Seroprevalence and Molecular Analysis of Bovine Leukemia Virus in Kazakhstan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population Characteristics
2.2. Study Design
2.3. Sampling
2.4. Laboratory Methods
2.5. Statistical Analysis
2.6. Ethical Considerations
2.7. Quality Control
2.8. Control Samples
3. Results
3.1. Seroprevalence of BLV in Cattle in Kazakhstan (2014–2024)
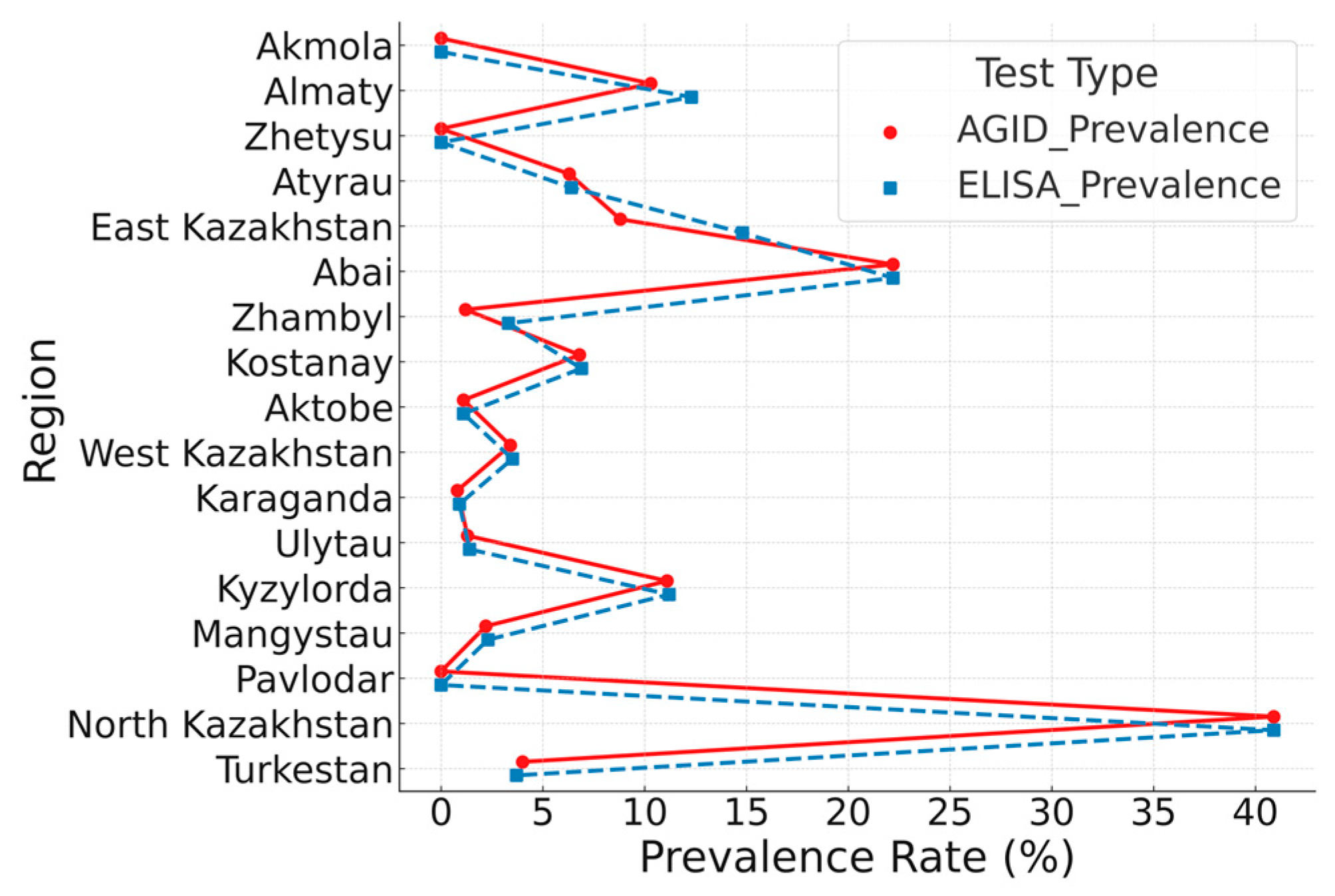
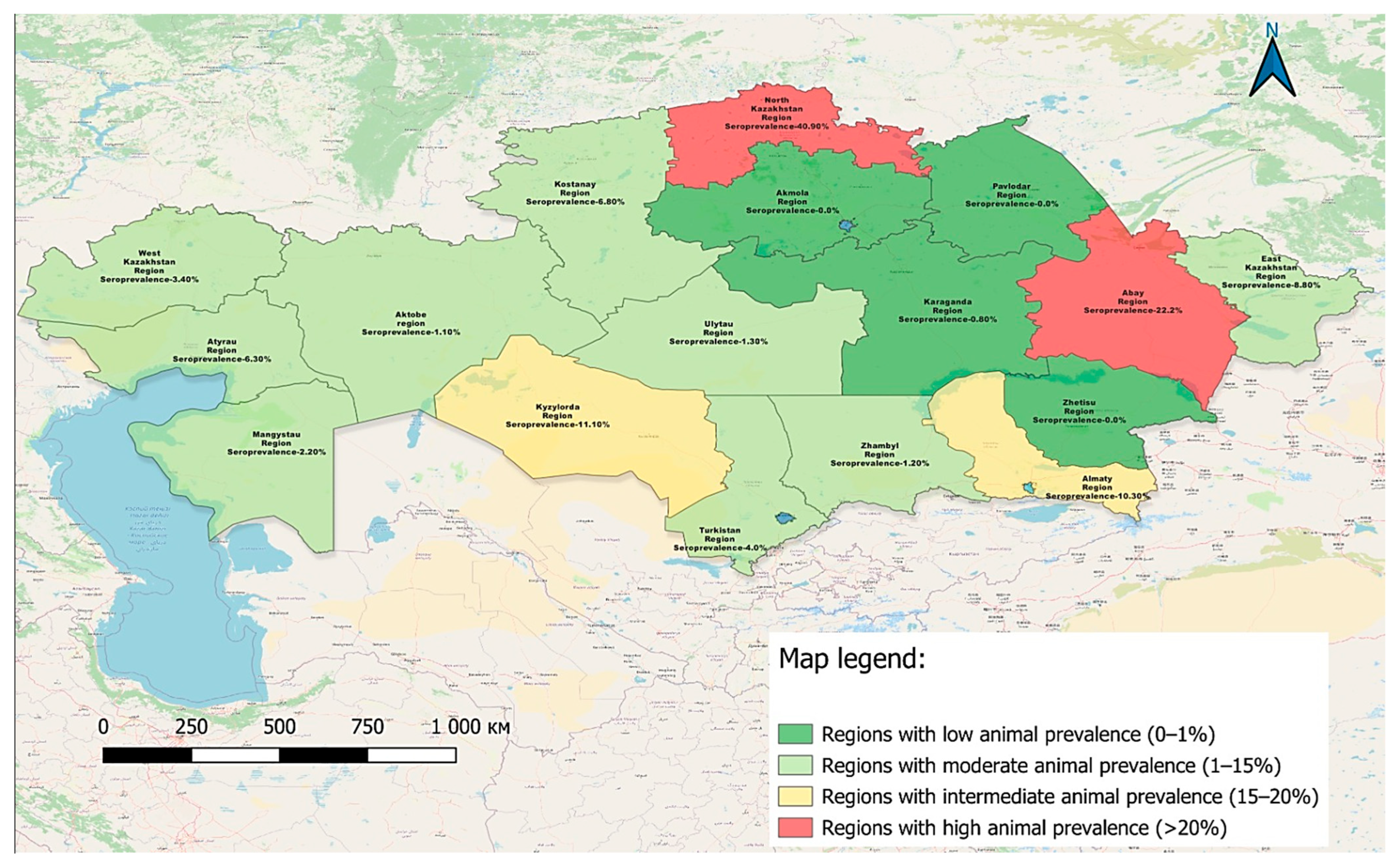
3.2. Serological Mapping and Test Performance Comparison for BLV (2024)
3.3. Diagnostic Validation of the KazSRVI AGID Test
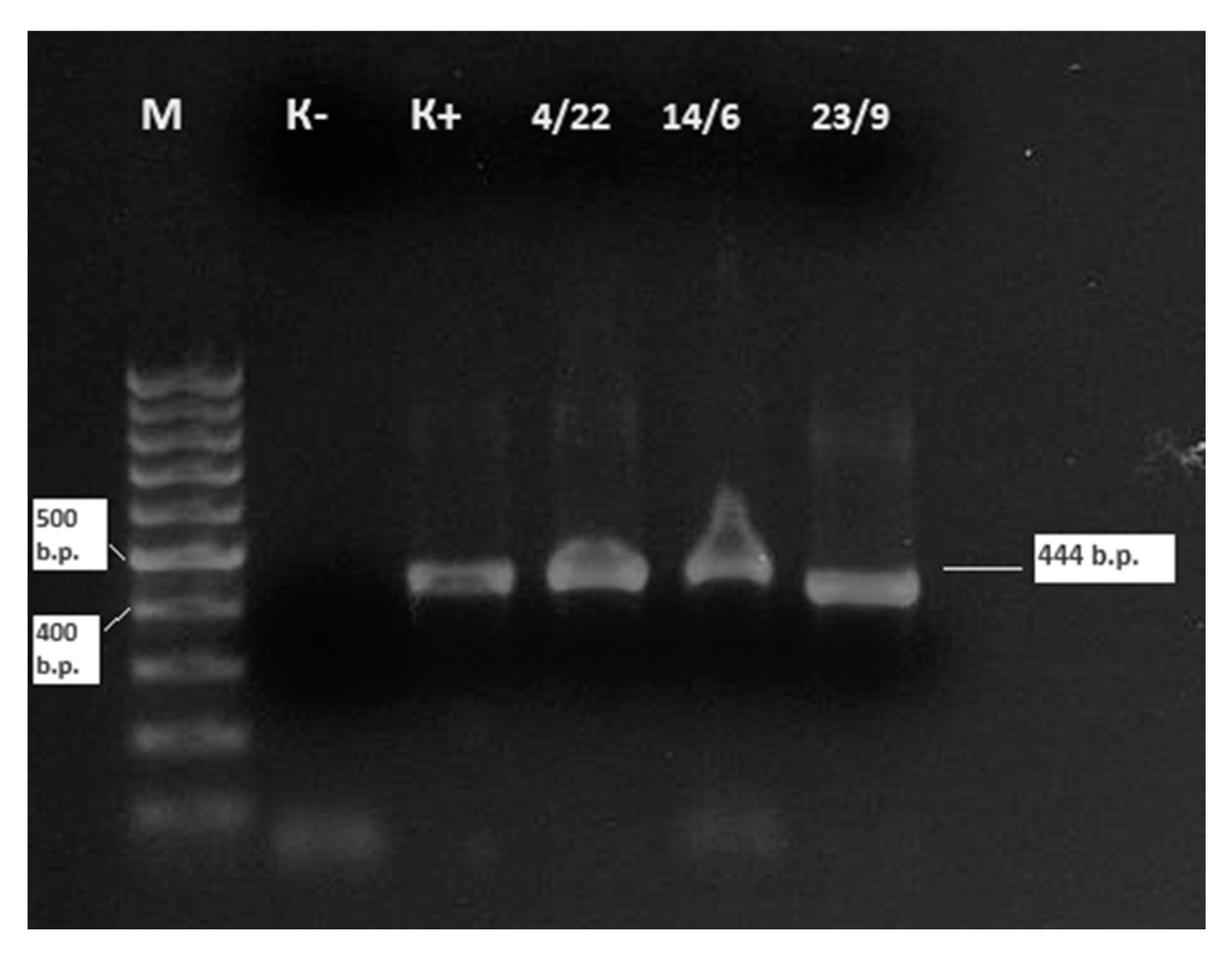
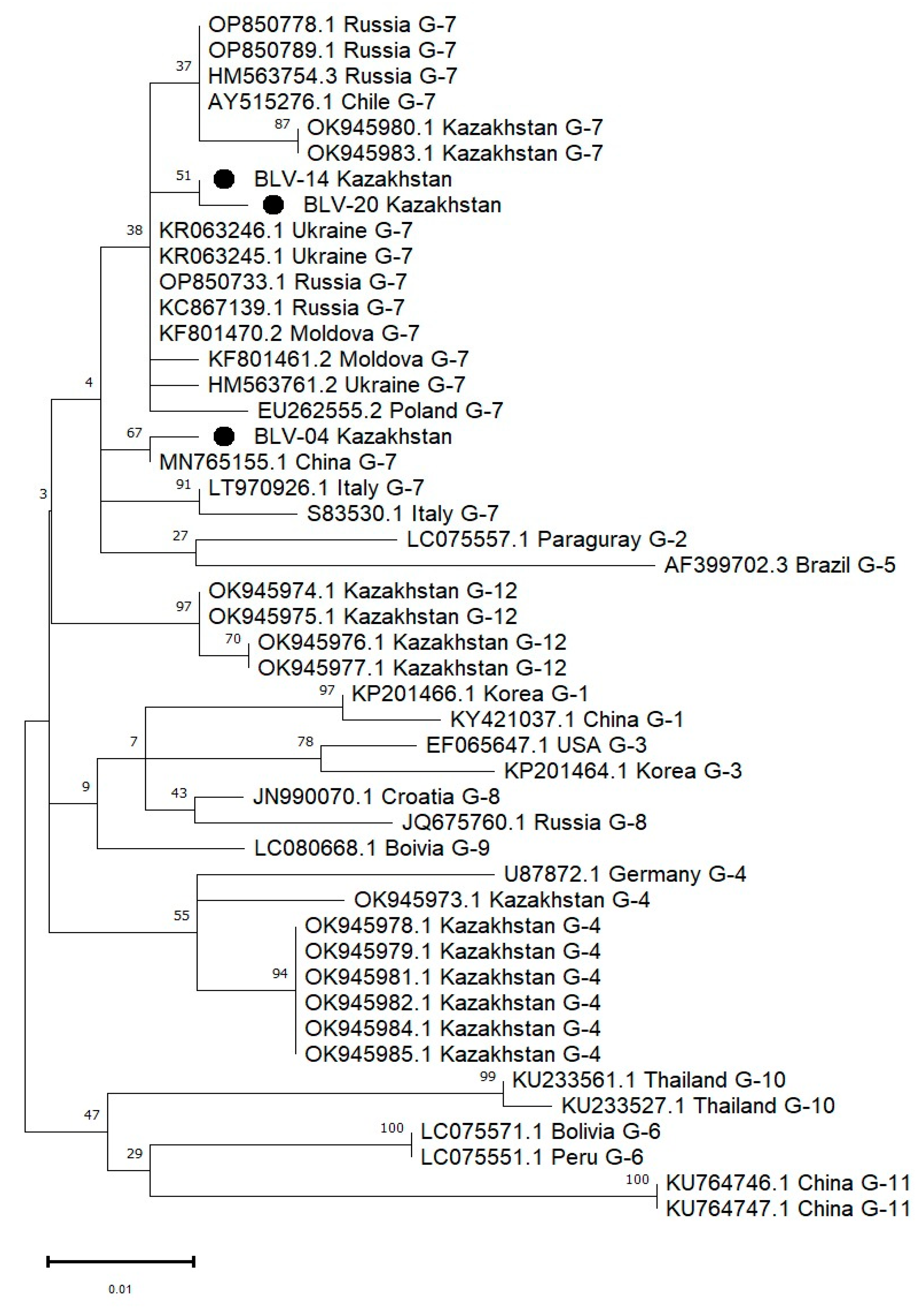
3.4. Molecular Detection and Genetic Characterization of BLV Isolates in 2024
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EBL | Enzootic Bovine Leukosis |
| AGID | Agar Gel Immunodiffusion |
| BLAST | Basic Local Alignment Search Tool |
| BLV | Bovine Leukemia Virus |
| bp | Base Pairs |
| Ct | Cycle Threshold |
| DNA | Deoxyribonucleic Acid |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| env | Envelope Gene |
| ML | Maximum Likelihood |
| NTC | No Template Control |
| OD | Optical Density |
| PCR | Polymerase Chain Reaction |
| RT-PCR | Real-Time Polymerase Chain Reaction |
