The Role of Comorbidities in COVID-19 Severity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
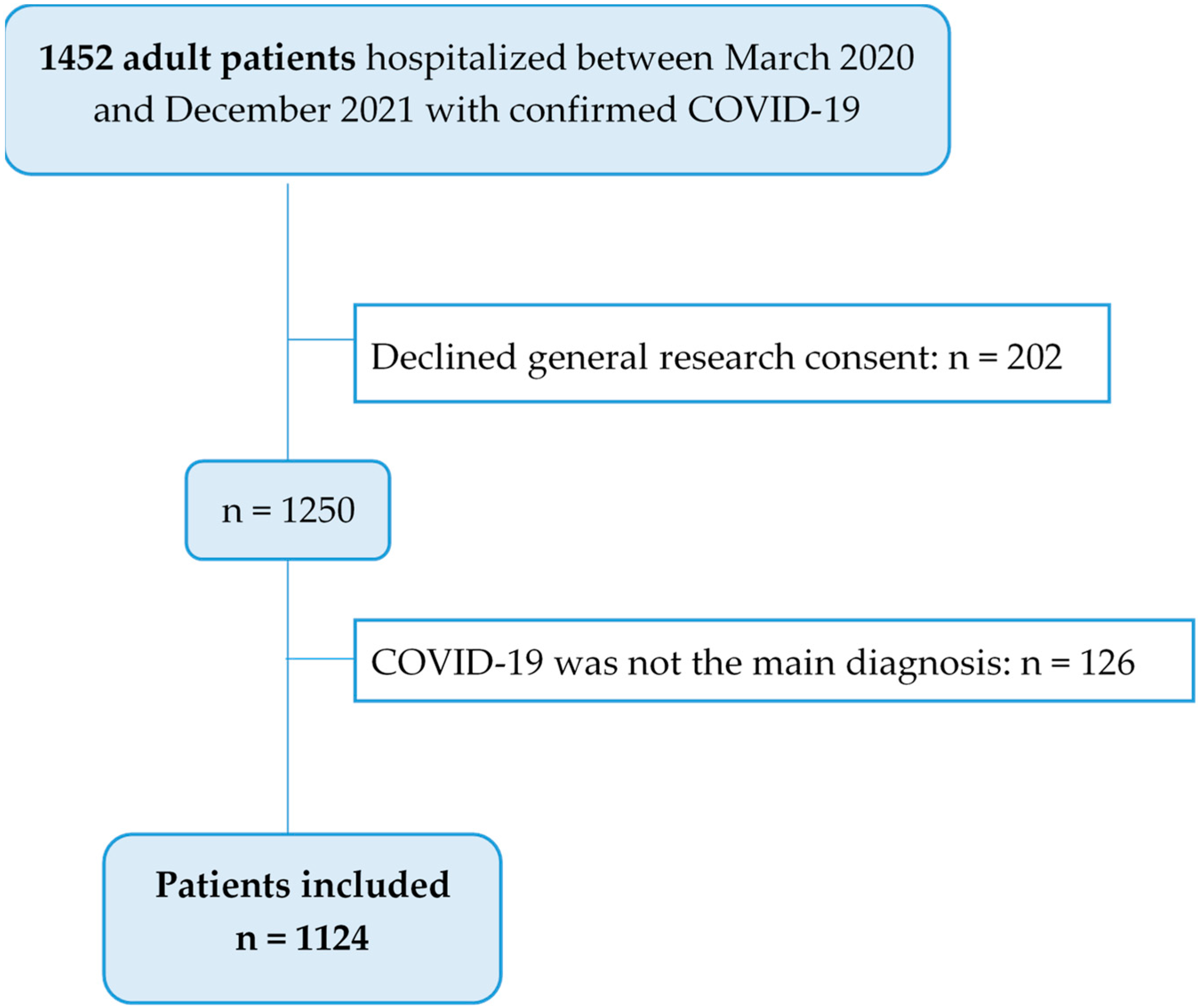
2.2. Study Population
2.3. Outcomes
- –
- Ventilator-associated pneumonia (VAP);
- –
- Acute kidney failure (AKF);
- –
- Multi-organ failure (MOF);
- –
- Thromboembolic complications, including:
- Pulmonary embolism
- Deep vein thrombosis
- Acute arterial occlusion
- Cerebrovascular insult
- Amputation
- Disseminated intravascular coagulation;
- –
- Cardiac complications, including:
- Heart failure
- Acute coronary syndrome
- Cardiac arrest;
- –
- Neurological complications, including:
- Delirium
- Encephalopathy
- Meningoencephalitis
- Neuromuscular disease
- Polyneuropathy.
