Phloroglucinol Oligomers from Callistemon rigidus as Novel Anti-Hantavirus Replication Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Isolation
2.4. Spectroscopic Data
2.5. ECD Calculations
2.6. Cell Lines and Virus
2.7. Cytotoxicity Assays
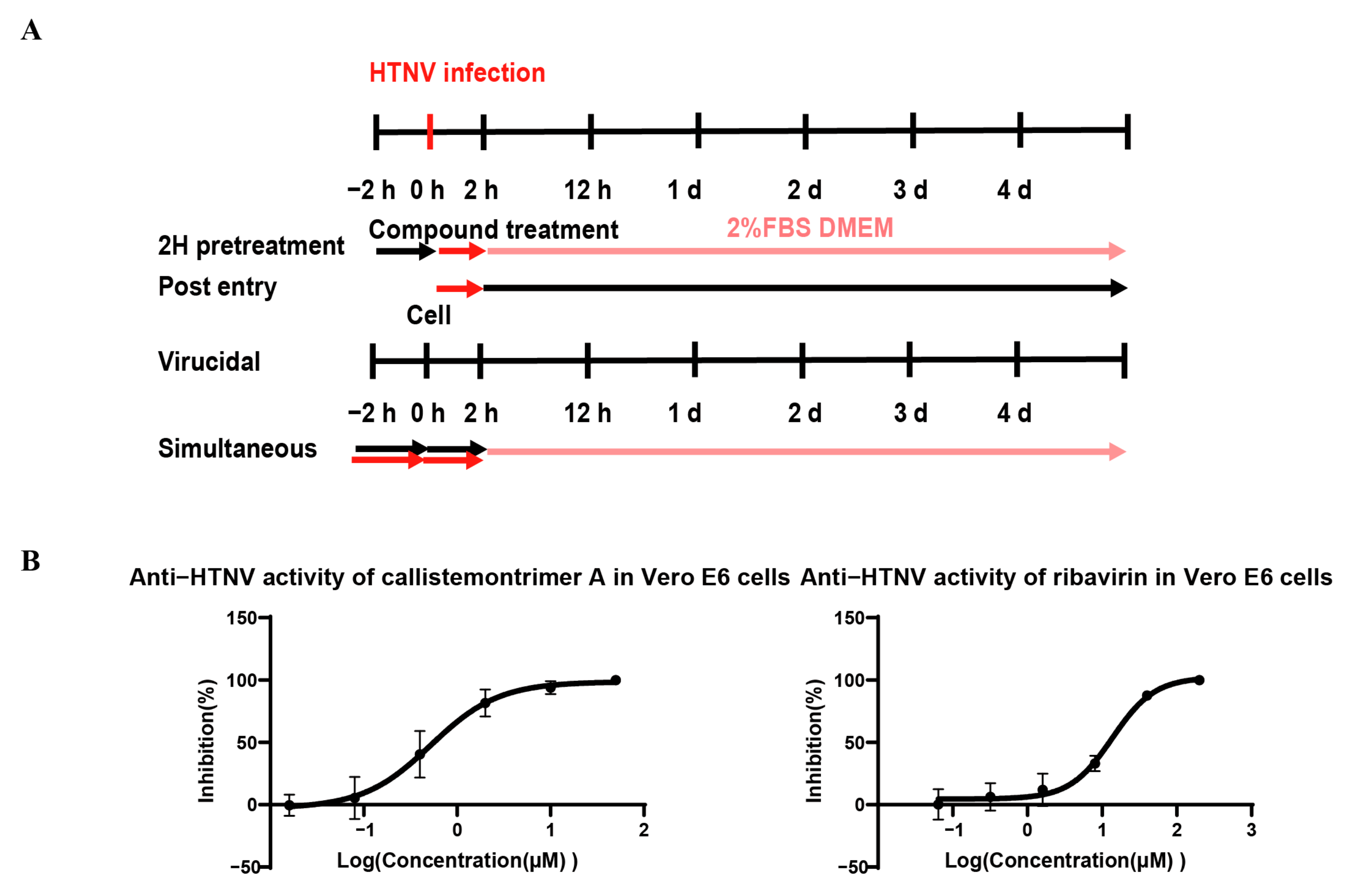
2.8. Time-of-Addition Assay (TOA)
2.9. Immunofluorescence Assay
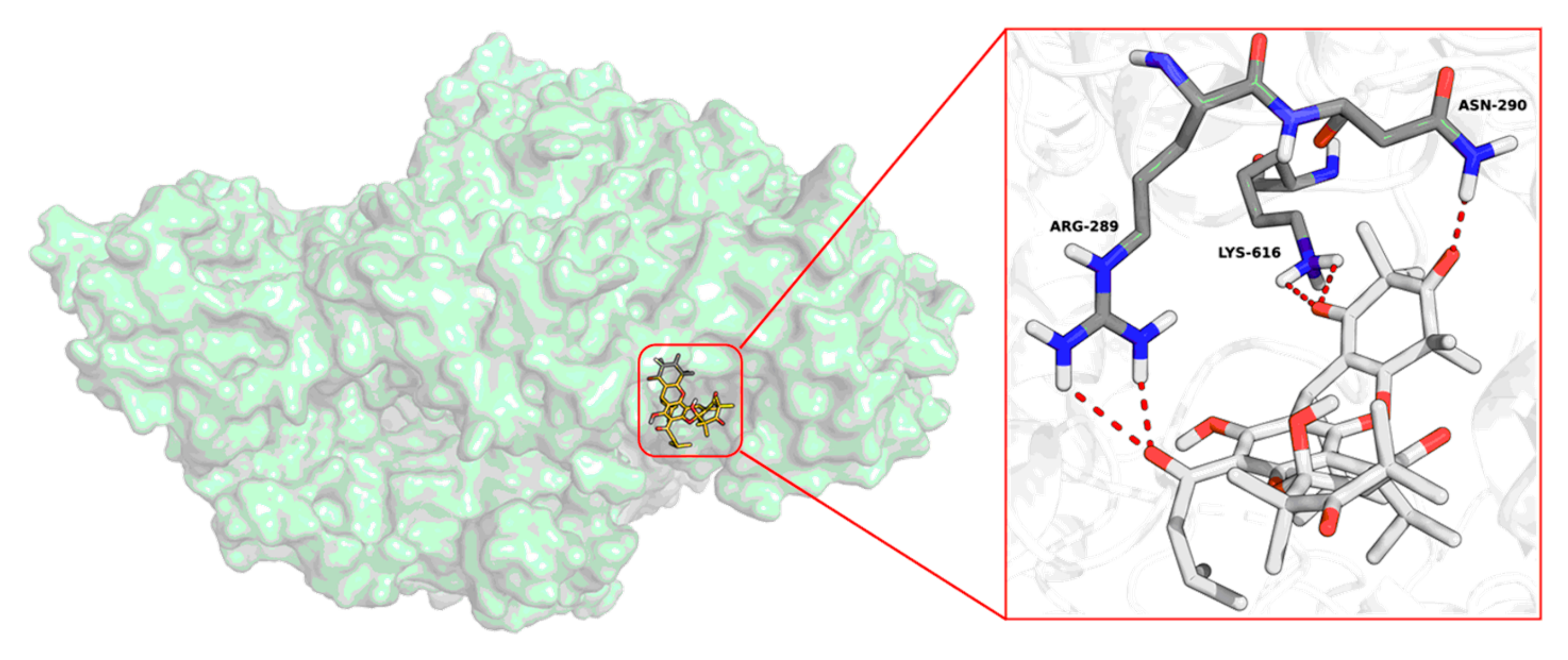
2.10. Molecular Docking
2.11. Statistical Analysis
3. Results
3.1. Isolation and Structural Characterization of the Isolated Compounds
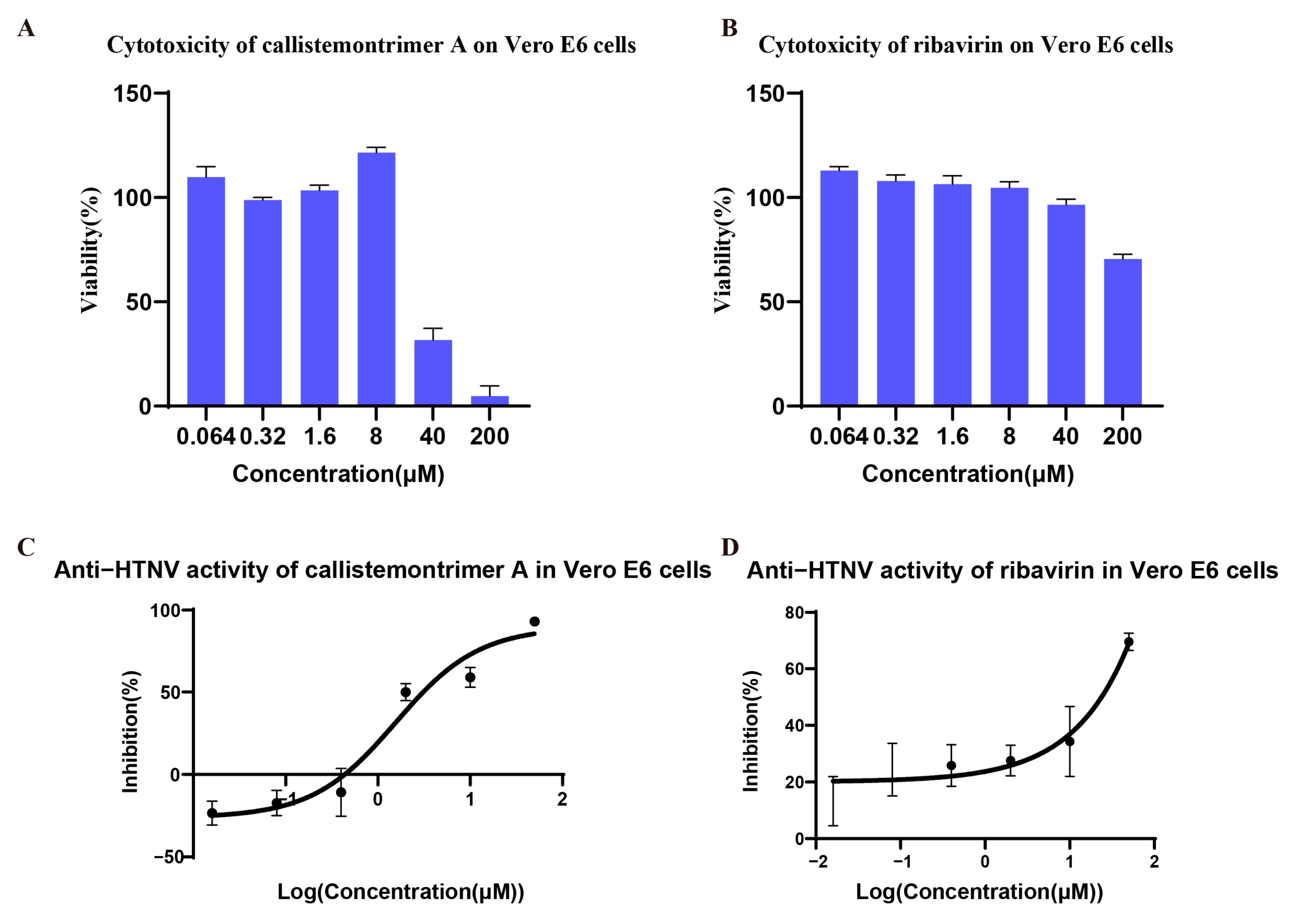
3.2. Cytotoxicity and Antiviral Activities of Isolated Compounds
3.3. Molecular Docking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, H.; Zheng, X.Y.; Wang, L.M.; Du, H.; Wang, P.Z.; Bai, X.F. Hantavirus infection: A global zoonotic challenge. Virol. Sin. 2017, 32, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Spiropoulou, C.F.; Bente, D.A. Orthohantavirus, Orthonairovirus, Orthobunyavirus and Phlebovirus. In Fields Virology: Emerging Viruses, 7th ed.; Howley, P.M., Knipe, D.M., Whelan, S., Eds.; Wolters Kluwer: New York, NY, USA, 2021; pp. 750–752. [Google Scholar]
- Wang, B.; Pei, J.W.; Zhang, H.; Li, J.; Dang, Y.M.; Liu, H.; Wang, Y.; Zhang, L.; Qi, L.B.; Yang, Y.W.; et al. Dihydropyridine-derived calcium channel blocker as a promising anti-hantavirus entry inhibitor. Front. Pharmacol. 2022, 13, 940178. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Cho, S.; Lee, S.H.; No, J.S.; Lee, G.Y.; Park, K.; Lee, D.; Jeong, S.T.; Song, J.W. Genomic epidemiology and active surveillance to investigate outbreaks of hantaviruses. Front. Cell Infect. Microbiol. 