| RNA | Ribonucleic Acid |
| SPSS | Statistical Package for the Social Sciences |
| WOAH | World Organisation for Animal Health (formerly OIE) |
References
- Bartlett, P.C.; Sordillo, L.M.; Byrem, T.M.; Norby, B.; Grooms, D.L.; Swenson, C.L.; Zalucha, J.; Erskine, R.J. Options for the Control of Bovine Leukemia Virus in Dairy Cattle. Javma 2014, 244, 914–922. [Google Scholar] [CrossRef]
- Marawan, M.A.; Alouffi, A.; El Tokhy, S.; Badawy, S.; Shirani, I.; Dawood, A.; Guo, A.; Almutairi, M.M.; Alshammari, F.A.; Selim, A. Bovine Leukaemia Virus: Current Epidemiological Circumstance and Future Prospective. Viruses 2021, 13, 2167. [Google Scholar] [CrossRef]
- Mamanova, S.; Kaimoldina, S.; Kassen, A.; Nissanova, R.; Bashenova, E. Study of Circulation of Bovine Leukaemia Virus Genotypes in Central Asia. CABI Rev. 2025, 20, 0045. [Google Scholar] [CrossRef]
- Asfaw, Y.; Sentsui, H.; Murakami, K.; Tsuduku, S.; Tsuboi, T.; Konishi, M.; Wu, D. Distribution and Superinfection of Bovine Leukemia Virus Genotypes in Japan. Arch. Virol. 2004, 150, 493–505. [Google Scholar] [CrossRef]
- Nobrega, D.B.; Miltenburg, C.; Séguin, G.; Kelton, D.F. Prevalence and Spatial Distribution of Infectious Diseases of Dairy Cattle in Ontario, Canada. J. Dairy Sci. 2024, 107, 5029–5040. [Google Scholar] [CrossRef]