2.4. Data Collection and Management
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
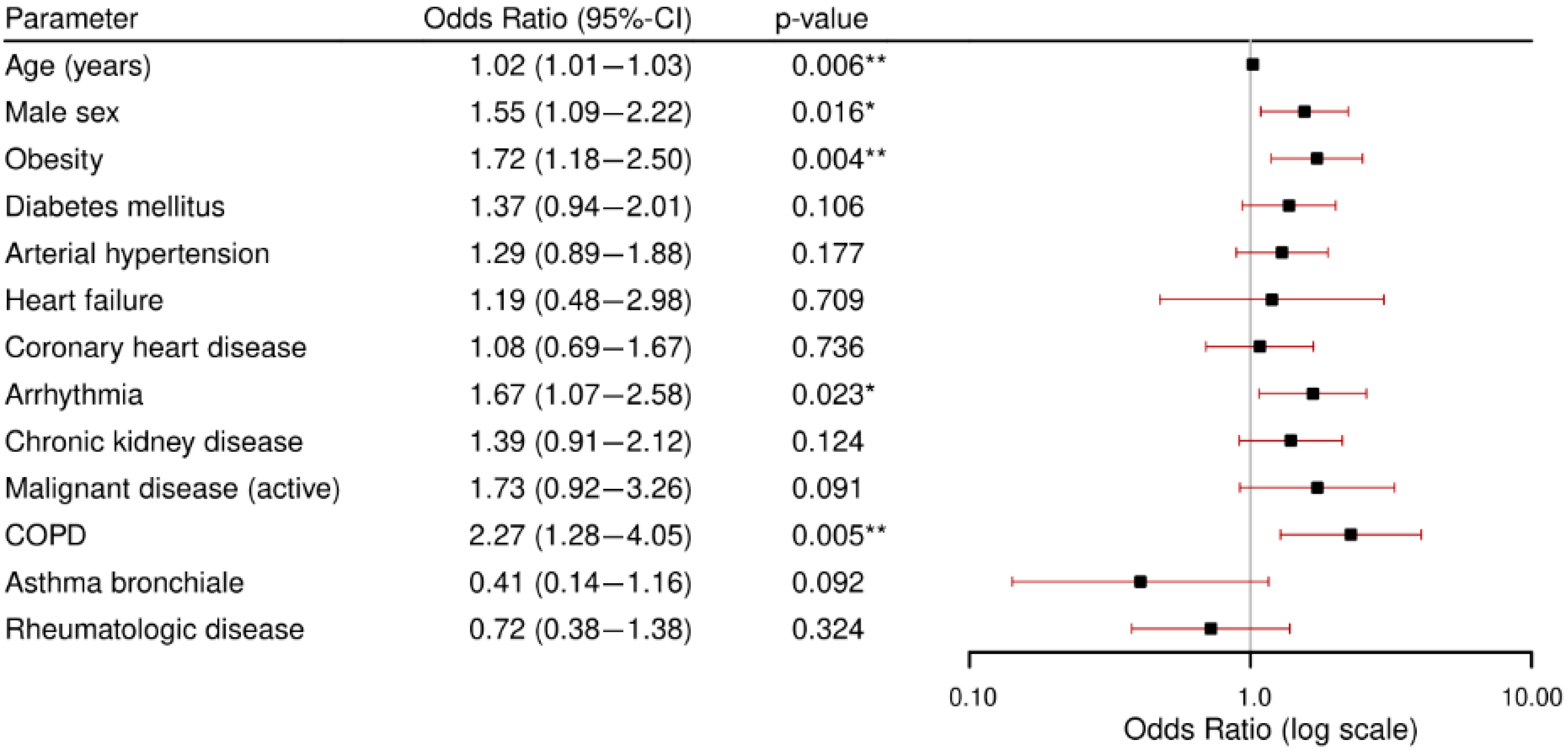
3.2. Primary Outcome: Comorbidities and Disease Severity
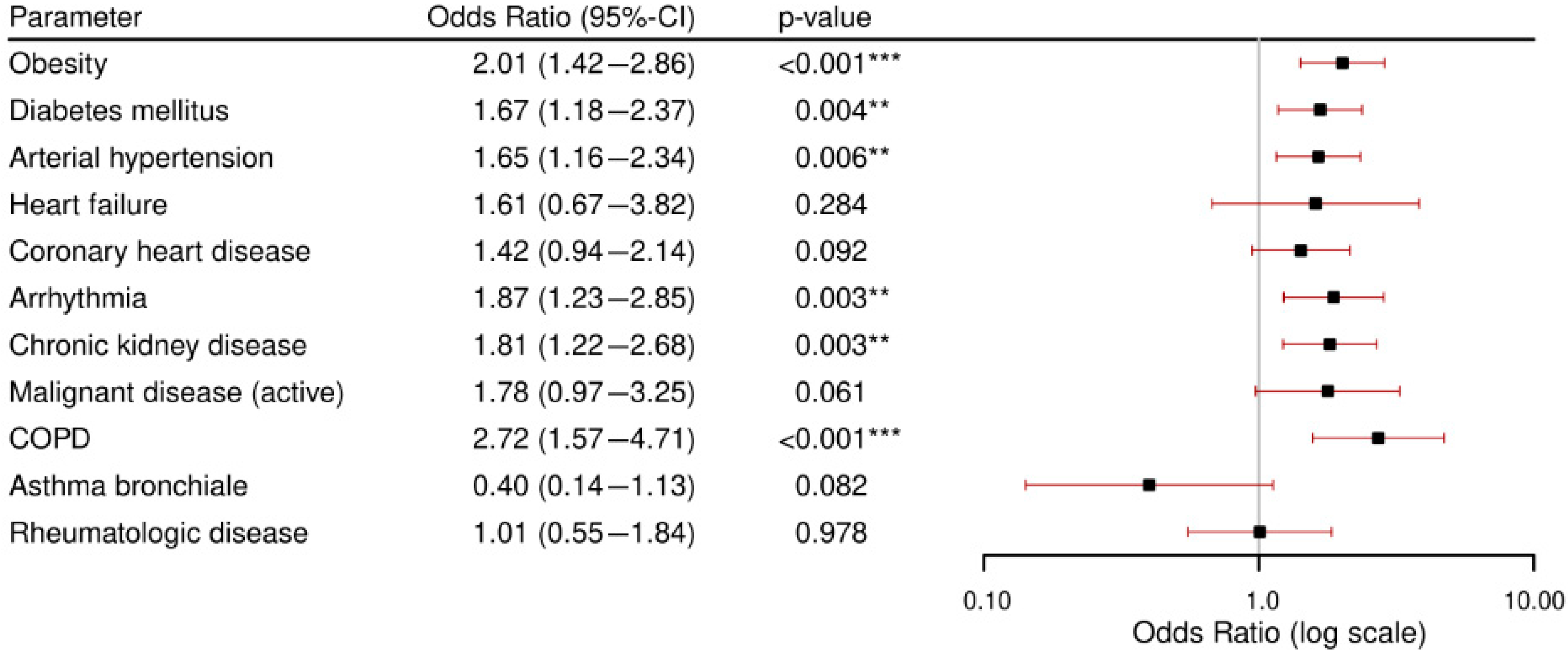
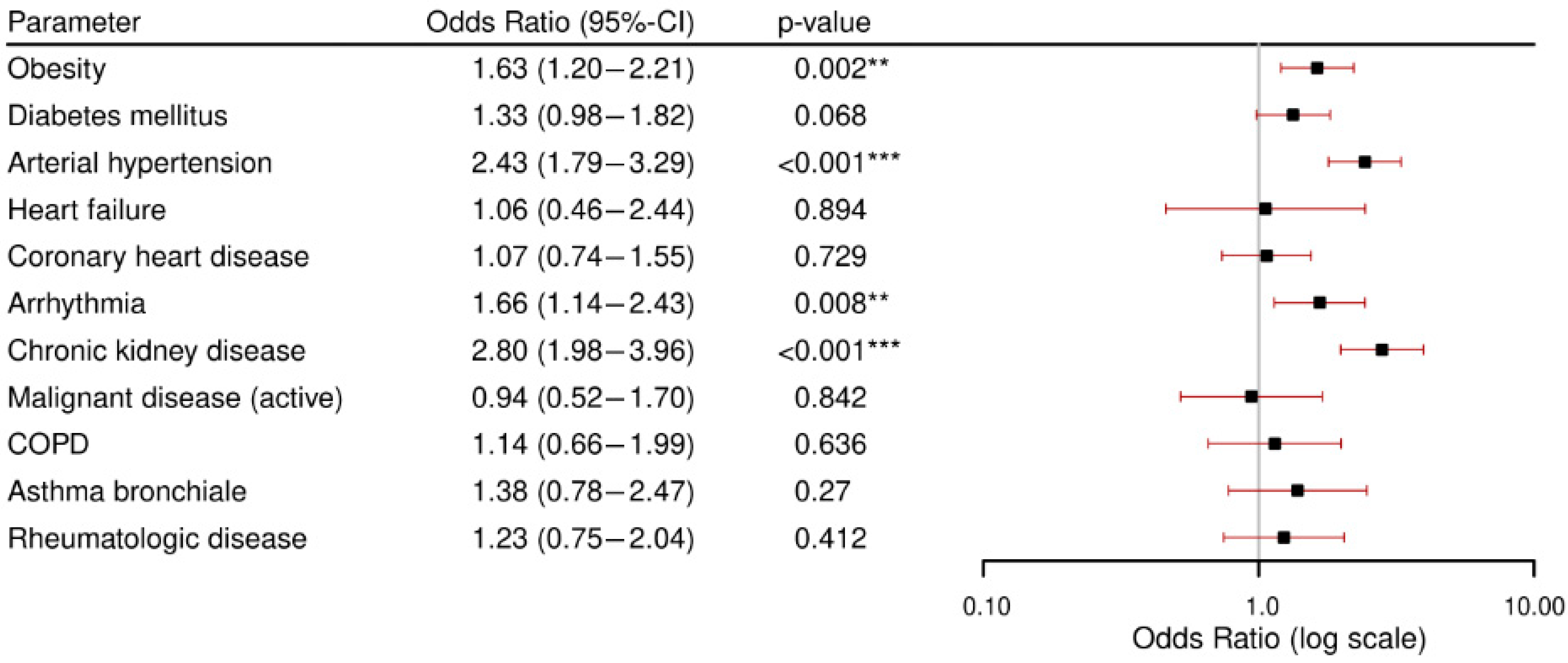
3.3. Secondary Outcome: Comorbidities and Complications.
4. Discussion
- Patients with comorbidities had a 1.4-fold rate of severe disease outcomes and a 20-fold in-hospital mortality rate compared to individuals without comorbidities, emphasizing the impact of pre-existing conditions. Moderate disease was the most frequent outcome across all observed sub-groups.
- Obesity, diabetes, and arterial hypertension were the most prevalent comorbidities and were significantly associated with a critical condition (severe disease or in-hospital death) after adjusting for age and gender. It should be noted that while comorbidities such as CKD and arrhythmia showed higher odds ratios, their lower prevalence means that their overall population-level impact differs from more common conditions like hypertension or obesity. Our focus in this section is, therefore, on conditions that combine both high prevalence and a significant risk increase. From a public health perspective, this dual perspective is critical for resource planning and preventive strategies.
