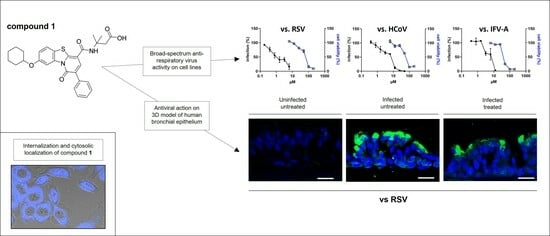
Broad-Spectrum Antiviral Activity of Pyridobenzothiazolone Analogues Against Respiratory Viruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Human-Derived Epithelia
2.2. Viruses
2.3. Chemistry
2.4. Virus Inhibition Assay
2.5. Cell Viability and Cytotoxicity Assays
2.6. Virus Inactivation Assay
2.7. Time-of-Addition Assay
2.8. Cellular Internalization via Confocal Laser Microscopy
2.9. Immunofluorescence Experiments
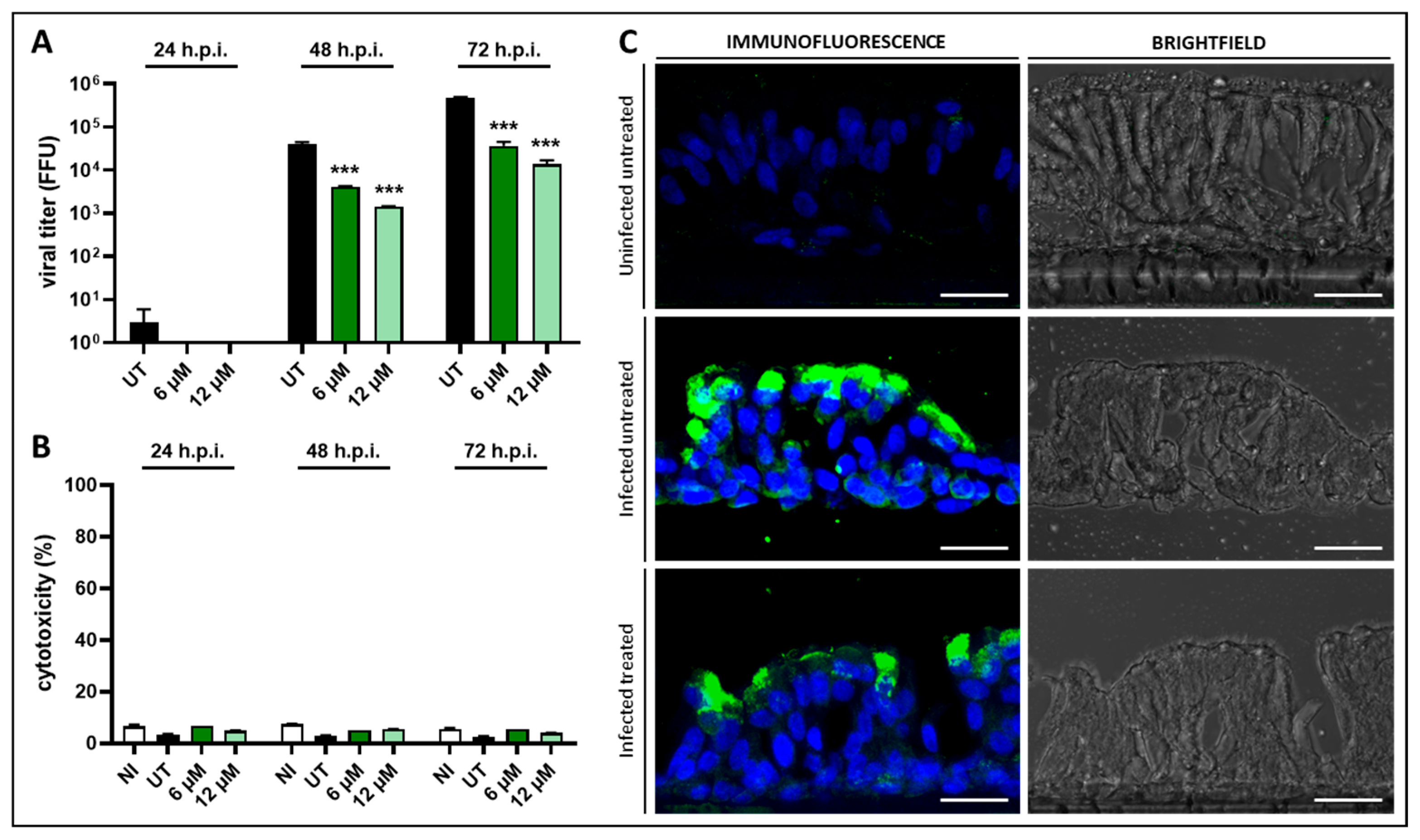
2.10. Experiments on a 3D Model of Human Bronchial Epithelium
2.11. Statistical Analyses
3. Results and Discussion
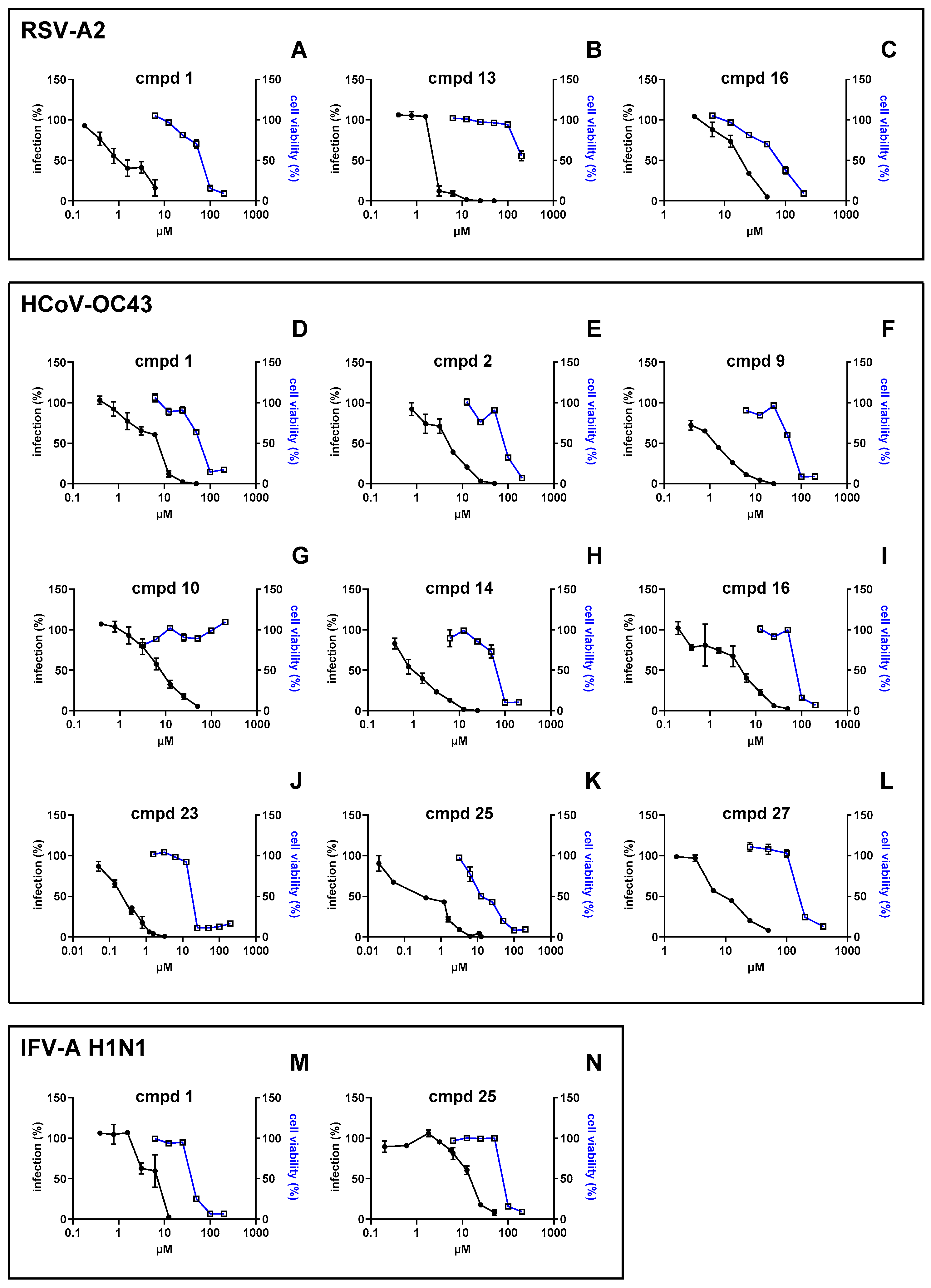
3.1. Investigation of the Antiviral Activity of a PBTZ Library Against Respiratory Viruses
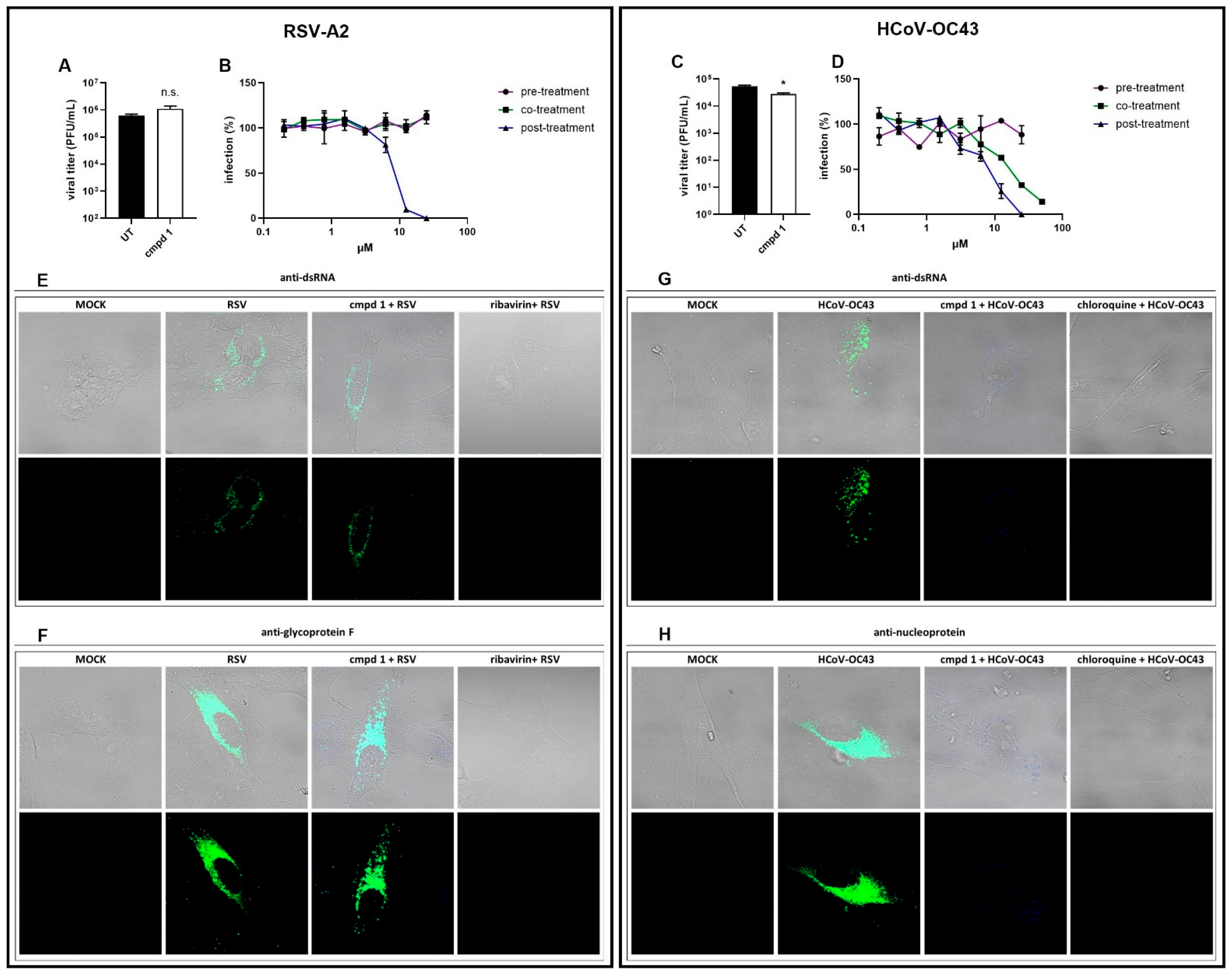