- Hamada, R.; Metwally, S.; Polat, M.; Borjigin, L.; Ali, A.O.; Abdel-Hady, A.A.A.; Mohamed, A.E.A.; Wada, S.; Aida, Y. Detection and Molecular Characterization of Bovine Leukemia Virus in Egyptian Dairy Cattle. Front. Vet. Sci. 2020, 7, 608. [Google Scholar] [CrossRef]
- Shrestha, S.; Orsel, K.; Barkema, H.W.; Martins, L.; Shrestha, S.; Meer, F.V.D. Effects of Bovine Leukemia Virus Seropositivity and Proviral Load on Milk, Fat, and Protein Production of Dairy Cows. J. Dairy Sci. 2023, 107, 530–539. [Google Scholar] [CrossRef]
- Bai, L.; Borjigin, L.; Sato, H.; Takeshima, S.-N.; Asaji, S.; Ishizaki, H.; Kawashima, K.; Obuchi, Y.; Sunaga, S.; Ando, A.; et al. Kinetic Study of BLV Infectivity in BLV Susceptible and Resistant Cattle in Japan from 2017 to 2019. Pathogens 2021, 10, 1281. [Google Scholar] [CrossRef]
- Frie, M.C.; Coussens, P.M. Bovine Leukemia Virus: A Major Silent Threat to Proper Immune Responses in Cattle. Vet. Immunol. Immunopathol. 2015, 163, 103–114. [Google Scholar] [CrossRef]
- Borjigin, L.; Watanuki, S.; Hamada, R.; Bai, L.; Hirose, T.; Sato, H.; Yoneyama, S.; Yasui, A.; Yasuda, S.; Yamanaka, R.; et al. Effectiveness of Integrated Bovine Leukemia Virus Eradication Strategies Utilizing Cattle Carrying Resistant and Susceptible Major Histocompatibility Complex Class II DRB3 Alleles. J. Dairy Sci. 2023, 106, 9393–9409. [Google Scholar] [CrossRef]