- Patients with chronic kidney disease and arterial hypertension were at particularly high risk for developing in-hospital complications. Across all analyzed comorbidities, acute kidney failure was the most frequently observed complication.Obstructive respiratory conditions (COPD and asthma bronchiale) were relatively uncommon. Notably, asthma bronchiale appeared to have a potentially protective effect against severe outcomes.
4.1. The Role of Comorbidities in Disease Severity and Complications
4.1.1. Disease Severity Across All Observed Comorbidities
4.1.2. Comorbidities in Metabolic Syndrome
4.1.3. Cardiovascular Comorbidities
4.1.4. Chronic Kidney Disease
4.1.5. Obstructive Respiratory Comorbidities
4.2. Strengths, Limitations, and Further Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| n | Vacc_Not_Available | Vacc_Yes | Vacc_No | Missing | |
|---|---|---|---|---|---|
| Overall | 1124 | 656 (69.1) | 108 (11.4) | 185 (19.5) | 175 |
| No comorbidity | 274 | 145 (62.5) | 16 (6.9) | 71 (30.6) | 42 |
| Obesity | 303 | 187 (71.4) | 34 (13) | 41 (15.6) | 41 |
| Diabetes mellitus | 268 | 155 (69.8) | 35 (15.8) | 32 (14.4) | 46 |
| Arterial hypertension | 525 | 319 (72.3) | 54 (12.2) | 68 (15.4) | 84 |
| Heart failure | 26 | 16 (69.6) | 4 (17.4) | 3 (13) | 3 |
| Coronary heart | 168 | 102 (75) | 19 (14) | 15 (11) | 32 |
| Arrhytmia | 153 | 84 (65.1) | 21 (16.3) | 24 (18.6) | 24 |