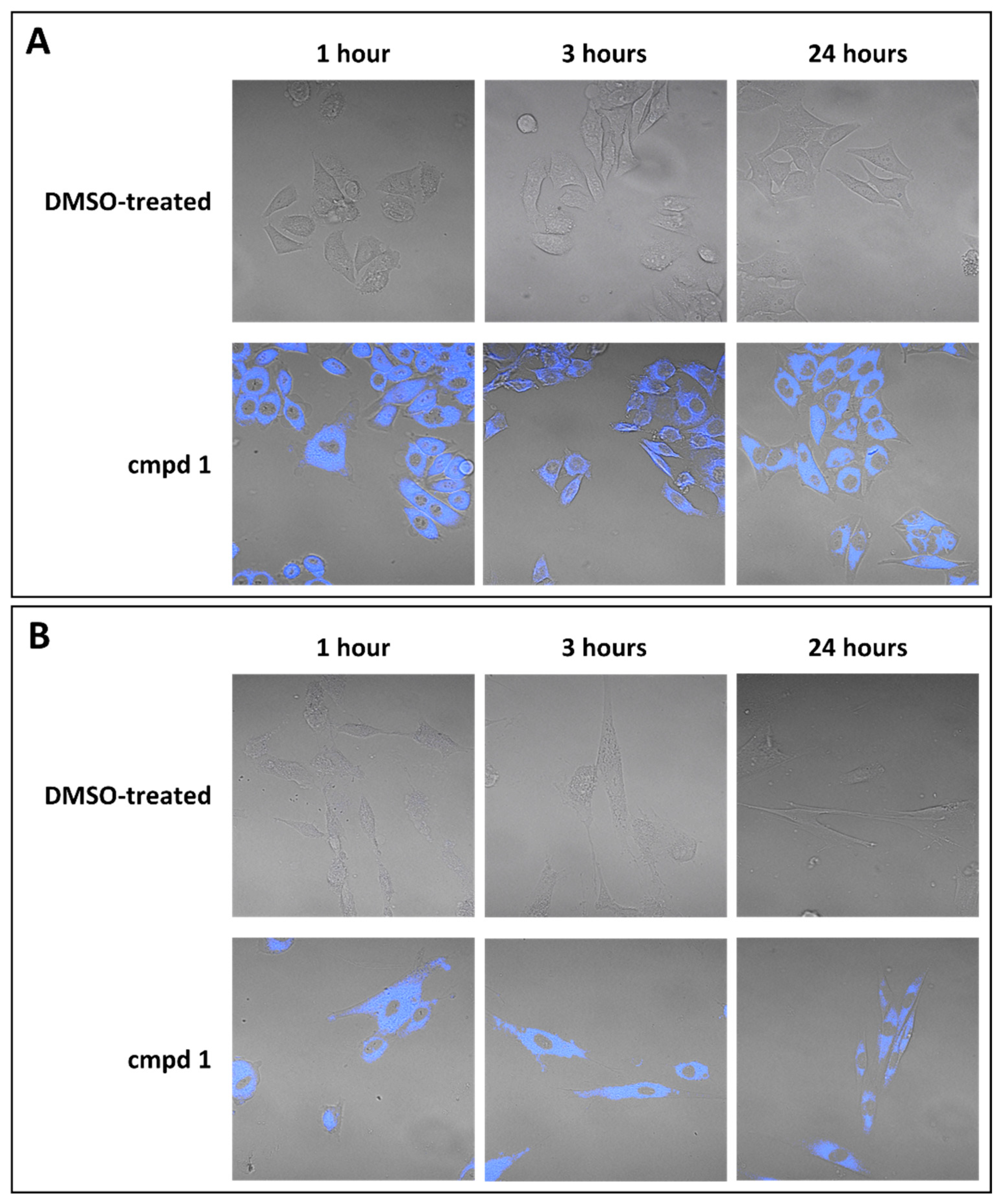
3.2. Focus on the Broad-Spectrum Antiviral Activity of Compound 1
3.3. Anti-RSV Activity of Compound 1 on a 3D Model of Human Bronchial Epithelium
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCoV | human coronavirus |
| RSV | respiratory syncytial virus |
| IFV | influenza virus |
| PBTZ | pyridobenzothiazolone |
| cmpd | compound |
| EC50 | 50% effective concentration |
| EC90 | 90% effective concentration |
| CC50 | Half-maximal cytotoxic concentration |
| SI | selectivity index |
| PFU | plaque-forming unit |
| PFU/mL | plaque-forming unit per mL |
| FFU | focus-forming unit |
| FFU/mL | focus-forming unit per mL |
References
- CDC About Respiratory Illnesses. Available online: https://www.cdc.gov/respiratory-viruses/about/index.html (accessed on 11 November 2024).