- Nakatsuchi, A.; Sato, R.; Borjigin, L.; Takeshima, S.-N.; Sato, H.; Watanuki, S.; Aida, Y.; Bai, L.; Murakami, H.; Asaji, S.; et al. BoLA-DRB3 Polymorphism Controls Proviral Load and Infectivity of Bovine Leukemia Virus (BLV) in Milk. Pathogens 2022, 11, 210. [Google Scholar] [CrossRef]
- Borjigin, L.; Takeshima, S.-N.; Yasuda, S.; Tanaka, N.; Yamanaka, R.; Shinozaki, Y.; Lo, C.-W.; Sato, H.; Yasui, A.; Bai, L.; et al. Risk Assessment of Bovine Major Histocompatibility Complex Class II DRB3 Alleles for Perinatal Transmission of Bovine Leukemia Virus. Pathogens 2021, 10, 502. [Google Scholar] [CrossRef]
- Kobayashi, S.; Konishi, M.; Yamamoto, T.; Kameyama, K.-I.; Hayama, Y.; Tsutsui, T.; Murakami, K. Risk Factors Associated with Within-Herd Transmission of Bovine Leukemia Virus on Dairy Farms in Japan. BMC Vet. Res. 2010, 6, 1. [Google Scholar] [CrossRef]
- Pereira, J.G.; de Assunção Silva, C.; Silva, L.D.; Lima, C.A.A.; do Rosário, C.J.R.M.; Silva, E.M.C.; do Socorro Costa Oliveira Oliveira, M.; dos Santos Ribeiro, L.S.; Santos, H.P.; Abreu-Silva, A.L.; et al. Diagnosis and Phylogenetic Analysis of Bovine Leukemia Virus in Dairy Cattle in Northeastern Brazil. Front. Vet. Sci. 2023, 9, 1080994. [Google Scholar] [CrossRef]