| Active cancer | 58 | 33 (64.7) | 10 (19.6) | 8 (15.7) | 7 |
| COPD | 62 | 39 (73.6) | 10 (18.9) | 4 (7.5) | 9 |
| Asthma bronchiale | 64 | 45 (81.8) | 8 (14.5) | 2 (3.6) | 9 |
| Rheumatologic disease | 77 | 50 (73.5) | 13 (19.1) | 5 (7.4) | 9 |
| Chronic kidney disease | 225 | 122 (67.8) | 37 (20.6) | 21 (11.7) | 45 |

References
- Rahman, M.M.; Bhattacharjee, B.; Farhana, Z.; Hamiduzzaman, M.; Chowdhury, M.A.B.; Hossain, M.S.; Siddiqee, M.H.; Islam, M.Z.; Raheem, E.; Uddin, M.J. Age-related risk factors and severity of SARS-CoV-2 infection: A systematic review and meta-analysis. J. Prev. Med. Hyg. 2021, 62, E329–E371. [Google Scholar] [PubMed]
- Bergeri, I.; Whelan, M.G.; Ware, H.; Subissi, L.; Nardone, A.; Lewis, H.C.; Li, Z.; Ma, X.; Valenciano, M.; Cheng, B.; et al. Global SARS-CoV-2 seroprevalence from January 2020 to April 2022: A systematic review and meta-analysis of standardized population-based studies. PLoS Med. 2022, 19, e1004107. [Google Scholar] [CrossRef] [PubMed]
- Nesteruk, I. Should we ignore SARS-CoV-2 disease? Epidemiol. Infect. 2024, 152, e57. [Google Scholar] [CrossRef] [PubMed]
- Federal Office of Public Health FOPH. COVID-19 Switzerland. Epidemiological Course, Switzerland and Liechtenstein. [Internet]. 2023. Available online: https://www.covid19.admin.ch/en/epidemiologic/case (accessed on 1 February 2025).
