang, Q.; Cheng, F.; Qiu, J. Internal polyadenylation of the parvovirus B19 precursor mRNA is regulated by alternative splicing. J. Biol. Chem. 2011, 286, 24793–24805. [Google Scholar] [CrossRef]
- Schrom, E.M.; Moschall, R.; Schuch, A.; Bodem, J. Regulation of retroviral polyadenylation. Adv. Virus Res. 2013, 85, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Schrom, E.M.; Moschall, R.; Hartl, M.J.; Weitner, H.; Fecher, D.; Langemeier, J.; Bohne, J.; Wohrl, B.M.; Bodem, J. U1snRNP-mediated suppression of polyadenylation in conjunction with the RNA structure controls poly (A) site selection in foamy viruses. Retrovirology 2013, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Di Giammartino, D.C.; Shi, Y.; Manley, J.L. PARP1 represses PAP and inhibits polyadenylation during heat shock. Mol. Cell 2013, 49, 7–17. [Google Scholar] [CrossRef]
- Peterson, M.L. Immunoglobulin heavy chain gene regulation through polyadenylation and splicing competition. Wiley Interdiscip. Rev. RNA 2011, 2, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Xiong, M.; Deng, X.; Engelhardt, J.F.; Yan, Z.; Qiu, J. A Comprehensive RNA-seq Analysis of Human Bocavirus 1 Transcripts in Infected Human Airway Epithelium. Viruses 2019, 11, 33. [Google Scholar] [CrossRef]
- Shao, L.; Shen, W.; Wang, S.; Qiu, J. Recent Advances in Molecular Biology of Human Bocavirus 1 and Its Applications. Front. Microbiol. 2021, 12, 696604. [Google Scholar] [CrossRef]
- Dong, Y.; Fasina, O.O.; Pintel, D.J. Minute Virus of Canines NP1 Protein Interacts with the Cellular Factor CPSF6 To Regulate Viral Alternative RNA Processing. J. Virol. 2019, 93, e01530-18. [Google Scholar] [CrossRef]
- Qin, S.; Chen, H.; Tian, C.; Chen, Z.; Zuo, L.; Zhang, X.; Hao, H.; Huang, F.; Liu, H.; Sun, X.; et al. NS1-mediated DNMT1 degradation regulates human bocavirus 1 replication and RNA processing. PLoS Pathog. 2024, 20, e1012682. [Google Scholar] [CrossRef]
- Seidler, J.F.; Sträßer, K. Understanding nuclear mRNA export: Survival under stress. Mol. Cell 2024, 84, 3681–3691. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, Y.; Ma, X.; Shang, G.; Liu, B.; Zhang, K. Virus Infection and mRNA Nuclear Export. Int. J. Mol. Sci. 2023, 24, 12593. [Google Scholar] [CrossRef]
- Li, L.; Pintel, D.J. Splicing of goose parvovirus pre-mRNA influences cytoplasmic translation of the processed mRNA. Virology 2012, 426, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Mohr, I. Viral subversion of the host protein synthesis machinery. Nat. Rev. Microbiol. 2011, 9, 860–875. [Google Scholar] [CrossRef]
- Kozak, M. Pushing the limits of the scanning mechanism for initiation of translation. Gene 2002, 299, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Firth, A.E.; Brierley, I. Non-canonical translation in RNA viruses. J. Gen. Virol. 2012, 93, 1385–1409. [Google Scholar] [CrossRef]
- Yueh, A.; Schneider, R.J. Selective translation initiation by ribosome jumping in adenovirus-infected and heat-shocked cells. Genes Dev. 1996, 10, 1557–1567. [Google Scholar] [CrossRef]
- Remm, M.; Remm, A.; Ustav, M. Human papillomavirus type 18 E1 protein is translated from polycistronic mRNA by a discontinuous scanning mechanism. J. Virol. 1999, 73, 3062–3070. [Google Scholar] [CrossRef] [PubMed]
- Stacey, S.N.; Jordan, D.; Williamson, A.J.; Brown, M.; Coote, J.H.; Arrand, J.R. Leaky scanning is the predominant mechanism for translation of human papillomavirus type 16 E7 oncoprotein from E6/E7 bicistronic mRNA. J. Virol. 2000, 74, 7284–7297. [Google Scholar] [CrossRef]
- Qiu, J.; Cheng, F.; Pintel, D. The abundant R2 mRNA generated by aleutian mink disease parvovirus is tricistronic, encoding NS2, VP1, and VP2. J. Virol. 2007, 81, 6993–7000. [Google Scholar] [CrossRef]
- Tijssen, P.; Li, Y.; El-Far, M.; Szelei, J.; Letarte, M.; Zadori, Z. Organization and expression strategy of the ambisense genome of densonucleosis virus of Galleria mellonella. J. Virol. 2003, 77, 10357–10365. [Google Scholar] [CrossRef]
- Becerra, S.P.; Rose, J.A.; Hardy, M.; Baroudy, B.M.; Anderson, C.W. Direct mapping of adeno-associated virus capsid proteins B and C: A possible ACG initiation codon. Proc. Natl. Acad. Sci. USA 1985, 82, 7919–7923. [Google Scholar] [CrossRef]
- Fasina, O.; Pintel, D.J. The adeno-associated virus type 5 small rep proteins expressed via internal translation initiation are functional. J Virol 2013, 87, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Somberg, M.; Li, X.; Backström Winquist, E.; Fay, J.; Ryan, F.; Pim, D.; Banks, L.; Schwartz, S. HPV-16 E2 contributes to induction of HPV-16 late gene expression by inhibiting early polyadenylation. Embo J 2012, 31, 3212–3227. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, X.; Forouzmand, E.; Jeong, J.; Qiao, F.; Sowd, G.A.; Engelman, A.N.; Xie, X.; Hertel, K.J.; Shi, Y. Molecular Mechanisms for CFIm-Mediated Regulation of mRNA Alternative Polyadenylation. Mol. Cell 2018, 69, 62–74.e64. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Norbury, C.J. Cleavage factor Im (CFIm) as a regulator of alternative polyadenylation. Biochem. Soc. Trans. 2016, 44, 1051–1057. [Google Scholar] [CrossRef]
- Scarborough, A.M.; Flaherty, J.N.; Hunter, O.V.; Liu, K.; Kumar, A.; Xing, C.; Tu, B.P.; Conrad, N.K. SAM homeostasis is regulated by CFI(m)-mediated splicing of MAT2A. Elife 2021, 10, e64930. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uhl, L.K.; Fasina, O.O. Parvovirus RNA Processing: Compact Genomic Organization and Unique Alternative mRNA Processing Mechanisms. Viruses 2025, 17, 984. https://doi.org/10.3390/v17070984
Uhl LK, Fasina OO. Parvovirus RNA Processing: Compact Genomic Organization and Unique Alternative mRNA Processing Mechanisms. Viruses. 2025; 17(7):984. https://doi.org/10.3390/v17070984
Chicago/Turabian StyleUhl, Lisa K., and Olufemi O. Fasina. 2025. "Parvovirus RNA Processing: Compact Genomic Organization and Unique Alternative mRNA Processing Mechanisms" Viruses 17, no. 7: 984. https://doi.org/10.3390/v17070984
APA StyleUhl, L. K., & Fasina, O. O. (2025). Parvovirus RNA Processing: Compact Genomic Organization and Unique Alternative mRNA Processing Mechanisms. Viruses, 17(7), 984. https://doi.org/10.3390/v17070984