- Úsuga-Monroy, C.; Díaz, F.J.; González-Herrera, L.G.; Echeverry-Zuluaga, J.J.; López-Herrera, A. Phylogenetic Analysis of the Partial Sequences of the Env and Tax BLV Genes Reveals the Presence of Genotypes 1 and 3 in Dairy Herds of Antioquia, Colombia. VirusDisease 2023, 34, 483–497. [Google Scholar] [CrossRef]
- Mendoza, W.; Isaza, J.P.; López, L.; López-Herrera, A.; Gutiérrez, L.A. Bovine leukemia virus Detection in Humans: A Systematic Review and Meta-Analysis. Virus Res. 2023, 335, 199186. [Google Scholar] [CrossRef]
- Bartlett, P.C.; Taxis, T.M.; Droscha, C.J.; Hutchinson, H.C.; Norby, B.; Sporer, K.R.B.; Ruggiero, V.J. Current Developments in the Epidemiology and Control of Enzootic Bovine Leukosis as Caused by Bovine Leukemia Virus. Pathogens 2020, 9, 1058. [Google Scholar] [CrossRef]
- Jaworski, J.P.; Willems, L.; Vahlenkamp, T.W.; Alvarez, I.; Rola-Łuszczak, M.; Carignano, H.A.; Murakami, K.; Kuźmak, J.; Pluta, A.; Choudhury, B.; et al. Interlaboratory Comparison of Six Real-Time PCR Assays for Detection of Bovine Leukemia Virus Proviral DNA. J. Clin. Microbiol. 2018, 56, e00304–18. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/PP (accessed on 1 April 2025).