- BAG COVID-19: Switzerland Can Start Vaccinating Vulnerable Groups Already in December [Internet]. 2024. Available online: https://www.bag.admin.ch/bag/en/home/das-bag/aktuell/medienmitteilungen.msg-id-81762.html (accessed on 1 February 2025).
- Sousa, F.M.; Roelens, M.; Fricker, B.; Thiabaud, A.; Iten, A.; Cusini, A.; Flury, D.; Buettcher, M.; Zucol, F.; Balmelli, C.; et al. Risk factors for severe outcomes for COVID-19 patients hospitalised in Switzerland during the first pandemic wave, February to August 2020: Prospective observational cohort study. Swiss Med. Wkly. 2021, 151, w20547. [Google Scholar] [CrossRef]
- Boesing, M.; Lüthi-Corridori, G.; Büttiker, D.; Hunziker, M.; Jaun, F.; Vaskyte, U.; Brändle, M.; Leuppi, J.D. The Predictive Performance of Risk Scores for the Outcome of COVID-19 in a 2-Year Swiss Cohort. Biomedicines 2024, 12, 1702. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Dong, X.; Liu, G.-H.; Gao, Y.-D. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin. Rev. Allergy Immunol. 2023, 64, 90–107. [Google Scholar] [CrossRef]
- Statsenko, Y.; Zahmi, F.A.; Habuza, T.; Almansoori, T.M.; Smetanina, D.; Simiyu, G.L.; Gorkom, K.N.-V.; Ljubisavljevic, M.; Awawdeh, R.; Elshekhali, H.; et al. Impact of Age and Sex on COVID-19 Severity Assessed from Radiologic and Clinical Findings. Front. Cell. Infect. Microbiol. 2022, 11, 777070. [Google Scholar] [CrossRef]
- Djaharuddin, I.; Munawwarah, S.; Nurulita, A.; Ilyas, M.; Tabri, N.A.; Lihawa, N. Comorbidities and mortality in COVID-19 patients. Gac. Sanit. 2021, 35, S530–S532. [Google Scholar] [CrossRef]
- Thiabaud, A.; Iten, A.; Balmelli, C.; Senn, L.; Troillet, N.; Widmer, A.; Flury, D.; Schreiber, P.W.; Vázquez, M.; Damonti, L.; et al. Cohort profile: SARS-CoV-2/COVID-19 hospitalised patients in Switzerland. Swiss Med. Wkly. 2021, 151, w20475. [Google Scholar] [CrossRef]
- Ning, Q.; Wu, D.; Wang, X.; Xi, D.; Chen, T.; Chen, G.; Wang, H.; Lu, H.; Wang, M.; Zhu, L.; et al. The mechanism underlying extrapulmonary complications of the coronavirus disease 2019 and its therapeutic implication. Signal Transduct. Target. Ther. 2022, 7, 57. [Google Scholar] [CrossRef]
- Głowacka, M.; Lipka, S.; Młynarska, E.; Franczyk, B.; Rysz, J. Acute kidney injury in COVID-19. Int. J. Mol. Sci. 2021, 22, 8081. [Google Scholar] [CrossRef] [PubMed]
- Leeuw, A.J.M.D.; Luttikhuis, M.A.M.O.; Wellen, A.C.; Müller, C.; Calkhoven, C.F. Obesity and its impact on COVID-19 The Obesity Pandemic. 2021. Available online: www.who.int (accessed on 1 April 2025).
- Gammone, M.A.; D’Orazio, N. COVID-19 and Obesity: Overlapping of Two Pandemics. Obes. Facts 2021, 14, 579–585. [Google Scholar] [CrossRef]
- Marshall, J.C.; Murthy, S.; Diaz, J.; Adhikari, N.; Angus, D.C.; Arabi, Y.M.; Baillie, K.; Bauer, M.; Berry, S.; Blackwood, B.; et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, B.; Seon, J.Y.; Oh, I.H. A National Study of Life-Sustaining Treatments in South Korea: What Factors Affect Decision-Making? Cancer Res. Treat. 2021, 53, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Diebold, M.; Martinez, A.E.; Adam, K.M.; Bassetti, S.; Osthoff, M.; Kassi, E.; Steiger, J.; Pargger, H.; Siegemund, M.; Battegay, M.; et al. Temporal trends of COVID-19 related in-hospital mortality and demographics in Switzerland—A retrospective single centre cohort study. Swiss Med. Wkly. 2021, 151, w20572. [Google Scholar] [CrossRef]
- Gregoriano, C.; Rafaisz, K.; Schuetz, P.; Mueller, B.; Fux, C.A.; Conen, A.; Kutz, A. Burden of disease in patients hospitalised with COVID-19 during the first and second pandemic wave in Switzerland: A nationwide cohort study. Swiss Med. Wkly. 2023, 153, 40068. [Google Scholar] [CrossRef]