- Weston, S.; Frieman, M.B. Respiratory Viruses. In Encyclopedia of Microbiology, 4th ed.; Schmidt, T.M., Ed.; Academic Press: Oxford, UK, 2019; pp. 85–101. ISBN 978-0-12-811737-8. [Google Scholar]
- Kumari, R.; Sharma, S.D.; Kumar, A.; Ende, Z.; Mishina, M.; Wang, Y.; Falls, Z.; Samudrala, R.; Pohl, J.; Knight, P.R.; et al. Antiviral Approaches against Influenza Virus. Clin. Microbiol. Rev. 2023, 36, e00040-22. [Google Scholar] [CrossRef] [PubMed]
- Langedijk, A.C.; Bont, L.J. Respiratory Syncytial Virus Infection and Novel Interventions. Nat. Rev. Microbiol. 2023, 21, 734–749. [Google Scholar] [CrossRef] [PubMed]
- Outteridge, M.; Nunn, C.M.; Devine, K.; Patel, B.; McLean, G.R. Antivirals for Broader Coverage against Human Coronaviruses. Viruses 2024, 16, 156. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, R.J.; Aliota, M.T.; Bonnac, L.F. Broad-Spectrum Antiviral Strategies and Nucleoside Analogues. Viruses 2021, 13, 667. [Google Scholar] [CrossRef]
- Cannalire, R.; Tarantino, D.; Piorkowski, G.; Carletti, T.; Massari, S.; Felicetti, T.; Barreca, M.L.; Sabatini, S.; Tabarrini, O.; Marcello, A.; et al. Broad Spectrum Anti-Flavivirus Pyridobenzothiazolones Leading to Less Infective Virions. Antivir. Res. 2019, 167, 6–12. [Google Scholar] [CrossRef]
- Felicetti, T.; Burali, M.S.; Gwee, C.P.; Ki Chan, K.W.; Alonso, S.; Massari, S.; Sabatini, S.; Tabarrini, O.; Barreca, M.L.; Cecchetti, V.; et al. Sustainable, Three-Component, One-Pot Procedure to Obtain Active Anti-Flavivirus Agents. Eur. J. Med. Chem. 2021, 210, 112992. [Google Scholar] [CrossRef]
- Smith, S.R.; Fallan, C.; Taylor, J.E.; McLennan, R.; Daniels, D.S.B.; Morrill, L.C.; Slawin, A.M.Z.; Smith, A.D. Asymmetric Isothiourea-Catalysed Formal [3+2] Cycloadditions of Ammonium Enolates with Oxaziridines. Chemistry 2015, 21, 10530–10536. [Google Scholar] [CrossRef]
- Tarantino, D.; Cannalire, R.; Mastrangelo, E.; Croci, R.; Querat, G.; Barreca, M.L.; Bolognesi, M.; Manfroni, G.; Cecchetti, V.; Milani, M. Targeting Flavivirus RNA Dependent RNA Polymerase through a Pyridobenzothiazole Inhibitor. Antivir. Res. 2016, 134, 226–235. [Google Scholar] [CrossRef]
- Manfroni, G.; Meschini, F.; Barreca, M.L.; Leyssen, P.; Samuele, A.; Iraci, N.; Sabatini, S.; Massari, S.; Maga, G.; Neyts, J.; et al. Pyridobenzothiazole Derivatives as New Chemotype Targeting the HCV NS5B Polymerase. Bioorg. Med. Chem. 2012, 20, 866–876. [Google Scholar] [CrossRef]
- Donalisio, M.; Quaranta, P.; Chiuppesi, F.; Pistello, M.; Cagno, V.; Cavalli, R.; Volante, M.; Bugatti, A.; Rusnati, M.; Ranucci, E.; et al. The AGMA1 Poly(Amidoamine) Inhibits the Infectivity of Herpes Simplex Virus in Cell Lines, in Human Cervicovaginal Histocultures, and in Vaginally Infected Mice. Biomaterials 2016, 85, 40–53. [Google Scholar] [CrossRef]
- Arduino, I.; Francese, R.; Civra, A.; Feyles, E.; Argenziano, M.; Volante, M.; Cavalli, R.; Mougharbel, A.M.; Kortz, U.; Donalisio, M.; et al. Polyoxometalate Exerts Broad-Spectrum Activity against Human Respiratory Viruses Hampering Viral Entry. Antivir. Res. 2024, 226, 105897. [Google Scholar] [CrossRef] [PubMed]
- Francese, R.; Civra, A.; Rittà, M.; Donalisio, M.; Argenziano, M.; Cavalli, R.; Mougharbel, A.S.; Kortz, U.; Lembo, D. Anti-Zika Virus Activity of Polyoxometalates. Antivir. Res. 2019, 163, 29–33. [Google Scholar] [CrossRef]