2021, 10, 532388. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.X.; Sun, L. Analysis of hemorrhagic fever with renal syndrome using wavelet tools in Mainland China, 2004–2019. Front. Public Health 2020, 8, 571984. [Google Scholar] [CrossRef]
- Mayor, J.; Engler, O.; Rothenberger, S. Antiviral efficacy of ribavirin and favipiravir against hantaan virus. Microorganisms 2021, 9, 1306. [Google Scholar] [CrossRef]
- Murphy, M.E.; Kariwa, H.; Mizutani, T.; Tanabe, H.; Yoshimatsu, K.; Arikawa, J.; Takashima, I. Characterization of in vitro and in vivo antiviral activity of lactoferrin and ribavirin upon hantavirus. J. Vet. Med. Sci. 2001, 63, 637–645. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Zhang, K.X.; Zheng, X.Y.; Lian, J.Q. Research progress of anti-hantavirus drugs. J. Pathog. Biol. 2023, 18, 482–485. [Google Scholar]
- Brocato, R.L.; Hooper, J.W. Progress on the prevention and treatment of hantavirus disease. Viruses 2019, 11, 610. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T.; International Natural Product Sciences Taskforce. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Liu, H.; He, X.Z.; Feng, M.Y.; Zeng, Y.; Rauwolf, T.J.; Shao, L.D.; Ni, W.; Yan, H.; Porco, J.A., Jr.; Hao, X.J.; et al. Acylphloroglucinols with acetylcholinesterase inhibitory effects from the fruits of Eucalyptus robusta. Bioorg. Chem. 2020, 103, 104127. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Q.; Wu, Y.; Zhong, Y.L.; Li, N.P.; Chen, M.; Li, M.M.; Ye, W.C.; Wang, L. Antiviral triketone-phloroglucinol-monoterpene adducts from Callistemon rigidus. Chem. Biodivers. 