- Sultanov, A.; Rola-Łuszczak, M.; Mamanova, S.; Ryło, A.; Osiński, Z.; Saduakassova, M.A.; Bashenova, E.; Kuźmak, J. Molecular Characterization of Bovine Leukemia Virus with the Evidence of a New Genotype Circulating in Cattle from Kazakhstan. Pathogens 2022, 11, 180. [Google Scholar] [CrossRef]
- Thrusfield, M.V. Veterinary Epidemiology, 3rd ed.; Blackwell Science: Oxford, UK, 2008; ISBN 978-1-4051-5627-1. [Google Scholar]
- WOAH—World Organisation for Animal Health. Codes and Manuals. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/ (accessed on 20 June 2025).
- Fechner, H.; Kurg, A.; Geue, L.; Blankenstein, P.; Mewes, G.; Ebner, D.; Beier, D. Evaluation of Polymerase Chain Reaction (PCR) Application in Diagnosis of Bovine Leukaemia Virus (BLV) Infection in Naturally Infected Cattle. J. Vet. Med. Ser. B 1996, 43, 621–630. [Google Scholar] [CrossRef]
- Rola-Łuszczak, M.; Finnegan, C.; Olech, M.; Choudhury, B.; Kuźmak, J. Development of an Improved Real Time PCR for the Detection of Bovine Leukaemia Provirus Nucleic Acid and Its Use in the Clarification of Inconclusive Serological Test Results. J. Virol. Methods 2013, 189, 258–264. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- KazNIVI. Bovine Leukemia Virus Isolate BLV-20 Envelope Glycoprotein (Env) Gene, Partial Cds 2025. GenBank: PV648327.1. Available online: https://www.ncbi.nlm.nih.gov/nuccore/PV648327.1 (accessed on 2 July 2025).
- KazNIVI. Bovine Leukemia Virus Isolate BLV-14 Envelope Glycoprotein (Env) Gene, Partial Cds 2025. GenBank: PV648326.1. Available online: https://www.ncbi.nlm.nih.gov/nuccore/PV648326.1 (accessed on 2 July 2025).
- KazNIVI. Bovine Leukemia Virus Isolate BLV-04 Envelope Glycoprotein (Env) Gene, Partial Cds 2025. GenBank: PV648325.1. Available online: https://www.ncbi.nlm.nih.gov/nuccore/PV648325.1 (accessed on 2 July 2025).