- Cannalire, R.; Ki Chan, K.W.; Burali, M.S.; Gwee, C.P.; Wang, S.; Astolfi, A.; Massari, S.; Sabatini, S.; Tabarrini, O.; Mastrangelo, E.; et al. Pyridobenzothiazolones Exert Potent Anti-Dengue Activity by Hampering Multiple Functions of NS5 Polymerase. ACS Med. Chem. Lett. 2020, 11, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, I.; Mora-Cardenas, E.; Aloise, C.; Carletti, T.; Segat, L.; Burali, M.S.; Chiarvesio, A.; Totis, V.; Avšič–Županc, T.; Mastrangelo, E.; et al. Comprehensive Response to Usutu Virus Following First Isolation in Blood Donors in the Friuli Venezia Giulia Region of Italy: Development of Recombinant NS1-Based Serology and Sensitivity to Antiviral Drugs. PLoS Negl. Trop. Dis. 2020, 14, e0008156. [Google Scholar] [CrossRef] [PubMed]
- Milan Bonotto, R.; Bonì, F.; Milani, M.; Chaves-Sanjuan, A.; Franze, S.; Selmin, F.; Felicetti, T.; Bolognesi, M.; Konstantinidou, S.; Poggianella, M.; et al. Virucidal Activity of the Pyridobenzothiazolone Derivative HeE1-17Y against Enveloped RNA Viruses. Viruses 2022, 14, 1157. [Google Scholar] [CrossRef]
- Milani, M.; Donalisio, M.; Bonotto, R.M.; Schneider, E.; Arduino, I.; Boni, F.; Lembo, D.; Marcello, A.; Mastrangelo, E. Combined in Silico and in Vitro Approaches Identified the Antipsychotic Drug Lurasidone and the Antiviral Drug Elbasvir as SARS-CoV2 and HCoV-OC43 Inhibitors. Antivir. Res. 2021, 189, 105055. [Google Scholar] [CrossRef]
- Huang, B.; Lin, B.; Zheng, H.; Zheng, B.; Xue, X.; Liu, M. Discovery of Natural Products as Influenza Neuraminidase Inhibitors: In Silico Screening, in Vitro Validation, and Molecular Dynamic Simulation Studies. Mol. Divers. 2025. [Google Scholar] [CrossRef]
- Florio Furno, M.; Laizé, V.; Arduino, I.; Pham, G.N.; Spina, F.; Mehiri, M.; Lembo, D.; Gavaia, P.J.; Varese, G.C. Bioprospecting Marine Fungi from the Plastisphere: Osteogenic and Antiviral Activities of Fungal Extracts. Mar. Drugs 2025, 23, 115. [Google Scholar] [CrossRef]
- Rajan, A.; Piedra, F.-A.; Aideyan, L.; McBride, T.; Robertson, M.; Johnson, H.L.; Aloisio, G.M.; Henke, D.; Coarfa, C.; Stossi, F.; et al. Multiple Respiratory Syncytial Virus (RSV) Strains Infecting HEp-2 and A549 Cells Reveal Cell Line-Dependent Differences in Resistance to RSV Infection. J. Virol. 2022, 96, e01904-21. [Google Scholar] [CrossRef]
- Felicetti, T.; Gwee, C.P.; Chan, K.W.K.; Pepe, G.; Milite, C.; Campiglia, P.; Watanabe, S.; Mazlan, M.D.B.M.; Sabatini, S.; Massari, S.; et al. New Pyridobenothiazolone Derivatives Display Nanomolar Pan-Serotype Anti-Dengue Virus Activity. ChemMedChem 2025, 2500163. [Google Scholar] [CrossRef]
- Boda, B.; Benaoudia, S.; Huang, S.; Bonfante, R.; Wiszniewski, L.; Tseligka, E.D.; Tapparel, C.; Constant, S. Antiviral Drug Screening by Assessing Epithelial Functions and Innate Immune Responses in Human 3D Airway Epithelium Model. Antivir. Res. 2018, 156, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Essaidi-Laziosi, M.; Brito, F.; Benaoudia, S.; Royston, L.; Cagno, V.; Fernandes-Rocha, M.; Piuz, I.; Zdobnov, E.; Huang, S.; Constant, S.; et al. Propagation of Respiratory Viruses in Human Airway Epithelia Reveals Persistent Virus-Specific Signatures. J. Allergy Clin. Immunol. 