2018, 15, e1800172. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.M.; Hu, L.J.; Bai, Y.T.; Wang, J.; Qin, G.Q.; Song, Q.Y.; Su, J.C.; Huang, X.J.; Jiang, R.W.; Tang, W.; et al. Rhodomentosones A and B: Two pairs of enantiomeric phloroglucinol trimers from Rhodomyrtus tomentosa and their asymmetric biomimetic synthesis. Org. Lett. 2021, 23, 4499–4504. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.J.; Rauwolf, T.J.; Li, P.P.; Liu, H.; McNeely, J.; Hua, Y.; Liu, H.Y.; Porco, J.A., Jr. Isolation and synthesis of novel meroterpenoids from Rhodomyrtus tomentosa: Investigation of a reactive enetrione intermediate. Angew. Chem. Int. Ed. Engl. 2019, 58, 4291–4296. [Google Scholar] [CrossRef]
- Qin, X.J.; Liu, H.; Li, P.P.; Ni, W.; He, L.; Khan, A.; Hao, X.J.; Liu, H.Y. Polymethylated acylphloroglucinols from Rhodomyrtus tomentosa exert acetylcholinesterase inhibitory effects. Bioorg. Chem. 2021, 107, 104519. [Google Scholar] [CrossRef]
- Yu, M.Y.; Liu, S.N.; Liu, H.; Meng, Q.H.; Qin, X.J.; Liu, H.Y. Acylphloroglucinol trimers from Callistemon salignus seeds: Isolation, configurational assignment, hAChE inhibitory effects, and molecular docking studies. Bioorg. Chem. 2021, 117, 105404. [Google Scholar] [CrossRef]
- Luo, E.E.; Liu, S.N.; Wang, Z.J.; Chen, L.Y.; Liang, C.Q.; Yu, M.Y.; Qin, X.J. Oligomeric phloroglucinols with hAChE inhibitory and antibacterial activities from tropic Rhodomyrtus tomentosa. Bioorg. Chem. 2023, 141, 106836. [Google Scholar] [CrossRef]