- Thiermann, A.B.; Babcock, S. Animal Welfare and International Trade; Edward Elgar Publishing Ltd.: Cheltenham, UK, 2021. [Google Scholar]
- Trono, K.G.; Pérez-Filgueira, D.M.; Duffy, S.; Borca, M.V.; Carrillo, C. Seroprevalence of Bovine Leukemia Virus in Dairy Cattle in Argentina: Comparison of Sensitivity and Specificity of Different Detection Methods. Vet. Microbiol. 2001, 83, 235–248. [Google Scholar] [CrossRef]
- Monti, G.E.; Frankena, K.; Engel, B.; Buist, W.; Tarabla, H.D.; de Jong, M.C.M. Evaluation of a New Antibody-Based Enzyme-Linked Immunosorbent Assay for the Detection of Bovine Leukemia Virus Infection in Dairy Cattle. J. Vet. Diagn. Investig. 2005, 17, 451–457. [Google Scholar] [CrossRef]
- Pluta, A.; Rola-Łuszczak, M.; Kubiś, P.; Balov, S.; Moskalik, R.; Choudhury, B.; Kuźmak, J. Molecular Characterization of Bovine Leukemia Virus from Moldovan Dairy Cattle. Arch. Virol. 2017, 162, 1563–1576. [Google Scholar] [CrossRef]
- Ochirkhuu, N.; Konnai, S.; Odbileg, R.; Nishimori, A.; Okagawa, T.; Murata, S.; Ohashi, K. Detection of Bovine Leukemia Virus and Identification of Its Genotype in Mongolian Cattle. Arch. Virol. 2016, 161, 985–991. [Google Scholar] [CrossRef]
- Wang, L.; Ning, C.; Ji, C.; Guo, Y.; Li, N.; Qiao, J.; Meng, Q.; Xia, X.; Zhang, X.; Liu, Y.; et al. Detection and Genetic Characteristics of Bovine Leukaemia Virus in Holstein Cows in China. Int. J. Agric. Biol. 2021, 25, 222–226. [Google Scholar]
| No | Region | Animal Type | Investigated (2014–2024) | Detected | Seroprevalence (%) | 95% CI |
|---|---|---|---|---|---|---|
| 1 | Akmola | Cattle | 40,103 | 1396 | 3.48 | 3.31–3.66 |
| 2 | Aktobe | Cattle | 28,596 | 72 | 0.25 | 0.20–0.32 |
| 3 | Almaty | Cattle | 82,704 | 607 | 0.73 | 0.68–0.79 |
| 4 | Atyrau | Cattle | 3678 | 151 | 4.11 | 3.51–4.80 |
| 5 | Zhambyl | Cattle | 40,103 | 1317 | 3.28 | 3.11–3.46 |
| 6 | East Kazakhstan | Cattle | 46,589 | 3909 | 8.39 | 8.14–8.65 |
| 7 | West Kazakhstan | Cattle | 18,436 | 1037 | 5.62 | 5.30–5.97 |
| 8 | Kyzylorda | Cattle | 3687 | 0 | 0.00 | 0.00–0.00 |
| 9 | Karaganda | Cattle | 12,349 | 56 | 0.45 | 0.35–0.59 |
| 10 | Kostanay | Cattle | 52,960 | 7183 | 13.56 | 13.27–13.86 |
| 11 | Mangystau | Cattle | 39 | 0 | 0.00 | 0.00–0.00 |
| 12 | Pavlodar | Cattle | 33,070 | 2211 | 6.69 | 6.42–6.96 |
| 13 | North Kazakhstan | Cattle | 43,007 | 7220 | 16.79 | 16.44–17.14 |
| 14 | Turkestan | Cattle | 17,011 | 117 | 0.69 | 0.57–0.82 |
| 15 | Ulytau | - | n/a * | - | - | - |
| 16 | Abai | Cattle | 7790 | 174 | 2.23 | 1.93–2.59 |
| 17 | Zhetysu | Cattle | 3415 | 0 | 0.00 | 0.00–0.00 |
| Total (Republic of Kazakhstan) | 433,537 | 25,450 | 5.87 | |||
| No. | Region | Tested Samples | Positive Results (n) | Seroprevalence (%) | 95% CI |
|---|---|---|---|---|---|
| 1 | Akmola | 171 | 0 | 0.0 | (0.0–2.2) |
| 2 | Almaty | 309 | 32 | 10.3 | (7.43–14.25) |
| 3 | Zhetysu | 465 | 0 | 0.0 | 0.0–0.82) |
| 4 | Atyrau | 47 | 3 | 6.3 | (2.19–17.16) |
| 5 | East Kazakhstan | 135 | 12 | 8.8 | (5.16–14.89) |
| 6 | Abai | 27 | 6 | 22.2 | (10.61–40.76) |
| 7 | Zhambyl | 235 | 3 | 1.2 | (0.44–3.69) |
| 8 | Kostanay | 276 | 19 | 6.8 | (4.45–10.5) |
| 9 | Aktobe | 89 | 1 | 1.1 | (3.13–13.94) |
| 10 | West Kazakhstan | 260 | 9 | 3.4 | (1.83–6.45) |
| 11 | Karaganda | 235 | 2 | 0.8 | (0.23–3.05) |
| 12 | Ulytau | 221 | 3 | 1.3 | (3.47–9.8) |