2018, 141, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Brookes, D.W.; Coates, M.; Allen, H.; Daly, L.; Constant, S.; Huang, S.; Hows, M.; Davis, A.; Cass, L.; Ayrton, J.; et al. Late Therapeutic Intervention with a Respiratory Syncytial Virus L-Protein Polymerase Inhibitor, PC786, on Respiratory Syncytial Virus Infection in Human Airway Epithelium. Br. J. Pharmacol. 2018, 175, 2520–2534. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.T.; Cagno, V.; Janeček, M.; Ortiz, D.; Gasilova, N.; Piret, J.; Gasbarri, M.; Constant, D.A.; Han, Y.; Vuković, L.; et al. Modified Cyclodextrins as Broad-Spectrum Antivirals. Sci. Adv. 2020, 6, eaax9318. [Google Scholar] [CrossRef]
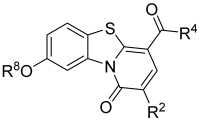 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmpd | R8 | R2 | R4 | Residual Virus Infectivity (%) | ||||||||
| RSV-A2 | HCoV-OC43 | IFV-A H1N1 | ||||||||||
| 33 µM | 3 µM | 0.3 µM | 33 µM | 3 µM | 0.3 µM | 33 µM | 3 µM | 0.3 µM | ||||
| 1 |  | 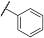 | 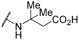 | - a | 20 | 38 | 0 | 73 | 100 | 2 | 56 | 100 |
| 2 |  | 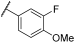 | 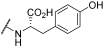 | - | 44 | 100 | 1 | 54 | 95 | 100 | 100 | 100 |
| 3 |  |  | 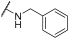 | 67 | 93 | 100 | 68 | 75 | 83 | 78 | 100 | 100 |
| 4 |  |  | 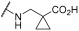 | - | 57 | 63 | - | 55 | 74 | 42 | 100 | 100 |
| 5 |  in C-7 in C-7 |  |  | - | 71 | 73 | - | 81 | 88 | 100 | 100 | 100 |
| 6 |  |  | 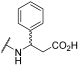 | - | - | 76 | - | 84 | 87 | 38 | 100 | 100 |
| 7 |  |  | 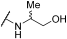 | - | - | 80 | - | 63 | 57 | - | 83 | 100 |
| 8 |  |  | 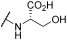 | 98 | 100 | 100 | 47 | 88 | 100 | 94 | 100 | 100 |
| 9 |  | 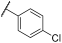 |  | - | 89 | 96 | 0 | 27 | 81 | 50 | 98 | 100 |
| 10 |  |  |  | - | 85 | 100 | 12 | 75 | 98 | 100 | 100 | 100 |
| 11 |  |  | 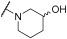 | - | - | 62 | - | 43 | 100 | - | 49 | 100 |
| 12 |  |  | 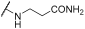 | - | 91 | 96 | 94 | 97 | 100 | 62 | 93 | 100 |
| 13 |  |  | 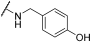 | 0 | 15 | 100 | 64 | 100 | 100 | 62 | 100 | 100 |
| 14 |  | 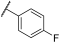 |  | - | 99 | 100 | 0 | 22 | 85 | 84 | 100 | 100 |
| 15 |  | 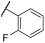 |  | 40 | 85 | 86 | 41 | 70 | 81 | 100 | 100 | 100 |
| 16 |  | 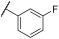 |  | 19 | 100 | 100 | 5 | 70 | 87 | 100 | 100 | 100 |
| 17 |  |  |  | - | 51 | 81 | 40 | 67 | 100 | 100 | 100 | 100 |
| 18 | 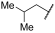 |  |  | 42 | 100 | 100 | 26 | 72 | 94 | 100 | 100 | 100 |
| 19 |  |  | 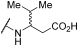 | - | 22 | 73 | - | 48 | 92 | - | 45 | 100 |
| 20 |  |  | 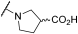 | 100 | 100 | 100 | 41 | 59 | 100 | 100 | 100 | 100 |
| 21 |  |  | 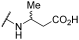 | - | 56 | 100 | - | 49 | 76 | - | 100 | 100 |
| 22 |  | 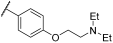 | 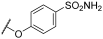 | - | 68 | 99 | 40 | 69 | - | 100 | 100 | |
| 23 |  |  |  | - | 100 | 100 | 81 | 100 | 100 | 52 | 73 | 100 |
| 24 |  | -H |  | - | 64 | 75 | - | 67 | 100 | - | 44 | 100 |
| 25 |  | 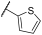 |  | - | 85 | 89 | 0 | 7 | 49 | 18 | 85 | 89 |
