First Molecular Evidence of Equine Herpesvirus Type 1 (EHV-1) in Ocular Swabs of Clinically Affected Horses
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Description of Critical Methods
2.2.1. Sample Collection
2.2.2. DNA Isolation and Molecular Analysis
2.2.3. Statistical Analysis
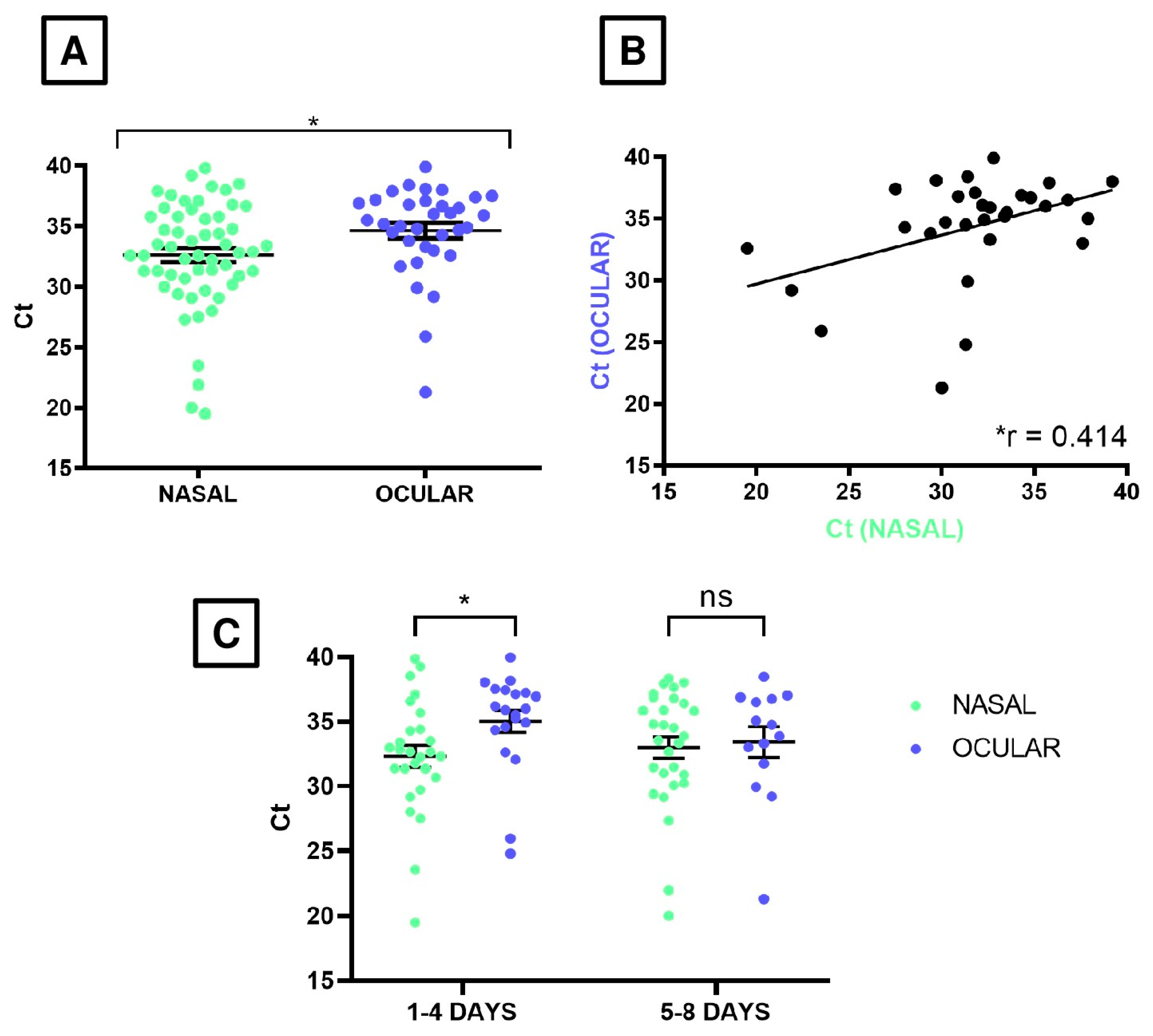
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EHV-1 | Equine Herpesvirus Type 1 |
| EHM | Equine herpesvirus myeloencephalopathy |
| RT-PCR | Real-time polymerase chain reaction |
| HCV-CEU | Veterinary Clinical Hospital UCH-CEU |
| FEI | International Equestrian Federation |
| Ct | Cycle Threshold |
| gB | Glycoprotein B |
| WOAH | World Organization for Animal Health |
References
- Pusterla, N.; Lawton, K.; Barnum, S.; Ross, K.; Purcell, K. Investigation of an Outbreak of Equine Herpesvirus-1 Myeloencephalopathy in a Population of Aged Working Equids. Viruses 2024, 16, 1693. [Google Scholar] [CrossRef] [PubMed]
- Couroucé, A.; Normand, C.; Tessier, C.; Pomares, R.; Thévenot, J.; Marcillaud-Pitel, C.; Legrand, L.; Pitel, P.-H.; Pronost, S.; Lupo, C. Equine Herpesvirus-1 Outbreak During a Show-Jumping Competition: A Clinical and Epidemiological Study. J. Equine Vet. Sci. 2023, 128, 104869. [Google Scholar] [CrossRef]
- Davison, A.J.; Eberle, R.; Ehlers, B.; Hayward, G.S.; McGeoch, D.J.; Minson, A.C.; Pellett, P.E.; Roizman, B.; Studdert, M.J.; Thiry, E. The order Herpesvirales. Arch. Virol. 2009, 154, 171–177. [Google Scholar] [CrossRef]
- Laval, K.; Poelaert, K.C.K.; Van Cleemput, J.; Zhao, J.; Vandekerckhove, A.P.; Gryspeerdt, A.C.; Garré, B.; van der Meulen, K.; Baghi, H.B.; Dubale, H.N.; et al. The Pathogenesis and Immune Evasive Mechanisms of Equine Herpesvirus Type 1. Front. Microbiol. 2021, 12, 662686. [Google Scholar] [CrossRef] [PubMed]
- Roizmann, B.; Desrosiers, R.C.; Fleckenstein, B.; Lopez, C.; Minson, A.C.; Studdert, M.J. The Family Herpesviridae: An Update. Arch. Virol. 1992, 123, 425–449. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Hussey, G.S. Equine Herpesvirus 1 Myeloencephalopathy. Vet. Clin. N. Am. Equine Pract. 2014, 30, 489–506. [Google Scholar] [CrossRef]
- Hussey, G.S. Key Determinants in the Pathogenesis of Equine Herpesvirus 1 and 4 Infections. Vet. Pathol. 2019, 56, 656–659. [Google Scholar] [CrossRef]
- Khusro, A.; Aarti, C.; Rivas-Caceres, R.R.; Barbabosa-Pliego, A. Equine Herpesvirus-I Infection in Horses: Recent Updates on Its Pathogenicity, Vaccination, and Preventive Management Strategies. J. Equine Vet. Sci. 2020, 87, 102923. [Google Scholar] [CrossRef]