- Chen, L.Y.; Luo, E.E.; Pan, Y.; Liang, C.Q.; Yu, M.Y.; Qin, X.J. Acetylcholinesterase inhibitory phloroglucinols from tropic Rhodomyrtus tomentosa. Phytochemistry 2024, 228, 114254. [Google Scholar] [CrossRef]
- Lu, S.; Zhu, N.; Guo, W.W.; Wang, X.; Li, K.J.; Yan, J.; Jiang, C.P.; Han, S.Y.; Xiang, H.M.; Wu, X.H.; et al. RNA-Seq revealed a circular RNA-microRNA-mRNA regulatory network in hantaan virus infection. Front. Cell Infect. Microbiol. 2020, 10, 97. [Google Scholar] [CrossRef]
- Kang, D.W.; Yang, J.X.; Kong, L.J.; Luo, R.H.; Huang, X.S.; Zhang, T.; Ma, M.D.; Feng, D.; Wang, Z.; Fang, H.; et al. Structure-based discovery and characterization of a preclinical drug candidate for the treatment of HIV-1 infection. Viruses 2022, 14, 2390. [Google Scholar] [CrossRef]
- Zheng, Y.G. The Pharmacodynamics Study of CCR5 Antagonists DC521042 and DC521043 Against HIV-1 In Vitro. Postgraduate. Master’s Thesis, Kunming Medical University, Kunming, China, 2019. [Google Scholar]
- Zhou, G.F. Anti-Zika Virus Activity and Mechanism Research of Compounds ZFD-10 and ZFD33A+B. Postgraduate. Master’s Thesis, Soochow University, Suzhou, China, 2022. [Google Scholar]
- Xue, J.X. The Screening and Mechanism of Anti-Dengue Virus Activity of the Indole and Pyrimidone Derivatives. Postgraduate. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2022. [Google Scholar]