- Reignier, J.; Dumont, R.; Katsahian, S.; Martin-Lefevre, L.; Renard, B.; Fiancette, M.; Lebert, C.; Clementi, E.; Bontemps, F. Patient-related factors and circumstances surrounding decisions to forego life-sustaining treatment, including intensive care unit admission refusal. Crit. Care Med. 2008, 36, 2076–2083. [Google Scholar] [CrossRef]
- Lloyd, C.B.; Nietert, P.J.; Silvestri, G.A. Intensive care decision making in the seriously ill and elderly. Crit. Care Med. 2004, 32, 649–654. [Google Scholar] [CrossRef]
- Anderegg, N.; Panczak, R.; Egger, M.; Low, N.; Riou, J. Survival among people hospitalized with COVID-19 in Switzerland: A nationwide population-based analysis. BMC Med. 2022, 20, 164. [Google Scholar] [CrossRef]
- Thakur, B.; Dubey, P.; Benitez, J.; Torres, J.P.; Reddy, S.; Shokar, N.; Aung, K.; Mukherjee, D.; Dwivedi, A.K. A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci. Rep. 2021, 11, 8562. [Google Scholar] [CrossRef]
- Xie, J.; Zu, Y.; Alkhatib, A.; Pham, T.T.; Gill, F.; Jang, A.; Radosta, S.; Chaaya, G.; Myers, L.; Zifodya, J.S.; et al. Metabolic syndrome and COVID-19 mortality among adult black patients in new orleans. Diabetes Care 2021, 44, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Denson, J.L.; Gillet, A.S.; Zu, Y.; Brown, M.; Pham, T.; Yoshida, Y.; Mauvais-Jarvis, F.; Douglas, I.S.; Moore, M.; Tea, K.; et al. Metabolic Syndrome and Acute Respiratory Distress Syndrome in Hospitalized Patients with COVID-19. JAMA Netw. Open 2021, 4, e2140568. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Rigatelli, G.; Bilato, C.; Cervellati, C.; Zuliani, G.; Roncon, L. Prognostic role of metabolic syndrome in covid-19 patients: A systematic review meta-analysis. Viruses 2021, 13, 1938. [Google Scholar] [CrossRef]
- Alexander, M.P.; Patel, T.V.; Farag, Y.M.K.; Florez, A.; Rennke, H.G.; Singh, A.K. Kidney Pathological Changes in Metabolic Syndrome: A Cross-sectional Study. Am. J. Kidney Dis. 2009, 53, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, P.; Sato, W.; Reungjui, S.; Heinig, M.; Gersch, M.; Sautin, Y.; Nakagawa, T.; Johnson, R.J. Uric acid, the metabolic syndrome, and renal disease. J. Am. Soc. Nephrol. 2006, 17, S165–S168. [Google Scholar] [CrossRef]
- Zhang, X.; Lerman, L.O. The metabolic syndrome and chronic kidney disease. Transl. Res. 2017, 183, 14–25. [Google Scholar] [CrossRef]
- Lin, L.; Tan, W.; Pan, X.; Tian, E.; Wu, Z.; Yang, J. Metabolic Syndrome-Related Kidney Injury: A Review and Update. Front. Endocrinol. 2022, 13, 904001. [Google Scholar] [CrossRef]
- Sabatino, J.; Rosa Sde Salvo, G.D.; Indolfi, C. Impact of cardiovascular risk profile on COVID-19 outcome. A meta-analysis. PLoS ONE 2020, 15, e0237131. [Google Scholar]
- Zhang, J.; Lu, S.; Wang, X.; Jia, X.; Li, J.; Lei, H.; Liu, Z.; Liao, F.; Ji, M.; Lv, X.; et al. Do underlying cardiovascular diseases have any impact on hospitalised patients with COVID-19? Heart 2020, 106, 1148–1153. [Google Scholar] [CrossRef]
- Peçanha-Pietrobom, P.M.; Leite, G.G.F.; Hunter, J.; Ferreira, P.R.A.; Burattini, M.N.; Bellei, N.; Ota-Arakaki, J.S.; Salomao, R. The clinical course of hospitalized moderately ill COVID-19 patients is mirrored by routine hematologic tests and influenced by renal transplantation. PLoS ONE 2021, 16, e0258987. [Google Scholar] [CrossRef]