- Allen, G.P.; Kydd, J.H.; Slater, J.D.; Smith, K.C. Equid Herpesvirus-1 (EHV-1) and -4 (EHV-4) Infections. In Infectious Diseases of Livestock, 2nd ed.; Coetzer, J.A.W., Tustin, R.C., Eds.; Oxford Press: Cape Town, South Africa, 2004; pp. 829–859. [Google Scholar]
- Gonzalez-Medina, S.; Newton, J.R. Equine Herpesvirus-1: Dealing Practically but Effectively with an Ever Present Threat. Equine Vet. J. 2015, 47, 142–144. [Google Scholar] [CrossRef]
- Del Piero, F.; Wilkins, P.A. Pulmonary vasculotropic EHV-1 infection in equids. Vet. Pathol. 2001, 38, 474. [Google Scholar] [CrossRef]
- Hussey, G.S.; Goehring, L.S.; Lunn, D.P.; Hussey, S.B.; Huang, T.; Osterrieder, N.; Powell, C.; Hand, J.; Holz, C.; Slater, J. Experimental infection with equine herpesvirus type 1 (EHV-1) induces chorioretinal lesions. Vet. Res. 2013, 44, 118. [Google Scholar] [CrossRef] [PubMed]
- McCartan, C.G.; Russel, M.M.; Wood, J.L.N.; Mumford, J.A. Clinical, serological and virological characteristics of an outbreak of paresis and neonatal foal disease due to equine herpesvirus-1 on a stud farm. Vet. Rec. 1995, 136, 7–12. [Google Scholar] [CrossRef] [PubMed]
- de la Cuesta-Torrado, M.; Velloso Alvarez, A.; Neira-Egea, P.; Cuervo-Arango, J. Long-term performance of show-jumping horses and relationship with severity of ataxia and complications associated with myeloencephalopathy caused by equine herpesvirus-1. J. Vet. Intern. Med. 2024, 38, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.R.; Heldens, J. Equine Herpesviruses 1 (EHV-1) and 4 (EHV-4)—Epidemiology, Disease and Immunoprophylaxis: A Brief Review. Vet. J. 2005, 170, 14–23. [Google Scholar] [CrossRef]
- Velloso-Alvarez, A.; Jose-Cunilleras, E.; Dorrego-Rodriguez, A.; Santiago-Llorente, I.; de la Cuesta-Torrado, M.; Troya-Portillo, L.; Rivera, B.; Vitale, V.; de Juan, L.; Cruz-Lopez, F. Detection of equine herpesvirus-1 (EHV-1) in urine samples during outbreaks of equine herpesvirus myeloencephalopathy. Equine Vet. J. 2024, 56, 456–463. [Google Scholar] [CrossRef]
- Hussey, S.B.; Clark, R.; Lunn, K.F.; Breathnach, C.; Soboll, G.; Whalley, J.M.; Lunn, D.P. Detection and Quantification of Equine Herpesvirus-1 Viremia and Nasal Shedding by Real-Time Polymerase Chain Reaction. J. Vet. Diagn. Investig. 2006, 18, 335–342. [Google Scholar] [CrossRef]
- Pusterla, N.; Barnum, S.; Young, A.; Mendonsa, E.; Lee, S.; Hankin, S.; Brittner, S.; Finno, C.J. Molecular Monitoring of EHV-1 in Silently Infected Performance Horses through Nasal and Environmental Sample Testing. Pathogens 2022, 11, 720. [Google Scholar] [CrossRef]