- Hiranrat, A.; Mahabusarakam, W. New acylphloroglucinols from the leaves of Rhodomyrtus tomentosa. Tetrahedron 2008, 64, 11193–11197. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Li, W.; Jiang, L.; Yang, L.; Chen, N.H.; Wu, Z.N.; Li, Y.L.; Wang, G.C. Cytotoxic and anti-inflammatory active phloroglucinol derivatives from Rhodomyrtus tomentosa. Phytochemistry 2018, 153, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Hägele, S.; Müller, A.; Nusshag, C.; Reiser, J.; Zeier, M.; Krautkrämer, E. Virus- and cell type-specific effects in orthohantavirus infection. Virus Res. 2019, 260, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Jeeva, S.; Mir, S.; Velasquez, A.; Weathers, B.A.; Leka, A.; Wu, S.; Sevarany, A.T.; Mir, M. Hantavirus RdRp requires a host cell factor for cap snatching. J. Virol. 2019, 93, e02088-18. [Google Scholar] [CrossRef]
- Faisal, S.; Badshah, S.L.; Sharaf, M.; Abdalla, M. Insight into the hantaan virus RNA-dependent RNA polymerase inhibition using in-silico approaches. Mol. Divers. 2023, 27, 2505–2522. [Google Scholar] [CrossRef]
- Shehzadi, K.; Saba, A.; Yu, M.J.; Liang, J.H. Structure-based drug design of RdRp inhibitors against SARS-CoV-2. Top. Curr. Chem. 2023, 381, 22. [Google Scholar] [CrossRef]
- Jiang, H.; Du, H.; Wang, L.M.; Wang, P.Z.; Bai, X.F. Hemorrhagic fever with renal syndrome: Pathogenesis and clinical picture. Front. Cell Infect. Microbiol. 2016, 6, 1. [Google Scholar] [CrossRef]
- Wen, X.J.; Zhang, L.; Liu, Q.; Xiao, X.Y.; Huang, W.J.; Wang, Y.C. Screening and identification of HTNV(pv) entry inhibitors with high-throughput pseudovirus-based chemiluminescence. Virol. Sin. 2022, 37, 531–537. [Google Scholar] [CrossRef]