| 13 | Kyzylorda | 251 | 28 | 11.1 | (7.83–15.65) |
| 14 | Mangystau | 88 | 2 | 2.2 | (0.63–7.91) |
| 15 | Pavlodar | 225 | 0 | 0.0 | (0.24–3.18) |
| 16 | North Kazakhstan | 430 | 176 | 40.9 | (36.38–45.64) |
| 17 | Turkestan | 272 | 11 | 4.0 | (2.27–7.1) |
| Total (Republic of Kazakhstan) | 3736 | 307 | 8.2 | ||
| Regions of the Republic of Kazakhstan | Age (Years)/Breed of Cattle | Number of Tested Samples (n Tested) | Diagnostic Systems | ||
|---|---|---|---|---|---|
| ID VET (n Positive) | IDEXX (n Positive) | Diagnostic AGID kit of “KazSRVI” LLP (n Positive) | |||
| East Kazakhstan region | 3–9/Simmental cattle | 100 | 20 | 20 | 20 |
| 3–4/Simmental cattle | 100 | 36 | 36 | 36 | |
| 3–10/Simmental cattle | 100 | 26 | 26 | 26 | |
| Abai region | 3–11/Simmental cattle | 100 | 16 | 16 | 16 |
| Abai region | 1–8/Kazakh Whiteheaded cattle | 60 | 0 | 0 | 0 |
| Kostanay region | 5–6/outbred cattle | 40 | 0 | 0 | 0 |
| 4–8/Kazakh Whiteheaded cattle | 28 | 12 | 12 | 12 | |
| 5–7/Kazakh Whiteheaded cattle | 60 | 37 | 37 | 37 | |
| 4–5/Black-and-white cow breeds | 26 | 9 | 9 | 9 | |
| 3–7/outbred cattle | 87 | 49 | 49 | 49 | |
| No. | Name of Region | Samples Tested | Positive by PCR | AGID Positive | ELISA Positive |
|---|---|---|---|---|---|
| 1 | Akmola | 17 | 0 | 0 | 0 |
| 2 | Almaty | 110 | 3 | 10 | 10 |
| 3 | Zhetysu | 130 | 0 | 0 | 0 |
| 4 | Atyrau | n/a * | n/a | n/a | n/a |
| 5 | East Kazakhstan | 27 | 15 | 10 | 11 |
| 6 | Abai | 26 | 5 | 6 | 8 |
| 7 | Zhambyl | 60 | 0 | 6 | 6 |
| 8 | Kostanay | n/a | n/a | n/a | n/a |
| 9 | Aktobe | 23 | 0 | 1 | 1 |
| 10 | West Kazakhstan | n/a | n/a | n/a | n/a |
| 11 | Karaganda | 24 | 2 | 2 | 2 |
| 12 | Ulytau | 22 | 0 | 3 | 4 |
| 13 | Kyzylorda | 26 | 0 | 26 | 26 |
| 14 | Mangystau | n/a | n/a | n/a | n/a |
| 15 | Pavlodar | 45 | 0 | 0 | 0 |
| 16 | North Kazakhstan | n/a | n/a | n/a | n/a |
| 17 | Turkestan | 26 | 0 | 25 | 25 |
| Total | 536 | 25 | 89 | 93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamanova, S.; Nurpeisova, A.; Bashenova, E.; Kaimoldina, S.; Kirpichenko, V.; Akshalova, P.; Karabassova, A.; Yussupov, M.; Mashzhan, A.; Tazhbayeva, D.; et al. Seroprevalence and Molecular Analysis of Bovine Leukemia Virus in Kazakhstan. Viruses 2025, 17, 956. https://doi.org/10.3390/v17070956
Mamanova S, Nurpeisova A, Bashenova E, Kaimoldina S, Kirpichenko V, Akshalova P, Karabassova A, Yussupov M, Mashzhan A, Tazhbayeva D, et al. Seroprevalence and Molecular Analysis of Bovine Leukemia Virus in Kazakhstan. Viruses. 2025; 17(7):956. https://doi.org/10.3390/v17070956
Chicago/Turabian StyleMamanova, Saltanat, Ainur Nurpeisova, Elvira Bashenova, Saira Kaimoldina, Vladimir Kirpichenko, Perizat Akshalova, Aiken Karabassova, Malik Yussupov, Akzhigit Mashzhan, Dauriya Tazhbayeva, and et al. 2025. "Seroprevalence and Molecular Analysis of Bovine Leukemia Virus in Kazakhstan" Viruses 17, no. 7: 956. https://doi.org/10.3390/v17070956
APA StyleMamanova, S., Nurpeisova, A., Bashenova, E., Kaimoldina, S., Kirpichenko, V., Akshalova, P., Karabassova, A., Yussupov, M., Mashzhan, A., Tazhbayeva, D., Abay, Z., Rola-Luszczak, M., Kuzmak, J., Nissanova, R., & Kassenov, M. (2025). Seroprevalence and Molecular Analysis of Bovine Leukemia Virus in Kazakhstan. Viruses, 17(7), 956. https://doi.org/10.3390/v17070956