- Paillot, R.; Prowse, L.; Montesso, F.; Stewart, B.; Jordon, L.; Newton, J.R. Duration of equine influenza virus shedding and infectivity in immunised horses after experimental infection with EIV A/eq2/Richmond/1/07. Vet. Microbiol. 2013, 166, 22–34. [Google Scholar] [CrossRef]
- Vandenberghe, E.; Boshuizen, B.; Delesalle, C.J.G.; Goehring, L.S.; Groome, K.A.; van Maanen, K.; de Bruijn, C.M. New insights into the management of an EHV-1 (Equine Hospital) outbreak. Viruses 2021, 13, 1429. [Google Scholar] [CrossRef]
- Pusterla, N.; Wilson, W.D.; Mapes, S.; Finno, C.; Isbell, D.; Arthur, R.M.; Ferraro, G.L. Characterization of viral loads, strain and state of equine herpesvirus-1 using real-time PCR in horses following natural exposure at a racetrack in California. Vet. J. 2009, 179, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Mapes, S.; Wilson, W.D. Use of viral loads in blood and nasopharyngeal secretions for the diagnosis of EHV-1 infection in field cases. Vet. Rec. 2008, 162, 728–729. [Google Scholar] [CrossRef]
- Pusterla, N.; Mapes, S.; Wademan, C.; White, A.; Estell, K.; Swain, E. Investigation of the Role of Mules as Silent Shedders of EHV-1 during an Outbreak of EHV-1 Myeloencephalopathy in California. Vet. Rec. 2012, 170, 465. [Google Scholar] [CrossRef] [PubMed]
- Sonis, J.M.; Goehring, L.S. Nasal Shedding of Equid Herpesvirus Type 1 and Type 4 in Hospitalized, Febrile Horses. J. Equine Vet. Sci. 2013, 33, 756–759. [Google Scholar] [CrossRef]
- Wilcox, A.; Barnum, S.; Wademan, C.; Corbin, R.; Escobar, E.; Hodzic, E.; Schumacher, S.; Pusterla, N. Frequency of Detection of Respiratory Pathogens in Clinically Healthy Show Horses Following a Multi-County Outbreak of Equine Herpesvirus-1 Myeloencephalopathy in California. Pathogens 2022, 11, 1161. [Google Scholar] [CrossRef]
- Pusterla, N.; Barnum, S.; Miller, J.; Varnell, S.; Dallap-Schaer, B.; Aceto, H.; Simeone, A. Investigation of an EHV-1 Outbreak in the United States Caused by a New H752 Genotype. Pathogens 2021, 10, 747. [Google Scholar] [CrossRef] [PubMed]
- Price, D.; Barnum, S.; Mize, J.; Pusterla, N. Investigation of the Use of Non-Invasive Samples for the Molecular Detection of EHV-1 in Horses with and without Clinical Infection. Pathogens 2022, 11, 574. [Google Scholar] [CrossRef]
- Mayhew, I.G.; Graham, M.; Waller, H. Congenital occipitoatlantoaxial malformations in the horse. Vet. Rec. 1978, 103, 125–128. [Google Scholar] [CrossRef]