- Li, Z.P.; Wang, F.; Liu, Y.S.; Zhai, D.S.; Zhang, X.X.; Ying, Q.K.; Jia, M.; Xue, X.Y.; Meng, J.R.; Li, J.; et al. Coumarin derivative N6 as a novel anti-hantavirus infection agent targeting AKT. Front. Pharmacol. 2021, 12, 745646. [Google Scholar] [CrossRef]
- Liang, W.Z.; Li, X.S.; Wang, H.; Nie, K.C.; Meng, Q.X.; He, J.L.; Zheng, C.F. Puerarin: A potential therapeutic for SARS-CoV-2 and hantavirus co-infection. Front. Immunol. 2022, 13, 892350. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Zhao, N.B.; Cai, Y.X.; Zhang, H.; Li, J.; Liu, J.Q.; Ye, C.T.; Wang, Y.; Dang, Y.M.; Li, W.Y.; et al. An algal lectin griffithsin inhibits hantaan virus infection in vitro and in vivo. Front. Cell Infect. Microbiol. 2022, 12, 881083. [Google Scholar] [CrossRef]
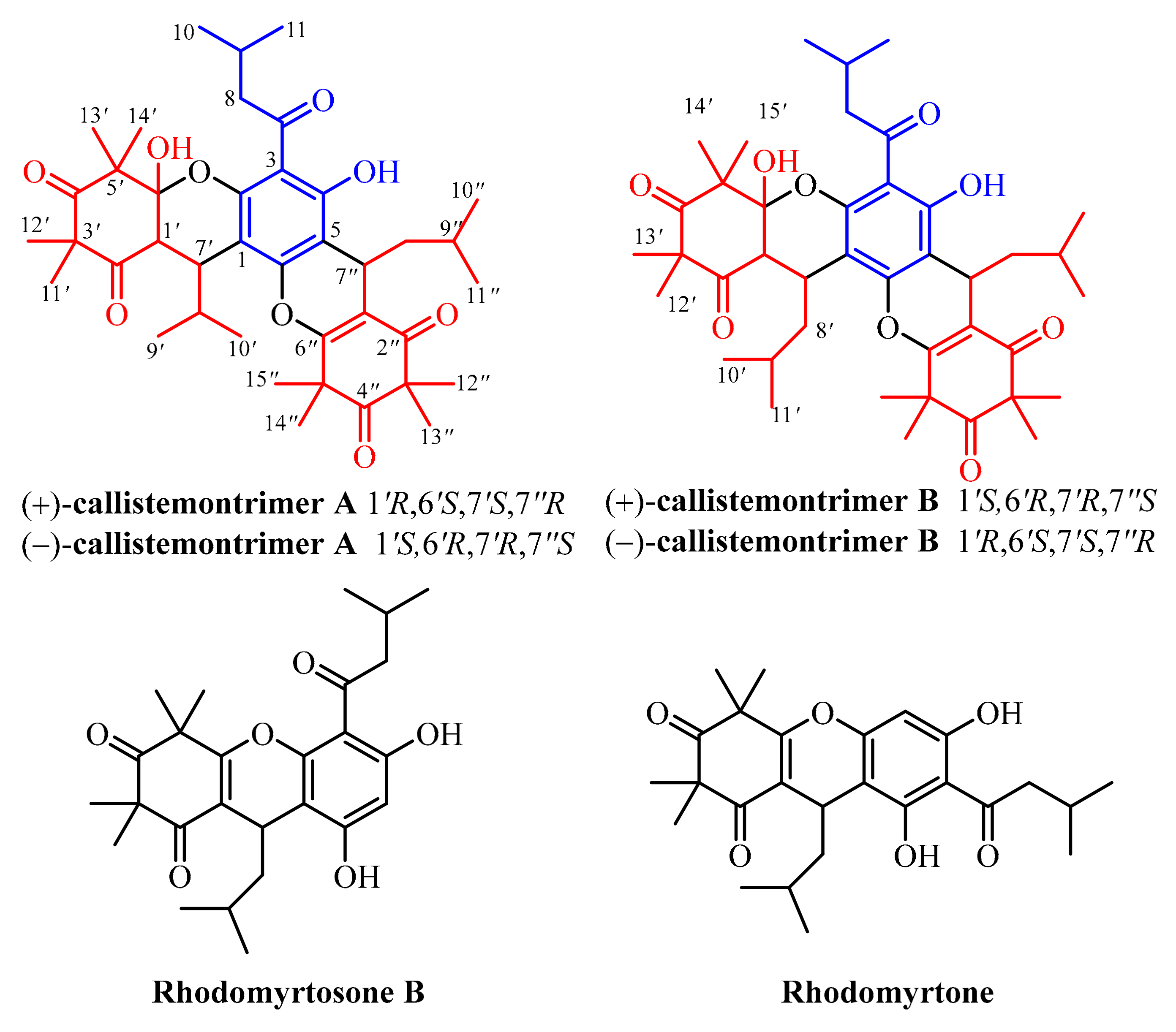
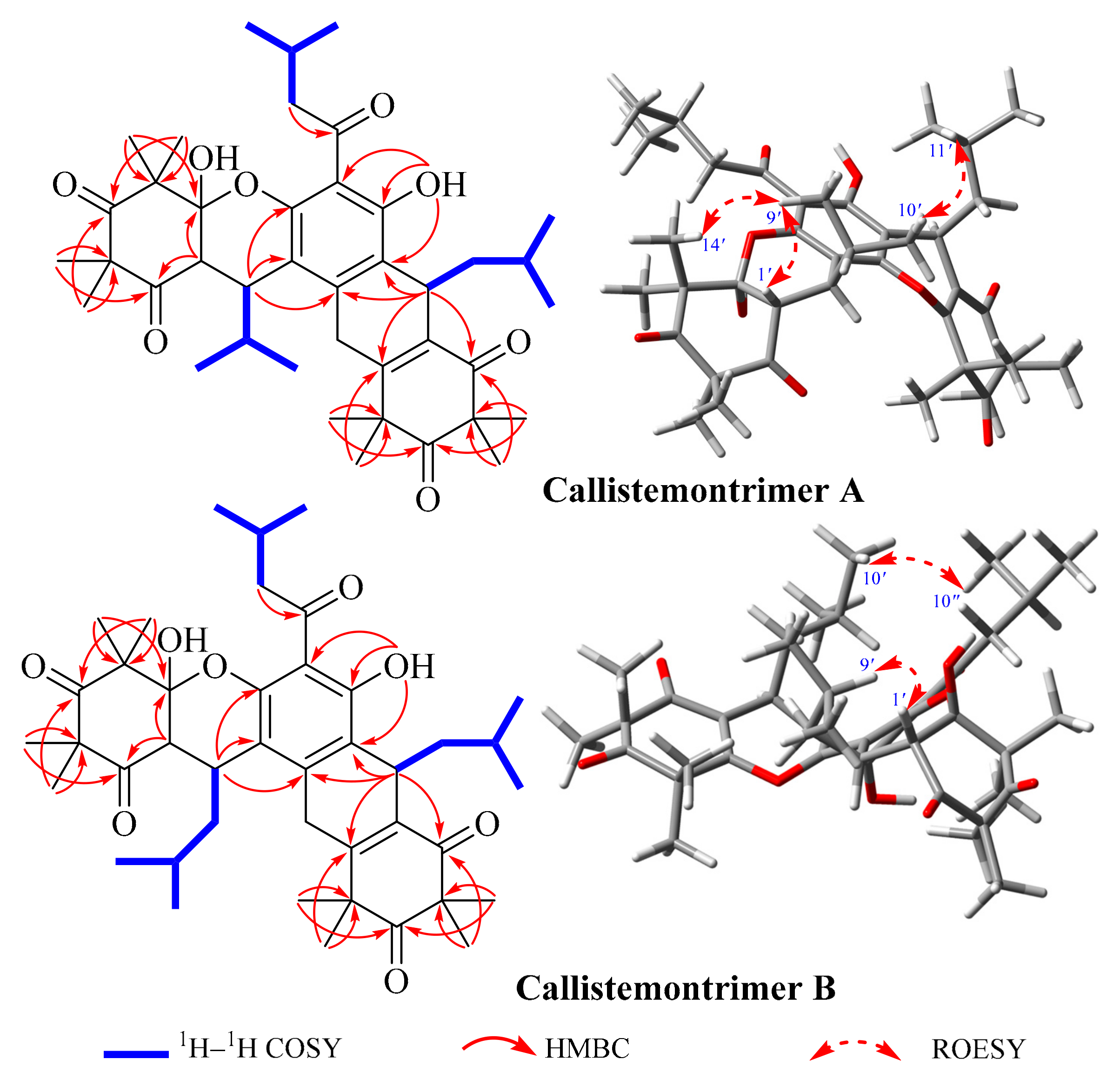
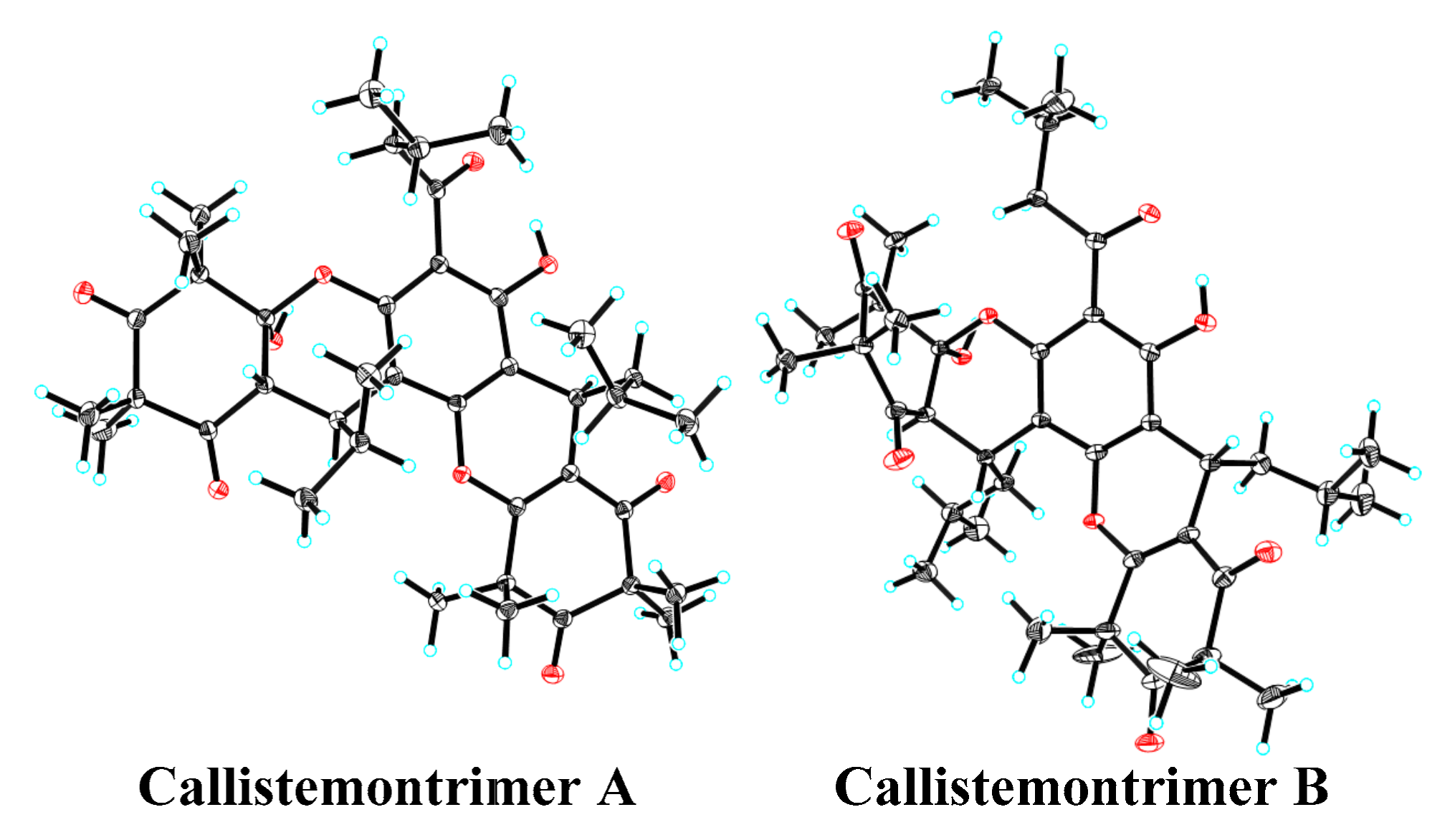
| No. | δC | δH (Mult, J in Hz) | No. | δC | δH (Mult, J in Hz) | No. | δC | δH (Mult, J in Hz) |
|---|---|---|---|---|---|---|---|---|
| 1 | 107.1 | 1′ | 45.8 | 3.66 (d, 5.9) | 1″ | 113.5 | ||
| 2 | 151.1 | 2′ | 205.1 | 2″ | 197.6 | |||
| 3 | 109.8 | 3′ | 57.8 | 3″ | 56.3 | |||
| 4 | 160.6 | 4′ | 214.0 | 4″ | 211.8 | |||
| 5 | 109.3 | 5′ | 54.9 | 5″ | 47.2 | |||
| 6 | 152.6 | 6′ | 100.2 | 6″ | 166.4 | |||
| 7 | 205.6 | 7′ | 29.0 | 4.17 (brdd, 5.9, 3.9) | 7″ | 25.7 | 4.19 (t, 6.5) | |
| 8 | 53.8 | 3.17 (dd, 16.9, 6.9) | 8′ | 32.1 | 2.35 (m) | 8″ | 44.3 | 1.58 (ddd, 18.5, 13.2, 6.5) |
| 2.80 (dd, 16.6, 6.9) | 1.41 (overlapped) | |||||||
| 9 | 24.9 | 2.26 (brheptet, 6.7) | 9′ | 15.9 | 0.68 (d, 6.9) | 9″ | 25.2 | 1.40 (overlapped) |