- Chung, E.Y.M.; Palmer, S.C.; Natale, P.; Krishnan, A.; Cooper, T.E.; Saglimbene, V.M.; Ruospo, M.; Au, E.; Jayanti, S.; Liang, A.; et al. Incidence and Outcomes of COVID-19 in People with CKD: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2021, 78, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, S.; Turgutalp, K.; Arici, M.; Odabas, A.R.; Altiparmak, M.R.; Aydin, Z.; Cebeci, E.; Basturk, T.; Soypacaci, Z.; Sahin, G.; et al. Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: A nationwide analysis from Turkey. Nephrol. Dial. Transplant. 2020, 35, 2083–2095. [Google Scholar] [CrossRef]
- Flythe, J.E.; Assimon, M.M.; Tugman, M.J.; Chang, E.H.; Gupta, S.; Shah, J.; Sosa, M.A.; Renaghan, A.D.; Melamed, M.L.; Wilson, F.P.; et al. Characteristics and Outcomes of Individuals with Pre-existing Kidney Disease and COVID-19 Admitted to Intensive Care Units in the United States. Am. J. Kidney Dis. 2021, 77, 190–203.e1. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, Y.; Wang, Z.; Xie, M.; Shi, Z.; Tang, Z.; Li, X.; Li, X.; Lei, C.; Li, Y.; et al. Clinical characteristics of COVID-19 infection in chronic obstructive pulmonary disease: A multicenter, retrospective, observational study. J. Thorac. Dis. 2020, 12, 1811–1823. [Google Scholar] [CrossRef]
- Alqahtani, J.S.; Oyelade, T.; Aldhahir, A.M.; Alghamdi, S.M.; Almehmadi, M.; Alqahtani, A.S.; Quaderi, S.; Mandal, S.; Hurst, J.R. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: A rapid systematic review and meta-analysis. PLoS ONE 2020, 15, e0233147. [Google Scholar] [CrossRef] [PubMed]
- Sunjaya, A.P.; Allida, S.M.; Tanna, G.L.D.; Jenkins, C. Asthma and risk of infection, hospitalization, ICU admission and mortality from COVID-19: Systematic review and meta-analysis. J. Asthma 2022, 59, 866–879. [Google Scholar] [CrossRef]
- Hou, H.; Xu, J.; Li, Y.; Wang, Y.; Yang, H. The Association of Asthma with COVID-19 Mortality: An Updated Meta-Analysis Based on Adjusted Effect Estimates. J. Allergy Clin. Immunol Pract. 2021, 9, 3944–3968.e5. [Google Scholar] [CrossRef]
- Assaf, S.M.; Tarasevych, S.P.; Diamant, Z.; Hanania, N.A. Asthma and severe acute respiratory syndrome coronavirus 2019: Current evidence and knowledge gaps. Curr. Opin. Pulm. Med. 2021, 27, 45–53. [Google Scholar] [CrossRef]
- Kompaniyets, L.; Pennington, A.F.; Goodman, A.B.; Rosenblum, H.G.; Belay, B.; Ko, J.Y.; Chevinsky, J.R.; Schieber, L.Z.; Summers, A.D.; Lavery, A.M.; et al. Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized with COVID-19, March 2020-March 2021. Prev. Chronic Dis. 2021, 18, E66. [Google Scholar] [CrossRef]
| Moderate | Hospitalized without oxygen therapy Hospitalized with oxygen by a nasal prong or mask |
| Severe | Hospitalized with oxygen by NIV or HFNO Hospitalized and intubated, incl. patients with additional organ support (dialysis, ECMO, vasoactive drugs) |
| In-hospital death |
| Overall n = 1124 (100.00%) | Missing Data | |
|---|---|---|
| Demographics | ||
| Age in years, median [IQR] (range) | 66 [54–79] (19–100) | |
| Male, n (%) | 669 (59.5) | |
| Female, n (%) | 455 (40.5) | |
| BMI, median [IQR] | 27.10 [23.9–30.8] | |
| Comorbidities | ||
| Obesity, n (%) | 303 (27.0) | |
| Diabetes mellitus, n (%) | 268 (23.8) | |
| Arterial hypertension, n (%) | 525 (46.7) | |
| Heart failure, n (%) | 26 (2.3) | |
| Coronary heart, n (%) | 168 (14.9) | |
| Arrhythmia, n (%) | 153 (13.6) | |
| Chronic kidney disease, n (%) | 225 (20) | |
| End-stage CKD | 9 (4) | |
| Malignant disease (active), n (%) | 58 (5.2) | |
| COPD, n (%) | 62 (5.5) | |
| Asthma bronchiale, n (%) | 64 (5.7) | |
| Rheumatologic disease, n (%) | 77 (6.9) | |
| No comorbidities, n (%) | 274 (24.4) | |
| COVID-19 vaccination status | ||