- Vereecke, N.; Carnet, F.; Pronost, S.; Vanschandevijl, K.; Theuns, S.; Nauwynck, H. Genome Sequences of Equine Herpesvirus 1 Strains from a European Outbreak of Neurological Disorders Linked to a Horse Gathering in Valencia, Spain, in 2021. Microbiol. Resour. Announc. 2021, 10, e00333-21. [Google Scholar] [CrossRef]
- Graham, D.A. Bovine herpes virus-1 (BoHV-1) in cattle-a review with emphasis on reproductive impacts and the emergence of infection in Ireland and the United Kingdom. Ir. Vet. J. 2013, 66, 15. [Google Scholar] [CrossRef]
- Lee, Y.; Maes, R.K.; Kruger, J.M.; Kiupel, M.; Giessler, K.S.; Soboll Hussey, G. Safety and Efficacy of Felid Herpesvirus-1 Deletion Mutants in Cats. Viruses 2021, 13, 163. [Google Scholar] [CrossRef]
- Ledbetter, E.C.; da Silva, E.C.; Kim, S.G.; Dubovi, E.J.; Schwark, W.S. Frequency of spontaneous canine herpesvirus-1 reactivation and ocular viral shedding in latently infected dogs and canine herpesvirus-1 reactivation and ocular viral shedding induced by topical administration of cyclosporine and systemic administration of corticosteroids. Am. J. Vet. Res. 2012, 73, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Goehring, L.S.; van Maanen, C.; Berendsen, M.; Cullinane, A.; de Groot, R.J.; Rottier, P.J.M.; Wesselingh, J.J.C.M.; van Oldruitenborh-Oosterbaan, M.M.S. Experimental infection with neuropathogenic equid herpesvirus type 1 (EHV-1) in adult horses. Vet. J. 2010, 186, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Petano-Duque, J.M.; Urueña-Martinez, E.; Cabezas-Callejas, L.L.; Perilla-Amaya, J.; Rueda-García, V.; Rondón-Barragán, I.S.; Lopera-Vásquez, R. Molecular and serological investigation of equine herpesvirus type 1 (EHV-1) and type 4 (EHV-4) in horses in Ibagué, Tolima. Vet. Med. Int. 2025, 2025, 1661949. [Google Scholar] [CrossRef]
- Seeber, P.A.; Dayaram, A.; Sicks, F.; Osterrieder, N.; Franz, M.; Greenwood, A.D. Noninvasive Detection of Equid Herpesviruses in Fecal Samples. Appl. Environ. Microbiol. 2019, 85, e02234-18. [Google Scholar] [CrossRef] [PubMed]
| ID Horse | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NS | OS | NS | OS | NS | OS | NS | OS | NS | OS | NS | OS | NS | OS | NS | OS | |
| 1 | NC | NC | NC | NC | - | - | 36.5 | - | - | - | 33.8 | - | 35.8 | - | - | - |
| 2 | NC | NC | NC | NC | - | - | 34.4 | - | 36.7 | - | 38.3 | - | - | - | - | - |
| 3 | 31.3 | 34.5 | 32.2 | 36.1 | NC | - | 32.9 | - | 29.1 | - | 33.3 | - | 30.2 | 34.7 | 30.9 | 36.8 |
| 4 | 33.5 | 35.5 | 32.8 | 39.9 | 38.5 | - | 33.4 | 35.2 | 35.8 | 37.9 | 31.4 | 29.9 | 37.6 | 33 | 29.4 | 33.8 |
| 5 | 28.0 | 34.3 | 19.5 | 32.6 | 23.5 | 25.9 | 32.6 | - | 31.0 | - | 20.0 | NC | 37.9 | 35 | 27.3 | - |