| 26 |  |  | 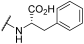 | - | 53 | 100 | - | 64 | 79 | 83 | 100 | 100 |
| 27 |  | -H |  | 100 | 100 | 100 | 17 | 92 | 100 | 81 | 99 | 100 |
| 28 |  |  |  | - | 97 | 100 | - | 0 | 32 | - | 83 | 100 |
| 29 |  | 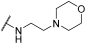 |  | - | 67 | 100 | - | 100 | 100 | - | 100 | 100 |
| Virus | Compound | EC50 a (µM) (95% CI b) | CC50 c (µM) (95% CI) | SI d |
|---|---|---|---|---|
| RSV-A2 | 1 | 1.30 (0.87–1.98) | 61.3 (52.3–71.3) | 47.2 |
| 13 | 2.87 (2.13–3.67) | >200 | >69.7 | |
| 16 | 18.6 (15.9–21.8) | 72.9 (64.2–82.8) | 3.91 | |
| ribavirin | 37.0 (24.8–52.9) | >2400 | >64.9 | |
| HCoV-OC43 | 1 | 4.83 (3.92–5.29) | 59.6 (49.5–72.0) | 12.3 |
| 2 | 4.67 (3.69–5.87) | 82.9 (75.1–98.2) | 17.8 | |
| 9 | 1.20 (1.04–1.37) | 55.6 (47.9–65.1) | 46.4 | |
| 10 | 7.97 (6.47–9.79) | >200 | >25.1 | |
| 14 | 1.09 (0.89–1.33) | 62.2 (51.7–74.2) | 57.1 | |
| 16 | 4.22 (2.82–6.21) | 84.7 (71.2–97.8) | 20.1 | |
| 23 | 0.23 (0.20–0.26) | 18.2 (12.7–23.2) | 79.1 | |
| 25 | 0.29 (0.18–0.47) | 16.5 (13.5–20.3) | 57.0 | |
| 27 | 10.0 (8.29–12.2) | 188.9 (154.2–223.0) | 18.9 | |
| chloroquine | 0.19 (0.13–0.26) | 169.3 (150.4–190.5) | 891.1 | |
| IFV-A H1N1 | 1 | 5.49 (3.85–7.83) | 41.1 (37.8–47.1) | 7.49 |
| 25 | 13.7 (11.8–15.7) | 86.1 (74.7–90.2) | 6.28 | |
| oseltamivir | 0.17 (0.04–0.42) | >200 | >1176 |
| Virus | Strain | Cell Line | EC50 a (µM) (95% CI b) | CC50 c (µM) (95% CI) | SI d |
|---|---|---|---|---|---|
| RSV | A2 | Hep-2 | 1.30 (0.87–1.98) | 61.3 (52.3–71.3) | 47.2 |
| A549 | 7.78 (6.92–9.10) | 30.8 (27.6–34.4) | 3.96 | ||
| B WV/14617/85 | Hep-2 | 4.23 (3.19–5.63) | 61.3 (52.3–71.3) | 14.5 | |
| HCoV | OC43 | MRC-5 | 4.83 (3.92–5.29) | 59.6 (49.5–72.0) | 12.3 |
| A549 | 8.14 (7.22–9.34) | 150.2 (142.0–158.9) | 18.5 | ||
| 229E | MRC-5 | 45.3 (37.3–49.2) | 59.6 (49.5–72.0) | 1.32 | |
| IFV | A-H1N1 | MDCK | 5.49 (3.85–7.83) | 41.1 (37.8–47.1) | 7.49 |
| A549 | 6.29 (4.50–8.42) | 57.6 (51.5–64.6) | 9.16 | ||
| A-H3N2 | MDCK | 5.96 (4.66–7.82) | 41.1 (37.8–47.1) | 6.90 | |
| B | MDCK | 4.21 (3.40–5.57) | 41.1 (37.8–47.1) | 9.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feyles, E.; Felicetti, T.; Arduino, I.; Rittà, M.; Civra, A.; Muratori, L.; Raimondo, S.; Lembo, D.; Manfroni, G.; Donalisio, M. Broad-Spectrum Antiviral Activity of Pyridobenzothiazolone Analogues Against Respiratory Viruses. Viruses 2025, 17, 890. https://doi.org/10.3390/v17070890
Feyles E, Felicetti T, Arduino I, Rittà M, Civra A, Muratori L, Raimondo S, Lembo D, Manfroni G, Donalisio M. Broad-Spectrum Antiviral Activity of Pyridobenzothiazolone Analogues Against Respiratory Viruses. Viruses. 2025; 17(7):890. https://doi.org/10.3390/v17070890
Chicago/Turabian StyleFeyles, Elisa, Tommaso Felicetti, Irene Arduino, Massimo Rittà, Andrea Civra, Luisa Muratori, Stefania Raimondo, David Lembo, Giuseppe Manfroni, and Manuela Donalisio. 2025. "Broad-Spectrum Antiviral Activity of Pyridobenzothiazolone Analogues Against Respiratory Viruses" Viruses 17, no. 7: 890. https://doi.org/10.3390/v17070890
APA StyleFeyles, E., Felicetti, T., Arduino, I., Rittà, M., Civra, A., Muratori, L., Raimondo, S., Lembo, D., Manfroni, G., & Donalisio, M. (2025). Broad-Spectrum Antiviral Activity of Pyridobenzothiazolone Analogues Against Respiratory Viruses. Viruses, 17(7), 890. https://doi.org/10.3390/v17070890