| 10 | 22.6 | 0.94 (d, 6.7) | 10′ | 20.2 | 0.93 (d, 6.9) | 10″ | 23.4 | 0.69 (d, 6.4) |
| 11 | 22.8 | 0.97 (d, 6.7) | 11′ | 23.8 | 0.84 (d, 6.3) | 11″ | 23.8 | 0.83 (d, 6.4) |
| OH-4 | 13.31 (s) | 12′ | 22.1 | 1.32 (s) | 12″ | 24.4 | 1.36 (s) | |
| OH-6′ | 3.42 (s) | 13′ | 26.9 | 1.42 (s) | 13″ | 24.7 | 1.39 (s) | |
| 14′ | 19.5 | 1.48 (s) | 14″ | 25.1 | 1.60 (s) | |||
| 15′ | 25.1 | 1.61 (s) | 15″ | 25.2 | 1.61 (s) |
| No. | δC | δH (Mult., J in Hz) | No. | δC | δH (Mult., J in Hz) | No. | δC | δH (Mult., J in Hz) |
|---|---|---|---|---|---|---|---|---|
| 1 | 104.3 | 1′ | 45.8 | 3.33 (brs) | 1″ | 114.6 | ||
| 2 | 150.6 | 2′ | 210.8 | 2″ | 197.3 | |||
| 3 | 108.5 | 3′ | 54.8 | 3″ | 56.2 | |||
| 4 | 161.4 | 4′ | 212.1 | 4″ | 210.6 | |||
| 5 | 109.2 | 5′ | 54.3 | 5″ | 47.2 | |||
| 6 | 153.5 | 6′ | 100.6 | 6″ | 166.7 | |||
| 7 | 205.4 | 7′ | 30.2 | 3.86 (brdd, 12.3, 4.5) | 7″ | 25.0 | 4.27 (t, 6.0) | |
| 8 | 54.0 | 2.80 (dd, 18.2, 6.3) | 8′ | 41.8 | 2.19 (td, 12.3, 3.2) | 8″ | 46.2 | 1.38 (m) |
| 2.66 (dd, 18.2, 7.1) | 1.73 (ddd, 13.5, 11.1, 4.5) | |||||||
| 9 | 23.9 | 2.27 (brheptet, 6.7) | 9′ | 25.5 | 1.87 (m) | 9″ | 25.2 | 1.44 (m) |
| 10 | 22.4 | 0.89 (d, 6.7) | 10′ | 20.8 | 1.16 (d, 6.4) | 10″ | 23.3 | 0.84 (d, 6.3) |
| 11 | 22.6 | 0.98 (d, 6.7) | 11′ | 24.0 | 1.00 (d, 6.5) | 11″ | 23.0 | 0.86 (d, 6.3) |
| OH-4 | 13.80 (s) | 12′ | 24.6 | 0.91 (s) | 12″ | 24.2 | 1.34 (s) | |
| 13′ | 26.4 | 1.34 (s) | 13″ | 24.4 | 1.39 (s) | |||
| 14′ | 22.1 | 1.37 (s) | 14″ | 24.7 | 1.51 (s) | |||
| 15′ | 15.8 | 1.54 (s) | 15″ | 24.9 | 1.62 (s) |
| Name of Compounds | EC50 (μM) | p Value | CC50 (μM) | SI |