| 6 | 29.1 | - | NC | 37.2 | NC | 32 | 31.3 | NC | 21.9 | 29.2 | 30.0 | 21.3 | 34.8 | 36.7 | 32.6 | 33.3 |
| 7 | 32.3 | 34.9 | 34.3 | 36.9 | 35.6 | 36 | NC | NC | 31.4 | 38.4 | - | - | - | - | 37.1 | NC |
| 8 | 39.2 | 38.0 | 32.6 | 35.9 | 27.5 | 37.4 | 39.8 | - | - | 31.7 | 36.8 | 36.5 | 35.8 | - | - | NC |
| 9 | - | - | - | - | 37.1 | NC | 31.3 | 24.8 | - | - | 38 | NC | 34.5 | NC | 34.7 | NC |
| 10 | 29.7 | 38.1 | 31.8 | 37.1 | - | 37.5 | 30.7 | - | 36.4 | NC | 33.5 | NC | - | NC | NC | NC |
| Day After the Onset of Disease (Stage of Infection) | qPCR Results Nasal Swab Samples | qPCR Results Ocular Swab Samples | ||
|---|---|---|---|---|
| Pos/Total (%) | Ct Range (Mean) | Pos/Total (%) | Ct Range (Mean) | |
| 1–8 days (Whole surveillance period) | 56/69 (81.16%) | 19.5–39.8 (32.63) | 34/63 (53.97%) | 21.3–39.9 (34.65) |
| 1–4 days (Early stage of infection) | 27/33 (81.81%) | 19.5–39.8 (32.31) | 20/33 (60.60%) | 24.8–39.9 (35.03) |
| 5–8 days (Advanced stage of infection) | 29/36 (80.56%) | 20.0–38.3 (33.01) | 14/30 (46.66%) | 21.3–38.4 (33.42) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musoles-Cuenca, B.; Padilla-Blanco, M.; Vitale, V.; Lorenzo-Bermejo, T.; de la Cuesta-Torrado, M.; Ballester, B.; Maiques, E.; Rubio-Guerri, C.; Velloso Alvarez, A. First Molecular Evidence of Equine Herpesvirus Type 1 (EHV-1) in Ocular Swabs of Clinically Affected Horses. Viruses 2025, 17, 862. https://doi.org/10.3390/v17060862
Musoles-Cuenca B, Padilla-Blanco M, Vitale V, Lorenzo-Bermejo T, de la Cuesta-Torrado M, Ballester B, Maiques E, Rubio-Guerri C, Velloso Alvarez A. First Molecular Evidence of Equine Herpesvirus Type 1 (EHV-1) in Ocular Swabs of Clinically Affected Horses. Viruses. 2025; 17(6):862. https://doi.org/10.3390/v17060862
Chicago/Turabian StyleMusoles-Cuenca, Beatriz, Miguel Padilla-Blanco, Valentina Vitale, Teresa Lorenzo-Bermejo, María de la Cuesta-Torrado, Beatriz Ballester, Elisa Maiques, Consuelo Rubio-Guerri, and Ana Velloso Alvarez. 2025. "First Molecular Evidence of Equine Herpesvirus Type 1 (EHV-1) in Ocular Swabs of Clinically Affected Horses" Viruses 17, no. 6: 862. https://doi.org/10.3390/v17060862
APA StyleMusoles-Cuenca, B., Padilla-Blanco, M., Vitale, V., Lorenzo-Bermejo, T., de la Cuesta-Torrado, M., Ballester, B., Maiques, E., Rubio-Guerri, C., & Velloso Alvarez, A. (2025). First Molecular Evidence of Equine Herpesvirus Type 1 (EHV-1) in Ocular Swabs of Clinically Affected Horses. Viruses, 17(6), 862. https://doi.org/10.3390/v17060862