| Not vaccinated, n (%) | 185 (16.5) | n = 175 (15.5%) |
| Vaccinated, n (%) | 108 (9.6) | |
| No vaccination available, n (%) | 656 (58.4) | |
| Smoking status | ||
| Never, n (%) | 127 (11.3) | n = 822 (73.1%) |
| Former smoker, n (%) | 138 (12.3) | |
| Current smoker, n (%) | 37 (3.3) | |
| Precise diagnosis of COVID-19 | ||
| Bilateral pneumonia with SARS-CoV-2, n (%) | 783 (69.7) | |
| Unilateral pneumonia with SARS-CoV-2, n (%) | 54 (4.8) | |
| Upper and lower respiratory tract infection with SARS-CoV, n (%) | 169 (15.0) | |
| ARDS in COVID-19, n (%) | 118 (10.5) |
| n | Moderate | Severe | Death | |
|---|---|---|---|---|
| Overall | 1124 | 943 (83.9) | 64 (5.7) | 117 (10.4) |
| No comorbidity | 274 | 260 (94.9) | 12 (4.4) | 2 (0.7) |
| Obesity | 303 | 238 (78.5) | 28 (9.2) | 37 (12.2) |
| Diabetes mellitus | 268 | 204 (76.1) | 21 (7.8) | 43 (16) |
| Arterial hypertension | 525 | 410 (78.1) | 37 (7) | 78 (14.9) |
| Heart failure | 26 | 18 (69.2) | 0 (0) | 8 (30.8) |
| Coronary heart | 168 | 124 (73.8) | 5 (3) | 39 (23.2) |
| Arrhythmia | 153 | 106 (69.3) | 8 (5.2) | 39 (25.5) |
| Chronic kidney disease | 225 | 160 (71.1) | 7 (3.1) | 58 (25.8) |
| Active cancer | 58 | 41 (70.7) | 1 (1.7) | 16 (27.6) |
| COPD | 62 | 38 (61.3) | 6 (9.7) | 18 (29) |
| Asthma bronchiale | 64 | 60 (93.8) | 4 (6.2) | 0 (0) |
| Rheumatologic disease | 77 | 62 (80.5) | 4 (5.2) | 11 (14.3) |
| n | Any | VAP | AKF | MOF | Thromb | Cardio | Neuro | |
|---|---|---|---|---|---|---|---|---|
| Overall | 1124 | 287 (25.5) | 49 (4.4) | 192 (17.1) | 13 (1.2) | 62 (5.5) | 54 (4.8) | 47 (4.2) |
| No comorbidity | 274 | 28 (10.2) | 6 (2.2) | 15 (5.5) | 1 (0.4) | 10 (3.6) | 2 (0.7) | 3 (1.1) |
| Obesity | 303 | 92 (30.4) | 22 (7.3) | 62 (20.5) | 6 (2) | 14 (4.6) | 19 (6.3) | 17 (5.6) |
| Diabetes mellitus | 268 | 87 (32.5) | 17 (6.3) | 59 (22) | 5 (1.9) | 14 (5.2) | 17 (6.3) | 12 (4.5) |
| Arterial hypertension | 525 | 192 (36.6) | 34 (6.5) | 129 (24.6) | 8 (1.5) | 37 (7) | 42 (8) | 32 (6.1) |
| Heart failure | 26 | 9 (34.6) | 0 (0) | 6 (23.1) | 1 (3.8) | 0 (0) | 8 (30.8) | 1 (3.8) |
| Coronary heart | 168 | 56 (33.3) | 5 (3) | 38 (22.6) | 3 (1.8) | 4 (2.4) | 20 (11.9) | 5 (3) |
| Arrhythmia | 153 | 64 (41.8) | 5 (3.3) | 43 (28.1) | 4 (2.6) | 12 (7.8) | 25 (16.3) | 8 (5.2) |
| Chronic kidney disease | 225 | 108 (48) | 6 (2.7) | 93 (41.3) | 6 (2.7) | 16 (7.1) | 28 (12.4) | 9 (4) |
| Active cancer | 58 | 17 (29.3) | 1 (1.7) | 13 (22.4) | 0 (0) | 3 (5.2) | 1 (1.7) | 1 (1.7) |
| COPD | 62 | 21 (33.9) | 5 (8.1) | 15 (24.2) | 2 (3.2) | 3 (4.8) | 7 (11.3) | 5 (8.1) |
| Asthma bronchiale | 64 | 18 (28.1) | 4 (6.2) | 12 (18.8) | 0 (0) | 4 (6.2) | 0 (0) | 4 (6.2) |
| Rheumatologic disease | 77 | 26 (33.8) | 2 (2.6) | 19 (24.7) | 2 (2.6) | 2 (2.6) | 7 (9.1) | 4 (5.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
König, S.; Vaskyte, U.; Boesing, M.; Lüthi-Corridori, G.; Leuppi, J.D. The Role of Comorbidities in COVID-19 Severity. Viruses 2025, 17, 957. https://doi.org/10.3390/v17070957
König S, Vaskyte U, Boesing M, Lüthi-Corridori G, Leuppi JD. The Role of Comorbidities in COVID-19 Severity. Viruses. 2025; 17(7):957. https://doi.org/10.3390/v17070957
Chicago/Turabian StyleKönig, Sandra, Ugne Vaskyte, Maria Boesing, Giorgia Lüthi-Corridori, and Joerg Daniel Leuppi. 2025. "The Role of Comorbidities in COVID-19 Severity" Viruses 17, no. 7: 957. https://doi.org/10.3390/v17070957
APA StyleKönig, S., Vaskyte, U., Boesing, M., Lüthi-Corridori, G., & Leuppi, J. D. (2025). The Role of Comorbidities in COVID-19 Severity. Viruses, 17(7), 957. https://doi.org/10.3390/v17070957