|---|---|---|---|---|
| Callistemontrimer A | 4.41 ± 3.40 | ns | 32.09 ± 6.00 | 7.28 |
| Callistemontrimer B | 2.79 ± 1.40 | ns | 16.89 ± 1.00 | 6.05 |
| Rhodomyrtosone B | 2.69 ± 0.32 | ns | 7.44 ± 1.15 | 2.77 |
| Rhodomyrtone | 0.30 ± 0.03 | * | 5.11 ± 2.49 | 17.03 |
| Ribavirin | 20.54 ± 1.63 | - | >200 | >9.74 |
| Time-of-Addition Assay | EC50(μM) | |
|---|---|---|
| Callistemontrimer A | Ribavirin | |
| 2H pretreatment | >50 | >200 |
| Post entry | 0.65 ± 0.45 | 13.16 ± 0.40 |
| Virucidal | >50 | >200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.-X.; Luo, E.-E.; Wu, Y.-C.; Zhao, K.; Hou, W.; Yu, M.-Y.; Qin, X.-J.; Yang, X.-L. Phloroglucinol Oligomers from Callistemon rigidus as Novel Anti-Hantavirus Replication Agents. Viruses 2025, 17, 916. https://doi.org/10.3390/v17070916
Yang J-X, Luo E-E, Wu Y-C, Zhao K, Hou W, Yu M-Y, Qin X-J, Yang X-L. Phloroglucinol Oligomers from Callistemon rigidus as Novel Anti-Hantavirus Replication Agents. Viruses. 2025; 17(7):916. https://doi.org/10.3390/v17070916
Chicago/Turabian StyleYang, Jin-Xuan, E-E Luo, Yue-Chun Wu, Kai Zhao, Wei Hou, Mu-Yuan Yu, Xu-Jie Qin, and Xing-Lou Yang. 2025. "Phloroglucinol Oligomers from Callistemon rigidus as Novel Anti-Hantavirus Replication Agents" Viruses 17, no. 7: 916. https://doi.org/10.3390/v17070916
APA StyleYang, J.-X., Luo, E.-E., Wu, Y.-C., Zhao, K., Hou, W., Yu, M.-Y., Qin, X.-J., & Yang, X.-L. (2025). Phloroglucinol Oligomers from Callistemon rigidus as Novel Anti-Hantavirus Replication Agents. Viruses, 17(7), 916. https://doi.org/10.3390/v17070916






